Summary
Chronic insomnia is a significant public health problem worldwide, and insomnia has considerable personal and social costs associated with serious health conditions, greater healthcare utilization, work absenteeism, and motor-vehicle accidents. Cognitive Behavioral Therapy for Insomnia (CBTI) is an efficacious treatment, yet attrition and suboptimal adherence may diminish its impact. Despite the increasing use of CBTI, surprisingly little attention has been devoted to understanding the role of adherence. This review describes a comprehensive literature search of adherence to CBTI. The search revealed 15 studies that evaluated adherence to CBTI in adults using valid and reliable measures of sleep, and measure of adherence other than study withdrawals. The primary purposes of this review were to (1) synthesize current study characteristics, methodology, adherence rates, contributing factors, and impact on outcomes, (2) discuss measurement issues, and (3) identify future practice and research directions that may lead to improved outcomes. Strong patterns and inconsistencies were identified among the studies, which complicate an evaluation of the role of adherence as a factor and outcome of CBTI success. The importance of standardized adherence and outcome measures is discussed. In light of the importance of adherence to behavior change, this systematic review may better inform future intervention efforts.
Keywords: adherence, cognitive behavioral therapy, insomnia, behavioral intervention
Introduction
Chronic insomnia is a public health crisis affecting approximately 10–15% of adults worldwide 1–3. In addition to the social and financial toll 4, insomnia is associated with serious health conditions 5, greater healthcare utilization 6, work absenteeism 7, and motor-vehicle accidents 8. With accumulating evidence of the effectiveness of Cognitive Behavioral Therapy for Insomnia (CBTI) 3, 9–11, adherence to this treatment is a fundamental public health, clinical, and scientific concern.
Little is known about adherence rates and factors related to patients’ adherence to CBTI. The lack of attention to adherence to CBTI stands in stark contrast to adherence to treatments for other sleep disorders 12 and other medical conditions, which have been the focus of theoretical and empirical research for decades13.The term compliance, used extensively in the past, has drawn criticism for its emphasis on medical authority and implication that patients are passive recipients of care. In response, the term “adherence” was introduced to call attention to the importance of patient agreement with medical recommendations 14. Adherence is generally referred to as the extent to which a person’s behavior coincides with medical or health advice 15, 16. For the purpose of this paper, adherence is defined as persistence in the practice and maintenance of desired health behaviors, and is the result of patient’s active participation in and agreement with treatment recommendations 17–19.
Insomnia, characterized by difficulty initiating, maintaining, or obtaining good quality sleep, often occurs in the presence of a medical or psychiatric condition (comorbid insomnia) or may stand alone as a single condition (primary insomnia) 1. Growing evidence supports the effectiveness of CBTI for the management of both primary and comorbid insomnia without the associated habituation, and cognitive and psychomotor impairments of pharmacotherapy 11, 20. CBTI, a multi-component intervention, most often features sleep restriction (SR), stimulus control (SC), sleep hygiene education (SHE), cognitive therapy (CT), and can include relaxation techniques. The specifics of these interventions are discussed in detail elsewhere 9, 21, 22. Based on substantial evidence primarily from randomized controlled trials (RCT), the American Academy of Sleep Medicine has established that combined SR, SC, relaxation training, and CT are efficacious therapies for chronic insomnia 10. Furthermore, CBTI, with and without relaxation therapy, is superior to pharmacological therapy in maintaining treatment gains beyond the completion of treatment 20, 23, 24. RCTs support the efficacy of CBTI under ideal controlled conditions, however, the effectiveness of CBTI in real world situations is less certain.
Adherence to medical treatment is a challenging problem for many clinicians, including behavioral therapists 25. Overall, adherence among patients with chronic conditions is disappointingly low compared to patients with acute medical problems, particularly after several months of therapy. It is not surprising that adherence is suboptimal to treatment for chronic sleep disorders. In a meta-analysis of 569 studies examining adherence to common medical treatments, the average adherence rate was 75% and the mean adherence to sleep-related treatments (65.5%) was the lowest of the disorders studied 25. Most studies reflect poor adherence to positive airway pressure (PAP) treatment for sleep apnea, but also include studies of adherence to behavioral treatment for insomnia. Poor adherence to sleep-related treatments may be due to barriers specific to PAP or to a low relative importance assigned to managing sleep problems in general.
In contrast to the PAP literature, CBTI studies rarely include adherence information. If included, it is often limited to average sessions attended or overall study attrition. Available estimates of withdrawal during CBTI suggest that 14–40% of study participants drop out of individual or group treatment before mid-treatment 26, thus potentially diminishing the opportunity for improvement in insomnia. Drop out rates for internet-based treatment fall within the same range as group or individual CBTI. For example, 17% of adults with chronic insomnia (n = 94) dropped out of weekly internet modules 27. In another study, 33% withdrew before the end of 5 weekly CBTI online modules 28.Research conducted in natural clinical settings may have even higher dropout rates 29, 30 compared with RCTs, which typically have more rigorous monitoring and homogenous participant samples 31, 32.
Examining the proportion of individuals who discontinue CBTI allows researchers to understand the characteristics of this group compared to treatment completers. Much can also be gained, however, by considering adherence beyond attrition rates, both in terms of factors that shape adherent behavior, and in refining CBTI to reflect the most effective treatment in a range of vulnerable subgroups. To date, previous reviews of CBTI have inadequately addressed the issue of adherence. The goal of this systematic review is to analyze the best available scientific evidence related to CBTI adherence and to identify gaps in the literature. Specifically, we will (1) describe current study characteristics, methodology, adherence rates, contributing factors, and impact on outcomes, (2) discuss measurement issues, and (3) identify future practice and research directions that may lead to improved outcomes.
Methods
Search methods and study selection
A systematic review of empirical literature having to do with adherence to CBTI was conducted with assistance from a trained health librarian. Databases included PubMed, Psychinfo, and MEDLINE. Search terms included “sleep disorders”, “insomnia”, “cognitive behavioral therapy”, “sleep restriction”, “stimulus control”, “sleep hygiene”, “sleep education”, “relaxation”, “cognitive therapy”, “adherence”, and “compliance” in all applicable combinations. The initial search was inclusive of all published articles written, regardless of date, to ensure a more comprehensive result. All CBTI studies that evaluated adherence as a primary or secondary outcome qualified for inclusion. Publications that met the following criteria were included in the final review: (a) study sample of adults with insomnia, (b) interventions comprised of any cognitive behavioral therapy components aimed at treating insomnia, (c) valid and reliable measures of sleep, and measure of adherence other than study withdrawals, (d) written in English, (e) published in peer reviewed journals. Case studies, opinions and editorials were not considered. Studies were evaluated for methodological and reporting quality using the Scottish Intercollegiate Guidelines Network (SIGN) checklists for controlled trials and cohort studies33. The checklist items were selected because they can be used in different study types to assess the study methodology and potential bias with respect to study question, participant selection, comparability of groups, clarity of outcomes, and statistical analysis, among other criteria 33.
The titles and abstracts of retrieved studies were independently reviewed by two authors (EEM and MSM) against the inclusion and exclusion criteria. The full text article was consulted if there were any aspects of the abstracts that were unclear and for the final selection of articles. A third author (MSA) arbitrated if there were disagreements between the initial reviewers; all authors reviewed the selected studies. Studies that did not explicitly report adherence data were excluded.
Evaluation of study characteristics, CBTI, and measures
Studies that met criteria were evaluated for sample characteristics, CBTI features, design, and measurement. CBTI was evaluated based on several components of the intervention, including whether CBTI was standardized or tailored, the mode of delivery (e.g., individual, group, internet modules), as well as the type of provider (e.g., nurse, psychologist). The dose (i.e., number of hours exposed to the intervention), duration (i.e., length of CBTI delivery in weeks) and presence of homework were also assessed. Study design, type of control group, theoretical underpinnings, and potential adherence factors were evaluated. We assessed the type and comprehensiveness of sleep and adherence measures (i.e., subjective/objective, daily log recording). The impact of adherence on sleep improvement was noted in available studies, however, no meta-analysis of adherence on sleep outcomes was conducted due to the paucity of intervention studies focused on adherence to CBTI in improving sleep quality.
Results and Synthesis
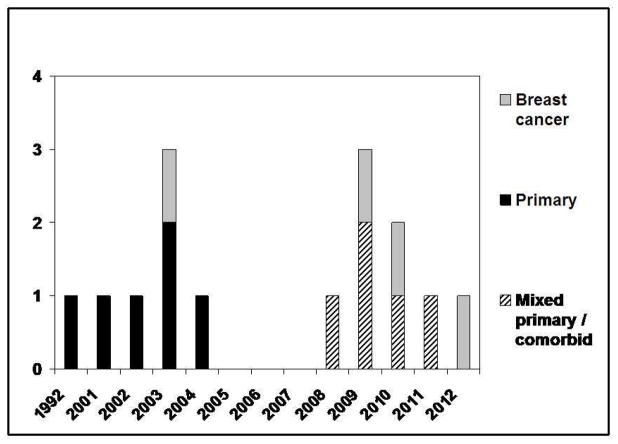
A total of 821 publications from 1981–2012 were identified from the database search. Of these, 227 articles were further reviewed; 212 articles were excluded based on the criteria listed above and duplicate results were noted. The most common reason for article exclusion was absence of adherence data. Table 1 summarizes the remaining 15 studies published between 1992 and 2012 that measured adherence to sleep restriction (SR), stimulus control (SC), and/or sleep hygiene education (SHE) using methods other than study withdrawals. As shown in Figure 1, the number of published studies increased in the last decade. Initial studies focused on adherence in primary insomnia. More recently, an increasing number of studies have included patients with insomnia in association with medical and psychiatric disorders. Fourteen out of 15 studies were published after 2001, which clearly demonstrates that research addressing adherence to CBTI is still in its infancy.
Table 1.
Summary of Studies Reviewed
| Study | Sample | Study Design | CBTI components | Total dose, description | Control group | Measures (bold = measures used for adherence) | Adherence Outcomes |
|---|---|---|---|---|---|---|---|
| Berger AM et al 200344 | 21 women with insomnia comorbid with breast cancer (stage I–II) (U.S.) Age range 43–66 years 100% female |
RCT pilot; secondary analysis of 1 group; RM | SR (modified) SC RT SHE |
5–6 ½ h over 4 CTX cycles; 30, 60, 90 day follow-up; tailored sleep promotion plan (ISPP) in home | Healthy eating education |
Sleep diary, 12-item questionnaire to record daily adherence to ISPP Pittsburgh Sleep Quality Index (PSQI), wrist actigraphy, Piper Fatigue Scale (PFS), |
↑ adherence for most components of ISPP; patterns of adherence stable over time (SC most variable); ↑adherence at the end of chemotherapy treatments; average nightly adherence for ISPP components at 30, 60, 90 days post intervention = SR (83–88%); SC (36–56%); RT (83–88%); SHE (77–88%) |
| Bouchard et al 200341 | 39 primary insomnia patients (Canada) Mean age = 41.44 years 59% female |
Correlation, descriptive; secondary analysis | SR SC CT SHE |
4–12 h over 8 weeks; various modalities: telephone (4 h); individual (7 h); group (12 h) | None |
Sleep diary Insomnia severity index (ISI), Beck anxiety inventory, Beck depression inventory (BDI), Self-efficacy Scale |
↑ self-efficacy associated with ↑ adherence behaviors; self-efficacy and adherence↑ from the 2nd to 8th and ↓ 1-week post CBTI |
| Chamber & Alexander 199234 | 103 primary insomnia patients; self or physician referred (U.S.) Mean age = 39.9 years 67 % female |
1 group; pre-post | SR SC CT SHE |
2–3 h individual initial session by a clinical psychologist; some patients returned for 1– 3 monitoring | None |
Questionnaire and Assessment of Wakefulness (SQAW) and items related to recall of compliance with CBTI Sleep history; at 6-month followup: current medications, other treatment strategies, change in sleep. |
Mean satisfaction with CBTI was 3.58/5 and ↑ satisfaction associated with ↑ adherence; self-reported adherence did not predict treatment outcomes |
| Edinger et al 2009 35 | 41 primary insomnia patients or insomnia associated with psychiatric disorders recruited from VAMC (U.S.) Mean age = 54.2 years 14% female |
RCT, parallel group | SR SC SHE |
2–4 h over 4 biweekly sessions; individual CBTI or SHE by clinical psychologists | SHE alone |
Electronic sleep diary, post – CBTI questionnaire assessing how many days/week 6 core elements of CBTI were enacted, and the usefulness of each strategy Polysomnography (PSG) (screening), actigraphy, Insomnia symptom questionnaire (ISQ), PSQI, Dysfunctional Attitudes about Sleep Scale (DBAS), Therapy Evaluation Questionnaire (TEQ), |
CBTI group adherence to 6 core elements averaged 6.23 days/week, compared with the 5.80 days/week in the control group (SHE alone); CBTI group reported ↑ average usefulness scores, compared with controls; post-treatment sleep diary data showed the CBTI group had significantly ↓ variability in bedtime and rise time than controls |
| Harvey et al 2002 37 | 90 primary insomnia patients recruited from a sleep clinic at 1 year post-CBTI (Scotland) Mean age = 53.9 years 73% female |
Secondary, longitudinal data analysis from large clinical effectivenes s trial | SR SC CT SHE |
5 h over 6 weeks; group sessions conducted by a primary care nurse |
Questionnaire related to the use of 10 CBTI components at 1 year follow-up post–CBTI (yes/no responses)
Sleep diary during CBTI |
Reported use of SC and SR was the best predictor of clinical improvement in SL and WASO. Use of CT contributed to a significant ↓ in WASO; relaxation was the most highly endorsed component (74%) but its use did not predict improvement in SL or WASO; imagery (19% used) was not associated with a ↓ in SL or WASO. Average % of self reported use of CBTI components: 49% altering lifestyle (e.g., caffeine, diet) 32% altering bedroom (e.g., temperature) 41% stimulus control/sleep restriction 59% not taking naps 52% pre-bed routine 74% relaxation (abbreviated method) 21% cognitive control 19% imagery 41% blocking thoughts 41% cognitive restructuring |
|
| Herbert et al 2010 27 | 94 primary insomnia patients (Canada); media or physician referred; 55.3% had a co-morbid medical or psychiatric condition age data unavailable 61.7% female |
RCT; secondary analysis of 1 group; RM | SR SC CT SHE RT medication tapering |
Total dose unknown; 5 weekly Internet modules with homework assignments | Wait control |
Sleep diary, weekly electronic homework checks (adherence defined as homework practice > 4 nights per week)
Medical history, mini- international neuropsychiatric interview (screening); Computer Attitudes Scale, Multidimensional Scale of Perceived Social Support, attitude toward completion of the internet program questionnaire (adapted), Pain Stages of Change Questionnaire (PSOCQ) (adapted to sleep) |
17% dropout rate; ↑ attrition predicted by ↑ symptom severity and psychiatric comorbidity; ↑ perceived behavioral control, social support, and intention to complete the program were significantly associated with ↑ adherence to sleep hygiene homework. Participant adherence to components = avoidance of clock-watching (70%), caffeine taper (60.9%), alcohol taper (95.6%), avoiding heavy meals at bedtime (87%), sleeping in separate bedroom from noisy bed partner (87%), exercising (39%), taper liquids before bed (60.9%), temperature control in bedroom (87%), avoidance of napping (82.6%), regular sleep schedule (82.6%), avoiding reading in bed (82.6%), abdominal breathing (60%), progressive muscle relaxation (44.4%), imagery induced relaxation (38.8%), hypnosis (41.2%), sleep restriction (44.4%). |
| Manber et al 201148 | 301 patients referred for insomnia, with comorbid psychiatric, sleep, and medical disorders (U.S.) age data unavailable 58% female | Pre-to post treatment case replication | SR SC SHE CT RT Scheduled worry time Relapse prevention |
10 ½ h over 7 sessions (1–5 weekly, 6–7 biweekly); group sessions | - |
Sleep diary, Treatment Components Adherence Scale (TCAS)
ISI, Beck Depression Inventory (BDI), Treatment Satisfaction Scale (TSS) |
Two behavioral element of CBTI (keeping a fixed rise time and restricting time in bed) and one cognitive element (changing expectations about sleep) were ↓ among patients with greater depression severity. |
| Matthews et al, 201245 | 34 women with stage I–III breast cancer recruited at cancer centers (U.S.) Mean age = 53.56 years 100% female |
RCT; secondary analysis of 1 group; RM | SR SC CT SHE |
3–4 h over 6 weeks; individual sessions conducted by a CBTI trained advanced practice nurse | Behavioral placebo (desensitiza tion) |
Sleep diary (sleep restriction coding grid calculation), motivation to change sleep behaviors (single item at baseline screening)
Demographic/medical characteristics; ISI, HADS, PFS |
Overall, adherence to prescribed bedtime remained constant but prescribed rise time and total time in bed ↓ in weeks 5–6 of CBTI. Adherence to rise time was poor, but ↑ for prescribed bedtime, which was associated with a ↓ number of nocturnal awakenings. ↑ adherence was associated with ↑ self-reported motivation to change sleep behaviors. And ↓ fatigue was associated with ↑ adherence. Past chemotherapy was related to ↑ adherence to prescribed bedtime, and prescribed total time in bed |
| McChargue et al 2010 46 | 113 women, stage I–IIIA breast cancer recruited at cancer centers (U.S.) Mean age = 51.57 years 100% female |
RCT; secondary data analysis | SR (modified) SC RT SHE |
5 –6 ½ h over 4 CTX cycles and 30, 60, 90 day follow-up; ISPP in participant’s home | Healthy eating education (HEC) |
12-item adherence questionnaire measured daily adherence to ISPP Demographic/ medical characteristics, Hospital Anxiety and Depression Scale (HADS), Symptom Experience Scale (SES) |
Average self-reported adherence rates to the ISPP were 51–52% across 4 CTX cycles but adherence varied by component with ↓ adherence to SR and ↑ adherence to RT; as sleep improved and depression ↑ across chemotherapy treatments, participants progressively adhered ↓ to the ISPP |
| Perlis et al 200442 | 27 primary insomnia patients (U.S.); physician or self- referred from ads Mean age = 41.3 years 70% female |
RCT; 3-arm | SR SC CT SHE relapse prevention |
4–12 h over 8 weeks; individual sessions varied in length between 30–90 minutes; by a single PhD- level clinician | Placebo +CBTI modafinil+ CBTI modafinil + control |
Sleep diary PSG, medical history inventory, and interview (screening), PSQI, Beck anxiety inventory; BDI; Schedule for Affective Disorders and Schizophrenia-Lifetime Version, computer-based Structure Clinical Interview for DSM-IV-TR, symptoms checklist |
Modafinil did not positively alter sleep outcomes, however, the modafinil + CBTI group tended to ↑ adherence (80% vs 51%, P = .059) for the first 4-week period of active treatment; consistently ↑ adherence to the prescribed delay in bedtime. Adherence was most robust in week 1–3 of CBTI. The placebo + CBTI group showed a linear trend toward ↑ adherence over time. |
| Riedel & Lichstein 200136 | 22 adults > 60 year old with primary insomnia recruited via media, senior gatherings (U.S.) Mean age = 67.96 years 73% female |
RCT; secondary analysis of 1 group; RM | SR SHE |
total dose unknown; 6 individual weekly sessions conducted by psychology graduate students | Unspecified behavioral treatment |
Sleep diary Eligibility interview, State-Trait Anxiety Inventory (STAI-T), Geriatric Depression Scale (GDS), Mini-mental state exam (screening), Epworth Sleepiness Scale |
Time spent in bed per night per night at post treatment exceeded therapist recommendations by an average of 27.89 minutes (SD = 31.72); 14 participants (64%) were within 30 minutes and 8 participants (36%) were within 15 minutes of prescribed time in bed. Mean adherence to SR was 68.99% Self-reported time in bed a post-CBTI was significantly ↓ than time in bed at baseline p < 0.01. Night to night consistency of time spent in bed and time arising variance were significantly ↑ at post treatment; ↑ consistency of time spent in bed per night and ↑ consistent arising time predicted sleep improvements |
| Tremblay et al 2009 47 | 57 women with breast cancer post- treatment (Canada) Mean age = 54.05 years 100% female |
RCT; secondary data analysis | SR SC CT SHE fatigue and stress management |
13.5 h over 8 weeks + booster session; group sessions of 4–6 patients 3 nights of PSG preceded CBTI. | Wait list control |
Sleep diary (adherence to behavioral strategies coding calculations) PSG, ISI, DBAS, HADS, Treatment Expectancies and Perceived Credibility Questionnaire (TEPCQ), Therapeutic Alliance Perception Questionnaire (TAPQ) |
Post-CBTI, subjective sleep improvements were best predicted by ↑ initial levels of treatment expectancies and ↓ dysfunctional beliefs about sleep. At 6-month follow-up, subjectively assessed sleep improvements were best predicted by adherence to behavioral strategies but not with PSG-assessed sleep improvements. |
| Vincent & Hameed 200343 | 50 primary insomnia patients (Canada) Mean age = 51.4 years 66% female |
1 group; RM | SR SC CT SHE medication taper relaxation training stress management and problem solving |
10 ½ h over 7 weeks; Groups sessions of 4–6 patients, by PhD level psychologist with sleep training | - |
Sleep diary; Therapist-rated adherence questionnaire (TRAQ), Spousal-Rated Adherence Questionnaire (SRAQ), attendance record Insomnia interview schedule (IIS) (screening), PSQI, Beliefs and Attitudes about Sleep Scale (BELIEF), DBAS, BDI, Structured Interview for DSM Disorders (SCID) |
Therapists' rated nearly half of the participants as "very much" to "extremely" adherent, which was the most consistent relationship to sleep outcomes (i.e., ↓ post- CBTI maladaptive beliefs, ↓ sleep-related impairment, and ↑ sleep quality). No adherence variables predicted post-CBTI sleep latency (SL), total sleep time (TST), or sleep efficiency (SE). Comorbid dysthymia was associated with ↓ adherence and ↓ improvement in SL and SE. There was a trend toward ↓ adherence associated with anxiety disorders. |
| Vincent & Lewycky 2009 49 | 118 primary insomnia patients (28%) or insomnia co-morbid with medical / psychiatric disorders (66.9%) (Canada) age data unavailable 67.8% female | 1 group; RM secondary adherence data | SR SC CT SHE medication tapering |
Total dose unknown; 5 weekly internet modules with homework assignments | - |
Weekly online homework checks of adherence to treatment components Sleep diary, ISI, Multi- Dimensional Fatigue Inventory (MFI) and general fatigue (GF) subscale, DBAS, Pre-Sleep Arousal Scale (PSAS), Clinical Global Improvement Scale (CGI), |
Reported adherence to treatment components (% of participants) was as follows: avoidance of clock-watching (73.9%), sleep hygiene (76.8%), stimulus control (64.2%), relaxation training (67.6%), sleep restriction (51.6%), hypnotic tapering (22.6%). There was a 33% attrition rate by 5 week and additional 8.5% dropped out at 1-month follow-up. Attrition was related to referral status (i.e., dropouts were more likely to have been referred for treatment rather than recruited from the community). TST did not predict attrition from online treatment. |
| Vincent et al 2008 28 | 40 adults with primary or comorbid insomnia (Canada) Mean age = 46.9 years 50% female |
Correlation aldescriptiv e design | SR SC CT SHE |
6 weekly small group (6–8 participants) sessions by psychologists |
Sleep Diary (adherence to wake-up time consistency), modified Medical Outcomes Study General Adherence Scale (MOS-A)
Group Therapy Questionnaire (GTQ) (assessed likes/dislikes and usefulness of SC/SRT), motivation survey (assessed beliefs about SC/SRT, perceived barriers, and perceived behavioral control_) |
↓ age was associated with ↑ wake-up time variance, which was associated with ↑ sleepiness; participants with ↓ perceived barriers were more likely to ↑ report adherence and discomfort, annoyance and boredom were robustly associated with ↓ adherence. Gender and educational level were not associated with adherence; a ↑ education was correlated with ↑ perceived control over SC/SRT ↓ perceived barriers to SC/SRT was associated with ↑ liking of SC/SRT and perceived usefulness of SC Participants with ↑ overall adherence had improved TST, ↓ nocturnal awakenings, and ↓ daytime impairment. Participants attended an average of 5.5 out of 6 sessions (91.7% attendance); Compared to non-users, sleep aid users attended fewer sessions |
Note: BDI = Beck depression inventory; BELIEF = Beliefs and attitudes about sleep scale; CBTI = cognitive behavioral therapy for insomnia; CGI = Clinical global improvement scale; CT = cognitive therapy; DBAS = Dysfunctional attitudes about sleep scale; GDS = Geriatric depression scale; HADS = Hospital anxiety and depression Scale; ISI = Insomnia severity index; ISPP = individualized sleep promotion plan; ISQ = Insomnia symptom questionnaire; MFI = Multi-dimensional fatigue inventory; PDAS = Pre-sleep arousal scale; PFS = Piper fatigue scale; PSG = Polysomnography; PSOCQ = Pain stages of change questionnaire; PSQI = Pittsburgh sleep quality index; RCT = randomized control trial, BT = behavioral therapy; RM = repeated measures; RT = relaxation therapy; SC = stimulus control; SE = sleep efficiency; SES = Symptom experience scale; SHE = sleep hygiene education; SL = sleep latency; SQAW = Sleep questionnaire and assessment of wakefulness; SR = sleep restriction; STAI-T = State-trait anxiety inventory; TCAS = Treatment components adherence scale; TEQ = Therapy evaluation questionnaire; TSS = Treatment satisfaction scale; TST = total sleep time.
Figure 1.
Studies by year and Insomnia Type
Sample characteristics
Sample size for the reviewed studies varied widely, with a range of 21–301 participants. The mean age ranged from 39.9 34 to 54.2 35. Only one study focused exclusively on older adults (M=67.96, SD=7.07, range 60–81) 36. Twelve studies included both genders, composed of 50% women 28 to 73% women 36, 37. Studies of insomnia comorbid with breast cancer (n=4) contained female participants only. Overall, study samples represented the higher reported incidence of insomnia in women 38. The one exception was a sample recruited from a Veteran’s Administration Medical Center (VAMC) in which only 14% of the participants were female 35. This is likely due to the largely male population of VAMCs. Interestingly, it has been suggested that psychoeducational intervention studies including a high proportion of women may produce larger effects 39 and preventive care utilization is higher in women than in men 40. Thus, gender disparity needs to be explored in greater detail to understand the role of gender in relation to adherence to CBTI.
Participants were described as having primary insomnia in six studies 34, 36, 37, 41–43, insomnia comorbid with breast cancer in four studies 44–47, and mixed samples of primary and insomnia comorbid with psychiatric or medical disorders in five studies 27, 28, 35, 48, 49 (Figure 1). Insomnia inclusion criteria for the majority of studies was based on either the research diagnostic criteria for an insomnia disorder 50,27, 28, 49, the International Classification of Sleep Disorders Criteria 51,37, 47, or criteria for primary insomnia based on the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) 52,43. Participants were self-referred or physician-referred and eligibility related to insomnia was typically determined through a clinical interview and/or the use of established measures such as the Insomnia Severity Index (ISI)41, 45, 47–49 or the Pittsburgh Sleep Quality Index (PSQI) 34, 36, 42, 45, 53. The ISI yields a total score from 0–28 and scores are interpreted as follows: absence of insomnia (0–7); sub-threshold insomnia (8–14); moderate insomnia (15–21); and severe insomnia (22–28). Only four studies of the reviewed studies41, 45, 48, 49 reported average baseline ISI assessments. In women with insomnia and breast cancer, scores ranged from 17.5 (4.21)45 to 17.9 (4.3)41. Slightly higher scores of 18.08 (.59)49 and 18.78 (5.0)48 were reported in patients with primary insomnia or insomnia comorbid with psychiatric and medical disorders. These baseline scores suggest many study participants experience moderate insomnia prior to therapy. It is difficult, however, to compare the severity of the insomnia symptoms among the reviewed studies due to the wide variety of screening instruments used to determine eligibility.
Evaluation of CBTI characteristics
The majority of studies combined at least three standard CBTI strategies; one study delivered SHE and SR only 36. CBTI sessions followed a standard protocol with the exception of one study, in which the therapy plan was individualized to meet the needs of women during chemotherapy treatment 44. The mode of CBTI delivery varied. Seven studies administered CBTI individually 34–36, 42, 44–46; and five studies utilized a small group format 28, 37, 43, 47, 48. Two studies used internet-based modules 27, 49. Due to small sample size in one study 41, telephone, individual and group CBTI participants were combined.
CBTI was delivered by nurses in four studies 37, 44–46; and psychologists 28, 34, 35, 43, PhD level clinicians 42, or psychology graduate students 36 in six studies. Five studies did not include a description of the therapy providers 27, 41, 47–49.
The total maximum CBTI dose and duration of the reviewed studies were assessed. Maximum doses were highly variable ranging from three hours 34 to 13.5 hours 47. The median dose was 6.5 hours. Most CBTI interventions were delivered in 6–8 sessions, however, some participants received two or fewer sessions 34, 35. Internet-based studies specifically required homework assignments in addition to the use of a daily sleep diary 27, 49. Homework assignments may enhance adherence, especially for longer interventions by consolidating acquired knowledge, improving skill acquisition, and bridging the gap between learning and real life 39.
Study design
Of the studies reviewed, over half were RCTs (8 out of 15; 53%) 27, 35, 36, 42, 44–47. Only one of these RCTs had three arms 42. Six out of the 15 studies (40%) used a one-group pre-post or repeated measures design 28, 34, 41, 43, 48, 49. One study reported cross sectional analysis of adherence to CBTI at 12 month post-treatment 37. Several studies were identified as pilots with 21–34 participants 36, 42, 44, 45. Adherence to CBTI was frequently a secondary aim of a larger study 27, 36, 37, 41, 44–47, 49, suggesting that adherence is rarely the primary focus of CBTI studies.
The control groups across the studies varied widely with respect to the type of intervention, including various active controls or wait list control 27, 47. Active controls included a healthy eating education 44, 46 , sleep hygiene education alone 35, desensitization 45, and an unspecified behavioral treatment 36.
A limited number of studies used explicit theoretical models to examine adherence to CBTI. Hebert and colleagues (2010) utilized the Theory of Planned Behavior (TPB) 54 and the Transtheoretical Model of Behavior Change (TTM) 55 as a framework to maximize adherence; however, their main findings suggest that neither TPB nor TTM did a good job of predicting participant attrition. Bouchard and colleagues (2003) proposed self-efficacy, a concept derived from Social Cognitive Theory 56, 57 is useful in predicting adherence to CBTI, while Vincent et al. (2008) indicate the Health Beliefs Model 58 may explain non-adherence to CBTI. Measures of adherence in three other studies 43, 45, 47 were based on the Cognitive Behavioral Model of Insomnia 59.
Potential predictors of CBTI adherence
Demographic, medical and psychological characteristics were not consistently associated with adherence in the studies in this review. The majority of studies failed to show any relationship between adherence and demographic factors (e.g., age, gender, education). In one study, gender and education had no significant impact on adherence, but younger participants were less adherent to wake time recommendations than older participants 28. Similarly, medical factors were not related to adherence in most studies. In a small pilot study, the use of stimulant medication (e.g., modafinil) in conjunction with CBTI enhanced adherence to prescribed bedtime compared to placebo plus CBTI 42. In women with breast cancer, better adherence was reported toward the end of chemotherapy treatments (when treatment adverse effects are less severe) 44 and in women with lower levels of subjective fatigue45.
Other factors such as depressive symptoms, may moderate the effect of sleep improvement on adherence and study attrition. A few studies identified potential psychological factors that decrease adherence including higher anxiety 43 and greater depressive symptoms 46, 48. Hebert and colleagues noted the presence of a psychiatric disorders (e.g., depression, generalized anxiety disorder) predicted attrition from internet-based CBTI.
Attitudes toward treatment and past experiences appear to impact willingness to follow CBTI recommendations. For example, greater pre-CBTI motivation to change sleep behaviors 45, higher treatment expectancies 47, higher levels of self-efficacy 41, and greater satisfaction with CBTI 34 were found to be related to better adherence. In one study, those with fewer perceived barriers (discomfort, annoyance, boredom) and less pretreatment sleepiness were more adherent, as measured by global self-report scale and wake time consistency from sleep diary 28. In a study of adults with primary insomnia, higher perceived behavioral control, social support, and intention were associated with better adherence as measured by online weekly reports (Herbert 2010). Overall, few studies have adequately addressed the impact of patient expectations and treatment barriers on adherence; these relationships merit further clinical consideration and investigation.
Proposed barriers to following CBTI recommendations may include ease of assimilation in daily life, adaptability in the home setting, perceived relevance, and perceived effectiveness 37. Adherence to sleep restriction recommendations and faithfulness to prescribed bedtime and wake-up times is difficult because it often requires a considerable alteration in lifestyle 36. It has been suggested that poor adherence to SR and treatment dropout may be due to 1) the fundamentally counterintuitive recommendation to limit sleep, 2) objections to increased daytime sleepiness early in treatment, 3) complaints of boredom and lack of activities because of delayed bedtime, and 4) resistance to prescribed time out of bed, especially on the weekends 36. Resistance to prescribed sleep times may also represent patient ambivalence about changing behavior. Few studies have investigated the role of these barriers or suitable interventions.
It is plausible that individuals become less adherent and stop attending appointments because they feel better or the symptoms have resolved. However, Ong et al.26 reported that the best predictors of early attrition from group CBTI were short sleep duration coupled with depressive symptoms. Other investigations have found mixed results regarding the impact of sleep improvement on adherence. Matthews et al.45 reported that subjective overall rating of sleep was inversely related to adherence to prescribed bedtime and total time in bed at the beginning of CBTI, however, significant correlations did not continue to the end of treatment. Conversely, McChargue and colleagues46 reported decreasing sleep disturbances contributed to lower total adherence rates during the course of chemotherapy. The level of sleep disturbance before treatment may be as important an indicator of adherence as how quickly sleep improves during treatment 37, 43, 46. Additional study is needed to understand how the severity of disease and symptoms affects adherence.
Measurement issues in adherence to CBTI
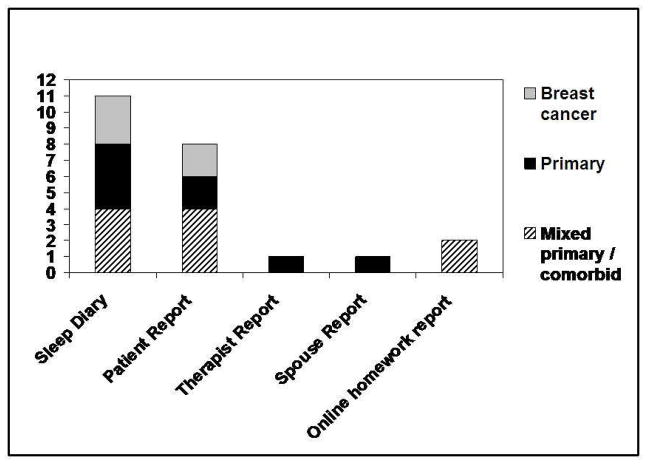
Measures of adherence to CBTI included multiple and single item patient-reported,27, 28, 34, 35, 37, 44, 46, 48 therapist/spouse-reported 43 adherence questionnaires, and adherence to prescribed sleep schedules as derived from sleep diaries 27, 28, 35–37, 41–45, 47, 48. Internet-based CBTI incorporated weekly homework checks of adherence 27, 49, 60. Combined self-reported adherence and sleep diary measures were used in four studies28, 35, 43, 44, thus providing a more complete assessment of adherence. Although several studies employed actigraphy or polysomnography35, 44–47, analysis of adherence using objective measures was rare. Figure 2 illustrates the type and frequency of use of adherence measures by study populations.
Figure 2.
Adherence Measures by Study Population
Participants in CBTI trials are frequently categorized as either adherent or non-adherent to a given behavior based on subjective responses. When adherence is measured as a dichotomous variable, the complexity inherent in adherence to a behavioral intervention may not be captured adequately. Behaviors related to stimulus control, sleep hygiene, and cognitive interventions are difficult to measure objectively. Some studies have included self-report items about the behaviors or derived information directly from sleep diaries using an operationally defined criterion for adherence. While inclusion of self-report adherence information appears obvious, it should be recognized that bias may result if self-report is the only source of adherence information. This bias underscores the need to compare adherence data from multiple sources. Information from bed partners and therapists may provide additional validation.
Actigraphy may be useful as an objective verification of daily reports of bedtime, rise time and may even improve self-report accuracy or adherence. Light monitors, available on some actiwatches, may provide insight into adherence to recommended behaviors. Even with actigraphy it is difficult to verify that patients are, in fact, getting out of bed during prolonged nocturnal awakenings. Future studies would benefit from comparing the degree to which various sources of information correspond.
Overall, measurement and reporting of adherence measures in the literature is inconsistent and insufficient, leading to challenges in interpreting treatment and process outcomes. Studies that attempt to predict or modify adherence often face the problem that adherence as a dependent variable is complex and non-normally distributed 61. It is apparent that both standardized definitions and measures of adherence are needed. To that end, the “Consensus Sleep Diary” was developed as the result of collaborations with insomnia experts and potential users. The adoption of this standardized patient-informed sleep diary will facilitate comparisons across studies and advance the field 62. Similarly, understanding the minimal level of adherence needed for positive outcomes and the time point in treatment (if any) at which adherence is most critical and discerning adherence to individual components will advance the field. For example, is it essential to have strict adherence during the initial phase of treatment when patients are learning the strategies and rationale, or toward the closing sessions to sustain treatment gains? One approach to measurement is to combine varying rates of adherence to the different components of treatment into a composite score. Morgenthaler et al., (2006) suggests SC is the most effective stand-alone behavioral intervention for insomnia, therefore, may need to be weighted heavily in a composite measure. As an alternative, more attention might be given to attendance at CBTI sessions. In the absence of other data, the percentage of treatment sessions attended could be regarded as a limited proxy for adherence. Confounding factors such as transportation would have to be considered.
Relationship between adherence and treatment outcome
Adherence to nonpharmacological regimens has been found to be strongly related to treatment outcome in other psychological settings, particularly when measures of adherence are continuous and when the disease is chronic 25. Evidence of the association between adherence to CBTI and sleep outcomes, however, is difficult to interpret due to the diverse measurement of both adherence and sleep improvement. Higher levels of adherence were associated with various sleep improvements in some studies 36, 37, 43 but not in all 34.
One study of adherence assessed by an author-constructed self-report scale showed greater adherence to CBTI components were associated with lower posttreatment Insomnia Severity Index (ISI) scores in 301 adults with insomnia 48. In contrast, Chambers et al. (1992) found that adherence assessed through recall of adherence to CBTI recommendations did not predict improvements in daytime sleepiness, sleep latency, total sleep time (TST), awakenings or wake after sleep onset (WASO) at six months after CBTI in 103 adults with chronic insomnia. This may be due in part to low variance in adherence, as patients reported high adherence to recommendations. Sleep improvements and adherence were subjectively assessed by patients, thus subject to bias and recall inaccuracy.
The majority of studies assessing diary-based sleep improvements relative to adherence, suggest that adherence to key therapy recommendations improves sleep outcomes in a variety of ways, however, divergent measures of adherence prevent definitive conclusions. For example, Harvey and colleagues (2002) used a self-report measure of adherence to 10 different CBTI components, administered one year after completion of online CBTI to adults with primary insomnia. They found that continued adherence to SC and SR was the best predictor of clinical improvement in sleep latency and nighttime wakefulness, and ongoing use of cognitive restructuring contributed significantly to reduction in nocturnal wakefulness 37. Similarly, in 40 outpatients with primary or comorbid insomnia participating in online CBTI, high levels of adherence to SC and SR using a global self-report scale and diary-based wake time consistency was associated with improvements in total sleep time, number of awakenings, Insomnia Severity Index ratings (ISI) and less daytime impairment 28.
In a study of group-based CBTI in 50 adults with chronic insomnia, adherence was measured by an investigator developed Therapist-Rated Adherence Questionnaire (TRAQ), a Spousal-Rated Adherence Questionnaire (SRAQ), and attendance record 43. Participants who were judged as more adherent by therapists reported subjective improvement in sleep-related impairment, sleep quality, and maladaptive beliefs/attitudes about sleep. Therapist-rated adherence, but not consistency of bedtime/wake-up time, attendance, or spousal-rated adherence, was positively associated with diary-based sleep outcomes.
In older adults with good overall adherence to 6-week CBTI, greater consistency with the recommended sleep schedule predicted less nocturnal wake time and higher sleep efficiency. A consistent rise time predicted better subjective sleep quality and fewer awakenings, however, three other sleep diary-based adherence calculations did not predict improvements in sleep diary measures of sleep latency, WASO, or number of awakenings 36. Using several methods to measure adherence to SR, these investigators concluded that the sleep schedule consistency that occurs by way of SR may underlie improved outcomes. Methods of measuring adherence consisted of (1) subtracting the amount of time in bed prescribed at the final treatment from mean reported time in bed at post-treatment, (2) calculating adherence percentage by dividing reported time in bed reduction since baseline by prescribed time in bed prediction X 100, (3) change in mean time in bed from baseline to post-treatment, (4) time in bed variance calculated by computing the variance of nightly time in bed (in minutes) for each participant at baseline and post-treatment, and (5) computing the variance of time arising (leaving bed) in the morning at baseline and post-treatment (arising variance) 36.
In breast cancer survivors (n = 58), five dichotomous indices of adherence to SR were calculated from sleep diary data 47. Indices included (1) adherence to prescribed bedtime hour (i.e., not more than 15 minutes before the set time), (2) adherence to prescribed arising time (i.e., not more than15 minutes after set time), (3) arising from bed within 30 minutes following a nocturnal awakening, (4) avoidance of daytime naps, and (5) adherence to total time spend in bed prescribed (i.e., not more than 30 minutes greater than the set amount of time. A proportion (%) of the number of days where the participant complied with CBTI recommendations was determined for each index. Higher levels of adherence to arising during night awakenings were associated with a reduction in clinician-rated ISI scores. Higher adherence to prescribed rise times was correlated with reduced WASO and total sleep time. In addition, actigraphy-based improvements in WASO, total sleep time, and sleep efficiency were significantly predicted by a greater adherence to the recommendation to avoid daytime napping 47. Using a similar sleep diary-based coding grid in breast cancer survivors, Matthews and colleagues 45 reported that higher adherence to prescribed bedtime in week 5–6 of CBTI was associated with fewer nighttime awakenings. Adherence to prescribed bedtime and prescribed total time in bed were associated with increased total sleep time throughout CBTI.
Conclusions
Insomnia is a serious health concern. CBTI is an efficacious treatment, yet attrition and suboptimal adherence potentially diminishes its impact. Although CBTI has grown in utility since the 1960s, there is a dearth of research devoted to measuring adherence to CBTI. General limitations of reviewed studies include small samples, reliance on self-reported data, and a restricted range of adherence scores. Inconsistency in adherence findings among the studies may be explained in part by diverse sample characteristics (e.g., education, socioeconomic status), varied CBTI components, and lack of a standardized definition and measurement of adherence.
The type of provider, mode of delivery, dose and duration of treatment are also inconsistent across studies, limiting the ability to compare findings. Although there have been several studies of insomnia in women with breast cancer, very little research has been done on other medical and psychiatric comorbidities. Expanding studies of adherence to CBTI to other chronically ill populations is warranted to fill this knowledge gap.
Despite the utility of behavioral change models, CBTI adherence studies are largely atheoretical. In fact, only three of the reviewed studies used an explicit conceptual model or theory. Some may argue that theory does not necessarily lead to better interventions and, indeed, this has not yet been the case with PAP adherence studies. Theories do, however, provide insights into the behavioral constructs that predict adherence. Advantages of theory guided research include the ability to make systematic predictions based on the theory, utilize measures derived from the theory, and interpret findings in support or against the theory. To that end, theory-based studies specifically designed with adherence as a primary outcome are needed. With this information, treatment approaches could be designed to target factors and processes that are most likely to impact treatment adherence.
Clinical and Research Implications
There is a paucity of evidence identifying the impact of adherence on sleep parameters and subjective sleep quality in both primary and comorbid insomnia. Few studies have evaluated the impact of adherence on sleep outcomes, yet some findings suggest that there may be an association between adherence to SR, SHE, SC and sleep improvements. Patients and providers invest substantial time and effort in CBTI , despite being in an environment of scarce healthcare resources. It is plausible that even modest improvements in adherence could result in better patient retention, greater patient and provider satisfaction, and most importantly, improved outcomes. It is possible that minimal investment in adherence may realize significant outcomes.
Initial steps to advance practice and research, therefore, include (1) establish standard measures and definitions of adherence; (2) develop standard measures and definitions of treatment response; (3) evaluate adherence and its relationship to treatment outcome across a wide-range of insomnia populations in different settings with different CBTI delivery modalities; and (4) consider the role of theory/models of behavior change in explaining relationship between adherence and outcomes.
Uniform measures of adherence and treatment outcomes are needed to enhance the comparability of studies. Standardized instruments are needed for screening and outcome assessment. For example, Morin and colleagues63 examined psychometric indices of the Insomnia Severity Index (ISI) to detect cases of insomnia in a population-based sample and to evaluate treatment response. A cutoff score of 10 was optimal for detecting insomnia cases in the community sample. In a clinical sample, a change score of −8.4 points was associated with moderate improvement63.
Investigations are warranted that evaluate adherence to CBTI protocols that contain additional treatment components, or are delivered via other modalities. While the studies reviewed here are informative, it is difficult to arrive at a definitive conclusion about the state of the science, in part due to the wide variation in study measures. To address measurement issues, adherence must be measured in a multiple ways, not simply inferred64, and future studies should include some type of adherence evaluation for all CBTI components. Studies that regularly measure perceived improvement, may discern whether participants’ declining attendance and adherence are due to mitigation of the problem or symptom.
Adherence metrics are largely based on clinical judgment of the allowable time before or after a prescribed bedtime or rise time that could be considered “adherent” to the therapist’s recommendation. The validity of different time intervals as the cut off for adherence to prescribed sleep times should be analyzed in large samples of those with comorbid as well as primary insomnia. Further analyses should determine whether the factors that predict sleep restriction adherence also predict adherence to stimulus control, sleep hygiene, and cognitive therapy. Larger adherence studies are needed to validate sleep diary data against objective data.
Large-scale RCTs focused on adherence are needed to fill the knowledge and clinical practice gaps identified in this review. It also would be valuable to target a variety of clinically heterogeneous groups of patients who experience insomnia. Studies guided by theories or models of behavior change to explain the relationship between adherence and outcomes, utilize measures derived from the theory, and interpret findings are needed to advance the field.
Finally, qualitative research with a focus on the individual meaning of adherence can provide important insight to the underlying adherence puzzle. Qualitative methods may uncover the answers to questions such as whether individuals do not adhere because they find CBTI is too difficult? or they do not believe it can help? or they give up after several weeks without improvement? Perhaps, they find one specific component of CBTI so effective that the other components are unnecessary. Understanding the nuances of behaviors and adherence to insomnia therapy and other sleep disorders is warranted.
Adherence factors and guidelines from other sleep disorders and populations utilizing cognitive behavioral therapy (CBT) may be helpful to frame the findings in this review. For example, a review of adherence to positive airway pressure (PAP) treatment for obstructive sleep apnea indicates that PAP is effective, but adherence is restricted by several disparate factors.12 Among these factors are those related to the efficacy of the treatment and its comfort as well as psychological and social factors that likely reflect a person’s approach to health management and behavior change. Interventions to improve adherence to PAP provide some insight to the limited effects of comfort management on adherence. The psychosocial aspects that contribute to behavior change provide promise for improving adherence, however, studies of social support, relationship-based approaches, and other theory-driven interventions, are warranted for PAP adherence as well as CBTI.12
Guidelines for adherence problems in those with serious and persistent mental illness65 suggest that psychological/programmatic interventions should include ongoing symptom/side effect monitoring and management; psychoeducation (e.g., counseling and written/audiovisual materials); and environmental supports (e.g., more frequent and/or longer visits with providers/therapists to improve therapeutic alliance). For example, adherence to internet CBT for anxiety and depression, as measured by “homework” completion, was improved with email/phone reminders and more frequent clinicians contact in a study of over 2,000 patients referred for generalized anxiety, depression, or social phobia.66 Investigators concluded that improved adherence to internet CBT is an important determinant of its effectiveness. Adherence to treatment for other sleep disorders, as well as CBT for mental illness and insomnia appear to involve many different factors, thus, a variety of strategies may be needed.
Steiner64 recently suggested that the design of adherence interventions has been guided by the mistaken assumption that adherence is a simple behavior that can be predicted from patient characteristics. Instead, adherence should be conceptualized as a set of interacting behaviors influenced by individual, social, and environmental forces. Thus, counseling for behavioral change is complex and needs to be complemented by identification and removal of multifaceted barriers to treatment.
Practice points.
Insomnia is a significant problem in adults worldwide
Evidence supports the efficacy of CBTI, however, evidence of the impact of adherence is inconclusive
CBTI interventions are highly variable in terms of components, mode of delivery, sleep measures used, and type of therapist
Methods of measuring adherence are also inconsistent across studies, contributing to difficulty in guiding practice
Evidence suggest that greater adherence to CBTI recommendations may lead to greater improvements in sleep
Research agenda.
Future CBTI studies should aim to:
Establish standard measures and definitions of adherence
Develop accepted measures and guidelines for treatment response
Target adherence and its relationship to treatment outcome across a wide-range of insomnia populations in different settings with different CBTI delivery modalities
Evaluate the role of theory/models of behavior change in explaining relationship between adherence and outcomes
Acknowledgments
This study was funded by the National Institute of Health and National Institute of Nursing Research (1K23NR010587) and the American Nurses Foundation (#2010-049).
Glossary of terms/abbreviations
- Actigraphy
a technique using a small device (i.e., actigraph) worn on the wrist or ankle to measure body movement and identify activity and rest patterns that is useful in assessing sleep-wake cycles across many consecutive days and nights
- BT
behavioral therapy
- CBTI
cognitive behavioral therapy for insomnia
- CT
cognitive therapy
- ISI
Insomnia severity index
- ISPP
individualized sleep promotion plan
- PAP
positive airway pressure
- PSG
polysomnography
- PSG
polysomnography
- PSQI
Pittsburgh sleep quality index
- RCT
randomized control trial
- RM
repeated measures
- RT
relaxation therapy
- SC
stimulus control
- SC
stimulus control
- Self-efficacy
one’s own judgment of competence to complete tasks and reach goals
- SHE
sleep hygiene education
- SR
sleep restriction
- TPB
Theory of Planned Behavior
- TST
total sleep time
- TTM
Transtheoretical Model of Behavior Change
- VAMC
Veteran’s administration medical center
- WASO
awakenings or wake after sleep onset
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kraus SS, Rabin LA. Sleep America: Managing the crisis of adult chronic insomnia and associated conditions. J Affect Disord. 2011;136:192–212. doi: 10.1016/j.jad.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 2.Morin CM, LeBlanc M, Belanger L, Ivers H, Merette C, Savard J. Prevalence of insomnia and its treatment in Canada. Can J Psychiatry. 2011;56:540–8. doi: 10.1177/070674371105600905. [DOI] [PubMed] [Google Scholar]
- 3.National Institutes of Health State of the Science Conference Statement. Manifestations and Management of Chronic Insomnia in Adults. Sleep. 2005;28:1049–57. doi: 10.1093/sleep/28.9.1049. [DOI] [PubMed] [Google Scholar]
- 4.Leger D, Bayon V. Societal costs of insomnia. Sleep Med Rev. 2010;14:379–89. doi: 10.1016/j.smrv.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Taylor DJ, Mallory LJ, Lichstein KL, Durrence HH, Riedel BW, Bush AJ. Comorbidity of chronic insomnia with medical problems. Sleep. 2007;30:213–8. doi: 10.1093/sleep/30.2.213. [DOI] [PubMed] [Google Scholar]
- 6.Kapur VK, Redline S, Nieto FJ, Young TB, Newman AB, Henderson JA. The relationship between chronically disrupted sleep and healthcare use. Sleep. 2002;25:289–96. [PubMed] [Google Scholar]
- 7.Kleinman NL, Brook RA, Doan JF, Melkonian AK, Baran RW. Health benefit costs and absenteeism due to insomnia from the employer's perspective: a retrospective, case-control, database study. J Clin Psychiatry. 2009;70:1098–104. doi: 10.4088/JCP.08m04264. [DOI] [PubMed] [Google Scholar]
- 8.Daley M, Morin CM, LeBlanc M, Gregoire JP, Savard J, Baillargeon L. Insomnia and its relationship to health-care utilization, work absenteeism, productivity and accidents. Sleep Med. 2009;10:427–38. doi: 10.1016/j.sleep.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Ebben MR, Spielman AJ. Non-pharmacological treatments for insomnia. J Behav Med. 2009;32:244–54. doi: 10.1007/s10865-008-9198-8. [DOI] [PubMed] [Google Scholar]
- 10.Morgenthaler T, Kramer M, Alessi C, Friedman L, Behlecke B, Brown T, et al. Practice parameters for the psychological and behavioral treatment of insomnia: an update. An American Academy of Sleep Medicine report. Sleep. 2006;29:1415–9. [PubMed] [Google Scholar]
- *11.Morin CM, Bootzin RR, Buysse DJ, Edinger JD, Espie CA, Lichstein KL. Psychological and behavioral treatment of insomnia: Update of the recent evidence (1998–2004) Sleep. 2006;29:1398–414. doi: 10.1093/sleep/29.11.1398. [DOI] [PubMed] [Google Scholar]
- 12.Matthews EE, Aloia MS. Continuous positive airway pressure treatment and adherence in obstructive sleep apnea. Sleep Medicine Clinics. 2009;4:473–85. [Google Scholar]
- 13.Dimatteo MR. Variations in patients' adherence to medical recommendations: a quantitative review of 50 years of research. Med Care. 2004;42:200–9. doi: 10.1097/01.mlr.0000114908.90348.f9. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization. Adherence to Long-term Therapies: Evidence for Action. Geneva: World Health Organization; 2003. [Google Scholar]
- 15.Haynes EB. Introduction. In: Haynes EB, Sackett DL, Taylor DW, editors. Compliance in Health Care. Baltimore, MD: The Johns Hopkins Press; 1978. pp. 1–18. [Google Scholar]
- 16.Sackett DL. Introduction and magnitude of compliance and noncompliance. In: Sackett DL, Haynes EB, editors. Compliance with Therapeutic Regimens. Baltimore, MD: The Johns Hopkins University Press; 1976. pp. 1–25. [Google Scholar]
- 17.Benner JS, Glynn RJ, Mogun H, Neumann PJ, Weinstein MC, Avorn J. Long-term persistence in use of statin therapy in elderly patients. JAMA. 2002;288:455–61. doi: 10.1001/jama.288.4.455. [DOI] [PubMed] [Google Scholar]
- 18.Carpenter R. Perceived threat in compliance and adherence research. Nursing Inquiry. 2005;12:192–9. doi: 10.1111/j.1440-1800.2005.00269.x. [DOI] [PubMed] [Google Scholar]
- 19.Chatterjee JS. From compliance to concordance in diabetes. J Med Ethics. 2006;32:507–10. doi: 10.1136/jme.2005.012138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morin CM, Vallieres A, Guay B, Ivers H, Savard J, Merette C, et al. Cognitive behavioral therapy, singly and combined with medication, for persistent insomnia: a randomized controlled trial. JAMA. 2009;301:2005–15. doi: 10.1001/jama.2009.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siebern AT, Manber R. Insomnia and its effective non-pharmacologic treatment. Med Clin North Am. 2010;94:581–91. doi: 10.1016/j.mcna.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 22.Yang CM, Spielman AJ, Glovinsky P. Nonpharmacologic strategies in the management of insomnia. Psychiatr Clin North Am. 2006;29:895–919. doi: 10.1016/j.psc.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 23.Jacobs GD, Pace-Schott EF, Stickgold R, Otto MW. Cognitive behavior therapy and pharmacotherapy for insomnia: a randomized controlled trial and direct comparison. Arch Intern Med. 2004;164:1888–96. doi: 10.1001/archinte.164.17.1888. [DOI] [PubMed] [Google Scholar]
- 24.Sivertsen B, Omvik S, Pallesen S, Bjorvatn B, Havik O, Kvale G, et al. Cognitive behavioral therapy vs zopiclone for treatment of chronic primary insomnia in older adults: a randomized controlled trial. JAMA. 2006;295:2851–8. doi: 10.1001/jama.295.24.2851. [DOI] [PubMed] [Google Scholar]
- 25.Dimatteo MR, Giordani PJ, Lepper HS, Croghan TW. Patient adherence and medical treatment outcomes: a meta-analysis. Med Care. 2002;40:794–811. doi: 10.1097/00005650-200209000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Ong JC, Kuo TF, Manber R. Who is at risk for dropout from group cognitive-behavior therapy for insomnia? J Psychosom Res. 2008;64:419–25. doi: 10.1016/j.jpsychores.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *27.Hebert EA, Vincent N, Lewycky S, Walsh K. Attrition and adherence in the online treatment of chronic insomnia. Behav Sleep Med. 2010;8:141–50. doi: 10.1080/15402002.2010.487457. [DOI] [PubMed] [Google Scholar]
- *28.Vincent N, Lewycky S, Finnegan H. Barriers to engagement in sleep restriction and stimulus control in chronic insomnia. J Consult Clin Psychol. 2008;76:820–8. doi: 10.1037/0022-006X.76.5.820. [DOI] [PubMed] [Google Scholar]
- *29.Espie CA, Inglis SJ, Tessier S, Harvey L. The clinical effectiveness of cognitive behaviour therapy for chronic insomnia: implementation and evaluation of a sleep clinic in general medical practice. Behav Res Ther. 2001;39:45–60. doi: 10.1016/s0005-7967(99)00157-6. [DOI] [PubMed] [Google Scholar]
- 30.Morgan K, Thompson J, Dixon S, Tomeny M, Mathers N. Predicting longer-term outcomes following psychological treatment for hypnotic-dependent chronic insomnia. J Psychosom Res. 2003;54:21–9. doi: 10.1016/s0022-3999(02)00569-x. [DOI] [PubMed] [Google Scholar]
- *31.Edinger JD, Wohlgemuth WK, Radtke RA, Marsh GR, Quillian RE. Cognitive behavioral therapy for treatment of chronic primary insomnia: a randomized controlled trial. JAMA. 2001;285:1856–64. doi: 10.1001/jama.285.14.1856. [DOI] [PubMed] [Google Scholar]
- 32.Morin CM, Colecchi C, Stone J, Sood R, Brink D. Behavioral and pharmacological therapies for late-life insomnia: a randomized controlled trial. JAMA. 1999;281:991–9. doi: 10.1001/jama.281.11.991. [DOI] [PubMed] [Google Scholar]
- 33.Harbour R, Miller J. A new system for grading recommendations in evidence based guidelines. BMJ. 2001;323:334–6. doi: 10.1136/bmj.323.7308.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *34.Chambers MJ, Alexander SD. Assessment and prediction of outcome for a brief behavioral insomnia treatment program. J Behav Ther Exp Psychiatry. 23:289–97. doi: 10.1016/0005-7916(92)90051-j. [DOI] [PubMed] [Google Scholar]
- 35.Edinger JD, Olsen MK, Stechuchak KM, Means MK, Lineberger MD, Kirby A, et al. Cognitive behavioral therapy for patients with primary insomnia or insomnia associated predominantly with mixed psychiatric disorders: a randomized clinical trial. Sleep. 2009;32:499–510. doi: 10.1093/sleep/32.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *36.Riedel BW, Lichstein KL. Strategies for evaluating adherence to sleep restriction treatment for insomnia. Behav Res Ther. 2001;39:201–12. doi: 10.1016/s0005-7967(00)00002-4. [DOI] [PubMed] [Google Scholar]
- *37.Harvey L, Inglis SJ, Espie CA. Insomniacs' reported use of CBT components and relationship to long-term clinical outcome. Behav Res Ther. 2002;40:75–83. doi: 10.1016/s0005-7967(01)00004-3. [DOI] [PubMed] [Google Scholar]
- 38.Phillips BA, Collop NA, Drake C, Consens F, Vgontzas AN, Weaver TE. Sleep disorders and medical conditions in women. J Womens Health (Larchmt ); Proceedings of the Women & Sleep Workshop, National Sleep Foundation; Washington, DC. March 5–6, 2007 ; 2008. pp. 1191–9. [DOI] [PubMed] [Google Scholar]
- 39.Van Daele T, Hermans D, Van Audenhove C, Van den Bergh O. Stress Reduction Through Psychoeducation: A Meta-Analytic Review. Health Educ Behav. 2012;39:474–85. doi: 10.1177/1090198111419202. [DOI] [PubMed] [Google Scholar]
- 40.Vaidya V, Partha G, Karmakar M. Gender Disparities in Utilization of Preventive Care Service in United States. J Womens Health (Larchmt ) 2012;21:140–5. doi: 10.1089/jwh.2011.2876. [DOI] [PubMed] [Google Scholar]
- *41.Bouchard S, Bastien C, Morin CM. Self-efficacy and adherence to cognitive-behavioral treatment of insomnia. Behav Sleep Med. 2003;1:187–99. doi: 10.1207/S15402010BSM0104_2. [DOI] [PubMed] [Google Scholar]
- 42.Perlis ML, Smith MT, Orff H, Enright T, Nowakowski S, Junquist C, et al. The effects of modafinil and cognitive behavior therapy on sleep continuity in patients with primary insomnia. Sleep. 2004;27:715–25. doi: 10.1093/sleep/27.4.715. [DOI] [PubMed] [Google Scholar]
- 43.Vincent NK, Hameed H. Relation between adherence and outcome in the group treatment of insomnia. Behav Sleep Med. 2003;1:125–39. doi: 10.1207/S15402010BSM0103_1. [DOI] [PubMed] [Google Scholar]
- *44.Berger AM, VonEssen S, Kuhn BR, Piper BF, Agrawal S, Lynch JC, et al. Adherence, sleep, and fatigue outcomes after adjuvant breast cancer chemotherapy: results of a feasibility intervention study. Oncol Nurs Forum. 2003;30:513–22. doi: 10.1188/03.ONF.513-522. [DOI] [PubMed] [Google Scholar]
- *45.Matthews EE, Schmiege SJ, Cook PF, Berger AM, Aloia MS. Adherence to cognitive behavioral therapy for insomnia (CBTI) among women following primary breast cancer treatment: A pilot study. Behavioral Sleep Medicine. 2012;10:217–29. doi: 10.1080/15402002.2012.666220. [DOI] [PubMed] [Google Scholar]
- 46.McChargue DE, Sankaranarayanan J, Visovsky CG, Matthews EE, Highland KB, Berger AM. Predictors of adherence to a behavioral therapy sleep intervention during breast cancer chemotherapy. Support Care Cancer. 2010;20:245–52. doi: 10.1007/s00520-010-1060-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *47.Tremblay V, Savard J, Ivers H. Predictors of the effect of cognitive behavioral therapy for chronic insomnia comorbid with breast cancer. J Consult Clin Psychol. 2009;77:742–50. doi: 10.1037/a0015492. [DOI] [PubMed] [Google Scholar]
- 48.Manber R, Bernert RA, Suh S, Nowakowski S, Siebern AT, Ong JC. CBT for insomnia in patients with high and low depressive symptom severity: adherence and clinical outcomes. J Clin Sleep Med. 2011;7:645–52. doi: 10.5664/jcsm.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vincent N, Lewycky S. Logging on for better sleep: RCT of the effectiveness of online treatment for insomnia. Sleep. 2009;32:807–15. doi: 10.1093/sleep/32.6.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Edinger JD, Bonnet MH, Bootzin RR, Doghramji K, Dorsey CM, Espiee CA, et al. Derivation of research diagnostic criteria for insomnia: report of an American Academy of Sleep Medicine Work Group. Sleep. 2004;27:1567–96. doi: 10.1093/sleep/27.8.1567. [DOI] [PubMed] [Google Scholar]
- 51.American Academy of Sleep Medicine. . The International Classification of Sleep Disorders: Diagnositic and Coding Manual. 2. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 52.American Psychiatric Association. . Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 53.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 54.Ajzen I. The Theory of Planned Behaviour. Organizational Behavior and Human Decision Processes. 1991;50:170–211. [Google Scholar]
- 55.Prochaska JO, Diclemente CC. Stages and processes of self–change of smoking: toward an integrative model of change. J Consult Clin Psychol. 1983;51:390–5. doi: 10.1037//0022-006x.51.3.390. [DOI] [PubMed] [Google Scholar]
- 56.Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev. 1977;84:191–215. doi: 10.1037//0033-295x.84.2.191. [DOI] [PubMed] [Google Scholar]
- 57.Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory. Englewood Cliffs, N.J: Prentice Hall; 1986. [Google Scholar]
- 58.Rosenstock IM. Historical origins of the health belief model. Health Education Monographs. 1974:1–8. doi: 10.1177/109019817800600406. [DOI] [PubMed] [Google Scholar]
- 59.Morin CM. Insomnia: Psychological Assessment and Management. New York: Guilford Press; 1993. [Google Scholar]
- 60.Watzke B, Rueddel H, Koch U, Rudolph M, Schulz H. Comparison of therapeutic action, style and content in cognitive-behavioural and psychodynamic group therapy under clinically representative conditions. Clin Psychol Psychother. 2008;15:404–17. doi: 10.1002/cpp.595. [DOI] [PubMed] [Google Scholar]
- 61.Aloia MS, Goodwin MS, Velicer WF, Arnedt TJ, Zimmerman M, Skrekas J, et al. Time series analysis of treatment adherence patterns in individuals with obstructive sleep apnea. Ann Behav Med. 2008;36:44–53. doi: 10.1007/s12160-008-9052-9. [DOI] [PubMed] [Google Scholar]
- 62.Carney CE, Buysse DJ, Ancoli-Israel S, Edinger JD, Krystal AD, Lichstein KL, et al. The consensus sleep diary: standardizing prospective sleep self-monitoring. Sleep. 2012;35:287–302. doi: 10.5665/sleep.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morin CM, Belleville G, Belanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34:601–8. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Steiner JF. Rethinking adherence. Ann Intern Med. 2012;157:580–5. doi: 10.7326/0003-4819-157-8-201210160-00013. [DOI] [PubMed] [Google Scholar]
- 65.Velligan DI, Weiden PJ, Sajatovic M, Scott J, Carpenter D, Ross R, et al. Strategies for addressing adherence problems in patients with serious and persistent mental illness: recommendations from the expert consensus guidelines. J Psychiatr Pract. 2010;16:306–24. doi: 10.1097/01.pra.0000388626.98662.a0. [DOI] [PubMed] [Google Scholar]
- 66.Hilvert-Bruce Z, Rossouw PJ, Wong N, Sunderland M, Andrews G. Adherence as a determinant of effectiveness of internet cognitive behavioural therapy for anxiety and depressive disorders. Behav Res Ther. 2012;50:463–8. doi: 10.1016/j.brat.2012.04.001. [DOI] [PubMed] [Google Scholar]