Abstract
SOD2 (superoxide dismutase 2) is one of the endogenous antioxidant enzymes that protect against reactive oxygen species. While explorations of SOD2 expression regulation are mainly focused on transcriptional and post-translational activation, there are few reports about the post-transcriptional regulation of SOD2. MicroRNAs (miRNAs) are 21nt-25nt (nucleotide) small noncoding RNAs that have emerged as indispensable regulators of gene expression. Here we show that miR-146a, a widely expressed miRNA, is up-regulated by H2O2-induced stress. By sequence analysis we found a binding site for miR-146a in the sod2 mRNA 3′UTR, and a luciferase reporter assay confirmed that miR-146a can interact with this sod2 regulatory region. Our results further show that miR-146a could down-regulate the SOD2 protein expression, and antisense-miR-146a could reverse the decrease of both the SOD2 level and cell viability in H2O2 treated PC12 cells. In conclusion, here we have identified a novel function of miR-146a in the post-transcriptional regulation of SOD2 expression.
Introduction
It is well known that the oxidative stress is involved in a lot of diseases like tumors [1], Alzheimer disease [2], aging [3], arthritis [4], neurodegenerative disease [5] and atherosclerosis [6]. The unbalance between oxidation and antioxidation is the mechanism of oxidative stress [7]. In vivo, major antioxidases include superoxide dismutase (SOD), glutathione peroxidase (GPx), catalase (CAT) and thioredoxin system [7], [8].
There are three subtypes of SOD in humans, Cu, Zn-SOD (SOD1), Mn-SOD (SOD2), and extra cellular SOD (SOD3) [9]. SOD2 is a mitochondrial manganese (Mn) containing enzyme, which is composed of a 96 kDa homotetramer and localized in the mitochondrial matrix [10]. Mn at the active site of SOD2 serves to catalyze the disproportionation of superoxide anion to oxygen and H2O2 in a similar fashion as SOD1 and SOD3 [11]. SOD2 was reported to be one of the antioxidases that have anti-tumor effect [12]. Studies have shown that nuclear factor κB (NF-κB) and activator protein (AP-1) can promote sod2 expression transcriptionally [13]. SIRT3 is a deacetylase located in mitochondria, and a few studies showed that SIRT3 could deacetylate two critical lysine residues on SOD2 and promote its antioxidative activity [14], [15]. But up to now, there have rare reports about the post transcriptional regulation of sod2 gene expression.
MicroRNAs (miRNAs) are evolutionarily conserved 21nt-25nt (nucleotide) small non-coding RNAs. They bind to partially complementary target sites in messenger RNA (mRNA) 3′-untranslated regions (3′-UTRs), which results in degradation of the target mRNA, or translational repression of the encoded protein [16]. It has been estimated that approximately 60% of all mRNA are under the control of miRNA [17]. MiRNAs play a significant role in various cellular processes including development, differentiation, cell growth, morphogenesis, apoptosis, and neurological disorders [18], [19]. MiR-146a is widely expressed in different species and tissues, and studies have shown that miR-146a was involved in immunity, inflammation and viral infections by regulating different target genes [20]. Lukiw et al., showed that miR-146a was significantly up-regulated in interleukin-1β, Aβ42-, and/or oxidatively-stressed human neural (HN) cells in primary culture, and a consequence of miR-146a up-regulation was the down-regulation of the important immune system regulator complement factor H (CFH) [21]. Another study shows that miR-146a was up-regulated significantly by ROS-generating metal sulfates (iron- plus aluminum-sulfate) in human astroglial (HAG) cells [22].
To reveal the possible involvement of miRNAs in the process of SOD2 expression regulation, we performed candidate sod2-targeting miRNAs predictions, reporter gene identification of putative complimentary region, gain-of-function and loss-of-function of miRNAs on SOD2 protein under H2O2 treatment, and identified a novel functional miRNA regulating SOD2 expression via repressing SOD2 protein translation.
Materials and Methods
Cell Culture
Rat PC12 cells and human SH-SY5Y cells (Shanghai Cell Bank, China) were separately cultured in high-glucose Dulbecco’s modified Eagle’s medium (DMEM) (Sigma, USA), supplemented with 10% calf serum (HyClone, USA), 100 U/ml of penicillin and 100mg/ml of streptomycin, and maintained in a humidified atmosphere of 5% CO2 at 37°C. Cells were subcultured every 2–3 days.
H2O2 Treatment
The PC12 was seeded to a density of 1×105/ml in 96-well plates, and 24 h later, the cell was treated with 12.5 µM, 25 µM, 50 µM, 100 µM and 200 µM H2O2 for 6 h, separately.
Viability Assay
Viability of PC12 cells was measured by using MTT (3-(4, 5-dimethylthiazol-2-yl) 2, 5-diphenyl tetrazolium bromide) assay. Following incubation, the cells was aspirated and washed with the medium, and treated with 0.5 mg/L MTT (Sigma, USA). After 4 h incubation at 37°C, 100 µl DMSO (dimethyl sulfoxide) (Sigma, USA) was added to each well, then the 96-well plates were being shaked for 10 minutes and the absorbance at 590 nm of solubilized MTT formazan products was measured using a microplate reader (BioTek, USA).
Real-time Polymerase Chain Reaction
Total RNA was extracted by TRIzol (Invitrogen, USA) according to the standard procedure. The RNA was treated with RNase-free DNase I (Takara, Japan), and subsequently reverse-transcribed with oligo dT and random 6mer primer. The expression of sod2 mRNA relative to β-actin mRNA was determined by SYBR green I real-time quantitative PCR assay. The primer sequences were as follow, sod2 forward: 5′-GCCTCCCTGACCTGCCTTAC-3′ reverse: 5′-GTGATTGATATGGCCCCCG-3′; β-actin forward: 5′-CCCTAAGGCCAACCGTGAA-3′, reverse: 5′-CCAGAGGCATACAGGGACAAC-3. The relative expression of miR-146a to 5sRNA was determined as described previously by Chen [23]. The primers were as follow, reverse transcription primer GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAACCCA; miR-146a forward primer: 5′-GCGTGAGAACTGAATTCCA-3′, reverse primer: 5′-GTGCAGGGTCCGAGGT-3′; 5sRNA forward primer: 5′-CAGCCATACCACCCTGAACG-3′, reverse primer: 5′-GGTATCCCAGGTGGTCTCCC-3′.
Recombinant Plasmid Construction and Dual Luciferase Reporter Assay
The entire sod2 mRNA 3′-UTR was PCR-amplified utilizing the sense (5′-GCTCTAGATCCGCCAGGCTGTGTGTC-3′) and antisense (5′-GCTCTAGAAGTAATGTGCATGCCTGGGG-3′) primers according to standard procedures and cloned into the XbaI site of the pGL3-Promoter plasmid (Promega, USA). Plasmid DNA was subsequently isolated from recombinant colonies and sequenced to ensure the authenticity and direction of the inserted sod2 3′UTR. For the luciferase reporter assays, PC12 cells were grown in DMEM with 10% FBS to 70% confluence in 48-well plates. Cells were transfected with 100 ng of firefly luciferase reporter vector containing the sod2 3′UTR (named pGL3-sod2-3′UTR) and miR-146a mimics (Genepharma, China) (final concentration was 100 nM) or negative control using lipofectamine 2000 (Invitrogen, USA). 10 ng pRL-TK vector (Promega, USA) was co-transfected as internal control for normalization of the transfection efficiency. After 24 hours, transfected cells were harvested with ice-cold phosphate-buffered saline, and dual luciferase assay was performed according to the manufacturer’s protocol (Promega, USA).
MiRNA Transfection
miR-146a (rno-miR-146a and hsa-miR-146a share the same sequence) was synthesized by GenePharma company (China). For functional analysis, miR-146a (miR-146a) mimics, antisense miR-146a mimics, non-targeting miRNA mimics (negative control), and scrambled antisense miR-146a (antisense miRNA negative control) were transfected into PC12 and SH-SY5Y cells with lipofectamine according to the manufacturer’s instruction, separately. The final concentration of miR-146a mimics and non-targeting miRNA mimics was 100 nM, while the concentration of antisense miR-146a and antisense miRNA negative control was 33 nM. Cells were incubated for 48 h until lysis.
Western Blot
After washing with cold PBS 3 times, cells were lysed in RIPA buffer of 150 mM NaCl, 10mM Tris-HCl, pH 7.4, 0.5% Triton X-100, and protease inhibitors (Sigma, USA), homogenized on ice, and centrifuged at 12,000 g at 4°C for 15 min. The supernatant was collected and stored at −80°C until use. Protein concentration was determined using the BCA Protein Assay Kit (Pierce, USA). 25 µg of protein extraction was loaded on 12% tris-polyacrylamide gels. The proteins were then transferred to nitrocellulose membrane (Milipore, USA). The membranes were blocked in 5% non-fat dry milk, washed in TBS with 0.05% Tween 20 (TBST), and incubated with mouse anti-SOD2 (Santa cruze, USA) in room temperature overnight at 4°C. Mouse anti-β-actin (Santa cruze, USA) was used for internal control. The membranes were washed three times in TBST and incubated for 1 hour with secondary antibodies conjugated to horseradish peroxidase, washed three times in TBST, and treated with Immun-Star HRP peroxide buffer and Luminol/Enhancer (Zhongshan, China) for chemiluminescence detection of protein bands.
Statistical Analysis
Statistical analysis was done with one way ANOVA. Differences with P<0.05 were considered statistically significant.
Results
H2O2 Decreased the Survival Rate of PC12 Cells
To investigate the survival rate changes of PC12 cells under the stimulation of H2O2, we treated the PC12 cells with different concentrations of H2O2 for 6 h. The results indicated that low concentrations of H2O2 (12.5 µM-25 µM) have no obvious effect on PC12 viability. While, with the increase of H2O2 concentrations, the viabilities of PC12 cells were gradually decreased significantly. (Fig. 1) Significant viability decrease was observed at 100 µM and 200 µM.
Figure 1. H2O2 decreased PC12 cells viability.
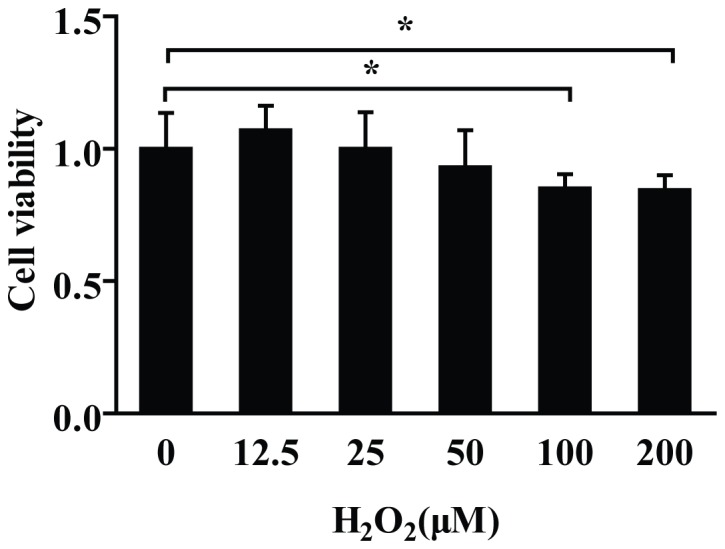
MTT assay showed that 12.5∼25 µM H2O2 treatment had no obvious effect, while 50 µM, 100 µM, and 200 µM H2O2 treatment resulted in cell viability decrease by 7%, 15% and 16%, respectively. When compared with control group (0 µM), 100 µM and 200 µM group showed significant difference. Data are shown mean ± SD (n = 6). *P<0.05, as compared with control.
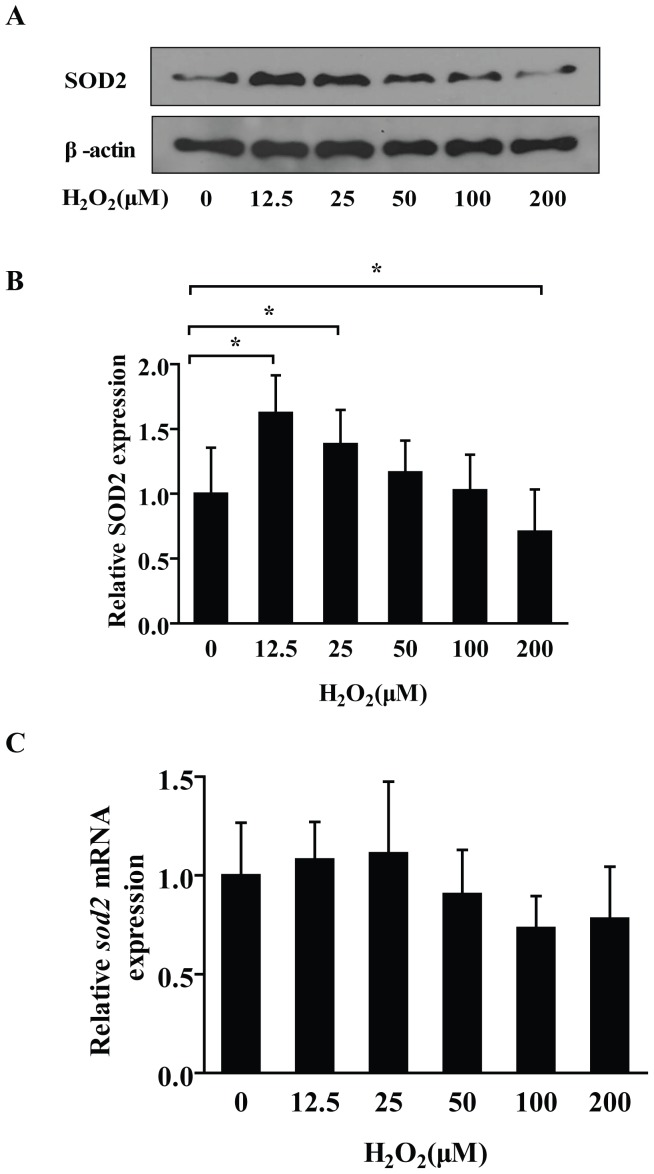
H2O2 Regulated the Expression of SOD2 Protein
Our results demonstrated that, after 6 h treatment, low concentration (12.5 µM) of H2O2 could result in a relative high level of SOD2 protein. Evidently, the SOD2 protein levels were decreased gradually along with the elevation of H2O2 concentrations, and the SOD2 level in 200 µM was lower than the control group (Fig. 2A and 2B). Interestingly, there were no differences of sod2 mRNA levels between each of the H2O2-treated groups (Fig. 2C).
Figure 2. H2O2 down-regulated the expression level of SOD2 protein in a dose-dependent manner.
(A) Along with the increase of H2O2 concentrations, the SOD2 protein was decreased gradually. Interestingly, the SOD2 protein showed an evident higher level with 12.5 µM and 25 µM H2O2 stimulation. With the higher concentration 200 µM H2O2 stimulation, it decreased significantly with a lower level than control group. (B) The relative amounts of SOD2, which were represented by the intensity ratio between SOD2 and β-actin in each lane. Data are shown mean ± SD from 3 independent experiments, each with 6 replicates. (C) H2O2 had no obvious effect on sod2 mRNA expression in PC12 cells. After 6 h treatment with different concentrations of H2O2, the RT-PCR assay demonstrated there were no statistical differences of PC12 sod2 mRNA expression levels between the groups. Data are shown the mean ± SD (n = 6). ANOVA (Dunnett) *P<0.05, as compared with control.
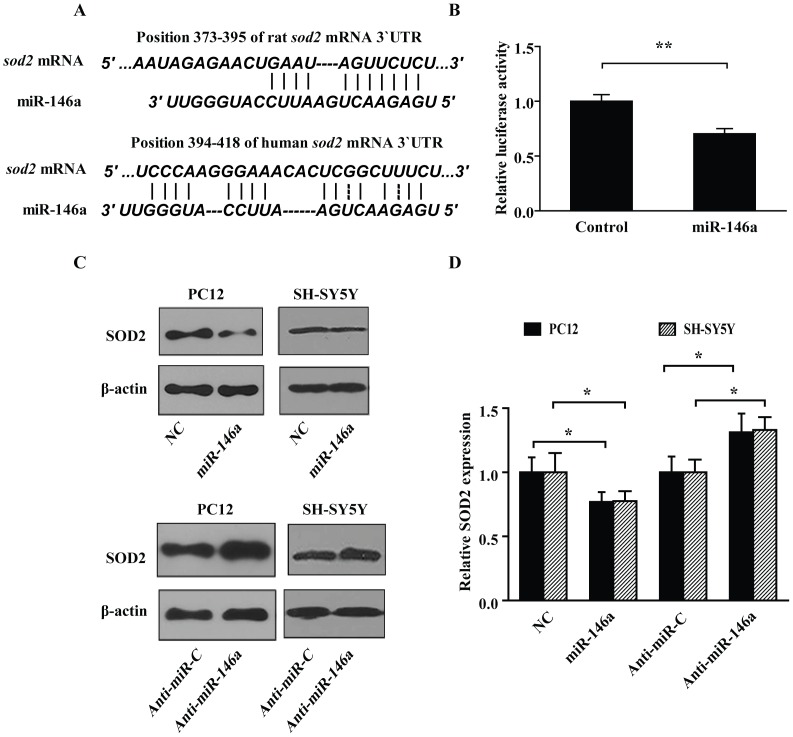
MiR-146a Interacted with the 3′UTR of sod2 mRNA
With bioinformatics sequence analysis, we found a binding site of miR-146a seed region both in Rattus norvegicus and Homo sapiens sod2 mRNA 3′UTR (Fig. 3A). To investigate whether miR-146a interacts with rat sod2 mRNA 3′UTR in vitro, we inserted the whole length region of rat sod2 mRNA 3′UTR into pGL3-promoter luciferase reporter plasmid 3′UTR region. The reporter constructs were co-transfected with miR-146a mimics in PC12 cells, and the results demonstrated that miR-146a mimics could down regulate the luciferase activity by 29.7% versus negative control group (Fig. 3B).
Figure 3. SOD2 was identified as a target of miR-146a in PC12 and SH-SY5Y cells.
(A) Prediction of the interaction between sod2 mRNA 3′UTR and miR-146a. Sequence analysis showed that rat sod2 mRNA 3′UTR 388-394 position mated with the rat miR-146a 2-8th nucleotides. (B) miR-146a down-regulated the luciferase activity of pGL3-Promoter-sod2-3′UTR. Dual luciferase reporter assay demonstrated that miR-146a mimics could down-regulate relative luciferase activity of pGL-sod2-3′UTR for 29.7%, compared with control group. Data are shown the mean ± SD (n = 6). **P<0.01, as compared with control. (C) miR-146a down-regulated the expression of SOD2 protein, while antisense-miR-146a reversely increased the amount of SOD2 protein. NC, non-targeting scrambled-miRNA control. Anti-miR-C, scrambled antisense miRNA control. Anti-miR-146a, antisense-miR-146a. (D) The relative amounts of SOD2 protein treated with miR-146a and antisense-miR-146a were presented by the intensity ratio between SOD2 and β-actin in each lane. Data are shown the mean ± SD from 3 independent experiments, *P<0.05.
MiR-146a Decreased SOD2 Expression
To investigate whether miR-146a participates in the regulation of SOD2 expression, we transfected miR-146a mimics or non-targeting miRNA mimics into PC12 and SH-SY5Y cells for 48 h, separately. Meanwhile, the antisense miR-146a mimics were transfected for the blockage of the internally originated miR-146a. The western blot assay showed that miR-146a mimics decreased the SOD2 protein expression significantly, while antisense miR-146a mimics up-regulated the SOD2 protein slightly, when compared with non-targeting scrambled-miRNAs and scrambled antisense miR-146a groups, respectively. Taken together, these results showed that miR-146a could regulate the SOD2 expression, and sod2 was a target gene of miR-146a. (Fig. 3C and 3D). Also, these results in Homo sapiens cells as well as in Rattus norvegicus cells revealed the novel possible anti-oxidative role of miR-146a.
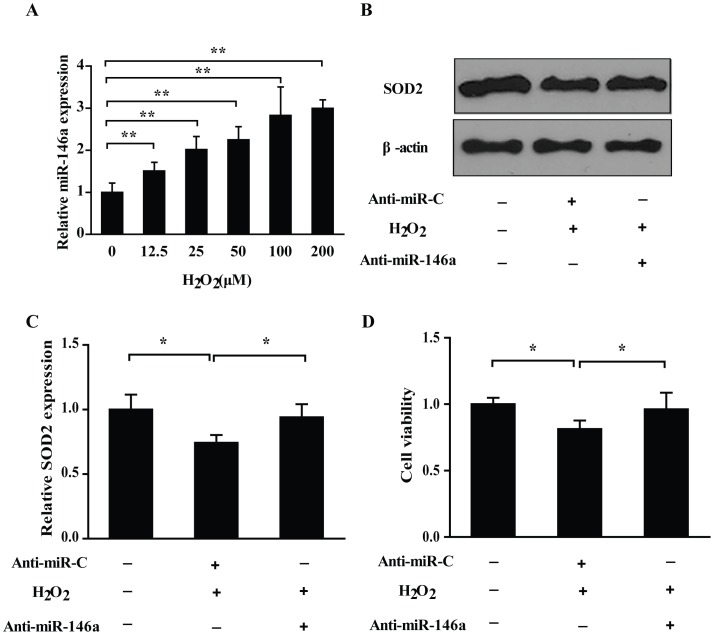
H2O2 Induced miR-146a Expression in PC12 Cells
As shown in Fig. 4A, miR-146a was up-regulated in a dose-dependent manner by H2O2 in PC12, which was inversely correlated with the SOD2 expression under H2O2 treatment. These results indicated the involvement of miR-146a in SOD2 protein expression regulation with H2O2 stimulation.
Figure 4. Identification of the regulatory role of miR-146a in the H2O2 induced changes of SOD2 protein and PC12 cells viability.
(A) H2O2 increased the miR-146a expression in PC12 cells. Real-time RT-PCR revealed that H2O2 enhanced the miR-146a relative expression level in a dose-dependent manner in PC12 cells after the stimulation with H2O2 for 6 h. Data are shown the mean ± SD (n = 6), **P<0.01, as compared with control. (B) Down-regulation of SOD2 was reversed by antisense-miR-146a. PC12 cells were stimulated with 200 µM H2O2 after a pretreatment with antisense-miR-146a for 24 h. Western blot assay showed antisense-miR-146a reversed the SOD2 protein decrease induced by H2O2. (C) The relative amounts of SOD2 protein are presented by the intensity ratio between SOD2 and β-actin in each lane. Data are shown the mean ± SD from 3 independent experiments, *P<0.05. (D) Antisense-miR-146a retrieved the decrease of PC12 cells viability induced by 200 µM H2O2. Data are shown the mean ± SD (n = 6), *P<0.05.
Antisense-miR-146a Reversed the Decreased SOD2 Expression and PC12 Cells Viability
To verify whether the decrease of miR-146a possesses an effect on SOD2 expression and cell viability with H2O2 treatment, we pretreated PC12 cells with antisense-miR-146a mimics for 24 h, and then stimulated with 200 µM H2O2 for 6 h. The results demonstrated that antisense-miR-146a mimics pretreatment could reverse the down-regulated SOD2 expression (Fig. 4B and 4C) and decreased cell viability (Fig. 4D) by H2O2 in PC12 cells.
Discussion
Our results indicate that high concentration of H2O2 causes cell viability decrease in PC12 cells, which is in accord with the previous study by Wei [24]. SOD2 plays an important role in maintenance of cell survival under stress conditions, and a lot of studies confirmed that cell viability decrease was correlated with SOD2 down-regulation under stress conditions [25], [26]. Meanwhile, when introduced exogenous SOD2 into cell the viability decrease could be improved more or less [27], [28]. To date, the efforts to decipher the mechanism of Sod2 gene expression are mainly focused on transcription and post-translational modification. Nevertheless, the post-transcriptional regulatory processes of Sod2 expression were seldom explored. Importantly, this regulatory process has been increasingly identified significant and necessary in the process of gene regulation, such as miRNAs down-regulation mechanism. Our studies demonstrate that H2O2 could significantly alter the SOD2 protein expression. Intriguingly, there are no differences of sod2 mRNA levels between H2O2-treated group and control group. These results indicate that the variation of SOD2 protein under H2O2 treatment is mainly caused in SOD2 protein repression process other than mRNA degradation.
MiRNAs are a class of non-coding RNA, and recent years it has been proved that they play a pivotal role in gene expression regulation. MiR-146a is a widely expressed miRNAs in animal, and recently it has been confirmed to be involved in immunity, inflammation, viral infections, etc. [20]. With the bioinformatics tools, including RegRNA and Miranda, Dharap found that SOD2 was possibly a target of miR-145. After transient middle cerebral artery occlusion (MCAO), the rat post-ischemic brain showed highly expressed miR-145. Meanwhile, miR-145 inhibitor pretreatment could induce SOD2 expression in reperfusion brain, indicating that elevated miR-145 could depress the SOD2 expression in MCAO brain [29]. In addition, a study using luciferase reporter assay revealed that miR-222 could bind the sod2 3′UTR, and the expression of SOD2 was reduced in a carcinoma cells when transfected with ectopic miR-222 [30]. Also, Kriegel and colleagues demonstrated that treatment of human renal epithelial cells with TGF-β1 resulted in up-regulation of miR-382 and down-regulation of SOD2. In this study, knockdown of miR-382 could attenuate TGF-β1 induced down-regulation of SOD2 protein, and 3′UTR reporter assay showed miR-382 could target sod2 mRNA [31]. Taken together, these results suggest that miR-382, miR-145 and miR-222 can regulate the expression of SOD2 in different pathological processes.
In our study, the results of reporter assay indicate the interaction between miR-146a and sod2 3′UTR, and both the gain-of-function and loss-of-function experiments demonstrate that miR-146a could regulate the expression of SOD2. Furthermore, we confirm that miR-146a is up-regulated by H2O2, and H2O2-caused cell viability decrease can be reversed by antisense-miR-146a. These results identify the post-transcriptional regulatory role of miR-146a in SOD2 expression, indicating the possible involvement of miR-146a in oxidative stress.
In conclusion, this study demonstrates that Sod2 expression can be regulated by miR-146a, and identifies a novel function of miR-146a in sod2 expression via inhibiting protein translation.
Funding Statement
This work was supported by the National Science and Techology Major Project (2012ZX09J12201), the National Key Techology R&D Program of China (2009BAK59B01), the Medicinal Science and Techology Research Project (BWS11J052), the National Natural Science Foundation of China (30973686), and the Foundation of State Key Laboratory of Space Medicine Fundamentals and Application, China Astronaut Research and Training Center(SMFA10B01, SMFA12B05, SMFA09A06). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Gerald D, Berra E, Frapart YM, Chan DA, Giaccia AJ, et al. (2004) JunD reduces tumor angiogenesis by protecting cells from oxidative stress. Cell 118: 781–794. [DOI] [PubMed] [Google Scholar]
- 2. Smith MA, Rottkamp CA, Nunomura A, Raina AK, Perry G (2000) Oxidative stress in Alzheimer’s disease. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease 1502: 139–144. [DOI] [PubMed] [Google Scholar]
- 3. Balaban RS, Nemoto S, Finkel T (2005) Mitochondria, oxidants, and aging. Cell 120: 483–495. [DOI] [PubMed] [Google Scholar]
- 4. Tak PP, Zvaifler NJ, Green DR, Firestein GS (2000) Rheumatoid arthritis and p53: how oxidative stress might alter the course of inflammatory diseases. Immunology today 21: 78–82. [DOI] [PubMed] [Google Scholar]
- 5. Lin MT, Beal MF (2006) Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443: 787–795. [DOI] [PubMed] [Google Scholar]
- 6. Harrison D, Griendling KK, Landmesser U, Hornig B, Drexler H (2003) Role of oxidative stress in atherosclerosis. The American journal of cardiology 91: 7–11. [DOI] [PubMed] [Google Scholar]
- 7. Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M (2006) Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact 160: 1–40. [DOI] [PubMed] [Google Scholar]
- 8. Fukai T, Ushio-Fukai M (2011) Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid Redox Signal 15: 1583–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mates JM, Perez-Gomez C, Nunez de Castro I (1999) Antioxidant enzymes and human diseases. Clin Biochem 32: 595–603. [DOI] [PubMed] [Google Scholar]
- 10. Fridovich I, Freeman B (1986) Antioxidant defenses in the lung. Annu Rev Physiol 48: 693–702. [DOI] [PubMed] [Google Scholar]
- 11. Hsu JL, Hsieh Y, Tu C, O’Connor D, Nick HS, et al. (1996) Catalytic properties of human manganese superoxide dismutase. J Biol Chem 271: 17687–17691. [DOI] [PubMed] [Google Scholar]
- 12. Behrend L, Henderson G, Zwacka RM (2003) Reactive oxygen species in oncogenic transformation. Biochem Soc Trans 31: 1441–1444. [DOI] [PubMed] [Google Scholar]
- 13. Kim HP, Roe JH, Chock PB, Yim MB (1999) Transcriptional activation of the human manganese superoxide dismutase gene mediated by tetradecanoylphorbol acetate. Journal of Biological Chemistry 274: 37455. [DOI] [PubMed] [Google Scholar]
- 14. Qiu X, Brown K, Hirschey MD, Verdin E, Chen D (2010) Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab 12: 662–667. [DOI] [PubMed] [Google Scholar]
- 15. Kong X, Wang R, Xue Y, Liu X, Zhang H, et al. (2010) Sirtuin 3, a New Target of PGC-1α, Plays an Important Role in the Suppression of ROS and Mitochondrial Biogenesis. PLoS One 5: e11707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bartel DP (2004) MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell 116: 281–297. [DOI] [PubMed] [Google Scholar]
- 17.Chan EK, Ceribelli A, Satoh M (2012) MicroRNA-146a in autoimmunity and innate immune responses. Ann Rheum Dis (Suppl 2): ii90–95. [DOI] [PMC free article] [PubMed]
- 18. Ambros V (2004) The functions of animal microRNAs Nature 431: 350–355. Proceedings of the National Academy of Sciences of the United States of America 103: 3687–3692. [DOI] [PubMed] [Google Scholar]
- 19. Mendell JT (2005) MicroRNAs: critical regulators of development, cellular physiology and malignancy. Cell cycle (Georgetown, Tex) 4: 1179. [DOI] [PubMed] [Google Scholar]
- 20. Li L, Chen XP, Li YJ (2010) MicroRNA-146a and human disease. Scand J Immunol 71: 227–231. [DOI] [PubMed] [Google Scholar]
- 21. Lukiw WJ, Zhao Y, Cui JG (2008) An NF-kB-sensitive micro RNA-146a-mediated inflammatory circuit in Alzheimer disease and in stressed human brain cells. Journal of Biological Chemistry 283: 31315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pogue AI, Percy ME, Cui JG, Li YY, Bhattacharjee S, et al. (2011) Up-regulation of NF-kB-sensitive miRNA-125b and miRNA-146a in metal sulfate-stressed human astroglial (HAG) primary cell cultures. J Inorg Biochem 105: 1434–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen C, Ridzon DA, Broomer AJ, Zhou Z, Lee DH, et al. (2005) Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res 33: e179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wei Z, Bai O, Richardson J, Mousseau D, Li X (2003) Olanzapine protects PC12 cells from oxidative stress induced by hydrogen peroxide. Journal of neuroscience research 73: 364–368. [DOI] [PubMed] [Google Scholar]
- 25. Van Remmen H, Williams MD, Guo Z, Estlack L, Yang H, et al. (2001) Knockout mice heterozygous for Sod2 show alterations in cardiac mitochondrial function and apoptosis. American Journal of Physiology-Heart and Circulatory Physiology 281: H1422–H1432. [DOI] [PubMed] [Google Scholar]
- 26. Sharma CS, Sarkar S, Periyakaruppan A, Barr J, Wise K, et al. (2007) Single-walled carbon nanotubes induces oxidative stress in rat lung epithelial cells. Journal of nanoscience and nanotechnology 7: 2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Plymate SR, Haugk KH, Sprenger CC, Nelson PS, Tennant MK, et al. (2003) Increased manganese superoxide dismutase (SOD-2) is part of the mechanism for prostate tumor suppression by Mac25/insulin-like growth factor binding-protein-related protein-1. Oncogene 22: 1024–1034. [DOI] [PubMed] [Google Scholar]
- 28. Fukui M, Zhu BT (2010) Mitochondrial superoxide dismutase SOD2, but not cytosolic SOD1, plays a critical role in protection against glutamate-induced oxidative stress and cell death in HT22 neuronal cells. Free Radical Biology and Medicine 48: 821–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dharap A, Bowen K, Place R, Li LC, Vemuganti R (2009) Transient focal ischemia induces extensive temporal changes in rat cerebral microRNAome. J Cereb Blood Flow Metab 29: 675–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu X, Yu J, Jiang L, Wang A, Shi F, et al. (2009) MicroRNA-222 regulates cell invasion by targeting matrix metalloproteinase 1 (MMP1) and manganese superoxide dismutase 2 (SOD2) in tongue squamous cell carcinoma cell lines. Cancer Genomics Proteomics 6: 131–139. [PMC free article] [PubMed] [Google Scholar]
- 31. Kriegel AJ, Fang Y, Liu Y, Tian Z, Mladinov D, et al. (2010) MicroRNA-target pairs in human renal epithelial cells treated with transforming growth factor beta 1: a novel role of miR-382. Nucleic Acids Res 38: 8338–8347. [DOI] [PMC free article] [PubMed] [Google Scholar]