Abstract
Background
CD22 expression occurs in > 90% of patients with ALL. Inotuzumab ozogamicin, a CD22 monoclonal antibody bound to calicheamicin, is active in ALL.
Methods
Patients with refractory-relapsed ALL were treated with inotuzumab. The first 49 patients received single-dose inotuzumab 1.3-1.8 mg/m2 IVq 3-4 weeks.In the next 41 patients, the schedule was modified to weekly, 0.8 mg/m2 D1, 0.5mg/m2 D8 and 15, q 3-4 weeks, based on higher invitro efficacy with more frequent exposure.
Results
Ninety patients were treated; 68% were in salvage 2 or beyond. Overall, 17 patients (19%) achieved complete response (CR), 27 (30%) had CRp (no platelet recovery), and 8 (9%) had marrow CR (no recovery of counts), for an overall response rate of 58%. Response rates were similar single-dose and weekly dose (57% versus 59%). The median survival was 6.2 months, 5.0 months with single-dose and 7.3 months with weekly dose. Median survival was 9.2 months in Salvage 1 (37% at 1 year), 4.3 months in Salvage 2, and 6.6 months in Salvage 3 or later. The median remission duration was 7 months. Reversible bilirubin elevation, fever and hypotension were observed less frequently on the weekly dose. Allogeneic stem cell transplant (SCT) was performed 36/90 patients (40%); veno-occlusive disease was noted in 6/36 patients post SCT (17%), less frequent post weekly schedule ( 7%), and with less alkylators in preparative regimen.
Conclusions
Inotuzumab single-agent therapy is highly active, safe, and convenient in refractory-relapsed ALL. Weekly dose appears to be equally effective and less toxic than single-dose.
INTRODUCTION
Modern multi-agent combination chemotherapy regimens in adults with acute lymphocytic leukemia (ALL) result in complete response rates of 80-90% and long-term survival rates of 35-50% (1-3). Improvement of adult ALL therapy is unlikely to result from further intensification therapy, since the current regimens are already associated with significant toxicities.
Leukemic ALL cells express CD20 in about 50% of cases, and CD22 and CD19 in 90% of cases. This provides opportunities to utilize new monoclonal antibodies in ALL, alone or in combinations with chemotherapy or with other monoclonal antibodies. Rituximab as a single agent had minimal activity in ALL, but improved survival when combined with chemotherapy in CD20 positive ALL (4-8). This encouraged investigational therapies with other monoclonal antibodies directed against ALL surface markers (9, 10).
Inotuzumab ozogamicin is a CD22 monoclonal antibody bound to calicheamicin, a natural product of Micromonospora echinospora, which is significantly more toxic than cytotoxic chemotherapy (11). Inotuzumab binds CD22 with subnanomolar affinity and is rapidly internalized, delivering the conjugated calicheamicin intracellularly. Calicheamicin binds to the minor DNA grove causing double strand DNA breaks, resulting in cell apoptosis. A phase 2 study of single-dose inotuzumab 1.8 mg/m2 every 3-4 weeks in refractory and relapsed ALL resulted in a marrow CR rate of 57%. Adverse events included fever, brief episodes of hypotension, and liver function abnormalities (12). Preclinical studies suggested that lower-dose more frequent schedules of inotuzumab may improve anti-ALL efficacy and reduce toxicities. This resulted in amending the study to change the inotuzumab dose schedule to weekly, 0.8 mg/m2 on Day 1, and 0.5 mg/m2 on Day 8 and 15 every 3-4 weeks, for the same total dose of inotuzumab 1.8 mg/m2 per course. This report updates our experience in 90 patients with refractory and relapsed ALL treated with weekly inotuzumab (n=41), and with the previously reported and now updated single-dose inotuzumab (n=49).
PATIENTS AND METHODS
Study Group
Patients with a confirmed diagnosis of refractory or relapsed ALL pre-B were eligible. Eligibility criteria were identical for the single-dose and weekly inotuzumab schedules (12). Inclusion criteria were ECOG performance status 0 to 3; adequate liver function (bilirubin ≤ 1.5 mg/dL and liver enzymes ≤ 3 × upper limit of normal, unless considered due to leukemia) and renal functions (creatinine ≤ 2.0 mg/dL); adequate cardiac functions (New York Heart Association class ≥ 3 or ejection fraction < 45% excluded). Exclusion criteria included allogeneic stem cell transplant (SCT) in the previous 4 months, pregnant or breast feeding women, and patients with known hepatitis B disease.
The study was a single institution study conducted at the MD Anderson Cancer Center. The study protocol was approved by the Institutional Review Board, in compliance with institutional guidelines. Patients signed informed consent in compliance with the Declaration of Helsinki.
Therapy
Single-dose inotuzumab was given at 1.3-1.8 mg/m2 intravenously as a short infusion once every 3-4 weeks. Weekly inotuzumab was given as 0.8 mg/m2 on Day 1 and 0.5 mg/m2 on Days 8 and 15, for a total dose of 1.8 mg/m2 per course. Courses were repeated every 3-4 weeks. Patients received the recommended pre-medication with acetaminophen 650 mg orally, diphenhydramine 10-25 mg IV, hydrocortisone 25 mg IV.
Inotuzumab was given as a short infusion over 1 hour. Courses were given every 4 weeks depending on the recovery of the counts and of the bone marrow status on Days 21 and 28. Briefly, if the bone marrow studies showed persistent or increasing leukemia on Day 21 and 28, a subsequent course of inotuzumab was given regardless of peripheral counts. If the blasts were reduced or 5% or less by Day 21-28, a subsequent course was given only after recovery of the counts to at least pre-treatment levels. Persistent thrombocytopenia was not a condition to delay therapy. In contrast to the previous study with single-dose inotuzumab (12), the weekly dose schedule study did not include the addition of rituximab in patients with stable disease, or no improvement or progression after 2 courses of inotuzumab. Patients achieving CR or marrow CR after one or two courses of therapy were allowed to receive two additional courses of therapy, for a maximum of four cycles. Additional treatments were based on response and liver toxicities in the previous four cycles. Patients achieving any response could also continue on treatment for up to 8 cycles. Subsequent cycles of inotuzumab were given at the same dose. Patients with grade 3 or worse toxicity and a favorable response to therapy could receive inotuzumab in subsequent cycles at a 25% dose reduction. Patients who developed CNS leukemia on inotuzumab and who had a positive response were allowed to continue on therapy and receive CNS-directed intrathecal chemotherapy after assessment of the benefit: risk ratio to the patient.
Patients received antibiotics, antifungals, and antiviral agents per institutional guidelines. Antifungal azoles prophylaxis was delayed for at least 24 hours after completion of inotuzumab. Patients with rapid increases in white blood cell counts could receive hydroxyurea or a short course of steroids at the beginning of the first cycle.
Suitability for allogeneic SCT was assessed in all patients. Eligible patients were considered for allogeneic SCT after achieving at least a marrow complete response, whenever possible. Minimal residual disease status was assessed by six-colour multiparameter flow cytometry of abnormal ALL surface antigen expression levels, according to standard protocols. The panel included 15 antigens: CD10, CD13, CD15, CD19, CD20, CD22, CD25, CD33, CD34, CD38, CD45, CD52, CD58, CD66c and CD81. 1 million cells were stained with a stain-lyse-wash technique, and 200,000 cells were analyzed per sample on FacsCanto II instruments (BD Biosciences, San Diego, CA, USA). Data were analyzed with FCS Express 3 software. Negative status was defined as fewer than 10-4 lymphocytic cells.
Statistical Considerations
Response criteria were standard. A CR was defined as disappearance of all disease with marrow blasts 5% or less, neutrophils ≥ 1.0 × 109 / L, and platelet count > 100 × 109 / L. A marrow CR with incomplete recovery of platelets (CRp) was defined as CR but without platelet recovery to ≥ 100 × 109 / L. A marrow CR with incomplete recovery (CRi) was defined as CR but without recovery of platelets to ≥ 100 × 109 / L or neutrophil counts to ≥ 109/L. Survival and response duration were calculated by the Kaplan-Meier method.
Early stopping rules for both single-dose and weekly inotuzumab were planned for futility if the CR + CRi + CRp + PR / total treated was: ≤ 0/10, 1/17, 2/24, 3/31, 4/37. A total of 40 patients were planned to be treated on single-dose inotuzumab. This was expanded to 60 patients because of high efficacy. However, the schedule was modified to a weekly schedule, which was IRB approved and modified after 49 patients were treated with single-dose inotuzumab. A total of 90 patients, including 41 patients on weekly inotuzumab were planned to be treated.
When comparing the weekly and single-dose inotuzumab study groups, comparison of parameters used the Chi-squared method. Univariate analyses were conducted using standard methods.
RESULTS
Study Group
The 49 patients treated with single-dose inotuzumab were accrued between June 2010 and March 2011; the 40 patients treated with weekly inotuzumab were accrued between March 2011 and September 2012.The median age patients in of the total study group was 39.5 years (range 4 to 84 yrs); 25 patients (28%) were 60 years or older and 6 (7%) were ≤ 18 years. Nine patients (10%) had a performance status of 2 to 3. Twenty-nine patients (32%) received inotuzumab as Salvage 1, 34(38%) as Salvage 2, and 27(30%) as Salvage 3 or greater. Ten patients (11%) had a prior allogeneic SCT. The study group characteristics indicate the heavily treated nature of this refractory-relapsed ALL study group (Table 1). All patients expressed high levels of CD22 positivity 50% or more on leukemic cells.
Table1.
Characteristics of the Study Group (n=90)
| No. (%) on Inotuzumab Schedule | ||||
|---|---|---|---|---|
| Characteristic | Category | Single-dose (n=49) | Weekly (n=41) | Overall (n=90) |
| Age (yrs) | ≤ 18 | 3 (6) | 3 (7) | 6 (7) |
| ≥ 60 | 12 (24) | 13 (32) | 25 (28) | |
| PS (ECOG) | 0-1 | 44 (90) | 37 (90) | 81 (90) |
| ≥ 2 | 5 (10) | 4 (10) | 9 (10) | |
| Salvage status | S1 | 13 (27) | 16 (39) | 29 (32) |
| S1, CRD1 < 12 mos | 3 (6) | 12 (29) | 15 1(7) | |
| S1, CRD1 ≥ 12 mos | 7 (14) | 2 (5) | 9 (10) | |
| S2 | 24 (49) | 10 (24) | 34 (38) | |
| ≥ S3 | 12 (24) | 15 (37) | 27 (30) | |
| Prior HCVAD regimen | Yes | 28 (57) | 29 (71) | 57 (63) |
| Karyotype | Diploid | 12 (24) | 9 (22) | 21 (23) |
| Ph-positive | 7 (14) | 8 (20) | 15 (17) | |
| T (4;11) | 5 (10) | 3 (7) | 8 (9) | |
| Other | 25 (51) | 21 (51) | 46 (51) | |
| Prior allo SCT | Yes | 7 (14) | 3 (7) | 10 (11) |
| % CD22-positive | > 90 | 28 (57) | 31 (76) | 59 (66) |
| 70-89 | 14 (29) | 8 (20) | 22 (24) | |
| 50-69 | 7 (14) | 2 (5) | 9 (10) | |
• Patients on weekly inotuzumab were more frequently in Salvage 1 with CRD1 < 12 months (p=.003) while patients on single-dose inotuzumab were more frequently in Salvage 2 (p=.016). No other significant differences were evident in the 2 study group characteristics.
Response
Overall, 17 patients (19%) achieved CR, 27 patients (30%) had CRp, and 8 patients (9%) had CRi. Thirty-four patients (38%) had resistant disease and 4 patients (4%) died within 4 weeks of start of therapy. Responses were thus observed in 52 of the 90 patients treated, for an overall response rate of 58%. Response rates with weekly and single-dose inotuzumab were similar (Table 2). The median number of cycles were 2 (range 1-6) with weekly and 2 (range 1-5) with single-dose inotuzumab.
Table 2.
Responses
| No. (%) on Inotuzumab Therapy | |||
|---|---|---|---|
| Response | Single-dose (n=49) | Weekly (n=41) | Overall (n=90) |
| CR | 9 (18) | 8(20) | 17 (19) |
| CRp | 14 (29) | 13(32) | 27 (30) |
| CRi (marrow CR) | 5 (10) | 3(7) | 8 (9) |
| PR | 0 | 0 | 0 |
| Resistant | 19(39) | 15(37) | 34 (38) |
| Death < 4 weeks | 2 (4) | 2(5) | 4 (4) |
Among the 52 patients achieving response with inotuzumab, 29 patients had chromosomal abnormalities at the start of therapy and sufficient metaphases for analysis at morphologic CR. Among them, a complete cytogenetic response was also observed in 26 patients (90%). The complete cytogenetic response by morphologic response was: 8 cytogenetic CR/9 morphologic CR (89%); 11 cytogenetic CR/ 13 morphology CRp (85 %);7 cytogenetic CR/7morphologic CRi (100 %). Multiparameter flow cytometric studies for minimal residual disease (MRD) were performed in 50 patients achieving morphologic marrow CR. A negative MRD status was observed in 36 of 50 responding patients (72 %). Considering the total study group, MRD negative status was achieved in 17/40 patients treated with weekly (42%) and in 19/49 patients (39%) treated with single-dose inotuzumab (p value not significant). The MRD negative status by morphologic response was: 14 MRD negative/17 CR (82 %);19 MRD negative / 25 CRp (76%);3 MRD negative /8 CRi (38%).
Most patients achieving CR obtained it early (14 CRs after one course; 3CRs after 2 or more courses). Among patients achieving CRp, 16 achieved it after one course and 11 after 2 or more courses. Among patients achieving CRi, 1 achieved it after one course and 7 after 2 or more courses.
In the total study group, lower response rates were observed among patients with Philadelphia chromosome positive ALL and those with translocation (4; 11) (38-40% versus 57-81% for others; p value 0.047). Response rate was also lower in patients treated in Salvage 2 or later (48-50% versus 76% in Salvage 1; p=0.056) (Table 3). Considering the small numbers, the trends of associations were similar with weekly and single-dose inotuzumab schedules.
Table 3.
Association of Characteristics with Response (n=90)
| Characteristic | Category | No. | No. Response (%) | PValue |
|---|---|---|---|---|
| Age (yrs) | < 60 | 65 | 37/(57) | P=.79 |
| ≥ 60 | 25 | 15/ (60) | ||
| PS (ECOG) | 0-1 | 81 | 48/ (59) | P=.39 |
| ≥ 2 | 9 | 4/ (44) | ||
| Salvage status | S1 | 29 | 22/ (76) | |
| S1, CRD1 < 12 mos | 15 | 11/ (73) | ||
| S1, CRD1 ≥ 12 mos | 9 | 7/78) | P=.056 | |
| S2 | 34 | 17/(50) | ||
| ≥ S3 | 27 | 13/ (48) | ||
| Karyotype | Diploid | 21 | 17/ (81) | |
| Ph – positive | 15 | 6/ (40) | ||
| T (4;11) | 8 | 3/(38) | P=.047 | |
| Other | 46 | 26/(57) | ||
| % CD22 - positive | > 90 | 59 | 33/(56) | |
| 70-89 | 22 | 13/(59) | P=.823 | |
| 50-69 | 9 | 6/(67) | ||
| Prior allogeneic SCT | Yes | 10 | 5/(50) | |
Outcome
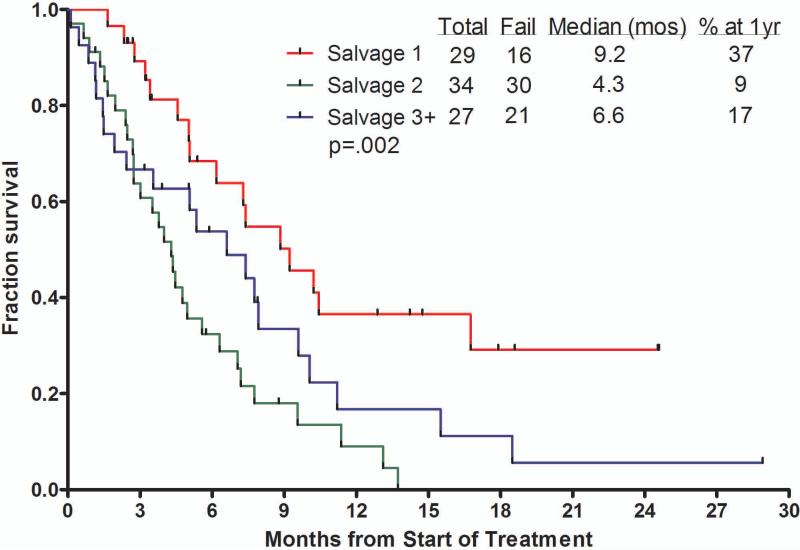
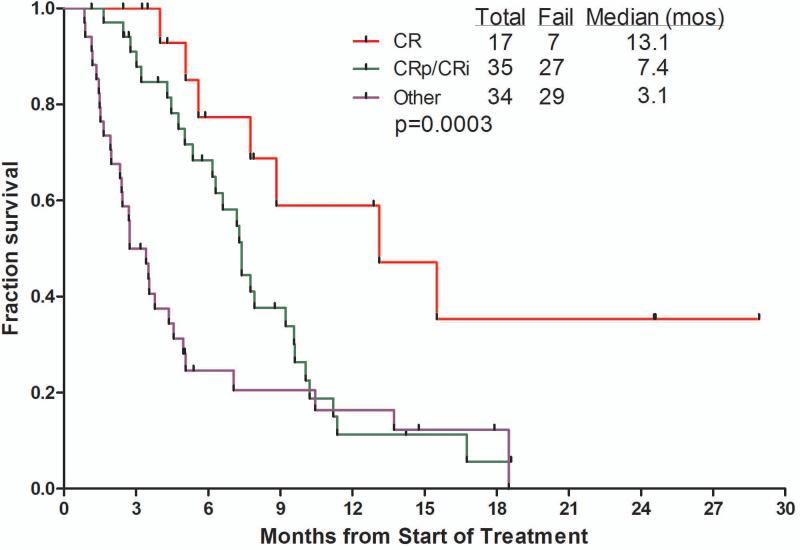
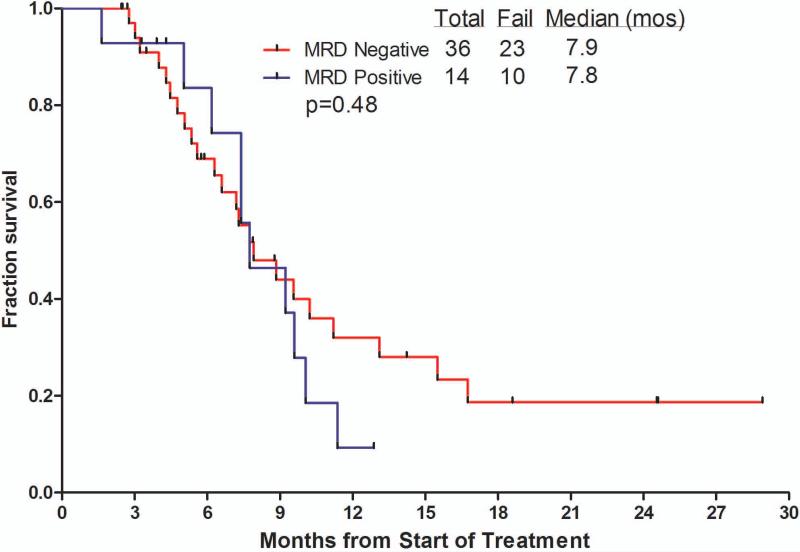
The median overall survival of patients receiving inotuzumab was 6.2 months (Figure 1). Censoring for the time of allogeneic SCT showed similar survivals, suggesting lack of benefit from allogeneic SCT. The median survival was 5.0 months with the single-dose schedule and 7.3months with the weekly schedule. The median remission duration was 7 months (1-year rate 42%). Survival by salvage number, response to treatment, and by MRD status among responders are shown in Figure 2A – C for the total study group of 90 patients. Patients treated in Salvage 1 had a median survival of 9.2 months with an estimated 1-year survival rate of 37%. Median survival for patients achieving CR, CRp + CRi, or with resistant disease were 13.1, 7.4 and 3.1 months, respectively. While survivals were not different with MRD-positive (n=14) versus MRD-negative patients (n=36) (median survivals 7.8 versus 7.9 months, 1-year rates 9% versus 32%; p=0.48), the remission durations tended to favor MRD-negative patients (median remission durations 5.1 versus 11.5 months, p=0.25).
Figure 1.
Survival of the study group with and without censoring for allogeneic stem cell transplantation.
Figure 2.
Survival by salvage status (A), by response to therapy (B), and by achievement of cytogenetic response and MRD status (C).
There were no differences in these 3 parameters by whether the patients received weekly or single-dose inotuzumab.
Pharmacokinetic studies
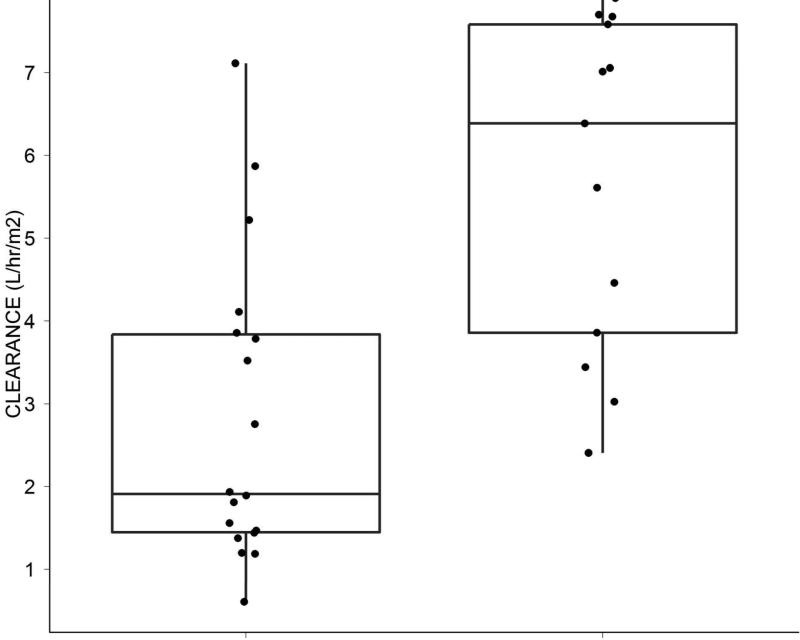
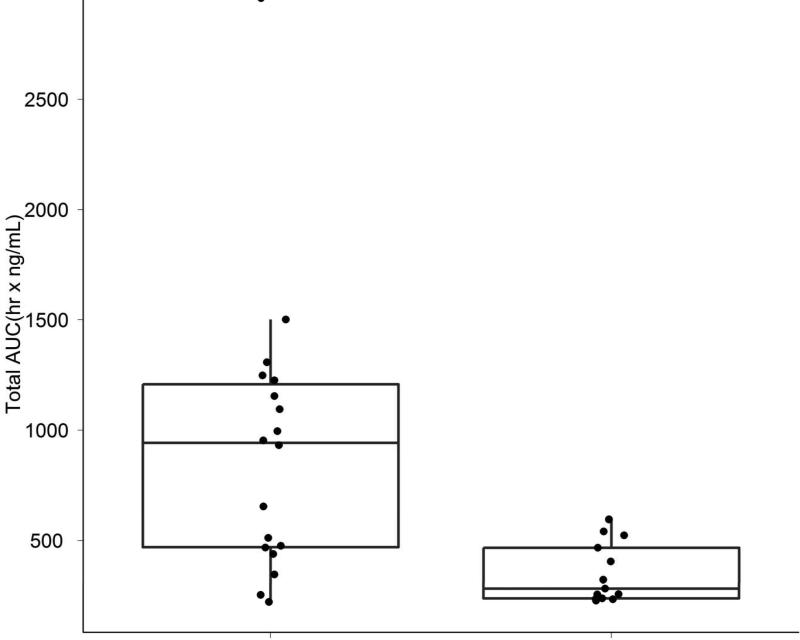
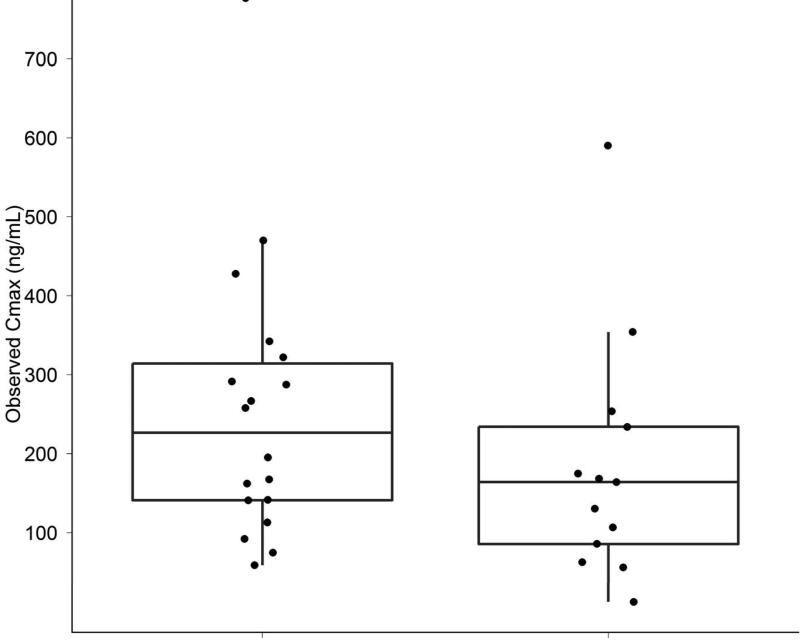
Measurement of inotuzumab levels were conducted at the end of infusion, 3 hours post end of infusion, and on Days 7-8. Patients achieving marrow CR had lower clearance rates and higher areas under the curve (AUC) levels compared with failures (Figure 3A). Higher inotuzumab peak levels were observed with single-dose inotuzumab (data not shown), but inotuzumab peak levels did not correlate with response rates. (Figure 3B).
Figure 3.
A & B. Slower clearance and higher AUCs were observed in patients achieving marrow CR (CR) versus others (A). Maximum inotuzumab plasma concentrations did not correlate with response. (B)
Treatment side effects
With weekly inotuzumab, previously observed prominent side effects associated with single-dose inotuzumab were less frequent, in particular fever and hypotension within 48 hours of drug administration, and liver function abnormalities. With weekly inotuzumab, 2 patients experienced Grade 1-2 bilirubin elevations and 0 had Grade 3 bilirubin elevations. Elevations of liver enzymes Grade 1-2 were observed in 9 patients and Grade 3 in 2 patients. All were reversible within 1-2 weeks. In contrast the single-dose inotuzumab which was associated with persistent liver function abnormalities in 2 of 49 patients; no such occurrences were noted with weekly inotuzumab. (Table 4)
Table 4.
Non-myelosuppressive Adverse Events during the first cycle of therapy
| Weekly | Single-dose | |||
|---|---|---|---|---|
| Grade 1-2 (n=41) | Grade 3-4 (n=41) | Grade 1-2 (n=49) | Grade 3-4 (n=49) | |
| Day 1-2 | 3 | 6 | 20 | 9 |
| Drug –related fever | ||||
| Drug-related hypotention Day 1-2 | 6 | 0 | 12 | 1 |
| Raised bilirubin concentration | 2 | 0 | 12 | 2 |
| Raised aminotransferase concentration | 9 | 2 | 27 | 1 |
| Raised lipase or amylase concentration | 1 | 0 | 0 | 1 |
| Nausea | 5 | 0 | 6 | 0 |
| Vomiting | 0 | 0 | 3 | 0 |
| Diarrhea | 1 | 0 | 3 | 0 |
| Mucositis | 0 | 0 | 0 | 1 |
| Anorexia | 0 | 0 | 1 | 0 |
| Headache | 1 | 0 | 1 | 0 |
| Constipation | 0 | 0 | 1 | 0 |
| Hypokalemia | 0 | 1 | 0 | 1 |
| Hypoalbuminemia | 0 | 0 | 1 | 0 |
• Grade 1-2 side-effects were significantly lower with weekly inotuzumab in relation to drug-related fever (p < 0.0001), increased bilirubin (p=0.01) and increased liver enzymes (p=0.001). No significant differences were observed in grade 3-4 side-effects with weekly versus single-dose inotuzumab schedules.
Feasibility of subsequent allogeneic SCT
The median follow up time is 4 months (range 1 to 19 months) on weekly inotuzumab and 21 months (range 5.4 to 28 months) on single-dose inotuzumab. Among the 41 patients with weekly inotuzumab, 14 patients (34%) so far were able to proceed to allogeneic SCT (6 related donors; 5 match unrelated donors, 2 haploidentical related, 1 cord). Among the 49 patients treated with single-dose inotuzumab, 22 patients (45%) were able to proceed to allogeneic SCT (7 related donors; 14 unrelated donors and one mismatched cord). At present, 9 of 14 patients on weekly inotuzumab and 4 of 22 patients on single-dose inotuzumab remain in remission and alive after allogeneic SCT. The median time from start of inotuzumab therapy to allogeneic SCT was 11 weeks (range 5 to 25 weeks). The median time from end of inotuzumab therapy to allogeneic SCT was 5 weeks (range 2 to 14 weeks). With the current follow-up, 13 patients are alive without evidence of disease. Veno-occlusive disease (VOD) was observed in 1 of 14 patients undergoing allogeneic SCT after weekly inotuzumab, and in 5 of 22 patients undergoing allogeneic SCT after single-dose inotuzumab. This may be also related to the preparative regimen: VOD was observed in 5 of 13 patients in whom the preparative regimen included 2 alkylating agents, but in only 1 of 21 patients where it included 1 alkylating agent (p=0.02).
DISCUSSION
In this study of 90 patients with refractory-relapsed ALL treated with inotuzumab in two different schedules, weekly (n= 41) and single-dose (n= 49), we identified inotuzumab as one of the most potent anti-ALL agents. Overall, 58 % of patients achieved marrow CRs. There was no difference in the response rates by whether patients received weekly or single-dose inotuzumab. Despite achievement of deep levels of remissions, as determined by cytogenetic CRs and negative MRDs, response durations were brief and the median overall survival 6.2 months (one year survival rate 20 %). Thus monoclonal antibody therapy with inotuzumab, and with monoclonal antibodies (blinotunumab; SAR3419) which target other ALL surface markers (CD19), have shown very promising anti-ALL activity with marrow CR rates of 50% to 75%, depending on the patient and leukemia characteristics. These monoclonal antibodies offer potentially the first highly active modalities that produce marrow CR rates equivalent or superior to those observed with intensive chemotherapy, without the notorious toxicities of the latter. Compared with our historical experience with intensive chemotherapy in 292 patients with refractory relapse ALL, the marrow CR rates with inotuzumab were 47% versus 29% overall. In Salvage 1, the marrow CR rates were 61% versus 40%. In Salvage 2 the marrow CR rates were 44% versus 16%. In Salvage 3 or later the marrow CR rates were 37% versus 16% (13, 14). Combining different monoclonal antibodies, or using combinations of chemotherapy with monoclonal antibodies, may in the future prolong survival in ALL salvage, and improve the cure rate in newly diagnosed ALL.
As expected, lower response rates were observed among patients known historically to have more refractory disease, including patients with Ph-positive ALL and those with ALL and translocation (4; 11). Similarly, response rates were lower in patients receiving inotuzumab in Salvage 2 or later compared to Salvage 1. However, even among the worst patient categories, inotuzumab was able to produce marrow CR rates substantially higher than what is expected with intensive chemotherapy, although these responses have been transient.
Single-dose inotuzumab therapy has been associated with liver function abnormalities, occasional VOD post allogeneic SCT, and transient febrile and hypotensive episodes. These were less frequent with the weekly schedule of inotuzumab, and were probably related to the peak levels of inotuzumab. Peak inotuzumab levels were not associated with differences in response rates, while inotuzumab cumulative AUC levels, which were equivalent with weekly and single-dose inotuzumab, were associated with significant differences in marrow response rates. Thus the weekly versus single-dose clinical experience, supported by the pharmacokinetic studies, indicates that weekly inotuzumab is as effective and less toxic than single-dose inotuzumab. Based on these experiences, a pivotal trial of weekly inotuzumab versus intensive chemotherapy in patients with refractory and relapsed ALL in first or second salvage is ongoing.
Despite the high response rates observed, responses were not durable, and median survival was modest. However, the responses obtained with inotuzumab allowed more than 40% of the patients to proceed to allogeneic SCT, compared with only 17% of patients achieving CR with intensive chemotherapy in our historical experience (5% of the total salvage population)(14). This suggests that, with proper modifications of the preparative regimens, and perhaps with combinations of inotuzumab and chemotherapy pre SCT and inotuzumab maintenance post SCT, we may achieve in the future long-term disease-free survival in a substantial proportion of patients using sequential combined modality strategies and allogeneic SCT. Future studies will also evaluate inotuzumab in combination with chemotherapy to improve the cure rates in newly-diagnosed adult ALL.
Acknowledgments
This research is supported in part by the National Institutes of Health through MD Anderson's Cancer Center Support Grant CA016672. This study was funded by a research grant from Pfizer Oncology.
Footnotes
Financial Disclosures: There are no financial disclosures from Drs. Thomas, Jorgensen, Kebriaei, Jabbour, Rytting,York,Ravandi,Faderl,Cortes,Champlin and O'Brien, Rebecca Garris and Monica Kwari. Hagop Kantarjian has research grants from MBS, Novartis, ARIAD, Pfizer. Consultant (non-paid) to Novartis.
REFERENCES
- 1.Gokbuget N, Hoelzer D, Arnold R, et al. Treatment of adult ALL according to protocols of the German multicenter study group for adult ALL (GMALL). Hematol Oncol Clin North Am. 2000;14:1307–1325. doi: 10.1016/s0889-8588(05)70188-x. [DOI] [PubMed] [Google Scholar]
- 2.Bassan R, Hoelzer D. Modern therapy of acute lymphoblastic leukemia. Journ of Clin Onc. 2011;29(5):532–543. doi: 10.1200/JCO.2010.30.1382. [DOI] [PubMed] [Google Scholar]
- 3.Faderl S, O'Brien S, Pui CH, et al. Adult acute lymphoblastic leukemia. Cancer. 2010;116(5):1165–1176. doi: 10.1002/cncr.24862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas DA, Faderl S, O'Brien S, et al. Chemoimmunotherapy with hyper-CVAD plus rituximab for the treatment of adult Burkitt and Burkitt-type lymphoma or acute lymphoblastic leukemia. Cancer. 2006;106:1569–1580. doi: 10.1002/cncr.21776. [DOI] [PubMed] [Google Scholar]
- 5.Gokbuget N, Hoelzer D. Rituximab in the treatment of adult ALL. Ann Hematol. 2006;85:117–119. [Google Scholar]
- 6.Rizzieri D, Johnson J, Byrd J, et al. Efficacy and toxicity of rituximab and brief duration, high intensity chemotherapy with filgrastim support for Burkitt or Burkitt-like leukemia/lymphoma: C ancer and Leukemia Group B (Calgh) study 10002. Blood ASH annual meeting abstracts. 2010;116(21) abst 858. [Google Scholar]
- 7.Thomas D, O'Brien S, Faderl S, et al. Chemoimmunotherapy with a modified hyper-CVAD and rituximab regimen improves outcome in de-novo Philadelphia chromosome-negative precursor B-liineage acute lymphoblastic leukemia. Journ of Clin Onc. 2010;28(24):3880–3889. doi: 10.1200/JCO.2009.26.9456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoelzer D, Huettmann A, Kaul F, et al. Immunochemotherapy with rituximab improves molecular CR rate and outcome in CD20+ B-lineage standard and high risk patients; results of 263 CD20+ patients studied prospectively in GMALL study 07/2003. Blood ASH annual meeting abstracts. 2010;116(21) abst 170. [Google Scholar]
- 9.Raetz E, Cairo M, Borowitz M, Blaney S, et al. Chemoimmunotherapy reinduction with epratuzumab in children with acute lymphoblastic leukemia in marrow relapse: a Children's Oncology Group pilot study. Journ of Clin Onc. 2008;26(22):3756–3762. doi: 10.1200/JCO.2007.15.3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Topp M, Kufer P, Gokbuget N, et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. Journ of Clin Onc. 2011;29(18):293–2498. doi: 10.1200/JCO.2010.32.7270. [DOI] [PubMed] [Google Scholar]
- 11.Thorson J, Sievers E, Ahlert J, et al. Understanding and exploiting nature's chemical arsenal: the past, present and future of calicheamicin research. Curr Pharm Des. 2000;6:1841–79. doi: 10.2174/1381612003398564. [DOI] [PubMed] [Google Scholar]
- 12.Kantarjian H, Thomas D, Jorgensen J, et al. Inotuzumab ozogamicin, an anti-CD22-calicheamicin conjugate, for refractory and relapsed acute lymphocytic leukaemia: a phase 2 study. The Lancet Oncology. 2012;13:403–11. doi: 10.1016/S1470-2045(11)70386-2. [DOI] [PubMed] [Google Scholar]
- 13.O'Brien S, Thomas D, Ravandi F, et al. Outcome of adults with acute lymphocytic leukemia after second salvage therapy. Cancer. 2008;113:3186. doi: 10.1002/cncr.23919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas D, Kantarjian H, Smith T, et al. Primary refractory and relapsed adult acute lymphoblastic leukemia. Cancer. 1999;86:1216–1230. doi: 10.1002/(sici)1097-0142(19991001)86:7<1216::aid-cncr17>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 15.Larson RA. Management of acute lymphoblastic leukemia in older patients. Semin Hematol. 2006;43:126–133. doi: 10.1053/j.seminhematol.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Rousselot P, Cayuela JM, Hayette S, et al. Dasatinib (Sprycel®) and low intensity chemotherapy for first-line treatment in elderly patients with De Novo Philadelphia positive ALL (EWALL-PH-01): kinetic of response, resistance and prognostic significance. Blood. 2010;116 abst 172. [Google Scholar]