Abstract
The pace of discovery of potentially actionable pharmacogenetic variants has increased dramatically in recent years. However, the implementation of this new knowledge for individualized patient care has been slow. The Pharmacogenomics Research Network (PGRN) Translational Pharmacogenetics Program seeks to identify barriers and develop real-world solutions to implementation of evidence-based pharmacogenetic tests in diverse health-care settings. Dissemination of the resulting toolbox of “implementation best practices” will prove useful to a broad audience.
Despite a number of important pharmacogenetic discoveries, substantial evidence supporting clinical utility, and US Food and Drug Administration labels recommending use of pharmacogenetic testing, few pharmacogenetic tests have made their way into routine clinical practice. Barriers to adoption of pharmacogenetic tests into practice are substantial and include (i) logistics of performing accurate and rapid turnaround genotyping in a Clinical Laboratory Improvement Amendments– approved laboratory setting; (ii) lack of a standardized format for the return of test results into the electronic health record; (iii) lack of prospective genotype-directed pharmacogenetic randomized clinical trials validating treatment algorithms; (iv) inexperience of many clinicians in interpreting and acting on pharmacogenetic information; (v) paucity of clear recommendations for pharmacogenetic testing by professional associations; (vi) lack of information infrastructure to provide decision support for genomic medicine; and (vii) cost considerations and reimbursement.
One barrier to clinical implementation addressed by the PGRN is the lack of clear, curated, peer-reviewed pharmacogenetic guidelines that translate laboratory test results into actionable prescribing decisions for specific drug–gene pairs. The PGRN Clinical Pharmacogenetics Implementation Consortium (CPIC) is a shared initiative between the Pharmacogenomics Knowledgebase (PharmGKB) and the PGRN. The CPIC produces clinical guidelines that are gene–drug pair specific, peer-reviewed, published, and posted to PharmGKB; the guidelines specifically do not consider how or why the genotype data were obtained but instead how to act on genotype data that have been obtained. CPIC guidelines contain information needed for clinical implementation, including tables that summarize the relevant functional gene variants and probable phenotypes, and recommendations regarding drug dosing or drug choice based on phenotype1 (http://www.pharmgkb.org/page/cpic). All CPIC recommendations are extensively annotated and supported by graded evidence; in addition the strength of the recommendations is indicated. The guidelines are freely available at PharmGKB (http://www.pharmgkb.org/page/cpicGeneDrugPairs), are updated on a regular basis, and are not linked to any commercial services, genotyping platforms, or financial interests. CPIC guidelines published to date include TPMT/thiopurines, CYP2C19/clopidogrel, CYP2D6/codeine, VKORC1-CYP2C9/warfarin, HLA-B*57:01/abacavir, SLCO1B1/simvastatin, and HLA-B*58:01/allopurinol; several others are under development.
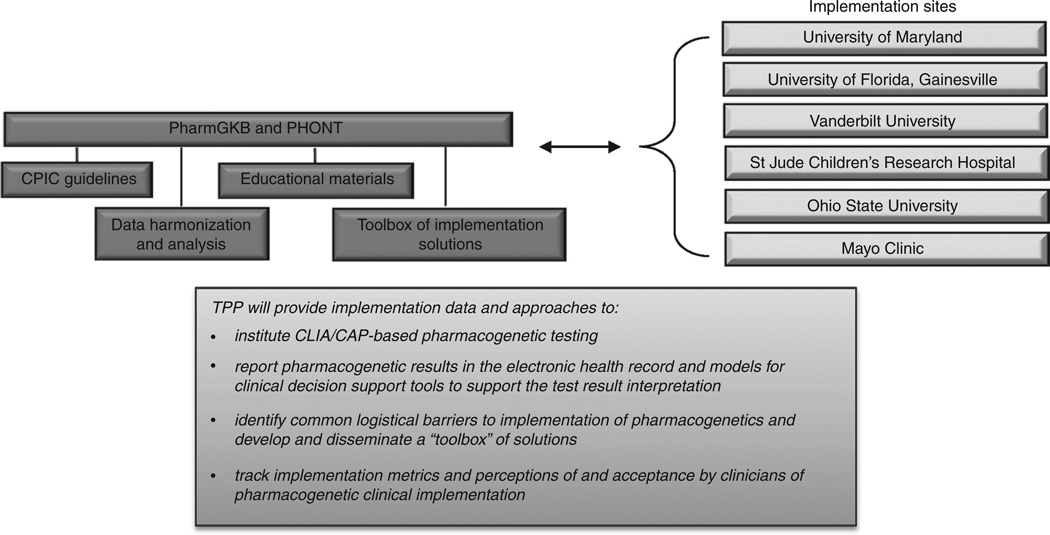
Each clinical practice setting has its own particular requirements for actual implementation, and few physicians and health systems actually use pharmacogenetics in clinical practice. In 2011, the PGRN established the Translational Pharmacogenomics Program to assess implementation of routine evidence-based pharmacogenetic testing, dosing, and drug selection within six diverse health-care systems. Participating implementation sites are the University of Maryland, University of Florida, St. Jude Children’s Research Hospital, Vanderbilt University, Mayo Clinic, and Ohio State University (Figure 1). PharmGKB (http://www.pharmGKB.org) at Stanford University serves a coordination and knowledge sharing/dissemination role. Pharmacogenomic Ontology at the Mayo Clinic provides data harmonization and standardization support to the Translational Pharmacogenetics Program.
Figure 1.
Organizational structure and goals of the Pharmacogenomics Research Network Translational Pharmacogenomics Program. CAP, College of American Pathologists; CLIA, Clinical Laboratory Improvement Amendments; CPIC, Clinical Pharmacogenetics Implementation Consortium; PharmGKB, The Pharmacogenomics Knowledgebase; PHONT, Pharmacogenomic Ontology; TPP, Translational Pharmacogenetics Program.
For this implementation science initiative, each Translational Pharmacogenetics Program site is implementing into clinical practice or designed clinical trials one or more pharmacogenetic tests, adapting evidence-based CPIC guidelines (e.g., CYP2C19 testing and antiplatelet therapy, TPMT and thiopurine dosing, and CYP2C9/VKORC1 and warfarin dosing) to local policies and practices, and identifying and attempting to overcome real-life policy, logistical, and translational barriers.2,3,4 Clinical Laboratory Improvement Amendments–licensed laboratories perform pharmacogenetic tests at each site. Both point-of-care (e.g., CYP2C19 testing in percutaneous coronary intervention patients with an immediate indication for antiplatelet therapy) and preemptive models (e.g., pharmacogenetic testing in patients likely to need a treatment that could be tailored by genomic data in the future) are being executed and studied.
Through monthly teleconferences, twice-annual in-person meetings, and other interactions, the Translational Pharmacogenetics Program is a coordinated effort that takes advantage of the diversity of participating health-care systems. Each implementation step will be evaluated scientifically to develop a practical evidence-based toolbox of best practices for pharmacogenetics implementation that will be useful across a wide spectrum of health-care systems. These tools will be disseminated widely through publication in peer-reviewed journals and updated regularly on the PharmGKB website.
Challenges in clinical implementation include the need to handle complex and sometimes ambiguous genetic results. Although there are inherent problems in the “star allele” nomenclature system, such a system does allow for the aggregation of several different variations in the same gene into a single test result. Creating computational rules for interpreting such results has been time consuming, and it is partly for this reason that Translational Pharmacogenetics Program investigators are sharing difficult-to-interpret test results and actions based on them in their Clinical Decision Support Tables posted on PharmGKB. These tables complement the existing CPIC guidelines by providing therapy recommendations for a more comprehensive coverage of pharmacogenetic test results. They represent the current state of knowledge and demonstrate the range of observed results and recommended actions across sites. A compilation of results and actions across sites helps to identify areas of consensus and areas in which there are differences in opinion for which additional research may be required. However, as DNA-sequencing technologies become more widely used, we acknowledge that despite the increased coverage, we cannot account for all (known and unknown) variations in the human genome, many of which may be functionally important. The tables are maintained and updated at PharmGKB (http://www.pharmgkb.org/page/tppTables) and can be adopted by other institutions. Other instruments that will be developed for dissemination include a best practices manual for implementation of pharmacogenetics testing, and questionnaires and surveys to assess implementation metrics and effectiveness.
All Translational Pharmacogenomics Program sites are tracking common summary descriptions and implementation metrics to objectively evaluate the effectiveness of implementation. Summary statistics include descriptive information regarding patient populations (age, sex, race, disease characteristics, medications, and health-care setting), frequencies of pharmacogenetic test results, and provider characteristics. Tracked standardized implementation metrics include number of tests ordered, average completion time for genetic tests (from phlebotomy to laboratory result posted within an electronic health record), genotype failure rates, tracking of who delivers the results and when (preemptive or on demand), average time from completion of laboratory test to availability of report to prescribers, utilization of test by clinician for treatment decisions, and costs of testing (Table 1). Health-care provider perceptions of pharmacogenetics in clinical decision making will be obtained through surveys and, at some sites, focus groups are planned.
Table 1.
Summary of Translational Pharmacogenomics Program implementation metrics
| Pharmacogenetic testing adoption | |
| Testing volume (cumulative total, by month) | |
| Test ordered and mode of order entry (e.g., computerized provider order entry, electronic health record, paper, automated rule) | |
| Role of provider | |
| Practice setting where order originated | |
| Cost of testing | |
| Number of tests ordered but not completed | |
| Other laboratory quality assurance measures: genotype failure rates | |
| Pharmacogenetic test adoption rates | |
| In patients receiving care within a specific clinical setting with a potential need for target drug | |
| In patients receiving care within a specific clinical setting and prescribed a target drug for specific indication(s) | |
| Pharmacogenetic results | |
| Timing of result | Time between pharmacogenetic test order and pharmacogenetic test report to prescribers |
| Time between target drug order/prescription and pharmacogenetic result | |
| Genotype distribution by haplotype | |
| Proportion of tested patients with actionable genotypes (meet criteria for consult or clinical decision support) | |
| Pharmacogenetic consultation and clinical decision support | |
| Preemptive/automated trigger vs. provider requested/on demand | |
| Automated clinical decision support delivered—vehicle (e-prescribing/computerized provider order entry/electronic health record), active (interruptive)/passive, recommendation, user response | |
| Manual consultation delivered—role, communication mode, successful contact with primary decision maker, response | |
| Provider genotype-guided prescription metrics | |
| Proportion of patients with pharmacogenetics consultation/clinical decision support leading to new/revised prescription for target drug | |
| Time between pharmacogenetic test result and new/revised target drug order | |
| Adherence to Clinical Pharmacogenetics Implementation Consortium Pharmacogenetic Guidelines | |
| Adherence to recommendation based on genotype | |
| Reason for nonadherence | |
| Communication of pharmacogenetic information to patients | |
| Role of provider communicating results | |
| Mode of communication (documented verbal discussion, messaging) | |
Efforts to make evidence-based pharmacogenetic laboratory tests available in the health-care system would be moot if practicing clinicians do not effectively use these tests. A critical barrier to adoption of new pharmacogenetic tests is the inexperience of many clinicians with interpretation and acting on pharmacogenetic information. Therefore, most centers are developing clinical decision support tools based on electronic health records to aid the clinician in interpretation of the pharmacogenetic data and to provide guidance on the clinical options based on the pharmacogenetic data. Although all sites are depositing pharmacogenetic test results into the electronic health record, a longer-term goal of the Translational Pharmacogenomics Program is to work with vendors of common electronic health records (e.g., Epic, Cerner) to develop templates for reporting results with clinical decision support. This innovation will enable broad plug-in dissemination to most electronic health record–enabled health-care organizations.
Another goal of the Translational Pharmacogenomics Program is to develop practical pharmacogenetic educational materials for clinicians. These include informal in-services, practice-based competencies for pharmacists and other clinicians, continuing education lectures/seminars, and Web-based programs. This education toolbox will be made available widely on the PharmGKB website.
Our experience to date indicates that key ingredients for successful implementation of pharmacogenetics include (i) recognition that involvement of many parties within the health-care system is required; (ii) early, persistent, and collaborative engagement with health-care providers, faculty, and administrative staff impacted by the drug–genome interaction; (iii) use of active clinical decision support that interactively interprets genetic data and guides providers through prescription options; and (iv) monitoring the uptake of pharmacogenomic testing and genotype-tailored prescriptions as an early signal for implementation barriers that need to be addressed.
During the preparation of this paper, the Food and Drug Administration listed more than 100 drugs for which the label includes information regarding pharmacogenetics (http://www.fda.gov/drugs/scienceresearch/researchareas/pharmacogenetics/ucm083378.htm). As the pace of pharmacogenomic discoveries accelerates, many more medications, both old and new, will carry such information. The PGRN Translational Pharmacogenetics Program is among the first coordinated efforts to traverse the real-world barriers to implementing evidence-based pharmacogenetic medicine. The program will generate important objective data that will guide best practices across a diversity of health-care settings and disseminate widely applicable tools for implementation of pharmacogenetics into patient care. In addition, this coordinated effort may make possible future clinical trials (both randomized and comparative effectiveness trials) as well as create a platform for ethical and legal issues in implementation of pharmacogenetic and other genomic information into the electronic health record. With growing implementation of pharmacogenetic test panels in clinical tests, and in particular a priori availability of pharmacogenetic information in an individual’s electronic health record, utility of such tests will be less burdened by the cost and effort to order the test when needed. Typically, pharmacogenetic information poses few ethical challenges because for the most part such genetic variants are not considered disease risk factors. Therefore, prospective broad genotyping to facilitate optimal drug therapy may rank among the frontline advances in personalized medicine.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health (NIH)/National Institute of General Medical Sciences Pharmacogenomics Research Network grants U01 HL105198 (A.R.S.), U01 GM92666 (M.V.R.), U01 HL105198 (M.V.R.), GM61374 (T.E.K.), U01 GM92655 (W.S.), U19 HL065962 (D.M.R.), U19 GM61388 (R.W., L.W.), KL2 RR024151 (N.L.P.), U01 GM074492(J.A.J.), and U19 GM61388 (R.R.F.), NIH/National Cancer Institute grants CA 36401 and CA 21765 (M.V.R.), and by American Lebanese Syrian Associated Charities (M.V.R.). Full list of authors in the Translational Pharmacogenetics Program Group: Pharmacogenomics of Anti-Platelet Intervention (PAPI) Study: University of Maryland School of Medicine, Baltimore, Maryland, USA: Alan R. Shuldiner, Mark Vesely, Shawn W. Robinson, Nicholas Ambulos Jr., Sanford A. Stass, Mark D. Kelemen, Lawrence A. Brown, Toni I. Pollin, Amber L. Beitelshees, Richard Y. Zhao, Ruth E. Pakyz, Kathleen Palmer, Tameka Alestock, Courtney O’Neill, Kristin Maloney, Amie Branham, and Danielle Sewell; Pharmacogenetics of Anticancer Agents Research in Children (PAAR4Kids): PG4KDS Protocol: Clinical Implementation of Pharmacogenetics, St. Jude Children’s Research Hospital, Memphis, Tennessee, USA: Mary V. Relling, Kristine Crews, James Hoffman, Shane Cross, Cyrine Haidar, Don Baker, J. Kevin Hicks, Gillian Bell, Fran Greeson, Aditya Gaur, Ulrike Reiss, Alicia Huettel, Cheng Cheng, Amar Gajjar, Alberto Pappo, Scott Howard, Melissa Hudson, Ching-Hon Pui, Sima Jeha, and William E. Evans; Medical College of Wisconsin: Ulrich Broeckel; The Pharmacogenomics Knowledgebase (PharmGKB), Stanford University, Stanford, California, USA: Russ B. Altman, Li Gong, Michelle Whirl-Carrillo, and Teri E. Klein; Expression Genetics in Drug Therapy (XGEN), The Ohio State University, Columbus, Ohio, USA: Wolfgang Sadee, Kandamurugu Manickam, Kevin M. Sweet, and Peter J. Embi; Pharmacogenomics of Arrhythmia Therapy (PAT), Vanderbilt University School of Medicine, Nashville, Tennessee, USA: Dan Roden, Josh Peterson, Josh Denny, Jonathan Schildcrout, Erica Bowton, Jill Pulley, Marc Beller, Jennifer Mitchell, Ioana Danciu, and Lisa Price; Pharmacogenetics of Phase II Drug Metabolizing Enzymes (PPII), Mayo Clinic, Rochester, Minnesota, USA: Naveen L. Pereira, Richard Weinshilboum, and Liewei Wang; Pharmacogenomic Evaluation of Antihypertensive Responses (PEAR), University of Florida, Gainesville, Florida, USA: Julie A. Johnson, David Nelson, Michael Clare-Salzler, Amanda Elsey, Benjamin Burkley, Taimour Langaee, Felix Liu, David Nessl, Hui-Jia Dong, and Larry Lesko; Pharmacogenomics Ontology (PHONT), Mayo Clinic, Rochester, Minnesota, USA: Robert R. Freimuth and Christopher G. Chute.
Footnotes
CONFLICT OF INTEREST
M.V.R. received patent royalties from TPMT genotyping tests. The other authors declared no conflict of interest.
References
- 1.Relling MV, Klein TE. CPIC: Clinical Pharmacogenetics Implementation Consortium of the Pharmacogenomics Research Network. Clin. Pharmacol. Ther. 2011;89:464–467. doi: 10.1038/clpt.2010.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crews KR, Hicks JK, Pui CH, Relling MV, Evans WE. Pharmacogenomics and individualized medicine: translating science into practice. Clin. Pharmacol. Ther. 2012;92:467–475. doi: 10.1038/clpt.2012.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson JA, Burkley BM, Langaee TY, Clare-Salzler MJ, Klein TE, Altman RB. Implementing personalized medicine: development of a cost-effective customized pharmacogenetics genotyping array. Clin. Pharmacol. Ther. 2012;92:437–439. doi: 10.1038/clpt.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lieb W, Völzke H, Pulley JM, Roden DM, Kroemer HK. Strategies for personalized medicine-based research and implementation in the clinical workflow. Clin. Pharmacol. Ther. 2012;92:443–445. doi: 10.1038/clpt.2012.119. [DOI] [PubMed] [Google Scholar]