Abstract
Objective
To investigate the clinical significance of the expression of MHC class I chain-related gene A (MICA) in patients with advanced non-small cell lung cancer and explore the relationship between MICA expression and the efficacy of cytokine-induced killer cell (CIK) therapy for treating advanced non-small cell lung cancer.
Methods
We obtained data on 222 patients with advanced non-small cell lung cancer, including data on MICA expression, age, gender, ECOG score, pathological type, stage, treatment history (including 38 patients who were given autologous CIK cell infusion), and overall survival (OS). MICA expression in lung cancer tissue was evaluated by immunohistochemical staining. Analyses of MICA expression, and CIK therapy association with survival outcomes were performed using Cox proportional models, Kaplan-Meier methods, and the log-rank test.
Result
s MICA was expressed in both membrane and cytoplasm. MICA expression correlated with the stage of lung cancer, ECOG score, gender and age. Multivariate COX regression analysis showed that the expression of MICA was an independent prognostic factor of advanced non-small cell lung cancer (p = 0.002). In subgroup analysis, we divided the 222 patients into CIK and control groups. In the CIK group, the medium OS (mOS) of patients with a high expression of MICA was longer than in those with low expression of MICA (27 months vs. 13 months). In the control group, the mOS in patients with a high expression of MICA was shorter than in patients with low MICA expression (9 months vs. 18 months). COX regression analysis showed that the MICA expression affects the effect of CIK therapy (p<0.0001).
Conclusion
1) The high expression of MICA is one of the indicators of a poor prognosis for advanced non-small cell lung cancer patients. 2) The high expression of MICA might be one of the predictive factors for successful CIK therapy.
Introduction
Lung cancer is still the leading cause of cancer-related mortality in the world, and about 1.4 million people are diagnosed with lung cancer every year [1]. Approximately, 85% of all lung cancer cases are categorized as non-small cell lung cancer (NSCLC), and more than 50% of NSCLC patients have advanced local invasion and/or distant metastases. In the last decade, many researches have focused on the immunological aspects of lung cancer. Immunotherapy has recently become the fourth important treatment modality for malignant tumors after surgery, radiotherapy, and chemotherapy [2], [3], [4], [5].
The genesis and progress of a tumor are closely related to the response of the immune system. The theory of “immunoediting” has attempted to describe the complex interaction of a developing tumor with an evolving immune response. This is thought to include three phases; an elimination phase where the immune system destroys the tumor, an equilibrium phase where the tumor and immune system coexist, and an escape phase where the tumor evolves mechanisms to evade destruction by the immune system. Molecules that are up or down regulated during immunoediting may represent potential prognostic markers [6]. The major histocompatibility complex class I chain-related (MIC) proteins represent a novel family of highly glycosylated, membrane-anchored MHC class I-like molecules. Although they share similar structure to classical class I heavy chains, MICA and MICB do not associate with β2-microglobulin or with the transporter associated with antigen processing (TAP) [7]. MIC proteins have a restricted tissue distribution and they are rarely expressed by normal cells. It has been reported that MICA is constitutively expressed by intestinal epithelial cells and is broadly expressed in a variety of malignancies. Therefore, it is considered to be a tumor associated antigen (TAA) [8], [9], [10].
MIC proteins function as ligands for the stimulatory C-type lectin-like NKG2D receptor, first identified on NK cells and subsequently shown to be expressed on CD8+, αβ and γδ T-cells. NKG2D is a type II transmembrane glycoprotein that is expressed as a disulfide linked homodimer on the cell surface. It acts as an activating receptor after ligand binding, supporting the cytotoxic activity of NK cells and T cells against tumor cells [11], [12]. Tumor cells stably transfected to express MICA and the murine versions of the NKG2D ligands RAE-1 or H60 at high levels, are removed by CD8+ T cells and NK cells [13], [14], which indicates that tumor cells over-expressing NKG2D ligands become more sensitive to immune cell-mediated cytolysis. Nevertheless, recent reports have identified a mechanism by which tumors appear to be able to subvert this surveillance mechanism. Soluble forms of MICA were identified to be shed by tumors and shown to cause downregulation of NKG2D by engaging with the receptor on tumor infiltrating lymphocytes (TILs), impairing T-cell function [15]. This study plans to examine advanced NSCLC cases to conduct a retrospective analysis exploring the association between MICA expression and the prognosis of advanced NSCLC patients.
Cytokine-induced killer (CIK) cells are a unique population of cytotoxic T lymphocytes with a characteristic CD3+/CD56+ phenotype, and they can be generated from cytokine cocktail-induced peripheral blood mononuclear cells (PBMC). These cells have shown higher proliferative and cytolytic activities compared with CD3-/CD56+ lymphokine-activated killer cells (LAK) [16]. At present, CIK cells are recognized as a new type of anti-tumor effector cell, with stronger anti-tumor activity and a broader spectrum of targeted tumors than other reported anti-tumor effector cells. Moreover, CIK cells can regulate and generally enhance immune functions in cancer patients [17], [18]. Current data from phase I/II studies on the anti-NSCLC effects of CIK cells are highly limited, and the therapeutic benefits of CIK cells are unknown in NSCLC patients. As targeted therapy and chemotherapy have made substantive breakthroughs in individualized treatment, we should explore the predictive factors of immune therapy to improve the effect of CIK therapy in NSCLC patients. Some studies have shown that the NKG2D receptor is highly expressed on the surface of CIK cells, and it is expected that a cytolytic effect can be mediated by the interaction between the NKG2D receptor on CIK cells and the MICA ligand on cancer cells [19], [20], [21]. So, theoretically, CIK cells can recognize MICA ligands on lung cancer cell membranes and kill the cancer cells. Whether high MICA expression can be used as a predictive factor of CIK therapy outcome still remains to be determined. We plan to study MICA expression and the effect of CIK therapy to determine if MICA expression is a predictive factor of successful CIK therapy.
Materials and Methods
Ethics Statement
All procedures were conducted in accordance with the Helsinki declaration, and with approval from the Ethics Committee of Fujian Provincial Cancer Hospital. Written informed consent was obtained from all participants.
Patients and Samples
Patients (n = 222) with advanced or locally advanced non-small cell lung cancer (III–IV phase) from the 12th Department of Fujian Provincial Cancer Hospital were enrolled between December 2005 and December 2010. The key exclusion criterion was lack of sufficient tissue for immunohistochemistry (IHC). Key clinicopathological data were collected from these patients, including TNM stage, ECOG score, gender, and age. General patient information is shown in Table 1. All tumors received following resection in the operating theatre or transbronchial lung biopsies or aspiration biopsies were immediately fixed and placed in 10% neutral buffered formalin, before processing and embedding in paraffin to ensure optimal tissue fixation and preservation for histological examination. The original histopathological slide sets and reports were obtained from each case, and these were reviewed to confirm the diagnosis and accuracy of the existing data, and to complete any information missing from the database wherever possible.
Table 1. Patient and tumor characteristics.
| Characteristics | Number | Constituent ratio | |
| Gender | Male | 160 | 72.10% |
| Female | 62 | 27.90% | |
| Histological type | Adenocarcinoma | 136 | 62.20% |
| squamous carcinoma | 60 | 27.00% | |
| other | 26 | 10.80% | |
| Stage* | IIIA | 24 | 10.80% |
| IIIB | 44 | 19.80% | |
| IV | 154 | 69.40% | |
| ECOG Score | 0–1 | 172 | 77.50% |
| 2–3 | 50 | 22.50% | |
Seventh edition of the AJCC/UICC TNM staging.
Treatments
Out of 222 patients 32 refused treatment after being diagnosed. The remaining 190 patients received treatments including chemotherapy, targeted therapy, radiotherapy, CIK treatment, and the best supportive symptomatic treatment. For cases of locally advanced lung cancer (22 cases with stage IIIA, 6 cases with stage IIIB) the treatment model consisted of preoperative induction chemotherapy, surgery, and postoperative adjuvant chemoradiotherapy. Four out of twenty-four patients received four cycles of supplementary CIK treatment (Table 2). Patients were given autologous CIK cell treatment after chemoradiotherapy. CIK cell treatment was given at 1-month intervals. The patients were eligible for CIK maintenance treatment until they no longer agreed to continue maintenance treatment or until disease progression occurred. For each treatment, patients were given an infusion of at least 1.0×1010 CIK cells.
Table 2. 38 patients treated with CIK.
| Stage\CIK cycles | Two cycle | Four cycle | Six cycle |
| Locally advanced (IIIA, fractional IIIB) | 0 | 4 | 0 |
| Advanced (fractional IIIB, IV) | 2 | 28 | 4 |
Advanced cases (34 cases with stage IIIB, 128 cases with stage IV), received treatment regimens of chemotherapy, EGFR-TKI targeted therapy, and CIK treatment. Chemotherapy included first-line, second-line, and third-line treatment. First-line chemotherapy used third-generation chemotherapeutic agents containing a platinum two-drug regimen. The second-line chemotherapy with single-agent docetaxel and pemetrexed was used more often than third-line chemotherapy and treatment was given according to the disease situation. EGFR-TKI therapy with 250 mg of gefitinib qd or 150 mg erlotinib was continued until disease progression or until toxicity could not be tolerated. There were 34 patients with advanced disease who received CIK treatment (Table 2). CIK cell treatment was given at 1-month intervals. For each treatment, patients were given an infusion of at least 1.0×1010 CIK cells.
CIK Cell Preparation
CIK cells were isolated and cultured according to standard protocol. Peripheral blood (50 ml) is drawn from patients using heparin as an anticoagulant. Mononuclear cells were isolated by Ficoll-Conray density gradient centrifugation and their viability assessed by trypan blue exclusion. About 2.0×106/ml mononuclear cells were plated onto six-well dishes and cultured with Medium I containing RPMI 1640 plus 1.0×106U/L human interferon gamma (IFN-γ), 5.0×105U/L recombinant human interleukin-2 (IL-2), 10% heat inactivated human serum, 25 mM HEPES, 2 mM L-glutamine, 100U/ml penicillin, and 100 µg/ml streptomycin. The cells were incubated in a humidified atmosphere with 5% CO2 at 37°C. After 24 h, 100 µg/L monoclonal antibody (MAb) against CD3 and 1.0×105U/L IL-1α were added. After another 48 h, the supernatant was aspirated and the cells were cultured in Medium II; Medium I in the absence of INF-γ. The medium was changed every three days. Cell viability was determined using trypan blue staining. CIK cells were transfused back into the donors following eight days of culture. All CIK cell cultures were tested for contamination (bacteria, fungi, and mycoplasma) throughout the study to assure culture quality and transfusion safety.
Phenotype analysis of CIK cells
The phenotypes of cultured cells were determined by flow cytometry (BD FACSCalibur). The cells were labeled with monoclonal antibodies (m Abs) that recognize human CD3, CD4, CD8, CD56, and NKG2D.
Immunohistochemistry
Immunohistochemical detection of MICA was performed by a routine streptavidin-biotin peroxidase technique employing the rabbit polyclonal anti-MICA antibody. Routine paraffin sectioning, dewaxing, and hydration using 3% hydrogen peroxide was performed to block endogenous peroxidase activity. Microwave antigen retrieval was done before blocking with fetal calf serum for 2 h. About 50 μl (1:25) of rabbit polyclonal anti-human MICA was added, and the mixture was incubated at 4°C overnight. About 50 μl of biotinylated goat anti-rabbit IgG secondary antibody working solution was added, and the mixture was incubated at 37°C for 30 min followed by diaminobenzidine coloration. The sample was counterstained with hematoxylin, washed with ethanol and hydrochloric acid, saturated with lithium carbonate until the color returned to blue, and then dehydrated with gradient alcohol and xylene before mounting with neutral resin.
The omission of primary antibody and its replacement with PBS was used as the negative control, and tumor infiltrating leukocytes were used as internal positive controls for MICA staining. A section of colorectal tissue which had been shown to be positive for MICA was also used as a positive control [22], [23].
Evaluation of immunostaining
Two independent pathologists examined the immunohistochemistry probed slides without any prior knowledge about the clinical history of the patients. MICA expression was evaluated according to staining intensity and scored as follows. The intensity of the immunostaining was classified into four categories: 0 = no staining or only a nonspecific background color, 1 = light yellow, 2 = yellow or deep yellow, and 3 = brown or tan. The percentage of positive cells was assessed semi-quantitatively and classified into five groups: 0 = ≤5% positive cells, 1 = 6%–25% positive cells, 2 = 26–50% positive cells, 3 = 51–75% positive cells, and 4 = 76%–100% positive cells. The histochemical score of immunoreactivity was obtained by adding the intensity and percentage scores. Histochemical scores were divided into two groups, cases with scores of 0–4 were defined as the low-expression group, and cases with scores of 5–7 were defined as the high-expression group. [23], [24], [25].
Prognosis evaluation
The overall survival (OS) serves as the gold standard in evaluation of the therapeutic efficacy of tumor therapy, so we adopted OS when evaluating the prognosis. Patients were followed from the date of diagnosis of Stage IIIB/IV disease to the date of death. Patients without a known date of death were censored at the time of the last follow-up.
Statistical analysis
Statistical analysis of the data was performed using the SPSS package (version 13 for Windows). The chi-square test (Fisher's exact test) and Student's t-test were used to evaluate the differences in categorical variables and continuous variables, respectively. Patients whose death related to their lung cancer were considered in the disease-specific survival calculations. Those who died from non-lung cancer related causes without evidence of recurrence were censored at the time of death. The survival curves were estimated by the Kaplan-Meier method, and differences in survival were assessed by the log-rank test. Multivariate analysis by the Cox's proportional hazards ratio model was used to test the significance of prognostic factors including MICA expression, gender, age, histology, ECOG score, and CIK therapy. A p-value<0.05 was considered as statistically significant.
Results
MICA expression
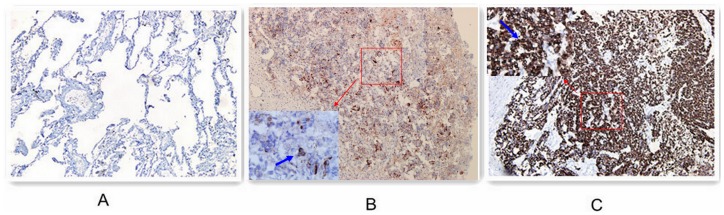
Immunohistochemical expression of MICA within the lung tumors was broadly homogenous. It combined granular cytoplasmic staining with cell membrane staining. No staining of stroma, extracellular matrix, or nuclei was observed, whereas tumor infiltrating leukocytes stained positive for MICA (Figure 1).
Figure 1. Immunohistochemical staining of MICA on human lung caner tissues (magnification ×200).
Expression of MICA in normal lung epithelium (A). MICA low-expression in lung tumor tissues (B), MICA high-expression in lung tumor tissues (C).
Analysis of MICA expression in the 222 NSCLC cases revealed that 98.2% of the lung tumors showed positive staining for MICA. Forty-five (20.3%) of the samples demonstrated light yellow staining, 33.8% (n = 75) were yellow or deep yellow, and 44.1% (n = 98) were brown or tan color (Table 3). A variable proportion of MICA-positive cells was also observed; 47.3% (n = 105) of the tumors showed extensive expression of MICA positive cells (76%–100%), whereas 1.8% (n = 4) of cases demonstrated MICA immunoreactivity in less than 5% of the tumor cells (Table 4).
Table 3. Intensity of MICA expression.
| Intensity of the immunostaining | Number (% of Tumors) |
| 0 score no staining | 4 (1.8%) |
| 1 score light yellow | 45 (20.3%) |
| 2 score yellow or deep yellow | 75 (33.8%) |
| 3 score brown or tan | 98 (44.1%) |
| Total | 222 |
Table 4. Proportion of MICA-positive lung carcinoma cells.
| Proportion of MICA-positive cells | Number (% of Tumors) |
| 0 score ≤5% | 4 (1.8%) |
| 1 score 6%∼25% | 9 (4.1%) |
| 2 score 26%∼50% | 18 (8.1%) |
| 3 score 51%∼75% | 86 (38.7%) |
| 4 score 76%∼100% | 105 (47.3%) |
| Total | 222 |
Characterization and phenotypes of CIK cells
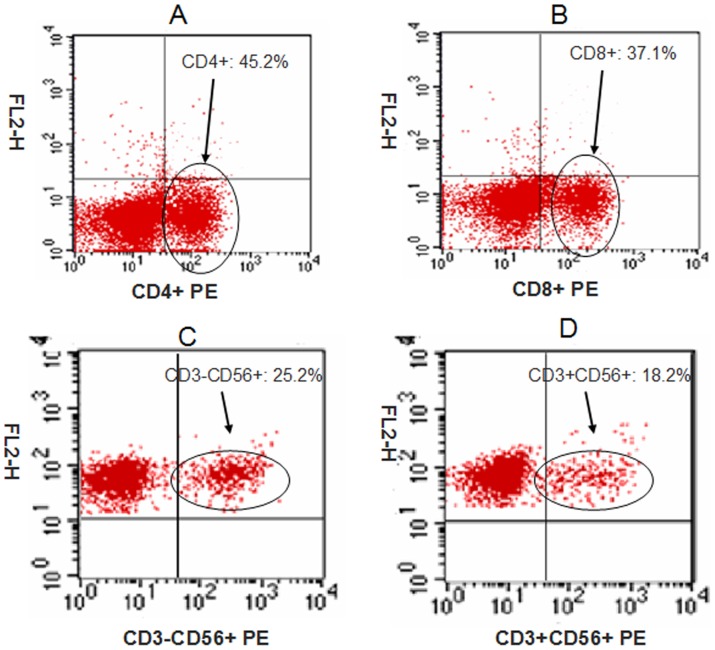
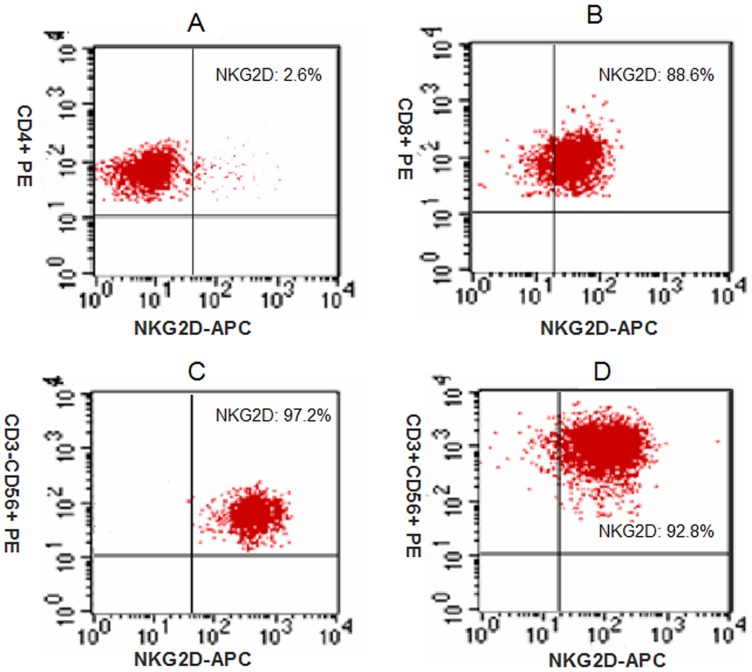
The expanded CIK cells were analyzed by flow cytometry. Phenotypic analysis of CIK cells from 38 patients after 14 days of culture demonstrated that the percentages of CD4+, CD8+, CD3-CD56+, CD3+CD56+ cells were 45.2±7.5%, 37.1±9.2%, 25.2±9.8%, and 18.2±5.6%, respectively (Figure 2). We analyzed the expression of NKG2D on in vitro expanded CIK cells. We found that only 2.6±1.2% of CD4+ T-cells were NKG2D+, while the other CIK cells expressed NKG2D. The percentage of NKG2D+ in CD8+, CD3-CD56+, and CD3+CD56+ populations were 88.6±5.4%, 97.2±2.1%, and 92.8±3.8%, respectively (Figure 3).
Figure 2. Percentages of different cells on bulk CIK cells were measured by flow cytometry (N = 38).
Figure 3. Expression of NKG2D on bulk CIK cells (N = 38).
Comparison of MICA expression with patient and tumor characteristics
The histochemical score of immunoreactivity was obtained by adding the intensity and percentage scores. Cases with scores of 0–4 were defined as the low-expression group, and cases with scores of 5–7 were defined as the high-expression group. The low-expression group included 84 cases (37.8%) and the high-expression group contained 138 cases (62.2%). Analysis utilizing the histochemical score method revealed statistically significant relationships between MICA expression and the staging of lung cancer, ECOG score, gender, and age. There was no significant correlation between MICA expression and pathological type (Table 5).
Table 5. Correlation between MICA expression and clinicopathologic features.
| Characteristics | Number | MICA low-expression | MICA high-expression | P value | |
| Gender | Male | 160 | 54 | 106 | 0.032 |
| Female | 62 | 30 | 32 | ||
| ECOG | 0–1 | 172 | 80 | 92 | 0.019 |
| 2–3 | 50 | 4 | 46 | ||
| Histology | Adenocarcinoma | 136 | 54 | 82 | 0.693 |
| Squamous carcinoma | 60 | 22 | 38 | ||
| other | 26 | 8 | 18 | ||
| Age | ≤60 | 126 | 58 | 68 | 0.003 |
| >60 | 96 | 26 | 70 | ||
| Stage | IIIA | 24 | 14 | 10 | 0.001 |
| IIIB | 44 | 24 | 20 | ||
| IV | 154 | 46 | 108 | ||
Univariate survival analysis
Among 222 patients, there were 160 males and 62 females and the median overall survival was 13 months, 95% CI (11.918–14.082), and the 1-year survival rate and 2-year survival rate were 52.3% and 19.8%, respectively (Figure 4A). By univariate analysis, MICA expression, gender, age, TMN stage, ECOG score, and CIK therapy were all predictors for overall survival (all p<0.05), while the histological subtype was not (p = 0.544) (Table 6). The median survival of patients with a high-expression of MICA was significantly shorter than those with a low-expression of MICA, p = 0.01 by log-rank test (Figure 4B).
Figure 4. Kaplan-Meier curves for overall survival (OS).
OS in 222 patients (A). OS in the overall population and by MICA expression (B). OS in the overall population by MICA expression and CIK therapy (C).
Table 6. Univariate analysis of factors associated with survival of patients with advanced non-small cell lung cancer.
| Variable | Number | OS MST(95%CI) | P value | |
| Gender | Male | 160 | 12.0 (10.5–13.5) | 0.002 |
| Female | 62 | 15 .0(12.7–17.3) | ||
| Stage | IIIA | 24 | 18.0 (15.6–20.4) | 0.001 |
| IIIB | 44 | 14.0 (12.0–15.9) | ||
| IV | 154 | 12.0 (10.7–13.3) | ||
| ECOG | 0–1 | 172 | 14.0 (13.1–14.9) | 0.000 |
| 2–3 | 50 | 7.0 (6.0–8.0) | ||
| Age | ≤60 | 126 | 15.0 (12.8–17.16) | 0.000 |
| >60 | 96 | 10.0 (7.9–12.1) | ||
| Histology | Adenocarcinoma | 136 | 14.0 (12.8–15.2) | 0.544 |
| Squamous carcinoma | 60 | 11.0 (8.8–13.2) | ||
| other | 26 | 15.0 (9.7–20.3) | ||
| MICA expression | High-expression | 138 | 10.0 (8.6–11.4) | 0.001 |
| Low-expression | 84 | 17.0 (14.4–19.6) | ||
| CIK therapy | Yes | 38 | 19.0 (113–26.7) | 0.040 |
| No | 184 | 12.0 (10.8–13.2) | ||
To confirm the importance of the CIK therapy in the treatment of advanced NSCLC with high expression of MICA, we divided the 222 patients into a CIK group and control group. In subgroup analysis the medium OS (mOS) of patients in the CIK group with high expression of MICA was longer than the OS for patients with low expression of MICA (27 months vs. 13 months). In the control group, the mOS of patients with high expression of MICA was shorter than in patients with low expression of MICA (9 months vs. 18 months) (Figure 4C). Furthermore, COX regression analysis showed that the MICA expression has a significant effect on CIK therapy (p = 0.001).
Multivariate survival analysis
In multivariate analysis, MICA expression, gender, age, ECOG score and CIK therapy were independent prognostic factors (Table 7). We could see that the risk of death for patients with high expression of MICA was 3.08 times higher than for patients with low expression of MICA. This suggests that the high expression of MICA was an indicator of poor prognosis in advanced NSCLC cancer patients. The interaction of MICA and CIK (p<0.0001), indicated that MICA overexpression was an independent predictor for successful CIK therapy. This was consistent with the results of the univariate subgroup analysis. We didn't introduce clinical stages into the multivariate analysis as the patients enrolled in our study were all advanced NSCLC cases.
Table 7. Multivariate analysis (Cox model) of factors associated with survival of patients with advanced non-small cell lung.
| Variable | SE | ?2 | P value | Hazard ratio | 95%CI |
| Gender | 0.160 | 10.18 | 0.001 | 1.67 | 1.22–2.28 |
| Age | 0.147 | 4.01 | 0.045 | 0.75 | 0.56–0.99 |
| ECOG | 0.180 | 19.14 | <0.001 | 0.46 | 0.32–0.648 |
| CIK therapy | 0.254 | 15.7 | <0.001 | 2.73 | 1.66–4.50 |
| MICA expression | 0.362 | 9.67 | 0.002 | 3.08 | 1.52–6.25 |
| MICA\CIK Interaction | 0.403 | 19.47 | <0.001 | 0.17 | 0.08–0.37 |
Discussion
Therapy for advanced NSCLC has entered the era of individualized treatment. As immunotherapy for treating lung cancer is becoming available, it is now important to identify factors that predict which patients will respond best to immune therapy.
MICA is rarely expressed by normal cells although it has been reported that MICA is constitutively expressed by intestinal epithelial cells, and is broadly expressed in a variety of malignancies, such as breast, ovarian, colon, kidney, pancreatic, prostate cancer, hepatocellular carcinoma, and melanoma [8], [9], [10]. This retrospective analysis of 222 NSCLC tumors investigated the role of the stress related protein MICA as a prognostic marker in advanced NSCLC and explored the relationship between MICA expression and the efficacy of CIK therapy for treating advanced NSCLC. Analysis of MICA expression in the 222 NSCLC tumors revealed that immunohistochemical expression of MICA within the lung tumors was broadly homogenous. The majority of lung tumors in this study showed strong cytoplasmic and cell membrane expression of MICA, but normal lung epithelium did not express them. These findings suggest that the expression of MICA only occurs after malignant transformation during lung cancer development, which is consistent with other findings for many other malignancies. Hence, these molecules have the potential to be used as tumor markers for lung cancer. Andreas Busche et al. [26] detected MICA in 40 NSCLC cases by immunohistochemistry, and found that 5 out of 17 adenocarcinoma cases and 5 out of 23 squamous carcinoma cases had positive MICA expression, which meant that 27.5% of the NSCLC cases had positive MICA expression. The higher MICA expression seen in our patients might result from differences between populations and races or that all patients enrolled in our study were advanced cases while the 40 cases enrolled in Busche's study covered the range from stage I to stage IV. By chi-square test and an exact probability test, we found that patients with stage IV disease did exhibit significantly higher MICA expression than patients with stage III disease. Considering the results above, we inferred that the positive rate of MICA expression would increase as the stage of lung cancer advanced. But we still need to enlarge the sample size and enroll patients in every stage to test the hypothesis.
It is well known that the cytotoxic function of NK cells is directly activated by tumor cells, whereas CD8+ T cells require simultaneous pre-sensitization and HLA stimulation. Inducible-surface expression of MICA in response to stress or malignant transformation is thought to mark dysfunctional cells for elimination by cytotoxic lymphocytes via NKG2D-mediated mechanisms [27], [28]. Indeed, ectopic expression of NKG2D ligands by tumors induces perforin-dependent strong NK and cytotoxic T lymphocyte (CTL) responses in vivo [13], [29]. Previous studies have shown that MICA expression is an independent marker of a good prognosis in colorectal cancers and osteosarcomas [22], [30]. Conversely, upregulation of MICA is an independent indicator of poor prognosis in breast cancers [23] and MICA expression did not correlate with prognoses in ovarian cancer [24]. However, our study of advanced NSCLC has demonstrated a significant correlation between MICA expression and the staging of lung cancer. In survival analysis, patients with a poor prognosis were more likely to have high levels of MICA on their tumors than those with a good prognosis.
MICA expression on lung cancer cells is a two-edged sword. MICA is a stress-induced molecule that has been associated with immune surveillance. However, recent reports have identified a mechanism by which tumors appear to be able to subvert the immune surveillance mechanism. Tumor cells are known to constitutively express MICA protein on the cell surface; however, these cells also shed MICA in a soluble form, possibly through proteolysis [8], [15]. The shedding of soluble MICA is coupled with a concomitant reduction in NK cell surface expression of NKG2DL, leading to diminished immunostimulatory signals for cytotoxic lymphocytes [15]. In addition, soluble MICA is associated with a systemic down regulation of NKG2D expression on the surface of CD8+ T-cells and on γδ T-cells, thereby further inhibiting the antitumor activity of these immune cells [31]. For lung cancer cells, MICA was mainly expressed in the membrane and cytoplasm. So it could be inferred that cancer cells with high expression of MICA in the membrane could block NKG2D receptors by releasing sMICA, and thereby evading immune rejection. This might be one of the reasons for the poor prognosis of lung cancer cases with high expression of MICA. Thereby, MICA may have variable effects on prognosis and on the biological behavior of tumors and influence tumor genesis and progression.
Cytokine-induced killer (CIK) cells are heterogeneous ex vivo-expanded T lymphocytes, with a mixed T-NK phenotype, that are able to exert a wide MHC-unrestricted antitumor activity against both solid and hematologic malignancies [18], [32], [33], However, clinical studies with CIK cells are still in their infancy and only report on a relatively small number of patients in most of these studies. Currently, accumulated evidence suggests that the amplification and immune response of CIK cells are related to activating the receptor NKG2D signaling pathway [19], [20], [21], [34]. It has been shown that the highly expressed NKG2D receptor on CIK cells can interact with ligands, like MICA, on tumor cells and activate second messenger signaling pathways downstream from the NKG2D receptor. CIK cells bind to tumor cells through increased expression of NKG2D and its ligation stimulates CIK cells to release perforin, which plays a role in lysing tumor cells. The important implication is that CIK immunotherapy may not be limited to a specific type of cancer or HLA haplotype, extending the range of potential patients who could benefit from such an approach. Furthermore, CIK cells can circumvent an important tumor immune escape mechanism when tumor cells down-regulate HLA expression. Our laboratory has also confirmed that, except for CD4+ T-cells, the other CIK cells express NKG2D. Therefore, we expect that the majority of CIK cells can recognize the MICA ligand on the lung tumor cell membrane through their NKG2D receptor and become activated to kill the tumor cells.
Through the single-factor analysis of 222 cases of advanced non-small-cell lung cancer in our study, it was found that the CIK treatment had a significant effect on OS (p = 0.040). COX regression analysis showed that CIK treatment can be used as one of the independent prognostic factors for advanced non-small cell lung cancer patients. This study further analyzed the cases in subgroups and found that patients with high MICA expression had a significantly greater survival benefit from CIK treatment. Further COX regression analysis showed that, at the intersection of MICA and the CIK efficacy, the p-value is <0.0001, which suggests that CIK treatment has a good effect in patients with high MICA expression and high expression of MICA can be used as a predictor for the efficacy of CIK treatment.
Our results are limited by the small number of CIK treated patients, the retrospective nature of the analysis, the variable number of treatment cycles, and the study lacks an evaluation of short-term effects. Therefore, a treatment model for MICA expression guided CIK for advanced non-small cell lung cancer patients needs further investigation with an expanded sample size in further prospective clinical studies.
In conclusion, the evaluation of MICA expression on tumors cells in patients with advanced non-small cell lung cancer would help to further determine the prognosis of the patient. Patients with high MICA expressing tumors have a relatively poorer prognosis, however, because of the interaction between the activating receptor NKG2D on CIK cells and MICA on the tumor cells, high expression of MICA can enhance the efficacy of CIK therapy and improve survival. This provides a new individualized treatment for future immunotherapy, and a theoretical basis for future prospective studies using an expanded sample size.
Acknowledgments
The authors wish to thank Drs Liyuan Zhang and Weifeng Zhu (Department of Pathology, Fujian Provincial Cancer Hospital) for their technical advice and histological observations.
Funding Statement
The project was supported by the Foundation for Young Scholars of Fujian Provincial Department of Health, China. (Grant No. 2011-1-24). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Jemal A, Bray F (2011) Center MM, Ferlay J, Ward E, et al (2011) Global cancer statistics. CA Cancer J Clin 61: 69–90. [DOI] [PubMed] [Google Scholar]
- 2. Stroncek D, Berlyne D, Fox B, Gee A, Heimfeld S, et al. (2010) Developments in clinical cell therapy. Cytotherapy 12: 425–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kakimi K, Nakajima J, Wada H (2009) Active specific immunotherapy and cell-transfer therapy for the treatment of non-small cell lung cancer. Lung Cancer 65: 1–8. [DOI] [PubMed] [Google Scholar]
- 4. Hontscha C, Borck Y, Zhou H, Messmer D, Schmidt-Wolf IG (2011) Clinical trials on CIK cells: first report of the international registry on CIK cells (IRCC). J Cancer Res Clin Oncol 137: 305–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dougan M, Dranoff G (2009) Immune therapy for cancer. Annu Rev Immunol 27: 83–117. [DOI] [PubMed] [Google Scholar]
- 6. Dunn GP, Old LJ, Schreiber RD (2004) The three Es of cancer immunoediting. Annu Rev Immunol 22: 329–360. [DOI] [PubMed] [Google Scholar]
- 7. Bauer S, Groh V, Wu J, Steinle A, Phillips JH, et al. (1999) Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science 285: 727–729. [DOI] [PubMed] [Google Scholar]
- 8. Salih HR, Antropius H, Gieseke F, Lutz SZ, Kanz L, et al. (2003) Functional expression and release of ligands for the activating immunoreceptor NKG2D in leukemia. Blood 102: 1389–1396. [DOI] [PubMed] [Google Scholar]
- 9. Groh V, Steinle A, Bauer S, Spies T (1998) Recognition of stress-induced MHC molecules by intestinal epithelial gammadelta T cells. Science 279: 1737–1740. [DOI] [PubMed] [Google Scholar]
- 10. Gleimer M, Parham P (2003) Stress management: MHC class I and class I-like molecules as reporters of cellular stress. Immunity 19: 469–477. [DOI] [PubMed] [Google Scholar]
- 11. Zamai L, Ponti C, Mirandola P, Gobbi G, Papa S, et al. (2007) NK cells and cancer. J Immunol 178: 4011–4016. [DOI] [PubMed] [Google Scholar]
- 12. Pardoll DM (2001) Immunology. Stress, NK receptors, and immune surveillance. Science 294: 534–536. [DOI] [PubMed] [Google Scholar]
- 13. Hayakawa Y, Kelly JM, Westwood JA, Darcy PK, Diefenbach A, et al. (2002) Cutting edge: tumor rejection mediated by NKG2D receptor-ligand interaction is dependent upon perforin. J Immunol 169: 5377–5381. [DOI] [PubMed] [Google Scholar]
- 14. Friese MA, Platten M, Lutz SZ, Naumann U, Aulwurm S, et al. (2003) MICA/NKG2D-mediated immunogene therapy of experimental gliomas. Cancer Res 63: 8996–9006. [PubMed] [Google Scholar]
- 15. Salih HR, Rammensee HG, Steinle A (2002) Cutting edge: down-regulation of MICA on human tumors by proteolytic shedding. J Immunol 169: 4098–4102. [DOI] [PubMed] [Google Scholar]
- 16. Shi M, Zhang B, Tang ZR, Lei ZY, Wang HF, et al. (2004) Autologous cytokine-induced killer cell therapy in clinical trial phase I is safe in patients with primary hepatocellular carcinoma. World J Gastroenterol 10: 1146–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schmidt-Wolf IG, Negrin RS, Kiem HP, Blume KG, Weissman IL (1991) Use of a SCID mouse/human lymphoma model to evaluate cytokine-induced killer cells with potent antitumor cell activity. J Exp Med 174: 139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schmidt-Wolf IG, Lefterova P, Mehta BA, Fernandez LP, Huhn D, et al. (1993) Phenotypic characterization and identification of effector cells involved in tumor cell recognition of cytokine-induced killer cells. Exp Hematol 21: 1673–1679. [PubMed] [Google Scholar]
- 19. Verneris MR, Karami M, Baker J, Jayaswal A, Negrin RS (2004) Role of NKG2D signaling in the cytotoxicity of activated and expanded CD8+ T cells. Blood 103: 3065–3072. [DOI] [PubMed] [Google Scholar]
- 20. Huang B, Sikorski R, Sampath P, Thorne SH (2011) Modulation of NKG2D-ligand cell surface expression enhances immune cell therapy of cancer. J Immunother 34: 289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guerra N, Tan YX, Joncker NT, Choy A, Gallardo F, et al. (2008) NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity 28: 571–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Watson NF, Spendlove I, Madjd Z, McGilvray R, Green AR, et al. (2006) Expression of the stress-related MHC class I chain-related protein MICA is an indicator of good prognosis in colorectal cancer patients. Int J Cancer 118: 1445–1452. [DOI] [PubMed] [Google Scholar]
- 23. Madjd Z, Spendlove I, Moss R, Bevin S, Pinder SE, et al. (2007) Upregulation of MICA on high-grade invasive operable breast carcinoma. Cancer Immun 7: 17. [PMC free article] [PubMed] [Google Scholar]
- 24. Li K, Mandai M, Hamanishi J, Matsumura N, Suzuki A, et al. (2009) Clinical significance of the NKG2D ligands, MICA/B and ULBP2 in ovarian cancer: high expression of ULBP2 is an indicator of poor prognosis. Cancer Immunol Immunother 58: 641–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McCarty KS Jr, Miller LS, Cox EB, Konrath J, McCarty KS (1985) Estrogen receptor analyses. Correlation of biochemical and immunohistochemical methods using monoclonal antireceptor antibodies. Arch Pathol Lab Med 109: 716–721. [PubMed] [Google Scholar]
- 26. Busche A, Goldmann T, Naumann U, Steinle A, Brandau S (2006) Natural killer cell-mediated rejection of experimental human lung cancer by genetic overexpression of major histocompatibility complex class I chain-related gene A. Hum Gene Ther. 17: 135–146. [DOI] [PubMed] [Google Scholar]
- 27. Raulet DH (2003) Roles of the NKG2D immunoreceptor and its ligands. Nat Rev Immunol 3: 781–790. [DOI] [PubMed] [Google Scholar]
- 28. Lanier LL (2001) A renaissance for the tumor immunosurveillance hypothesis. Nat Med 7: 1178–1180. [DOI] [PubMed] [Google Scholar]
- 29. Diefenbach A, Jensen ER, Jamieson AM, Raulet DH (2001) Rae1 and H60 ligands of the NKG2D receptor stimulate tumour immunity. Nature 413: 165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xiao P, Xue L, Che LH, Peng JJ, Wu HX, et al. (2008) Expression and roles of MICA in human osteosarcoma. Histopathology 52: 640–642. [DOI] [PubMed] [Google Scholar]
- 31. Groh V, Wu J, Yee C, Spies T (2002) Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature 419: 734–738. [DOI] [PubMed] [Google Scholar]
- 32. Wang Y, Dai H, Li H, Lv H, Wang T, et al. (2011) Growth of human colorectal cancer SW1116 cells is inhibited by cytokine-induced killer cells. Clin Dev Immunol 2011: 621414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Helms MW, Prescher JA, Cao YA, Schaffert S, Contag CH (2010) IL-12 enhances efficacy and shortens enrichment time in cytokine-induced killer cell immunotherapy. Cancer Immunol Immunother 59: 1325–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mesiano G, Todorovic M, Gammaitoni L, Leuci V, Giraudo DL, et al. (2012) Cytokine-induced killer (CIK) cells as feasible and effective adoptive immunotherapy for the treatment of solid tumors. Expert Opin Biol Ther 12: 673–684. [DOI] [PubMed] [Google Scholar]