Abstract
Background
Tamarindus indica (T. indica) is a medicinal plant with many biological activities including anti-diabetic, hypolipidaemic and anti-bacterial activities. A recent study demonstrated the hypolipidaemic effect of T. indica fruit pulp in hamsters. However, the biochemical and molecular mechanisms responsible for these effects have not been fully elucidated. Hence, the aims of this study were to evaluate the antioxidant activities and potential hypocholesterolaemic properties of T. indica, using in vitro and in vivo approaches.
Methodology/Principal Findings
The in vitro study demonstrated that T. indica fruit pulp had significant amount of phenolic (244.9±10.1 mg GAE/extract) and flavonoid (93.9±2.6 mg RE/g extract) content and possessed antioxidant activities. In the in vivo study, hamsters fed with high-cholesterol diet for ten weeks showed elevated serum triglyceride, total cholesterol, HDL-C and LDL-C levels. Administration of T. indica fruit pulp to hypercholesterolaemic hamsters significantly lowered serum triglyceride, total cholesterol and LDL-C levels but had no effect on the HDL-C level. The lipid-lowering effect was accompanied with significant increase in the expression of Apo A1, Abcg5 and LDL receptor genes and significant decrease in the expression of HMG-CoA reductase and Mtp genes. Administration of T. indica fruit pulp to hypercholesterolaemic hamsters also protected against oxidative damage by increasing hepatic antioxidant enzymes, antioxidant activities and preventing hepatic lipid peroxidation.
Conclusion/Significance
It is postulated that tamarind fruit pulp exerts its hypocholesterolaemic effect by increasing cholesterol efflux, enhancing LDL-C uptake and clearance, suppressing triglyceride accumulation and inhibiting cholesterol biosynthesis. T. indica fruit pulp has potential antioxidative effects and is potentially protective against diet-induced hypercholesterolaemia.
Introduction
Hyperlipidaemia refers to increased levels of lipids in the blood, including cholesterol and triglycerides. This increase is one of the significant risk factors involved in the development of cardiovascular disease (CVD) [1] and diabetes [2]. CVD remains an important cause of mortality and morbidity worldwide [3]. Research in the area of CVD is progressing rapidly and evidence and clinical trials for new drugs to treat CVD are continuously emerging. It is well established that increased levels of blood cholesterol especially low density lipoprotein cholesterol (LDL-C) is an important risk factor for cardiovascular complications since it favours lipid deposition in blood vessels. Epidemiological studies have clearly established that reduction of total cholesterol or LDL-C is associated with decreased risk of atherosclerosis and coronary heart disease [4], [5].
Treatment of hyperlipidaemia includes dietary changes, weight reduction, exercise and lipid-lowering drugs. Nonetheless, the use of these oral medications can lead to side effects [6]. Therefore, there continues to be a high demand for new, more effective and less toxic oral hypolipidaemic drugs. Plant products are frequently considered to be less toxic and relatively free from side effects than synthetic drugs. Hence, plants play a major role in the introduction of new therapeutic agents and have received much attention as sources of biologically active substances including antioxidative, hypoglycaemic and hypolipidaemic agents.
Tamarindus indica, commonly known as tamarind belongs to the family Leguminosae. It is native to tropical Africa, but is now grown all over the tropics. The tamarind tree is a large, frost-tender evergreen tropical tree which can grow to a height of about 30 m. T. indica is slow-growing but long-lived and can remain productive for 150 years or longer. The brown sticky pulp is a much-valued food ingredient in many Asian recipes due to its sweet and sour taste. T. indica has been used in folk medicine for treating diarrhea, stomach disorder, cold, fever, jaundice and as a skin cleanser [7]. Scientifically, the extract of T. indica fruit pulp has been shown to have anti-fungal and anti-inflammatory activities [8]. T. indica fruit pulp extract has been reported to possess hypolipidaemic effects when fed to hypercholesterolaemic hamsters [9]. However, this study did not report on the potential mechanisms of actions for the hypolipidaemic effects, particularly at the molecular levels. In vitro analysis carried out by our group suggest that the hypolipidaemic activity of T. indica fruit extract involves the regulation of expression of genes associated with lipid metabolism [10]. This study was aimed to corroborate the in vitro findings in order to further understand the hypolipidaemic effects of T. Indica and its protective action against oxidative damage.
Materials and Methods
Reagents and Chemicals
HPLC grade solvents, acetonitrile, ethanol and methanol were purchased from Fisher Scientific (United Kingdom). Polyphenol standards were purchased from Sigma Chemical Co. (St. Louis, USA) and had purities above 95%. Fast SYBR® Green Master Mix and High Capacity RNA-to-cDNA Master Mix was sourced from Applied Biosystems (California, United States). RNAlater™ RNA stabilization reagent, RNase-free DNase set and RNeasy mini kit were obtained from Qiagen (Hilden, Germany). Catalase, glutathione peroxidase and superoxide dismutase assay kits were purchased from Cayman Chemical Co. (Michigan, USA). 2,2-diphenyl-1-1 picrylhydrazyl (DPPH), butylated hydroxytoluene (BHT), dimethylsulphoxide (DMSO), 2,4,6-tripyridyl-s-triazine (TPTZ), Trolox and cholesterol were purchased from Sigma Chemical Co. (St. Louis, USA). Potassium persulphate and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) were sourced from Fluka Chemical (Steinheim, Germany). Sodium carbonate and Folin-Ciocalteu reagent were purchased from Merck (Darmstadt, Germany).
Preparation of the Extract of T. indica fruit pulp
T. indica fruit pulp was collected at Universiti Putra Malaysia and identified by comparison with the Voucher Specimen (KLU 45976), deposited at the Herbarium of the Institute of Biological Sciences, University of Malaya, Kuala Lumpur. The fruit pulp was air dried, ground to powder and stored at −20°C until further analyses. Extraction of the fruit pulp was performed by mixing the dried powder with 95% ethanol at a ratio of 1∶20 (g:ml), stirred for one hour, and subsequently incubated in the dark for 48 h at room temperature [11]. The resulting extract was then filtered twice using Macherey-Nagel filter paper and the ethanol was evaporated to dryness using a rotary evaporator at 37°C. For in vitro analyses of polyphenolic content and antioxidant activities, the extract was dissolved in 10% DMSO and stored at −20°C.
Polyphenolic Content Analyses
Polyphenolic content was determined using Folin-Ciocalteau assay developed by Singleton and Rossi [12]. Folin-Ciocalteau reagent (1 N) was mixed with the plant extract (1.0 mg/ml) and incubated at room temperature for 5 min before the addition of a sodium carbonate (Na2CO3) solution (60 g/l). The mixture was subsequently incubated at room temperature for 2 h. Absorbance readings were taken spectrophotometrically at 765 nm. Gallic acid was used as standard and was analyzed as above. Phenolic content was expressed as mg gallic acid equivalents (GAE)/g extract.
Flavonoid Content Analyses
Flavonoid content of the plant extract was determined using the aluminium chloride colorimetric method described by Liu and Qiu [13]. Briefly, 100 µl of plant extract (1 mg/ml) was mixed with 10 µl of 5% sodium nitrate and 10 µl of 10% aluminium chloride. The mixture was incubated for 6 min at room temperature. One hundred microlitres sodium hydroxide (1 M) was then added to the mixture and brought to a volume of 250 µl using distilled water. Absorbance of the mixture was measured at 510 nm using a spectrophotometer. Rutin was used as standard and was analyzed as above. Flavonoid content was expressed as mg of rutin equivalents (RE)/g extract.
Analysis of Polyphenols using High Performance Liquid Chromatography (HPLC)
The T. indica fruit pulp extract was subjected to acid hydrolysis before analyses of polyphenolic content using HPLC [11]. The hydrolysis process was performed by mixing 20 mg of sample with 1.6 ml of methanol and 0.4 ml of 1.2 M HCl. The mixture was then hydrolyzed at 90°C for 2 h in a tightly-sealed glass reactive vial. Subsequently, the hydrolyzed sample was centrifuged for 5 min at 5000 x g. The supernatant was collected and diluted with trifluoroacetic acid (TFA) (pH 2.6) and 200 µl was injected into the HPLC.
The HPLC system consisted of a Shimadzu dual wavelength absorbance detector (SPD-20A UV-VIS), a Rheodyne 7725i manual injector that comprised a 200 µl sample loop and column oven (CTO-10AS VP) and two pumps (LC 20AC). The mobile phase consisted of TFA (pH 2.6) as solvent A and acetonitrile as solvent B. The flow rate was 0.5 ml/min. Separation of polyphenols in the extract was achieved using the following gradient condition: 7% to 40% B for 20 min, 40% to 100% B for 6 min and 100% to 7% B for 9 min, using a reversed-phased column (NovaPak C18, 150×3.0 mm, inner diameter 4 µm). Absorbance of the sample was monitored at 260 nm. Data acquisition and processing were performed using the Lab Solution Chromatography Manager.
DPPH Radical Scavenging Activity
The 2,2-diphenyl-1-1picrylhydrazyl (DPPH) radical scavenging activity of the plant extract was determined according to Cos and Rajan [14]. Various concentrations of 25 µl of the ethanol extract of T. indica fruit pulp was mixed with 150 µl of a 0.04 mg/ml methanolic solution of DPPH. The mixture was vortexed and then incubated in the dark at room temperature for 20 min. Absorbance of the mixture was measured at 515 nm. Trolox was used as the standard and was analysed in the same manner as the sample. DPPH radical scavenging activities of the plant extracts were expressed as mmole Trolox equivalents antioxidant capacity (TEAC) per gram of extract. Ascorbic acid, butylated hydroxytoluene (BHT) and quercetin were used as positive controls. The percentage inhibition of the DPPH radicals by the plant extract was calculated using the following equation:
where, 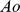 is the absorbance of the reaction mixture without the plant extract and
is the absorbance of the reaction mixture without the plant extract and 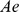 is the absorbance of the reaction mixture containing plant extract.
is the absorbance of the reaction mixture containing plant extract.
ABTS Radical Scavenging Activity
The 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic) acid (ABTS) radical scavenging activity of the plant extract was estimated according to the method of Re and Pellegrini [15]. ABTS+ cation chromophore was generated by reacting 7 mM ABTS with 2.45 mM potassium persulfate for 16 h in the dark at room temperature, followed by dilution with methanol to give an absorbance of 0.70±0.02 at 734 nm. For the antioxidant assay, 10 µl of plant extract (0−125 µg/ml) was added to 1.0 ml of the ABTS reagent, incubated in the dark for 15 min before absorbance was read at a wavelength of 734 nm. Trolox was used as the standard and analysed in the same manner as the sample. ABTS radical-scavenging activities of the plant extracts were expressed as mmole Trolox equivalents antioxidant capacity (TEAC) per gram of extract. Ascorbic acid, BHT and quercetin were used as positive controls. The percentage inhibition of the ABTS radicals by the plant extract was calculated using similar equation as the DPPH radical scavenging activity.
Ferric Reducing Antioxidant Power (FRAP) Activity
The ferric reducing activity of the plant extract was estimated using the method developed by Benzie and Strain [16]. Reagents for the assay consisted of 300 mmol/l acetate buffer, 10 mmol/l 2,4,6-tripyridyl-s-triazine (TPTZ) in 40 mmol/l of HCl and 20 mmol/l of FeCl3.6 H2O. The working FRAP reagent was prepared fresh by mixing 25 ml of acetate buffer, 2.5 ml of TPTZ solution and 2.5 ml of FeCl3.6 H2O. The freshly prepared mixture was incubated at 37°C in a water bath for 5 min before a blank reading was taken spectrophotometrically at 593 nm. After that, 30 µl of plant extract or standard and 90 µl of distilled water were added to 900 µl of the FRAP reagent. Absorbance of the mixture was measured immediately and thereafter at an interval of 15 sec for 4 min. The change in absorbance in the 4 min time reaction was calculated. A calibration curve was generated using ferrous sulphate as the standard. Ascorbic acid, BHT and quercetin were used as positive controls and analysed as above. Ferric reducing activity was expressed as µmole Fe(II) per gram of extract.
Ethics Statement for Animal Study
The protocol for the in vivo study was conducted with the approval of the ethics committee for animal experiments, Faculty of Medicine, University of Malaya {ethics no: PM/26/08/2009/AAZ(R)}.
Animal Diet
T. indica fruit pulp extract (500 mg/kg body weight) for the feeding study was prepared by diluting the dried fruit pulp powder with distilled water. High-cholesterol diet (1%) was prepared by mixing the standard chow with cholesterol. Briefly, standard chow (99 g) was ground into powder and mixed with 1 g of cholesterol, after which 100 ml of distilled water was added and the mixture was shaped into small pellets. The pellets were dried in an oven at 50°C and kept at 4°C before use.
Treatment of Hamsters with T. indica Fruit pulp Extract
Six week-old male Syrian hamsters (n = 20) were randomly divided into four treatment groups and housed individually in well-ventilated cages under a 12-h light:dark cycle. Diet and water were provided ad libitum. Animals were treated for ten weeks with the following diet; Group I: standard chow plus distilled water (5 ml/kg body weight); Group II: standard chow plus T. indica fruit pulp extract (500 mg/kg body weight); Group III: high-cholesterol diet plus distilled water (5 ml/kg body weight) and Group IV: high-cholesterol diet plus T. indica fruit pulp extract (500 mg/kg body weight). Feeding of T. indica fruit pulp extract was conducted through the gavage method once a day.
Collection and Preparation of Blood and Liver Samples
At the end of the feeding experiment, the animals were anaesthetized with a ketamine-xylazine cocktail (90 mg/kg and 2 mg/kg body weight, respectively) and blood was collected through cardiac puncture after an overnight fast. The livers of the animals were excised, homogenized with cold phosphate buffered saline, centrifuged at 2000 x g at 4°C for 15 min and the supernatant stored at −80°C before analysis. For the gene expression study, 1 g of the liver tissues were immediately washed with saline, kept in RNAlater™ RNA Stabilization Reagent (Qiagen) and stored at −80°C until further analysis.
Biochemical Analysis
Serum samples were analysed for lipid profiles, fasting blood glucose, alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels using commercially available kits on an automated analyser.
Analyses of Antioxidant Enzyme Activities
Activities of the antioxidant enzymes, catalase (CAT), glutatione peroxidase (GPx) and superoxide dismutase (SOD) were determined using commercially available assay kits (Cayman, USA). The assays were performed according to the manufacturer’s instructions.
Determination of Antioxidant Activities and TBARS in Serum and Liver
The ABTS radical scavenging and FRAP activities of serum and liver homogenate were analysed as described above. Thiobarbituric acid reactive substance (TBARS) assay was used to assess lipid peroxidation in serum and hepatic samples by measuring levels of malondialdehyde (MDA) [17]. A mixture of 100 µl sample and 100 µl trichloroacetic acid (10%) was centrifuged at 3500 rpm at 25°C for 5 min. One hundred and twenty microlitres of 0.67% thiobarbituric acid was added to the clear supernatant and heated at 100°C for 15 min and then cooled at room temperature. Absorbance of the mixture was read at 550 nm. 1,1,3,3-tetraethoxypropane was used as standard. Lipid peroxidation was expressed as nmol MDA/ml.
Gene Expression Analysis
The expression of selected genes that were associated with antioxidant activity and lipid metabolism (Table 1) were quantitated using real time relative quantitative polymerase chain reaction (qRT-CR) as previously described [10].
Table 1. Primer sequences used for qRT-PCR analysis of selected genes in hamsters.
| Gene (GenBank accession no.) | Forward primer (5′–3′) | Reverse primer (5′–3′) | Size of PCR product |
| Apo A1 (AF046919.1) | CGCCACCACGTTGACGCTCT | GGTCAGCGGCCTTGGTGTGG | 133 |
| Beta actin (AJ312092.1) | CGACAACGGCTCCGGCATGT | TCACGCCCTGGTGCCTAGGG | 101 |
| Abcg5 (NM_053754.2) | GGAAGGGGAGGTGTTTGT | GCCAGCATCGCCGTGTAG | 138 |
| Abcg8 (NM_130414.2) | CATCATTGGCTTCCTTTA | CCGCTCCGAGTGACATTT | 139 |
| Cyp1A1 (D12977.1) | TAAAGCACGCCCGCTGCGAA | AGC CCCCTGCTCTGGTGACC | 103 |
| Gstm1 (M59772.1) | AGCTGGGCCTGGACTTCCCC | ACACAGGTCGTGCTTGCGGG | 107 |
| HMG-CoA Reductase (M12705.1) | GCTGTCTGGTGGCCAGCACC | GGAAGACGCACCACTGGGCC | 112 |
| LDL-C Receptor (M94387.1) | GGCAGCGCTGACTGCAAGGA | TTCACGGTCACACTGGCGGC | 123 |
| Mtp (U14995.1) | AGCTGGCCTGGAGAGCAGGG | GCCCGGTCCATCTGCATGCA | 105 |
Apo A1, Apolipoprotein A1; Abcg5, ATP-binding cassette, subfamily G (WHITE), member 5; Abcg8, ATP-binding cassette, subfamily G (WHITE), member 8; Cyp1A1, Cytochrome P450, family 1, subfamily A, polypeptide 1; Gstm1, Glutathione S-transferase mu 1; Mtp, Microsomal triglyceride transfer protein.
RNA Extraction, Cleanup and Quantitation
Total cellular RNA (tcRNA) was extracted from 30 mg of the liver of control and treated hamsters using the RNeasy Mini Kit and its accompanying QIAshredder (Qiagen) in accordance with the manufacturer’s instructions. Briefly, the tissues were thoroughly ground to fine powder in liquid nitrogen with a mortar and pestle. Highly denaturing guanidine thiocyanate-containing buffer, buffer RLT was then added to the powder and the cell suspension was homogenized using QIAshredder. The homogenate was centrifuged and the lysate was then transferred to a microcentrifuge tube. The lysate was centrifuged and the supernatant was collected. Ethanol (50%) was then added to the supernatant and mixed well by pipetting. The mixture was then transferred to an RNeasy Spin Column and washed with RW1 and RPE buffers (QIAGEN; composition not declared). RNase-free water (40 µl) was added to the RNeasy Spin column and the eluent containing the tcRNA was treated with DNase I for 10 min to remove contaminating DNA using RNase-Free DNase Set (Qiagen). Then, buffer RLT and absolute ethanol were added to the samples and the mixture was transferred to an RNeasy Spin Column. Buffer RPE was added to wash the spin column membrane. In the final step, tcRNA was eluted with RNase-free water into collection tubes. Absorbance of the extracted tcRNA was measured at 260 and 280 nm using GeneQuantpro spectrophotometer. The concentration of the tcRNA was quantified using the 260 nm absorbance reading while its purity was evaluated from the 260∶280 ratio.
Reverse Transcription of the tcRNA
TcRNA (1000 ng) was reverse-transcribed into complementary DNA (cDNA) using a High Capacity RNA-to-cDNA Master Mix (Applied Biosystems) following the manufacturer’s instructions. The final reaction volume for the reverse transcription was 20 µl, consisted of 1000 ng TcRNA and 1 X Master Mix which contained magnesium chloride (MgCl2), deoxyribonucleotide triphosphate (dNTPs), recombinant RNase inhibitor protein, reverse transcriptase, random primers, oligo(dT) primer and stabilizers. The mixture was pipetted into a microcentrifuge tube and then placed in a thermocycler with the following program: 25°C for 5 min, 42°C for 30 min and 85°C for 5 min. The cDNA was kept at −80°C until further analysis.
Real-time Relative Quantitative RT–PCR (qRT–PCR)
Oligonucleotide primers used for qRT-PCR are listed in Table 1. All cDNA samples were diluted to a final concentration of 5 ng/µl. The final reaction volume for real time PCR was 20 µl, consisted of 10 µl of 2 X Fast SYBR® Green Master Mix (Applied Biosystem) which contained SYBR® Green I Dye, AmpliTaq® Fast DNA Polymerase, UP (Ultra Pure), Uracil-DNA Glycosylase (UDG), ROX™ dye Passive Reference, dNTPs and optimized buffer components, 200 nM of forward primer, 200 nM of reverse primer and 10 ng of cDNA. qRT-PCR was performed using Applied Biosystems StepOne Plus™ Real Time PCR System with a thermal profile consisted of 20 sec of Taq DNA Polymerase activation at 95°C, followed by 40 cycles of denaturation at 95°C for 3 sec, primer annealing at 60°C for 30 sec. The melting curves of the real-time PCR products were analysed from 65°C to 95°C to aid in product identification. Each measurement was carried out in triplicate. Differences in gene expression, expressed as fold-change, were calculated using the 2–ΔΔCt method where beta actin was used as the reference gene.
Statistical Analyses
All analyses were done in triplicate and data were expressed as means ± standard error of means. Significant differences among the groups were determined by one-way ANOVA using SPSS followed by the post hoc Tukey’s Honestly Significant Different test. Values corresponding to p<0.01 or p<0.05 were considered to be statistically significant.
Results
Polyphenolic and Flavonoid Content and Antioxidant Activities of T. indica Fruit pulp
The T. indica fruit pulp extract contains considerable amounts of polyphenols and flavonoids (Table 2) and showed moderate antioxidant activities, which were comparable to the positive control BHT but lower than ascorbic acid and quercetin.
Table 2. Polyphenol, flavonoid and antioxidant activities of the ethanol extract of T. indica fruit pulp.
| Polyphenolic content | Flavonoid content | DPPH | ABTS | FRAP | |
| T. indica fruit pulp | 244.93±10.07 | 93.93±2.57 | 0.08±0.01c | 0.07±0.04b | 0.72±0.03b |
| Ascorbic acid | NA | NA | 4.05±0.02a | 1.13±0.04a | 6.52±0.10a |
| BHT | NA | NA | 0.09±0.02c | 0.14±0.04b | 0.70±0.07b |
| Quercetin | NA | NA | 3.94±0.02b | 2.93±0.06a | 6.46±0.08a |
Values are expressed as mean ± standard deviation (n = 3). Means with different superscript letters within the same column are significantly different (p<0.05). Polyphenolic content is expressed as mg GAE/g extract; flavonoid content is expressed as mg RE/g extract; DPPH, 1,1-diphenyl-2-picryl hydrazyl radical scavenging activity and ABTS, 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) radical scavenging activity are expressed as mmole TE/g extract; FRAP, ferric reducing antioxidant power is expressed as mmole of Fe(II)/g extract; NA, not available.
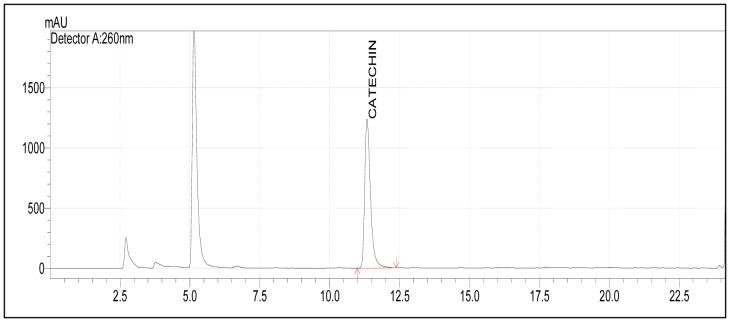
Figure 1 shows the chromatogram of hydrolysed T. indica fruit pulp analysed by HPLC. Catechin was identified in the fruit pulp sample by comparing the retention times of the peak with the authentic standard.
Figure 1. Chromatogram of T. indica fruit pulp analysed by HPLC.
HPLC analysis was carried out on the hydrolysed sample of T. indica fruit pulp using a reversed-phase column (NovaPak C18, 150 x 3.0 mm, inner diameter 4 µm). Separation of polyphenols was achieved using a linear gradient system comprising of acetonitrile in trifluoroacetic acid (pH2.6) as the mobile phase. Absorbance was measured at 260 nm.
Body Weight and Liver Weight of Control and Treated Hamsters
Table 3 shows the food consumption, body weight and weight of livers of the hamsters. No significant differences were seen in the amount of food consumption among the four groups. With the exception of the hypercholesterolaemic group (Gp. III), the remaining groups showed reduced weight after the 10-week experimental period. There were no significant differences in weight of livers among the control hamsters, hamsters treated with T. indica fruit pulp and hypercholesterolaemic hamsters treated with T. indica fruit pulp. On the contrary, the hypercholesterolaemic hamsters showed significant increase in liver weight, with the weight twice those of the control group (p<0.01). However, administration of T. indica fruit pulp to hypercholesterolaemic hamsters reduced liver weight by approximately 35% relative to the hypercholesterolaemic hamsters.
Table 3. Food consumption, body weight and liver weight of control and hamsters treated with T. indica fruit pulp extracts, in the presence or absence of cholesterol.
| Group | Group I | Group II | Group III | Group IV |
| Food Consumption (g/day) | 8.71±0.32 | 8.72±0.21 | 8.51±0.40 | 8.22±0.24 |
| Intial body weight(g) | 128.80±5.70 | 137.00±5.15 | 127.80±1.39 | 135.40±2.23 |
| Final body weight (g) | 121.60±5.26 | 130.80±3.99 | 139.00±4.14 | 127.00±1.34 |
| Weight change (g) | - 7.20±1.69b | - 6.20±4.60b | +7.00±0.71a | - 5.20±0.45b |
| Liver weight (g) | 3.40±0.24b | 3.60±0.24b | 6.80±0.20a | 4.40±0.24b |
Values are given as mean ± standard error of mean (n = 5). Values not sharing a common superscript letter within the same row differ significantly at p<0.01. Group I: standard chow plus distilled water (5 ml/kg body weight); Group II: standard chow plus T. indica fruit pulp extract (500 mg/kg body weight); Group III: high-cholesterol diet plus distilled water (5 ml/kg body weight) and Group IV: high-cholesterol diet plus T. indica fruit pulp extract (500 mg/kg body weight).
Effect of T. indica Fruit pulp on Biochemical Parameters in Hamsters
Table 4 summarizes the serum lipid profiles, glucose, AST and ALT levels of control and hamsters treated with T. indica fruit pulp. There was no significant difference in levels of serum triglyceride, total cholesterol, HDL, LDL, glucose, AST and ALT between control hamsters (Gp. I) and hamsters treated with T. indica fruit pulp (Gp. II). Feeding a high-cholesterol diet to the hamsters significantly increased all the biochemical parameters. On the contrary, when T. indica fruit pulp was fed together with the high-cholesterol diet, levels of triglyceride and LDL-C reduced to amounts similar to the normal untreated group whereas the level of total cholesterol reduced to roughly 50% compared to the control group. However, there was no significant change in HDL levels between the treated and untreated hypercholesterolaemic groups.
Table 4. Analyses of biochemical parameters of control and hamsters treated with T. indica fruit pulp extracts, in the presence or absence of cholesterol.
| Group | Group I | Group II | Group III | Group IV |
| Triglyceride (mmol/l) | 0.88±0.05b | 1.00±0.10b | 1.98±0.21a | 0.96±0.09b |
| Total choleste-rol (mmol/l) | 2.08±0.12c | 1.94±0.08c | 5.74±0.55a | 3.74±0.14b |
| HDL-C (mmol/l) | 1.34±0.07b | 1.25±0.07b | 2.20±0.23a | 2.27±0.19a |
| LDL-C (mmol/l) | 0.34±0.08b | 0.21±0.07b | 2.38±0.56a(*) | 0.95±0.15b(*) |
| Glucose (mmol/l) | 8.38±0.62ab | 5.18±0.27b | 10.00±2.00a | 8.66±0.78ab |
| AST (IU/l) | 95.20±13.38a | 70.20±12.73a | 142.00±29.62a | 87.00±9.47a |
| ALT (IU/l) | 51.40±5.81b | 62.80±11.21b | 243.00±84.10a | 104.60±22.69ab |
Values are given as mean ± standard error of mean (n = 5). Values not sharing a common superscript letter within the same row differ significantly at p<0.01 and * p<0.05. Group I: standard chow plus distilled water (5 ml/kg body weight); Group II: standard chow plus T. indica fruit pulp extract (500 mg/kg body weight); Group III: high-cholesterol diet plus distilled water (5 ml/kg body weight) and Group IV: high-cholesterol diet plus T. indica fruit pulp extract (500 mg/kg body weight).
The levels of glucose only increased in hypercholesterolaemic hamsters (Gp. III) and remained unchanged in the other groups. Although hamsters treated with T. indica fruit pulp showed reduced glucose levels compared to control group, the values were not significantly different. There was no significant difference in AST activities among the four groups. Hypercholesterolaemic hamsters showed significant increase in serum ALT compared to control hamsters. Administration of T. indica fruit pulp to the hypercholesterolaemic hamsters appeared to reduce ALT levels relative to the hypercholesterolaemic hamsters, however this was not statistically significant.
Effects of T. indica Fruit pulp on Serum and Liver Oxidative Status in Hamsters
Serum and liver antioxidant enzymes, antioxidant activities and lipid peroxidation levels are presented in Table 5 and 6, respectively. Hamsters fed the high-cholesterol diet showed reduced activities of CAT, SOD and GPx in serum and liver. Interestingly, treatment of either the control or hypercholesterolaemic hamsters with T. indica fruit pulp significantly increased activities of CAT and SOD in both serum and liver. In contrast, activity of GPx was unchanged in the serum of control hamsters fed T. indica fruit pulp although there was increased GPx activities in the hypercholesterolaemic hamsters fed T. indica fruit pulp in both serum and liver and also in the liver of control group fed T. indica fruit pulp.
Table 5. Serum antioxidant enzymes, antioxidant activities and lipid peroxidation in control and hamsters treated with T. indica fruit pulp extracts, in the presence or absence of cholesterol.
| Group | Group I | Group II | Group III | Group IV |
| Catalase (µmol/min/ml) | 3.54±0.21b | 4.48±0.19a | 2.67±0.04c | 3.24±0.04b |
| Superoxide dismutase (U/ml) | 13.34±0.63b | 18.67±0.47a | 6.25±0.67c | 12.00±0.45b |
| Glutathione peroxidase(µmol/min/ml) | 0.60±0.02a | 0.63±0.05a | 0.22±0.01c | 0.43±0.02b |
| ABTS (µmol/ml serum) | 2.53±0.08b | 2.93±0.03a | 2.14±0.05c | 2.41±0.05b |
| FRAP (µmol Fe (II)/ml serum) | 0.32±0.02b | 0.40±0.01a | 0.28±0.01b | 0.30±0.01b |
| MDA (nmol/ml serum) | 0.033±0.001 | 0.033±0.001 | 0.036±0.002 | 0.034±0.001 |
Values are given as means ± standard error of mean (n = 5). Values not sharing a common superscript letter within the same row are significantly different at p<0.05. Group I: standard chow plus distilled water (5 ml/kg body weight); Group II: standard chow plus T. indica fruit pulp extract (500 mg/kg body weight); Group III: high-cholesterol diet plus distilled water (5 ml/kg body weight) and Group IV: high-cholesterol diet plus T. indica fruit pulp extract (500 mg/kg body weight). DPPH, 1,1-diphenyl-2-picryl hydrazyl radical scavenging activity; ABTS, 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) radical scavenging activity; FRAP, ferric reducing antioxidant power; MDA (malondialdehyde).
Table 6. Hepatic antioxidant enzymes, antioxidant activities and lipid peroxidation in control and hamsters treated with T. indica fruit pulp extracts, in the presence or absence of cholesterol.
| Group | Group I | Group II | Group III | Group IV |
| Catalase (µmol/min/ml) | 1279.25±34.48b | 1857.16±49.07a | 958.65±44.48c | 1197.41±19.54b |
| Superoxide dismutase(U/ml) | 3616.48±67.98b | 4452.17±139.81a | 3153.66±110.03c | 3592.95±87.34b |
| Glutathione peroxidase (µmol/min/ml) | 8.48±0.63b | 10.70±0.33a | 6.91±0.20c | 8.70±0.43b |
| ABTS (µmol/g liver) | 16.93±0.52b | 19.67±0.38a | 11.35±0.56d | 13.32±0.27c |
| FRAP (µmol Fe (II)/g liver) | 3.26±0.07a | 3.43±0.08a | 2.07±0.10c | 2.48±0.09b |
| MDA (nmol/g liver) | 0.48±0.04c | 0.46±0.03c | 1.82±0.06a | 1.18±0.07b |
Values are given as means ± standard error of mean (n = 5). Values not sharing a common superscript letter within the same row differ significantly at p<0.05. Group I: standard chow plus distilled water (5 ml/kg body weight); Group II: standard chow plus T. indica fruit pulp extract (500 mg/kg body weight); Group III: high-cholesterol diet plus distilled water (5 ml/kg body weight) and Group IV: high-cholesterol diet plus T. indica fruit pulp extract (500 mg/kg body weight). DPPH, 1,1-diphenyl-2-picryl hydrazyl radical scavenging activity; ABTS, 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) radical scavenging activity; FRAP, ferric reducing antioxidant power; MDA (malondialdehyde).
The serum and liver of hypercholesterolaemic hamsters showed reduced ABTS radical scavenging activities although treatment of either the control or hypercholesterolaemic group with T. indica fruit pulp significantly increased ABTS radical scavenging activities in both serum and liver. In serum, FRAP values did not differ among the control, hypercholesterolaemic and hypercholesterolaemic group treated with T. indica fruit pulp. However, hamsters treated with T. indica fruit pulp (Gp. II) showed 20% higher FRAP activities compared to control (Gp. I). In the liver, administration of T. indica fruit pulp did not cause any significant change in FRAP activities compared to control group. However, in the hypercholesterolaemic hamsters fed T. indica fruit pulp, FRAP activities increased by approximately 20% compared to hypercholesterolaemic hamsters (p<0.05).
There was no significant difference in serum MDA levels among all the groups. Similarly, lipid peroxidation in the liver of the control (Gp. I) and T. indica fruit pulp-treated (Gp. II) hamsters was not significantly altered. High-cholesterol diet induced lipid peroxidation in the liver while treatment of T. indica fruit pulp to hypercholesterolaemic hamsters lowered lipid peroxidation by approximately 35%.
Effects of Ethanolic Extract of T. indica Fruit pulp on the Expression of Selected Hepatic Genes Associated with Lipid Metabolism and Antioxidant Activity in Hamsters
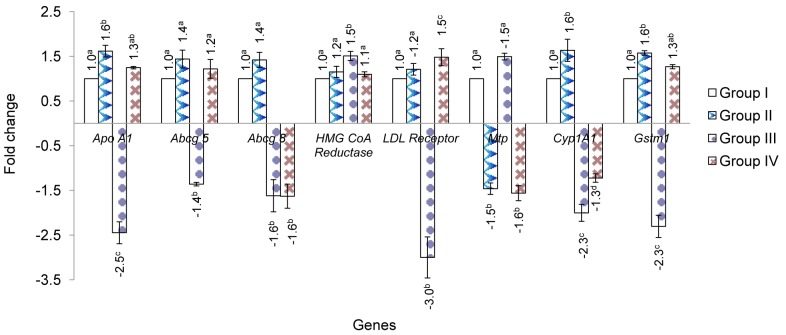
Figure 2. The effect of ethanolic extract of T. indica fruit pulp on the expression of selected hepatic genes in hamster (n = 3).
Each bar represents mean ± standard error of mean. Values not sharing a common superscript letter differ significantly at p<0.05. Group I: standard chow plus distilled water (5 ml/kg); Group II: standard chow plus 500 mg/kg T. indica fruit pulp extract; Group III: high-cholesterol diet plus distilled water (5 ml/kg). Group IV: high-cholesterol diet plus 500 mg/kg T. indica fruit pulp extract.
In hypercholesterolaemic hamsters, the expression of the Abcg5 was significantly down-regulated by 1.4-fold and the suppression of the ApoA-I gene expression was also significantly reversed when the hypercholesterolaemic hamsters were co-treated with T. indica extract. Significant down-regulation was observed for Abcg8 gene expression in the hypercholesterolaemic hamsters. Unlike the Abcg5, the expression of Abcg8 in the hypercholesterolaemic hamsters was unchanged in the presence of T. indica extract. Both Abcg5 and Abcg8 were up-regulated by 1.4-fold in hamsters treated with only T. indica fruit pulp extract (Gp. II). Besides that, HMG-CoA reductase gene expression was significantly up-regulated by 1.5-fold with the hypercholesterolaemic diet relative to that of control. T. indica extract managed to significantly suppress the HMGCoA reductase gene expression in the presence of cholesterol. The expression of HMG-CoA reductase was not significantly altered in hamsters receiving only T. indica fruit pulp extract.
LDL receptor gene was significantly down-regulated by 3.0-fold (p<0.05) in the presence of high cholesterol diet. When given together with T. indica fruit extract, the regulation of gene expression was reversed. However, treatment with only T. indica fruit extract did not significantly regulate the expression of LDL receptor gene.
The expression of microsomal triglyceride transfer protein (Mtp) was down-regulated by 1.5-fold in T. indica-treated hamsters (Gp. II) compared with control hamsters (Gp. I). Meanwhile, hamsters fed the high-cholesterol diet had increased hepatic expression level of Mtp gene by 1.5-fold compared with control hamsters, whereas, T. indica fruit pulp extract administration to hypercholesterolaemic hamsters caused down-regulation of Mtp gene expression level by 1.6-fold relative to the control hamsters.
In T. indica fruit pulp extract supplemented hamsters, the expression level of Cyp1A1 gene was significantly (p<0.05) increased by 1.6-fold relative to control hamsters. In contrast, hepatic Cyp1A1 expression was down-regulated by 2.3-fold in hamsters fed the high-cholesterol diet. Interestingly, treatment of T. indica fruit pulp extract to hypercholesterolaemic hamsters for ten weeks significantly (p<0.05) increased Cyp1A1 level compared to hypercholesterolaemic hamsters. In addition, the gene expression of Gstm1 was significantly up-regulated by 1.6-fold with the feeding of T. indica fruit pulp extract to control hamsters. In hypercholesterolaemic hamsters, the hepatic gene expression of Gstm1 was significantly (p<0.05) reduced (2.3-fold) compared with control. Surprisingly, the administration of T. indica fruit pulp extract along with high-cholesterol diet up-regulated Gstm1 gene expression level by 1.3-fold relative to control hamsters.
Discussion
A few studies have reported various biological activities of T. indica [7], [8]. Our group recently reported the high antioxidant activities of various parts of T. indica [11] as well as its ability to influence expression of genes and proteins which may explain its reported biological activities [10], [18]. Although the antioxidant activity of the fruit pulp in this study was lower than that of the leaves [13], the fruit pulp was chosen for the animal study as it was the part of the plant that was edible and commonly consumed. T. indica fruit pulp contains considerable amounts of phenolics and flavonoids as well as antioxidant activities. The antioxidant activities demonstrated by the fruit pulp could be due to the presence of phenolic compounds. Catechin, which was detected in the fruit pulp by HPLC, had been reported to have strong antioxidant activity [19]. In addition to catechin, Sudjaroen and Haubner [20] identified other phenolics in T. indica fruit pulp, including epicatechin, taxifolin, apigenin, eriodictyol, luteolin, naringenin and different classes of procyanidins. These compounds were reported to exhibit strong antioxidant properties and lipid-lowering effects [21], [22].
Epidemiological studies have shown inverse relationships between the intake of flavonoids from vegetables and fruits and the incidence of heart disease [23], [24]. Hence, many plants have been investigated for their potential hypocholesterolaemic properties. Hamsters are a suitable animal model for studying cholesterol metabolism due to their ability to develop hypercholesterolaemia when fed a high-cholesterol diet [9] in addition to having similar characteristics of cholesterol metabolism as humans [25].
The increase in liver weight of cholesterol-fed hamsters could be due to accumulation of cholesterol and triglyceride as a result of increased absorption from the diet. Liver is the major site for cholesterol metabolism in hamsters as well as humans [26] and a high-cholesterol diet had been reported to increase liver weight of hamsters [27], [28]. However, feeding of T. indica fruit pulp along with the high-cholesterol diet reversed this condition, possibly as a result of increased excretion and/or metabolism of cholesterol. The increased body weight of hypercholesterolaemic hamsters was likely contributed by the increase in liver weight. Serum AST, indicators of hepatotoxicity, were unchanged in hamsters treated with T. indica fruit pulp. Although ALT increased in hypercholesterolaemic hamsters, treatment with T. indica fruit pulp reduced the levels back to normal. Increased ALT in hamsters fed a high-cholesterol diet is commonly observed [29], [30]. Martinello and Soares [9] reported that administration of T. indica fruit pulp to hamsters did not lead to changes in AST and ALT levels, indicating that T. indica fruit pulp is not hepatotoxic.
In this study, hamsters fed on the high-cholesterol diet (Gp. III) showed increased triglyceride, total cholesterol, HDL-C and LDL-C compared to normal hamsters (Gp. I). A high-cholesterol diet can reduce the LDL-C clearance rate of hamsters causing increased LDL-C in the circulation [26]. Decreasing cholesterol and triglyceride levels in blood prevent the risk of cardiovascular diseases and atherosclerosis [31]. In this study, administration of T. indica fruit pulp together with high-cholesterol diet decreased triglyceride, total cholesterol and LDL-C but did not alter HDL-C levels. It is interesting to note that feeding T. indica fruit pulp to hypercholesterolaemic hamsters reduced LDL-C by more than two-fold. This suggests that the fruit pulp has cholesterol-lowering effect but it does not involve HDL-C directly.
Taxifolin, apigenin, naringenin and luteolin, phenolic compounds identified in T. indica fruit pulp had been reported to have hypolipidaemic properties, including inhibiting the production of cholesterol, suggesting their possible contribution towards improving the serum lipid profiles in cholesterol-fed hamsters [21], [22], [32].
Serum HDL-C levels were significantly higher in the hypercholesterolaemic hamsters (Gp. III) compared to those on the normal diet (Gp. I). This observation is in agreement with previous studies in humans and hamsters where high-cholesterol diet caused HDL-C elevation [33], [34]. In addition, the increased HDL-C levels in cholesterol-fed hamsters could also be an adaptive mechanism showing the need to enhance cholesterol efflux through the reverse cholesterol transport pathway in response to the high-cholesterol diet. Moreover, administration of T. indica fruit pulp along with the high-cholesterol diet did not produce significant variation in the serum HDL-C levels compared to hamsters fed on the high-cholesterol diet which suggests that T. indica fruit pulp had no effect on HDL-C.
A few genes that may contribute to the hypocholesterolaemic effect of T. indica fruit pulp were chosen and their expressions were determined by qRT-PCR to evaluate the molecular mechanisms underlying its lipid-lowering effects. We had previously shown that the T. indica fruit pulp extracts were able to regulate the expression of genes associated with lipid metabolism in an in vitro model, liver HepG2 cells [10]. Amongst the regulated genes were those encoding ABCG5, ApoA-1 and MTP. In the same study, the T. indica fruit pulp extract did not alter the expression of genes encoding LDL receptor which facilitates cholesterol uptake from the blood circulation as well as HMG-CoA reductase, the rate-limiting enzyme in the de novo synthesis of cholesterol. In this study, the HMG-CoA reductase gene expression in hypercholesterolaemic hamsters increased in parallel with serum total cholesterol concentration. This result is consistent with previous reports where animals fed a high-cholesterol diet had elevated HMG-CoA reductase expression [35], [36]. This finding points out the potential role of exogenous cholesterol as a cause for the up-regulation of hepatic HMG-CoA reductase expression. The observed increase in gene expression could also be due to the elevated glucose concentration in hamsters fed the high-cholesterol diet since insulin function to increase hepatic HMG-CoA reductase activity [37], [38].
Consistent with our in vitro analysis, hamsters treated with only T. indica fruit pulp extract did not show significant changes in the expression of HMG-CoA reductase gene compared to the control group. However, hamsters fed on the high-cholesterol diet along with T. indica fruit pulp had decreased expression level of HMG-CoA reductase compared to hypercholesterolaemic hamsters. This group of animals also had decreased levels of serum triglyceride, total cholesterol and LDL-C. This indicates that in the presence of cholesterol, the hypolipidaemic effect of T. indica fruit pulp could have possibly reduced cholesterol levels by reducing hepatic HMG-CoA reductase gene expression. Taxifolin had also been shown to have lipid-lowering effects through inhibition of the HMG-CoA reductase activity in HepG2 cells [21]. Therefore, the lipid lowering effect of T. indica fruit pulp could be due to the presence of taxifolin through the inhibition of HMG-CoA reductase expression.
The metabolism of LDL-C is greatly influenced by the number and activity of LDL receptors [39], [40]. Hence, functional LDL receptors are vital to take up LDL-C in order to decrease their circulating levels in the blood circulation which is one of the contributing factors towards the development of atherosclerosis. The effect of the plant extract on the expression of LDL receptor gene was studied to ascertain if the cholesterol-lowering effect of the plant extract occurred through increased production of LDL receptors. In the present study, the gene expression of LDL receptor in T. indica fruit pulp-fed hamsters was not significantly different from control hamsters. Similarly, the serum concentration of LDL-C in both groups of animals was not significantly different. The expression of LDL receptor in hamsters fed on the high-cholesterol diet was significantly down-regulated, signifying the possibility that high intracellular cholesterol reduced and suppressed synthesis of LDL receptors. LDL-C in excess was not efficiently taken up by the limited amounts of LDL receptors, hence decreasing LDL-C clearance from peripheral tissues [35], [41]. This could explain the elevation of serum LDL-C observed in the present study. This finding is consistent with another study in hamsters [42] where high-cholesterol diet caused down-regulation of LDL receptor expression. On the other hand, administration of T. indica fruit pulp to hypercholesterolaemic hamsters significantly up-regulated the hepatic mRNA levels of LDL receptor. This result suggests that T. indica fruit pulp could have induced LDL receptor gene expression, causing increased uptake and clearance of LDL-C from peripheral tissues, thus decreasing serum LDL-C concentration in hypercholesterolaemic hamsters. Indeed, plant extracts have been reported to be able to lower LDL-C levels by increasing LDL receptor activities [43]. It was reported that HepG2 cells treated with cacao polyphenols such as catechin elevated LDL receptor activity [44]. Catechin which is also present in the T. indica fruit pulp could have contributed towards reducing LDL-C in the hypercholesterolaemic hamsters by inducing the expression of LDL receptor.
Another gene that was measured in this study was Apo A1 which codes for Apolipoprotein A1 (Apo A1). Apo A1 is one of the major lipoprotein constituents of HDL-C and is involved in cholesterol efflux from tissues to liver for excretion [45]. Some studies have reported inverse relationship between serum Apo A1 levels and CHD [46], [47]. Similarly, over-expression of Apo A1 in Apo A1 transgenic mice has been shown to increase HDL-C and reduce the risk against diet-induced atherosclerosis [48]. The possible mechanism exerted by Apo A1 to provide cardioprotective effects include its role in reversing cholesterol transport which is the main pathway to eliminate excess cholesterol from peripheral tissues and to transport them to the liver. Cholesterol in the liver is then partially converted into bile acids and subsequently excreted in bile. Thus, the concentration of HDL-C is highly correlated with Apo A1 levels and Apo A1 gene expression may be an essential determinant of HDL-C. Therefore, increasing the production of Apo A1 by enhancing its gene transcription in the liver would be greatly advantageous.
In this study, hepatic expression of Apo A1 was down-regulated in hamsters fed with high-cholesterol diet compared with control, indicating that the cholesterol-enriched diet suppressed the Apo A1 expression which could have led to the observed elevated total cholesterol and triglyceride levels. A report showed that high-cholesterol diet decreased the levels of Apo A1 in rabbits [49]. However, in this study, the down-regulation of Apo A1 expression was not accompanied by a decreased in HDL-C levels in the hypercholesterolaemic hamsters. This is unexpected because suppression of Apo A1 gene is predicted to also decrease HDL-C levels. It is possible that other mechanisms maybe involved in regulating HDL-C levels. For instance, overexpression of lecithin cholesterol acyltransferase (LCAT) in hamsters had been reported to increase HDL-C levels [50]. T. indica fruit pulp supplementation to normal hamsters neither altered the expression of hepatic Apo A1 gene nor the levels of HDL-C. However, in hypercholesterolaemic hamsters, T. indica fruit pulp was able to up-regulate Apo A1 gene expression as well as increase HDL-C levels suggesting the role of the extract in reverse cholesterol transport mechanism.
Reverse cholesterol transport also includes the secretion of excess cholesterol in the liver into the bile. The efflux of the cholesterol is controlled by adenosine triphosphate (ATP)-binding cassette sub-family G member 5 (ABCG5) and (ATP)-binding cassette sub-family G member 8 (ABCG8) encoded by Abcg5 and Abcg8 genes, respectively. They are mainly expressed in the liver and are up-regulated in response to high-cholesterol diet [51]. In this study, adding cholesterol to the diet significantly down-regulated hepatic Abcg5 and Abcg8 expressions. The suppression of these two genes by cholesterol would limit efflux of cholesterol to the bile duct, resulting in the elevation of serum cholesterol concentrations which was observed in the serum of the hamsters. Interestingly, feeding of T. indica fruit pulp to hypercholesterolaemic hamsters significantly up-regulated expression of Abcg5. This result suggests that T. indica fruit pulp stimulates Abcg5 in response to the high-cholesterol diet intake to increase cholesterol secretion into the bile and inhibit cholesterol absorption, thus lowering serum cholesterol levels. Yu et al. [52] reported that over-expression of Abcg5 and Abcg8 in mice promoted cholesterol efflux and lowered plasma cholesterol concentrations. Transgenic mice expressing both human and mouse Abcg5 and Abcg8 genes had 50% reduction in cholesterol absorption and marked increased in biliary cholesterol and faecal neutral sterol secretion [53]. It has been suggested that Abcg5 and Abcg8 may work in concert as an apical sterol export pump to promote cholesterol and plant sterol efflux [51]. However, data from this study did not support this hypothesis as only the expression of Abcg5 was up-regulated but there was no significant difference in Abcg8 expression between hypercholesterolaemic hamsters and hypercholesterolaemic hamsters supplemented with the T. indica fruit pulp. Our findings support our previous in vitro study [10] where only ABCG5 and not ABCG8 was up-regulated in response to T. indica fruit pulp treatment of HepG2 cells.
Another gene that was studied was Mtp which encodes the heterodimeric microsomal triglyceride transfer protein (MTP). MTP is rate-limiting for assembly and secretion of apoB-containing lipoproteins such as very low density lipoprotein (VLDL-C) and chylomicrons [54], [55]. Diet enriched with cholesterol induced Mtp gene expression [56], [57]. In the present study, expression of Mtp gene was up-regulated in hypercholesterolaemic hamsters and this was accompanied by elevated triglyceride levels. It is possible that increased amounts of MTP are needed for the clearance of elevated triglyceride accumulated in the liver of hypercholesterolaemic hamsters. Bennett and Bruce [58] showed that hepatic Mtp gene expression and triglyceride levels increased in hamsters fed with cholesterol in a dose-dependent manner. Interestingly, the administration of T. indica fruit pulp to hypercholesterolaemic hamsters down regulated the expression of Mtp gene. This suggests that T. indica fruit pulp could provide lipid-lowering effect partially by decreasing hepatic Mtp gene expression, hence suppressing the assembly, accumulation and secretion of apo B-containing lipoproteins and triglyceride from liver to blood circulation. One of the active compounds in T. indica, naringenin, had been shown to reduce apoB accumulation in HepG2 cells via the inhibition of MTP activity and expression [59] and this may explain the reduction of triglyceride in the T. indica fruit pulp-treated hypercholesterolaemic hamsters.
In this study, we also tested the effects of T. indica fruit pulp on markers of oxidative stress and antioxidant activities as plants rich in antioxidants have the potential to protect against oxidative damage. CAT, SOD and GPx are antioxidant enzymes which play a significant role in the cellular defense against deleterious effects of free radicals. A decrease in the activities of these enzymes could increase the availability of O2 • and H2O2, which can lead to the production of OH•, subsequently initiating oxidative damage.
The reduced antioxidant activities as well as CAT, SOD and GPx activities in hypercholesterolaemic hamsters indicated possible oxidative stress conditions, leading to increased levels of O2 • and H2O2 CAT and SOD had been reported to be inactivated by ROS and lipid peroxides [60], [61]. Administration of T. indica fruit pulp to either control or hypercholesterolaemic hamsters increased antioxidant activities together with activities of CAT, SOD and GPx, although for GPx, this was only significant in the liver. Increased SOD implies increased capacity to detoxify O2 •, leading to increased production of H2O2. Concurrently, increased CAT and GPx allowed for the effective removal of excess H2O2. Thus T. indica fruit pulp could prevent the cholesterol-induced oxidative stress by enhancing and restoring the antioxidant defense systems.
Malondialdehyde (MDA), the end product in the oxidative breakdown of unsaturated fatty acids, is a marker used frequently to evaluate lipid peroxidation in tissues. In the present study, the high-cholesterol diet induced lipid peroxidation, suggesting increased oxidative stress. This could be due to increased free radical formation accompanied by reduced antioxidant enzyme and antioxidant activities. Hypercholesterolaemia has been reported to elevate the levels of lipid peroxidation in hamsters [9]. Administration of T. indica fruit pulp along with the high-cholesterol diet inhibited lipid peroxidation, demonstrating the protective effects of T. indica fruit pulp against oxidative damage. The increased antioxidant activities and antioxidant enzymes could have also contributed towards the protective effects against lipid peroxidation. Phenolics such as catechin, procyanidins and naringenin that are present in T. indica fruit pulp have been reported to improve the antioxidative defense mechanism by increasing antioxidant activities, SOD and CAT and inhibiting lipid peroxidation in rats [62], [63].
Besides measuring the expression of genes in cholesterol metabolism, two genes (Cyp1A1 and Gstm1) involved in xenobiotic metabolism were also evaluated. Their differential expression can provide beneficial information on the effect of the fruit pulp on antioxidant activities and oxidative stress. Xenobiotics and carcinogens are generally detoxified by phase I and phase II enzymes. Phase I detoxifying enzymes mainly composed of cytochrome P450 (CYP) supergene family of enzymes while phase II detoxifying enzymes comprised of glutathione S-transferase (GST) family. Variations in the expression of phase I and phase II detoxification enzymes may explain the carcinogenic effects leading to cancer. Hence, the influence of T. indica fruit pulp and cholesterol on the expression of genes related to detoxification was measured by qRT-PCR.
Cytochrome P450 Cyp1A1 is one of the members of the CYP family involved in the metabolism of drugs, environmental pollutants and carcinogens as well as a small number of endogenous substrates [64], [65]. In this study, hypercholesterolaemic hamsters showed down-regulation of hepatic Cyp1A1 expression. However, T. indica fruit pulp administration to the hypercholesterolaemic hamsters reversed this condition. The induction of Cyp1A1 might be essential to provide protection from the harmful effects of disruptors in the environment. Our study also showed that feeding of T. indica fruit pulp to hamsters induced Cyp1A1 gene expression as compared to control hamsters. Quiñones and Lucas [66] reported that people carrying CYP1A1 polymorphism could be more susceptible to lung cancer induced by environmental pollutants. This may indicate that T. indica fruit pulp has the potential to defend against carcinogenic risk via the induction of Cyp1A1 gene expression. Although some studies have reported the carcinogenic effects of Cyp1A1 [67], [68], more recent studies have implied potential detoxication as well as chemoprevention activities of Cyp1A1 [69]. Several phytochemicals including the flavonoids quercetin was reported to induce the expression of Cyp1A1 in MCF-7 breast cancer cells [70].
Gstm1 encodes the glutathione S-transferase Mu 1, an enzyme involved in phase II detoxification of electrophilic compounds such as products of oxidative stress, environmental toxins and carcinogens and its impairment is associated with increased cancer risk [71]. The hepatic Gstm1 gene expression level was significantly lowered in hamsters fed cholesterol diet compared with control. This signifies that cholesterol potentially inhibits the expression of Gstm1, thus enhancing the susceptibility of hypercholesterolaemic hamsters to environmental and carcinogenic challenges. Administration of T. indica fruit pulp to hypercholesterolaemic hamsters induced Gstm1 expression to a level similar to control hamsters. In addition, administration of T. indica fruit pulp to control hamsters also increased the hepatic Gstm1 gene expression significantly. These results suggest that T. indica fruit pulp could reverse the detrimental effects of high-cholesterol diet and contribute to the prevention of carcinogenesis by inducing the phase II detoxifying enzyme. Together, the regulation of both Cyp1A1 and Gstm1 by T. indica fruit pulp seems to be in a coordinated manner.
Conclusion
In conclusion, this study shows that T. indica fruit pulp is a natural health food with hypocholesterolaemic and antioxidant properties. T. indica fruit pulp exerts its potential hypocholesterolaemic action by increasing hepatic gene expression of Apo A1, Abcg5 and LDL receptor while suppressing HMG-CoA reductase and Mtp gene expressions. Hence, T. indica fruit pulp could potentially enhance cholesterol efflux, inhibit cholesterol biosynthesis, increase uptake and clearance of LDL-C from peripheral tissues and suppress triglyceride accumulation in the liver. On the other hand, supplementation of T. indica fruit pulp to hamsters did not lead to notable changes in lipid profiles compared to control hamsters. These observations imply that the fruit pulp is only beneficial under hypercholesterolaemic conditions. In addition, T. Indica fruit pulp can also prevent oxidative damage, particularly oxidation of LDL-C, which is one of the risk factors for atherosclerosis. These beneficial effects may be attributed to the phytochemical constituents, including the many phenolic and flavonoid compounds in the T. indica fruit pulp. T. indica fruit pulp may have clinical applications particularly in individuals with hypercholesterolaemia who may benefit from the cholesterol-lowering effect of this plant as well as its potential protection against oxidative damage. However, further work is needed to confirm the efficacy and proper dosage of the T. indica fruit pulp as a potential cholesterol-lowering agent including human trials to gain further insight into its therapeutic effect.
Funding Statement
This research was financially supported by the High Impact Research Grant (H-20001-00-E000009), University of Malaya Research Grant (RG458-12HTM) and Postgraduate Research Fund (PV116-2012A) from University of Malaya, Malaysia. The funders had no role in the study design, data collection and analysis and preparation of the manuscript.
References
- 1. Frishman W (1998) Biologic markers as predictors of cardiovascular disease. The American Journal of Medicine 104 (6 Supplement 1)18S–27S. [DOI] [PubMed] [Google Scholar]
- 2. Pushparaj P, Tan CH, Tan BKH (2000) Effects of Averrhoa bilimbi leaf extract on blood glucose and lipids in streptozotocin-diabetic rats. Journal of Ethnopharmacology 72 (1–2): 69–76. [DOI] [PubMed] [Google Scholar]
- 3.WHO (2012) Cardiovascular disease. Available: http://www.who.int/cardiovascular_diseases/en/. Accessed 2012 Jul 17.
- 4. Sever PS, Dahlöf B, Poulter NR, Wedel H, Beevers G, et al. (2003) Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial–Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. The Lancet 361 (9364): 1149–1158. [DOI] [PubMed] [Google Scholar]
- 5. Wilson PWF, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, et al. (1998) Prediction of Coronary Heart Disease Using Risk Factor Categories. Circulation 97 (18): 1837–1847. [DOI] [PubMed] [Google Scholar]
- 6. Davidson MH, Armani A, McKenney JM, Jacobson TA (2007) Safety Considerations with Fibrate Therapy. The American Journal of Cardiology 99 (6 Supplement 1)S3–S18. [DOI] [PubMed] [Google Scholar]
- 7. Doughari JH (2006) Antimicrobial Activity of Tamarindus indica Linn. Tropical Journal of Pharmaceutical Research 5 (2): 597–603. [Google Scholar]
- 8. Rimbau V, Cerdan C, Vila R Iglesias J (1999) Antiinflammatory activity of some extracts from plants used in the traditional medicine of north-African countries (II). Phytotherapy Research 13 (2): 128–132. [DOI] [PubMed] [Google Scholar]
- 9. Martinello F, Soares SM, Franco JJ, Santos AC, Sugohara A, et al. (2006) Hypolipemic and antioxidant activities from Tamarindus indica L. pulp fruit extract in hypercholesterolemic hamsters. Food and Chemical Toxicology 44 (6): 810–818. [DOI] [PubMed] [Google Scholar]
- 10. Razali N, Aziz AA, Junit SM (2010) Gene expression profiles in human HepG2 cells treated with extracts of the Tamarindus indica fruit pulp. Genes and Nutrition 5 (4): 331–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Razali N, Mat-Junit S, Abdul-Muthalib AF, Subramaniam S, Abdul-Aziz A (2012) Effects of various solvents on the extraction of antioxidant phenolics from the leaves, seeds, veins and skins of Tamarindus indica L. Food Chemistry 131. (2): 441–448. [Google Scholar]
- 12. Singleton VL, Rossi JA (1965) Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. American Journal of Enology and Viticulture 16 (3): 144–158. [Google Scholar]
- 13. Liu H, Qiu N, Ding H, Yao R (2008) Polyphenols contents and antioxidant capacity of 68 Chinese herbals suitable for medical or food uses. Food Research International 41 (4): 363–370. [Google Scholar]
- 14. Cos P, Rajan P, Vedernikova I, Calomme M, Pieters L, et al. (2002) In vitro antioxidant profile of phenolic acid derivatives. Free Radical Research 36 (6): 711–716. [DOI] [PubMed] [Google Scholar]
- 15. Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, et al. (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology & Medicine 26 (9–10): 1231–1237. [DOI] [PubMed] [Google Scholar]
- 16. Benzie IF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of "antioxidant power": the FRAP assay. Analytical Biochemistry 239 (1): 70–76. [DOI] [PubMed] [Google Scholar]
- 17. Mengel CE, Kann HE (1966) Effects of in vivo hyperoxia on erythrocytes. 3. In vivo peroxidation of erythrocyte lipid. The Journal of Clinical Investigation 45 (7): 1150–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chong URW, Abdul-Rahman PS, Abdul-Aziz A, Hashim OH, Mat Junit S (2012) Tamarindus indica Extract Alters Release of Alpha Enolase, Apolipoprotein A-I, Transthyretin and Rab GDP Dissociation Inhibitor Beta from HepG2 Cells. PLoS ONE 7 (6): e39476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Katalinić V, Milos M, Modun D, Musić I, Boban M (2004) Antioxidant effectiveness of selected wines in comparison with (+)-catechin. Food Chemistry 86 (4): 593–600. [Google Scholar]
- 20. Sudjaroen Y, Haubner R, Würtele G, Hull WE, Erben G, et al. (2005) Isolation and structure elucidation of phenolic antioxidants from Tamarind (Tamarindus indica L.) seeds and pericarp. Food and Chemical Toxicology 43 (11): 1673–1682. [DOI] [PubMed] [Google Scholar]
- 21. Theriault A, Wang Q, Van Iderstine SC, Chen B, Franke AA, et al. (2000) Modulation of hepatic lipoprotein synthesis and secretion by taxifolin, a plant flavonoid. Journal of Lipid Research 41 (12): 1969–1979. [PubMed] [Google Scholar]
- 22. Bundy R, Walker AF, Middleton RW, Wallis C, Simpson HCR (2008) Artichoke leaf extract (Cynara scolymus) reduces plasma cholesterol in otherwise healthy hypercholesterolemic adults: A randomized, double blind placebo controlled trial. Phytomedicine 15 (9): 668–675. [DOI] [PubMed] [Google Scholar]
- 23. Katan MB (1997) Flavonoids and heart disease. American Journal of Clinical Nutrition 165: 1542–1543. [DOI] [PubMed] [Google Scholar]
- 24. He FJ, Nowson CA, MacGregor GA (2006) Fruit and vegetable consumption and stroke: meta-analysis of cohort studies. Lancet 367 (9507): 320–326. [DOI] [PubMed] [Google Scholar]
- 25. Hoffmann A, Schmalz M, Leineweber M (1996) Cholesterol lowering action of HOE 402 in the normolipidemic and hypercholesterolemic golden Syrian hamster. Biochimica et Biophysica Acta (BBA) - Bioenergetics 1299 (1): 95–102. [DOI] [PubMed] [Google Scholar]
- 26. Spady DK, Turley SD, Dietschy JM (1985) Rates of low density lipoprotein uptake and cholesterol synthesis are regulated independently in the liver. Journal of Lipid Research 26 (4): 465–472. [PubMed] [Google Scholar]
- 27. Alsaif MA, Khan LK, Alhamdan AAH, Alorf SM, Harfi SH, et al. (2007) Effect of Dates and Gahwa (Arabian Coffee) Supplementation on Lipids in Hypercholesterolemic Hamsters. International Journal of Pharmacology 3 (2): 123–129. [Google Scholar]
- 28. Kahlon TS, Chow FI, Wood DF (1999) Cholesterol Response and Foam Cell Formation in Hamsters Fed Rice Bran, Oat Bran, and Cellulose+Soy Protein Diets With or Without Added Vitamin E. Cereal Chemistry Journal 76. (5): 772–776. [Google Scholar]
- 29. Yazdanparast R, Bahramikia S, Ardestani A (2008) Nasturtium officinale reduces oxidative stress and enhances antioxidant capacity in hypercholesterolaemic rats. Chemico-Biological Interactions 172 (3): 176–184. [DOI] [PubMed] [Google Scholar]
- 30. Ben Khaled H, Ghlissi Z, Chtourou Y, Hakim A, Ktari N, et al. (2012) Effect of protein hydrolysates from sardinelle (Sardinella aurita) on the oxidative status and blood lipid profile of cholesterol-fed rats. Food Research International 45 (1): 60–68. [Google Scholar]
- 31. Ling J, Wei B, Lv G, Ji H, Li S (2012) Anti-hyperlipidaemic and antioxidant effects of turmeric oil in hyperlipidaemic rats. Food Chemistry 130 (2): 229–235. [Google Scholar]
- 32. Kim K-T, Yeo E-J, Moon S-H, Cho S-C, Han Y-S, et al. (2008) Inhibitory effects of naringenin, kaempherol and apigenin on cholesterol biosynthesis in HepG2 and MCF-7 cells. Food Science and Biotechnology 17 (6): 1361–1364. [Google Scholar]
- 33. Brinton EA, Eisenberg S, Breslow JL (1990) A low-fat diet decreases high density lipoprotein (HDL) cholesterol levels by decreasing HDL apolipoprotein transport rates. The Journal of Clinical Investigation 85 (1): 144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hayek T, Ito Y, Azrolan N, Verdery RB, Aalto-Setälä K, et al. (1993) Dietary fat increases high density lipoprotein (HDL) levels both by increasing the transport rates and decreasing the fractional catabolic rates of HDL cholesterol ester and apolipoprotein (Apo) A-I. Presentation of a new animal model and mechanistic studies in human Apo A-I transgenic and control mice. The Journal of Clinical Investigation 91 (4): 1665–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Huang X, Tang J, Zhou Q, Lu H, Wu Y, et al. (2010) Polysaccharide from Fuzi (FPS) Prevents Hypercholesterolemia in Rats. Lipids in Health and Disease 9 (1): 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nammi S, Kim MS, Gavande NS, Li GQ, Roufogalis BD (2010) Regulation of low-density lipoprotein receptor and 3-hydroxy-3-methylglutaryl coenzyme A reductase expression by Zingiber officinale in the liver of high-fat diet-fed rats. Basic Clin Pharmacol Toxicol 106 (5): 389–395. [DOI] [PubMed] [Google Scholar]
- 37. Ingebritsen TS, Geelen MJ, Parker RA, Evenson KJ, Gibson DM (1979) Modulation of hydroxymethylglutaryl-CoA reductase activity, reductase kinase activity, and cholesterol synthesis in rat hepatocytes in response to insulin and glucagon. Journal of Biological Chemistry 254 (20): 9986–9989. [PubMed] [Google Scholar]
- 38. Lakshmanan MR, Nepokroeff CM, Ness GC, Dugan RE, Porter JW (1973) Stimulation by insulin of rat liver -hydroxy- -methylglutaryl coenzyme A reductase and cholesterol-synthesizing activities. Biochemical and Biophysical Research Communications 50 (3): 704–710. [DOI] [PubMed] [Google Scholar]
- 39. White DA, Bennett AJ, Billett MA, Salter AM (1997) Genetic determinants of plasma lipoprotein levels and their dietary response. Prostaglandins, Leukotrienes and Essential Fatty Acids 57 (4–5): 455–462. [DOI] [PubMed] [Google Scholar]
- 40. Patalay M, Lofgren IE, Freake HC, Koo SI, Fernandez ML (2005) The lowering of plasma lipids following a weight reduction program is related to increased expression of the LDL receptor and lipoprotein lipase. Journal of Nutrition 135 (4): 735–739. [DOI] [PubMed] [Google Scholar]
- 41. Pitman WA, Osgood DP, Smith D, Schaefer EJ, Ordovas JM (1998) The effects of diet and lovastatin on regression of fatty streak lesions and on hepatic and intestinal mRNA levels for the LDL receptor and HMG CoA reductase in F1B hamsters. Atherosclerosis 138 (1): 43–52. [DOI] [PubMed] [Google Scholar]
- 42. Horton JD, Cuthbert JA, Spady DK (1993) Dietary fatty acids regulate hepatic low density lipoprotein (LDL) transport by altering LDL receptor protein and mRNA levels. Journal of Clinical Investigation 92 (2): 743–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bursill CA, Abbey M, Roach PD (2007) A green tea extract lowers plasma cholesterol by inhibiting cholesterol synthesis and upregulating the LDL receptor in the cholesterol-fed rabbit. Atherosclerosis 193 (1): 86–93. [DOI] [PubMed] [Google Scholar]
- 44. Yasuda A, Natsume M, Osakabe N, Kawahata K, Koga J (2011) Cacao Polyphenols Influence the Regulation of Apolipoprotein in HepG2 and Caco2 Cells. Journal of Agricultural and Food Chemistry 59 (4): 1470–1476. [DOI] [PubMed] [Google Scholar]
- 45. Shoulders CC, Kornblihtt AR, Munro BS, Baralle FE (1983) Gene structure of human apolipoprotein Al. Nucleic Acids Research 11 (9): 2827–2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fruchart JC, Castro G, Duriez P (1996) Apolipoprotein-AI-containing particles and atherosclerosis. Israel Journal of Medical Sciences 32 (6): 498–502. [PubMed] [Google Scholar]
- 47. Miller NE (1987) Associations of high-density lipoprotein subclasses and apolipoproteins with ischemic heart disease and coronary atherosclerosis. Am Heart J 113 (2, Part 2): 589–597. [DOI] [PubMed] [Google Scholar]
- 48. Rubin EM, Krauss RM, Spangler EA, Verstuyft JG, Clift SM (1991) Inhibition of early atherogenesis in transgenic mice by human apolipoprotein AI. Nature 353 (6341): 265–267. [DOI] [PubMed] [Google Scholar]
- 49. Zhao J, Xiao L, Zhu H, Shu Y, Cheng N (1998) Changes of lipid metabolism in plasma, liver and bile during cholesterol gallstone formation in rabbit model. World Journal of Gastroenterol 4 (4): 337–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhang A-H, Gao S, Fan J-l, Huang W, Zhao T-Q, et al. (2004) Increased plasma HDL cholesterol levels and biliary cholesterol excretion in hamster by LCAT overexpression. FEBS Letters 570 (1–3): 25–29. [DOI] [PubMed] [Google Scholar]
- 51. Berge KE, Tian H, Graf GA, Yu L, Grishin NV, et al. (2000) Accumulation of Dietary Cholesterol in Sitosterolemia Caused by Mutations in Adjacent ABC Transporters. Science 290 (5497): 1771–1775. [DOI] [PubMed] [Google Scholar]
- 52. Yu L, Li-Hawkins J, Hammer RE, Berge KE, Horton JD, et al. (2002) Overexpression of ABCG5 and ABCG8 promotes biliary cholesterol secretion and reduces fractional absorption of dietary cholesterol. The Journal of Clinical Investigation 110 (5): 671–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wilund KR, Yu L, Xu F, Hobbs HH, Cohen JC (2004) High-level expression of ABCG5 and ABCG8 attenuates diet-induced hypercholesterolemia and atherosclerosis in Ldlr−/− mice. Journal of Lipid Research 45 (8): 1429–1436. [DOI] [PubMed] [Google Scholar]
- 54. Gregg RE, Wetterau JR (1994) The molecular basis of abetalipoproteinemia. Current Opinion in Lipidology 5 (2): 81–86. [DOI] [PubMed] [Google Scholar]
- 55. Wetterau, Aggerbeck L, Bouma M, Eisenberg C, Munck A, et al. (1992) Absence of microsomal triglyceride transfer protein in individuals with abetalipoproteinemia. Science 258 (5084): 999–1001. [DOI] [PubMed] [Google Scholar]
- 56. Du EZ, Wang SL, Kayden HJ, Sokol R, Curtiss LK, et al. (1996) Translocation of apolipoprotein B across the endoplasmic reticulum is blocked in abetalipoproteinemia. Journal of Lipid Research 37 (6): 1309–1315. [PubMed] [Google Scholar]
- 57. Gao S, He L, Ding Y, Liu G (2010) Mechanisms underlying different responses of plasma triglyceride to high-fat diets in hamsters and mice: Roles of hepatic MTP and triglyceride secretion. Biochemical and Biophysical Research Communications 398 (4): 619–626. [DOI] [PubMed] [Google Scholar]
- 58. Bennett AJ, Bruce JS, Salter AM, White DA, Billett MA (1996) Hepatic microsomal triglyceride transfer protein messenger RNA concentrations are increased by dietary cholesterol in hamsters. FEBS Letters 394 (3): 247–250. [DOI] [PubMed] [Google Scholar]
- 59. Wilcox LJ, Borradaile NM, de Dreu LE, Huff MW (2001) Secretion of hepatocyte apoB is inhibited by the flavonoids, naringenin and hesperetin, via reduced activity and expression of ACAT2 and MTP. Journal of Lipid Research 42 (5): 725–734. [PubMed] [Google Scholar]
- 60. Daniel RS, Mathew BC, Devi KS, Augusti KT (1998) Antioxidant effect of two flavonoids from the bark of Ficus bengalensis Linn in hyperlipidemic rats. Indian Journal of Experimental Biology 36 (9): 902–906. [PubMed] [Google Scholar]
- 61. Halliwell B, Gutteridge JM (1984) Oxygen toxicity, oxygen radicals, transition metals and disease. Biochemistry Journal 219 (1): 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ramiro-Puig E, Urpi-Sarda M, Perez-Cano FJ, Franch A, Castellote C, et al. (2007) Cocoa-enriched diet enhances antioxidant enzyme activity and modulates lymphocyte composition in thymus from young rats. Journal of Agricultural and Food Chemistry 55 (16): 6431–6438. [DOI] [PubMed] [Google Scholar]
- 63. Lee MK, Bok SH, Jeong TS, Moon SS, Lee SE, et al. (2002) Supplementation of naringenin and its synthetic derivative alters antioxidant enzyme activities of erythrocyte and liver in high cholesterol-fed rats. Bioorganic and Medicinal Chemistry 10 (7): 2239–2244. [DOI] [PubMed] [Google Scholar]
- 64. Kittichanun C, Phivthong-ngam L, Niwattisaiwong N, Piyachaturawat P, Suksamran A, et al. (2010) Effects of curcuma comosa extracts on hepatic cytochrome P450 activities in rats. Journal of Health Research 24 (1): 1–6. [Google Scholar]
- 65. Uno S, Dalton T, Derkenne S, Curran C, Miller M, et al. (2004) Oral exposure to benzo[a]pyrene in the mouse: Detoxication by inducible cytochrome P450 is more important than metabolic activation. Molecular Pharmacology 65: 1225–1237. [DOI] [PubMed] [Google Scholar]
- 66. Quiñones L, Lucas D, Godoy J, Cáceres D, Berthou F, et al. (2001) CYP1A1, CYP2E1 and GSTM1 genetic polymorphisms. The effect of single and combined genotypes on lung cancer susceptibility in Chilean people. Cancer Letters 174 (1): 35–44. [DOI] [PubMed] [Google Scholar]
- 67. Surekha D, Rao D, Raghunadharao D, Sailaja K, Vishnupriya S, et al. (2009) Association of CYP1A1*2 Polymorphisms with breast cancer risk : A case control study. Indian Journal of Medical Sciences 63 (1): 13–20. [PubMed] [Google Scholar]
- 68. Suzuki K, Matsui H, Nakazato H, Koike H, Okugi H, et al. (2003) Association of the genetic polymorphism in cytochrome P450 (CYP) 1A1 with risk of familial prostate cancer in a Japanese population: a case-control study. Cancer Letters 195 (2): 177–183. [DOI] [PubMed] [Google Scholar]
- 69. Androutsopoulos V, Tsatsakis A, Spandidos D (2009) Cytochrome P450 CYP1A1: wider roles in cancer progression and prevention. BMC Cancer 9 (1): 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ciolino H, Daschner P, Yeh G (1999) Dietary flavonols quercetin and kaempferol are ligands of the aryl hydrocarbon receptor that affect CYP1A1 transcription differentially. Biochemical Journal 340: 715–722. [PMC free article] [PubMed] [Google Scholar]
- 71. Masood N, Malik FA, Kayani MA (2011) Expression of xenobiotic metabolizing genes in head and neck cancer tissues. Asian Pacific Journal of Cancer Prevention 12 (2): 377–382. [PubMed] [Google Scholar]