Abstract
Phospholipid membranes are thought to be one of the main inducers of hemozoin formation in Plasmodia and other blood-feeding parasites. The “membrane surrounding hemozoin” has been observed in infected cells but has not been observed in in vitro experiments. This study focused on observing the association of phospholipid membranes and synthetic β-hematin, which is chemically identical to hemozoin, and on a further exploration into the mechanism of phospholipid membrane-induced β-hematin formation. Our results showed that β-hematin formation was induced by phospholipids in the fluid phase but not in the gel phase. The ability of phospholipids to induce β-hematin formation was inversely correlated with gel-to-liquid phase transition temperatures, suggesting an essential insertion of heme into the hydrocarbon chains of the phospholipid membrane to form β-hematin. For this study, a cryogenic transmission electron microscope was used to achieve the first direct observation of the formation of a monolayer of phospholipid membrane surrounding β-hematin.
Background
Malaria is one of the most common diseases in tropical countries. Each year, there are an estimated 225 million new malaria infections and almost a million deaths due to malaria world-wide [1]. Spreading resistance to current quinoline antimalarials and artemisinine has made malaria a major global problem [2]. Since a vaccine for malaria is not available, it is essential to study the molecular, biochemical, and immunological aspects of malarial parasites to develop vaccines and new antimalarial drugs.
Host protein digestion has two aspects: to obtain amino acids and to regulate osmotic pressure. This hemoglobin digestion takes place in the parasites’ food vacuoles and is carried out by multiple proteases including four aspartic versions [3]: three cysteine proteases [4] and a zinc metalloprotease (falsilysin) [5]. These proteases digest hemoglobin into small fragments consisting of about 20 different amino acids and free ferrous protoporphyrin IX (Fe(II)PPIX), which is rapidly oxidized to Fe(III)PPIX (heme). Heme is the deep red, oxygen-carrying, non-protein, ferrous component of hemoglobin in which the iron is Fe(II) (ferrous iron) and also called reduced hematin. Iron(III), ferriprotoporphyrin IX, (Fe(III)PPIX) is known to be present in solution as hematin (H2O/OH-Fe(III)PPIX). The free heme is oxidatively active and toxic to both the host cell and the malarial parasite, however free heme is rapidly oxidized to hematin and sequestered into hemozoin (malarial pigment). Due to the absence of heme oxygenase, the parasite is unable to cleave heme into an open-chain tetrapyrrole, which is necessary for cellular excretion [6]. To protect itself, the malarial parasite detoxifies free heme via neutralization with a histidine-rich protein 2 [7], [8], degradation with reduced glutathione [9], [10], [11], or crystallization into hemozoin which is a water-insoluble malarial pigment that is not lethal to biological cells [7], [12]. However, at least 95% of free heme in P. falciparum is reportedly converted to hemozoin [13], [14]. Hemozoin is known to be structurally and chemically identical to in vitro synthetic β-hematin (BH), which is a crystal of the heme (Fe(III)PPIX) dimer of the hematin Fe (III) PPIX dimer [15], [16], [17]. It has been used for parasite concentration and detection [18], [19], [20], [21], [22]. It is also suggested that the blocking of BH formation is an ideal target for antimalarial screening [23], [24], [25], [26]; thus, it is important to understand the mechanism of BH formation.
Several factors such as histidine-rich protein [7], [8], elevated temperature [27], lipids [28], [29], pre-formed BH [25], alcohols [30], detergent [31], and malarial heme detoxification protein [32] are reportedly responsible for heme crystallization. Among these factors, lipid droplets and phospholipid membranes are proposed as the main inducers of hemozoin formation in Plasmodia and other blood-feeding parasites including Schistosoma and Rhodnius [33], [34], [35], [36], [37], [38], [39], [40], [41]. The mechanism of BH formation induced by neutral lipid droplets both in vivo and in vitro has been well documented [34], [35], [36], [37], [38]. The “membrane surrounding hemozoin” has been found in the in vivo ultrastructure [39], [41], [42], but it has not been observed in in vitro experiments. In the present study, we aimed to observe the association of phospholipid membranes and BH crystals, and to further explore the mechanism of phospholipid membrane-induced BH formation.
Materials and Methods
Materials
Hemin chloride (heme) was purchased from Sigma. L-α-Phosphatidylcholine dilauroyl (dilauroyl-PC), L-α-phosphatidylcholine dimyristoyl (dimyristoyl-PC), L-α-phosphatidylcholine dipalmitoyl (dipalmitoyl-PC), L-α-phosphatidylcholine distearoyl (distearoyl-PC), L-α-phosphatidylcholine dioleoyl (dioleoyl-PC), L-α-phosphatidylserine dipalmitoyl (dipalmitoyl-PS), L-α-phosphatidylethanolamine dimyristoyl (dimyristoyl-PE), L-α-phosphatidylethanolamine dipalmitoyl (dipalmitoyl-PE), dimethyl sulfoxide, and chloroform were provided by Wako Pure Chemicals (Osaka, Japan). The remaining reagents were also acquired from Wako Pure Chemicals.
Preparation of Lipid Vesicles
Phospholipids were dissolved in 1 ml chloroform at a concentration of 2 mM. Then they were sprayed and dried on the walls of 1.5 ml micro-tubes under a nitrogen gas flush to create a thin layer, which was then suspended in 1 ml of distilled water. The lipid suspension (2 mM) was sonicated for 10 s and used for BH formation assay, as previously described [43], and for cryo-TEM observation.
Assay of BH Formation Initiated by Phospholipids
Stock heme solution (10 mM) was prepared using hemin chloride in dimethyl sulfoxide as described previously [10]. Heme (100 µM) was incubated with various concentrations of phospholipids in 1 ml of 50 mM acetate buffer at pH 4.8. For quantification of BH, after incubating at 37°C for 16 h, the sample was centrifuged for 5 min at 7,000×g, and the supernatant was discarded. The obtained BH was purified and quantified as previously described [44], [45]. Values obtained from triplicate assays were plotted, and the EC20 values (M), along with the concentration of lipids required for crystallizing 20% of the heme, were calculated graphically. The characteristics of BH were confirmed by infrared spectroscopy with expected infrared spectra peaks at 1210 and 1664 cm−1, confirming the presence of BH.
For cryo-TEM observation, the heme (100 µM) in the acetate buffer (0.5 M) and that in the phospholipid suspension (0.5 mM) was mixed and incubated at 37°C for 6 h. Then, 2.5 µl of the BH precipitate that had formed in the bottom of the tube was used.
Physical and Chemical Properties of Phospholipids
The gel-to-fluid phase of the transition temperature of phospholipids (T m) was derived from a procedure established by Cevc et al. [46]. The molecular weight was recorded from the supplier (Wako Pure Chemicals). Other physical properties of the phospholipids, including total net charge, number of anion charge, octanol-water partition coefficient (logP), distribution coefficient (logD at pH 5.5), hydrogen bond acceptors, hydrogen bond donors, freely rotating bonds, polar surface area, and polarizability were retrieved from ChemSpider (www.chemspider.com), as predicted by Advanced Chemistry Development (ACD/Laboratories) software.
Cryo-transmission Electron Microscopy (cryo-TEM)
The analysis was performed as described previously [47], [48]. Briefly, samples (2.5 µL) were applied to glow-discharged microgrids supported by 5-nm-thick carbon films (JEOL, Tokyo, Japan). After removing excess samples with pre-water-soaked filter paper, the samples were quickly frozen by liquid ethane cooled by liquid Nitrogen (EM CPC, LEICA Microsystems, Vienna). The grid was then transferred into a JEOL cryo-electron microscope (JEM3000SFF) and kept at 4.2 K and observed at 300 kV.
Statistical Analysis
Data analysis was performed using the SPSS Version 14. The Pearson correlation was analyzed to evaluate the relationship between the abilities of phospholipids to induce BH formation and their physical properties. Differences in BH formation induced by lipids were analyzed for statistical significance using the nonparametric Mann–Whitney U test. Values were considered significant at p<0.05.
Results
BH Formation Induced by Phospholipids
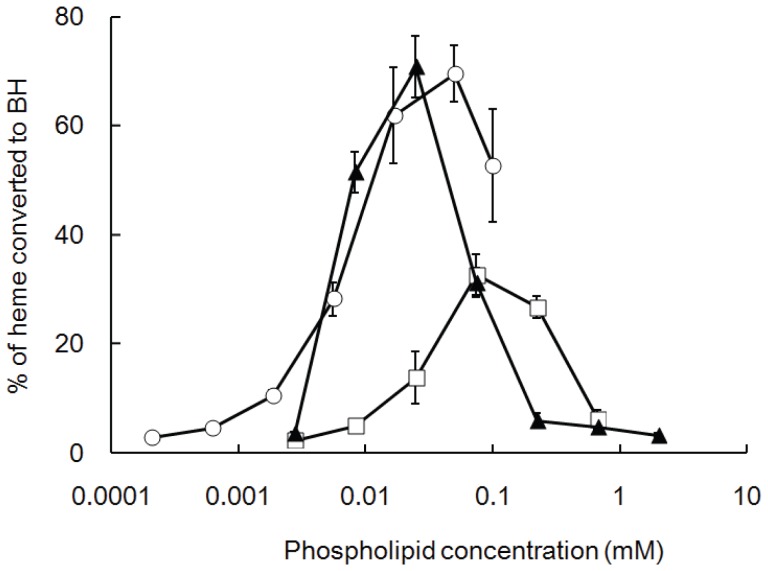
The abilities of various phospholipids to induce BH formation in vitro at 37°C are shown in Fig. 1. BH formation was induced by phospholipids in a biphasic dose-dependent manner. Two phosphatidylcholines, dilauroyl-PC and dioleoyl-PC, were catalyzed by BH formation at low molar concentrations and converted a maximum of 70–80% of the heme into BH, indicating a relatively high efficiency. Dimyristoyl-PC converted a maximum of 30–40% of the heme into BH at a slightly higher concentration of the inducer. The concentration that is required to convert 20% of heme into BH (EC20 values) for these phospholipids varied from 5 to 12 µM (Table 1). We also observed that the maximal yield of BH in the presence of lipids was negatively correlated with the EC20 values (Fig. 1). In contrast, dipalmitoyl-PC, distearoyl-PC, dimyristoyl-PE, dipalmitoyl-PE, and dipalmitoyl-PS could not induce BH formation under our experimental conditions.
Figure 1. BH formation induced by various concentrations of phospholipids at 37°C.
The ordinate shows the amount of BH produced by incubation with various concentrations of phospholipids for 16 h, as expressed as the percentage of heme converted to BH. Values represent the mean ± SD (n = 3). The results were reproducible. Circle; dioleoyl-PC, triangle; dilauroyl-PC, square; dimyristoyl-PC.
Table 1. BH-forming ability and the physical properties of phospholipids.
| Phospholipids | Acyl groups | T m (°C)a | MWb | Total net charge | No. of anion charge | logP c | logD c (pH 5.5) | Hydrogen bond acceptorsc | Hydrogen bonddonorsc | Freely rotating bondsc | Polar surface area (angstrom2)c | Polarizabilityc (×10−24) | BH induction (EC20 values, mM)d |
| dilauroyl-PC | 12∶0 | 0 | 621.8 | 0 | 1 | 6.62 | 7.23 | 9 | 1 | 32 | 118.17 | 1 | 6 |
| dimyristoyl-PC | 14∶0 | 23 | 677.9 | 0 | 1 | 8.05 | 8.65 | 9 | 1 | 36 | 121 | 1 | 12 |
| dipalmitoyl-PC | 16∶0 | 42 | 734.0 | 0 | 1 | 10.09 | 10.69 | 9 | 1 | 40 | 121 | 1 | ND |
| distearoyl-PC | 18∶0 | 55 | 790.1 | 0 | 1 | 12.12 | 12.73 | 9 | 1 | 44 | 121 | 1 | ND |
| dioleoyl-PC | 18∶1 | −22 | 788.1 | 0 | 1 | 11.96 | 12.57 | 9 | 1 | 42 | 121 | 1 | 5 |
| dimyristoyl-PE | 14∶0 | 48 | 635.8 | 0 | 1 | 11.00 | 8.51 | 9 | 3 | 36 | 144.190 | 68.93 | ND |
| dipalmitoyl-PE | 16∶0 | 63 | 691.9 | 0 | 1 | 13.70 | 11.20 | 9 | 3 | 40 | 144.19 | 76.276 | ND |
| dipalmitoyl-PS | 16∶0 | 51 | 735.9 | −1 | 2 | 13.04 | 9.55 | 11 | 4 | 41 | 181.49 | 78.717 | ND |
Tm: Phase transition temperature was derived from Cevc et al (1993) in Phospholipids Handbook (Cevc, G., ed.), pp. 939–956, Marcel Dekker, New York.
given by supplier.
Values were retrieved from ChemSpider (www.chemspider.com), predicted by Advanced Chemistry Development (ACD/Laboratories) software.
performed by this study.
Effects of Physical and Chemical Properties of Phospholipids on BH Formation
To further understand the mechanism of phospholipid-mediated BH formation, Pearson correlation analysis between the abilities of phospholipids to induce BH and their physical properties was performed. All phospholipids were tested at the same temperature (37°C). Our results showed that the ability of phospholipids to induce BH formation was inversely correlated with the gel-to-fluid phase transition temperature (T m) of phospholipids (Table 1, p>0.05). Moreover, only phospholipids with a T m that was lower than the experimental temperature (37°C) could induce BH formation. Phospholipids such as dioleoyl-PC with a lower T m were more effective in inducing BH formation than phospholipids such as dilauroyl-PC, which had a higher T m.
In contrast, other physical properties of phospholipids were not significantly associated with the ability of phospholipids to induce BH formation: molecular weight, total net charge, number of anion charge, octanol-water partition coefficient (logP), distribution coefficient at pH 5.5, hydrogen bond acceptors, hydrogen bond donors, freely rotating bonds, polar surface area, and polarizability (Table 1, p>0.05).
Effect of Reaction Temperature on Phospholipid-mediated BH Formation
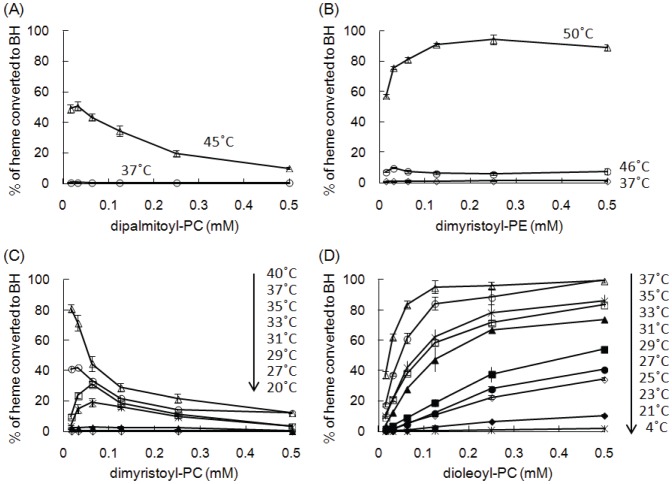
To further clarify the relationship between the phase transition temperature of phospholipids and their abilities to induce BH, we performed a BH formation assay at temperatures ranging from below to above the T m of a particular phospholipid. Since BH formation spontaneously occurs with no inducer at high temperature [49], distearoyl-PC, dipalmitoyl-PE, and dipalmitoyl-PS could not be tested in this experiment because of their high T m values. We further confirmed that spontaneous BH formation was not observed at 50°C in the absence of phospholipids. The results shown in Fig. 2 indicate that all tested phospholipids could induce BH formation at a temperature higher than the T m of a particular phospholipid in the liquid phase. On the other hand, no BH was formed with a reaction temperature below the T m of a particular phospholipid in the solid phase. Furthermore, the maximal yield of BH was positively correlated with the reaction temperature. These results suggested that the ability of phospholipid vesicles to induce BH formation was correlated with their membrane fluidity.
Figure 2. Effect of reaction temperature on phospholipid-mediated BH formation.
Dipalmitoyl-PC (A), dimyristoyl-PE (B), dimyristoyl-PC (C), and dioleoyl-PC (D) were used to induce BH formation at various temperatures, and the reaction was dependent on the phase transition temperature of phospholipids. T m values are 42°C for dipalmitoyl-PC (A), 48°C for dimyristoyl-PE (B), 23°C for dimyristoyl-PC (C), and −22°C for dioleoyl-PC (D). It is noted that BH was not formed at 50°C in the absence of phospholipids. Values represent the mean ± SD (n = 3).
Cryo-TEM Analysis
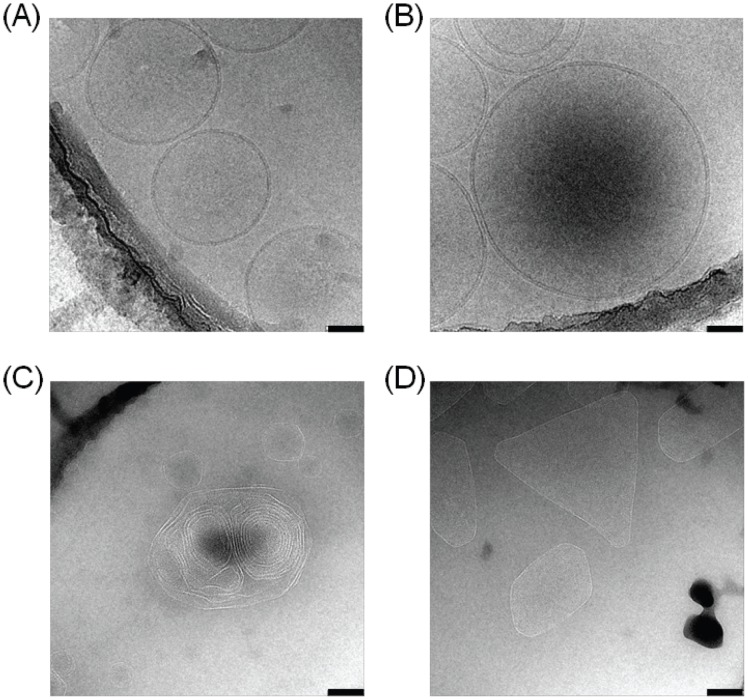
To examine whether the BH-inducing ability of phospholipid vesicles was related to structure, observations by cryo-TEM were performed. The dioleoyl-PC liposome was mostly observed as spherical vesicles with a bilayer membrane (Fig. 3A), while a few multilamellar liposomes were also observed by cryo-TEM (Fig. 3B). However, dipalmitoyl-PC liposome was also observed, but the large liposome (diameter >100 nm) was irregular and angular, probably indicating less fluidity compared with dioleoyl-PC liposome (Figs. 3C and D).
Figure 3. Cryo-TEM images of lipid liposomes prepared in water at 37°C.
Liposome of dioleoyl-PC (A and B) or dipalmitoyl-PC (C and D) prepared in water by ultrasonication was observed by cryo-TEM. Scale bars are 50 nm in Figs. A and B and 100 nm in Figs. C and D.
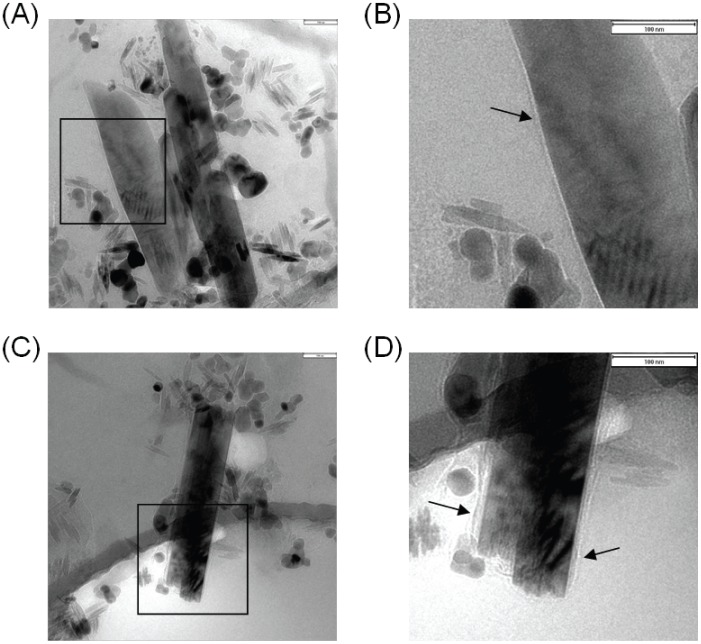
The formation of BH catalyzed by dioleoyl-PC liposome was also observed using cryo-TEM. The cryo-TEM images of BH showed morphologies that were almost identical (Fig. 4). The BH appeared as variable-sized crystals with long thin shapes, smooth surfaces and tapered ends. These morphological characteristics were similar to those seen in previous reports of BH both in vivo and in vitro [50], [51], which further confirmed the BH formation. The dioleoyl-PC liposome was not observed around the BH, but a monolayer membrane-like structure that surrounded the BH was observed, and is indicated by arrows in Figs. 4B and 4D. With longer irradiation from an electron beam, this membrane-like structure was burned and developed a white color, as observed under cryo-TEM. Given the fact that only the dioleoyl-PC in the mixture had the potential to form a monolayer structure, this observation strongly suggests that the membrane-like structure was formed by a phospholipid.
Figure 4. Cryo-TEM images of BH catalyzed by dioleoyl-PC liposome.
Heme (100 µM) was incubated with dioleoyl-PC liposome in 1 ml of acetate buffer at pH 4.8. After incubation for 16 h at 37°C, the samples were observed by cryo-TEM as described in the Methods section. Figs. B and D are magnified images of the squared area in Figs. A and C, respectively. Black arrows in Figs. B and D indicate the monolayers of the phospholipids. Scale bars are 100 nm.
Discussion
Numerous studies have suggested that neutral lipid droplets are the main templates for hemozoin formation [34], [35], [36], [37], [38]. For many years, phospholipid membranes have also been proposed as the site of hemozoin formation in malaria [39], [40]. Recently, Kapishnikov et al. used cryogenic soft X-ray tomography to demonstrate the hemozoin formation templates on the inner layer of a digestive vacuole [41]. However, very few studies have investigated BH formation induced by phospholipid membranes in an in vitro experiment [40], [52]. As far as could be ascertained, the present study is the first to observe a membrane surrounding BH that was induced by phospholipid vesicles (Fig. 4), which was a structure that was similar to that observed in vivo by Hempelmann et al. [39]. The advantage of cryo-TEM was that it allowed the direct observation of phospholipid membranes and BH formation with no fixation or dehydration of the sample on the grid. In addition, cryo-TEM was suitable for the detection of phospholipid membranes because the phosphorus groups have a low permeability to the electron beam. Moreover, the association of polar lipids with malarial hemozoin observed by the thin layer chromatography also supports our results [53].
The differences in morphology between dioleoyl-PC and dipalmitoyl-PC vesicles, as observed by cryo-TEM, suggested that a smooth regular shape of vesicles is required for the site of a BH template. The differences in morphology between dioleoyl-PC and dipalmitoyl-PC vesicles were probably due to their different physical properties, in particular the different states – fluid or gel – caused by the differences in T m values. Moreover, since the hydrophobic interactions between their inducers and heme have been proposed as an important force in the creation of a precursor heme dimer [38], [45], [54], [55], it was interesting to explore the correlation between the physical properties of phospholipids and their ability to induce BH. Our results demonstrated that phospholipids could induce BH formation only in the fluid phase, and could not do so in the gel phase. Recently, Hoang et al. showed that a blending of five neutral lipids lower the melting temperature of lipid droplets compared to the homogeneous samples and the blending of five neutral lipids produced more hemozoin compared to that of homogeneous lipids, further supporting the role of lipid fluidity in the hemozoin formation.
Evidence showed that an increase in membrane fluidity results in the membrane insertion of heme [56], which is positively correlated with an increase in the BH induction of phospholipids (Fig. 2). These observations suggest that the mechanism of BH formation involves the acyl chains of the phospholipid membranes. Furthermore, free heme can be quickly and easily inserted into phospholipid vesicles as monomeric heme at a ratio of 1 heme per 4–5 phospholipid molecules in the fluid phase [57], [58]. Taken together, these observations suggest that the free heme inserts its vinyl groups deeply into the hydrophobic acyl chains of phospholipids while the charged propionate groups are exposed to the aqueous solution. The hydrophobic environment of acyl chains helps to form monomeric heme, which favors the formation of a BH dimer. However, other mechanism cannot be excluded, and further studies are needed to clarify this issue.
A biphasic dose-dependent manner of BH formation induced by phospholipids was also observed in the assay induced by a detergent [31], probably due to the over dilution of the heme molecules in the high number of vesicles. Further studies are required to clarify this mechanism.
Conclusions
Our results showed that the abilities of phospholipids to induce BH is inversely correlated with the phase transition temperatures, suggesting a required insertion of heme into the hydrophobic acyl chains of a phospholipid membrane in order to form BH. Finally, a monolayer of membrane surrounding BH was observed using cryo-TEM.
Funding Statement
The authors have no support or funding to report.
References
- 1. Cibulskis RE, Aregawi M, Williams R, Otten M, Dye C (2011) Worldwide incidence of malaria in 2009: estimates, time trends, and a critique of methods. PLoS Med 8: e1001142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Muller O, Sie A, Meissner P, Schirmer RH, Kouyate B (2009) Artemisinin resistance on the Thai-Cambodian border. Lancet 374: 1419. [DOI] [PubMed] [Google Scholar]
- 3. Banerjee R, Liu J, Beatty W, Pelosof L, Klemba M, et al. (2002) Four plasmepsins are active in the Plasmodium falciparum food vacuole, including a protease with an active-site histidine. Proc Natl Acad Sci U S A 99: 990–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rosenthal PJ, Sijwali PS, Singh A, Shenai BR (2002) Cysteine proteases of malaria parasites: targets for chemotherapy. Curr Pharm Des 8: 1659–1672. [DOI] [PubMed] [Google Scholar]
- 5. Eggleson KK, Duffin KL, Goldberg DE (1999) Identification and characterization of falcilysin, a metallopeptidase involved in hemoglobin catabolism within the malaria parasite Plasmodium falciparum. J Biol Chem 274: 32411–32417. [DOI] [PubMed] [Google Scholar]
- 6. Eckman JR, Modler S, Eaton JW, Berger E, Engel RR (1977) Host heme catabolism in drug-sensitive and drug-resistant malaria. J Lab Clin Med 90: 767–770. [PubMed] [Google Scholar]
- 7. Sullivan DJ Jr, Gluzman IY, Goldberg DE (1996) Plasmodium hemozoin formation mediated by histidine-rich proteins. Science 271: 219–222. [DOI] [PubMed] [Google Scholar]
- 8. Huy NT, Serada S, Trang DT, Takano R, Kondo Y, et al. (2003) Neutralization of toxic heme by Plasmodium falciparum histidine-rich protein 2. J Biochem (Tokyo) 133: 693–698. [DOI] [PubMed] [Google Scholar]
- 9. Atamna H, Ginsburg H (1995) Heme degradation in the presence of glutathione. A proposed mechanism to account for the high levels of non-heme iron found in the membranes of hemoglobinopathic red blood cells. J Biol Chem 270: 24876–24883. [DOI] [PubMed] [Google Scholar]
- 10. Huy NT, Kamei K, Yamamoto T, Kondo Y, Kanaori K, et al. (2002) Clotrimazole binds to heme and enhances heme-dependent hemolysis: proposed antimalarial mechanism of clotrimazole. J Biol Chem 277: 4152–4158. [DOI] [PubMed] [Google Scholar]
- 11. Huy NT, Kamei K, Kondo Y, Serada S, Kanaori K, et al. (2002) Effect of antifungal azoles on the heme detoxification system of malarial parasite. J Biochem (Tokyo) 131: 437–444. [DOI] [PubMed] [Google Scholar]
- 12. Francis SE, Sullivan DJ Jr, Goldberg DE (1997) Hemoglobin metabolism in the malaria parasite Plasmodium falciparum. Annu Rev Microbiol 51: 97–123. [DOI] [PubMed] [Google Scholar]
- 13. Combrinck JM, Mabotha TE, Ncokazi KK, Ambele MA, Taylor D, et al. (2013) Insights into the role of heme in the mechanism of action of antimalarials. ACS Chem Biol 8: 133–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Egan TJ, Combrinck JM, Egan J, Hearne GR, Marques HM, et al. (2002) Fate of haem iron in the malaria parasite Plasmodium falciparum. Biochem J 365: 343–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bohle DS, Dinnebier RE, Madsen SK, Stephens PW (1997) Characterization of the products of the heme detoxification pathway in malarial late trophozoites by X-ray diffraction. J Biol Chem 272: 713–716. [DOI] [PubMed] [Google Scholar]
- 16. Pagola S, Stephens PW, Bohle DS, Kosar AD, Madsen SK (2000) The structure of malaria pigment beta-haematin. Nature 404: 307–310. [DOI] [PubMed] [Google Scholar]
- 17. Wood BR, Langford SJ, Cooke BM, Glenister FK, Lim J, et al. (2003) Raman imaging of hemozoin within the food vacuole of Plasmodium falciparum trophozoites. FEBS Lett 554: 247–252. [DOI] [PubMed] [Google Scholar]
- 18. Men TT, Huy NT, Trang DT, Shuaibu MN, Hirayama K, et al. (2012) A simple and inexpensive haemozoin-based colorimetric method to evaluate anti-malarial drug activity. Malar J 11: 272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Trang DT, Huy NT, Kariu T, Tajima K, Kamei K (2004) One-step concentration of malarial parasite-infected red blood cells and removal of contaminating white blood cells. Malar J 3: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rebelo M, Sousa C, Shapiro HM, Mota MM, Grobusch MP, et al. (2013) A Novel Flow Cytometric Hemozoin Detection Assay for Real-Time Sensitivity Testing of Plasmodium falciparum. PLoS One 8: e61606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Saha RK, Karmakar S, Roy M (2012) Computational investigation on the photoacoustics of malaria infected red blood cells. PLoS One 7: e51774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thomas V, Gois A, Ritts B, Burke P, Hanscheid T, et al. (2012) A novel way to grow hemozoin-like crystals in vitro and its use to screen for hemozoin inhibiting antimalarial compounds. PLoS One 7: e41006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Egan TJ, Hunter R, Kaschula CH, Marques HM, Misplon A, et al. (2000) Structure-function relationships in aminoquinolines: effect of amino and chloro groups on quinoline-hematin complex formation, inhibition of beta-hematin formation, and antiplasmodial activity. J Med Chem 43: 283–291. [DOI] [PubMed] [Google Scholar]
- 24. Adams PA, Berman PA, Egan TJ, Marsh PJ, Silver J (1996) The iron environment in heme and heme-antimalarial complexes of pharmacological interest. J Inorg Biochem 63: 69–77. [DOI] [PubMed] [Google Scholar]
- 25. Sullivan DJ (2002) Theories on malarial pigment formation and quinoline action. Int J Parasitol 32: 1645–1653. [DOI] [PubMed] [Google Scholar]
- 26. Ridley RG, Dorn A, Vippagunta SR, Vennerstrom JL (1997) Haematin (haem) polymerization and its inhibition by quinoline antimalarials. Ann Trop Med Parasitol 91: 559–566. [DOI] [PubMed] [Google Scholar]
- 27. Egan TJ, Hempelmann E, Mavuso WW (1999) Characterisation of synthetic beta-haematin and effects of the antimalarial drugs quinidine, halofantrine, desbutylhalofantrine and mefloquine on its formation. J Inorg Biochem 73: 101–107. [DOI] [PubMed] [Google Scholar]
- 28. Dorn A, Stoffel R, Matile H, Bubendorf A, Ridley RG (1995) Malarial haemozoin/beta-haematin supports haem polymerization in the absence of protein. Nature 374: 269–271. [DOI] [PubMed] [Google Scholar]
- 29. Tripathi AK, Gupta A, Garg SK, Tekwani BL (2001) In vitro beta-hematin formation assays with plasma of mice infected with Plasmodium yoelii and other parasite preparations: comparative inhibition with quinoline and endoperoxide antimalarials. Life Sci 69: 2725–2733. [DOI] [PubMed] [Google Scholar]
- 30. Blauer G, Akkawi M (2002) Alcohol-water as a novel medium for beta-hematin preparation. Arch Biochem Biophys 398: 7–11. [DOI] [PubMed] [Google Scholar]
- 31. Huy NT, Uyen DT, Maeda A, Trang DT, Oida T, et al. (2007) Simple colorimetric inhibition assay of heme crystallization for high-throughput screening of antimalarial compounds. Antimicrob Agents Chemother 51: 350–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jani D, Nagarkatti R, Beatty W, Angel R, Slebodnick C, et al. (2008) HDP-a novel heme detoxification protein from the malaria parasite. PLoS Pathog 4: e1000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stiebler R, Soares JB, Timm BL, Silva JR, Mury FB, et al. (2011) On the mechanisms involved in biological heme crystallization. J Bioenerg Biomembr 43: 93–99. [DOI] [PubMed] [Google Scholar]
- 34. Kapishnikov S, Berthing T, Hviid L, Dierolf M, Menzel A, et al. (2012) Aligned hemozoin crystals in curved clusters in malarial red blood cells revealed by nanoprobe X-ray Fe fluorescence and diffraction. Proc Natl Acad Sci U S A 109: 11184–11187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hoang AN, Sandlin RD, Omar A, Egan TJ, Wright DW (2010) The neutral lipid composition present in the digestive vacuole of Plasmodium falciparum concentrates heme and mediates beta-hematin formation with an unusually low activation energy. Biochemistry 49: 10107–10116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Correa Soares JB, Maya-Monteiro CM, Bittencourt-Cunha PR, Atella GC, Lara FA, et al. (2007) Extracellular lipid droplets promote hemozoin crystallization in the gut of the blood fluke Schistosoma mansoni. FEBS Lett 581: 1742–1750. [DOI] [PubMed] [Google Scholar]
- 37. Pisciotta JM, Coppens I, Tripathi AK, Scholl PF, Shuman J, et al. (2007) The role of neutral lipid nanospheres in Plasmodium falciparum haem crystallization. Biochem J 402: 197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hoang AN, Ncokazi KK, de Villiers KA, Wright DW, Egan TJ (2010) Crystallization of synthetic haemozoin (beta-haematin) nucleated at the surface of lipid particles. Dalton Trans 39: 1235–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hempelmann E, Motta C, Hughes R, Ward SA, Bray PG (2003) Plasmodium falciparum: sacrificing membrane to grow crystals? Trends Parasitol 19: 23–26. [DOI] [PubMed] [Google Scholar]
- 40. Orjih AU (2001) On the mechanism of hemozoin production in malaria parasites: activated erythrocyte membranes promote beta-hematin synthesis. Exp Biol Med (Maywood) 226: 746–752. [DOI] [PubMed] [Google Scholar]
- 41. Kapishnikov S, Weiner A, Shimoni E, Guttmann P, Schneider G, et al. (2012) Oriented nucleation of hemozoin at the digestive vacuole membrane in Plasmodium falciparum. Proc Natl Acad Sci U S A 109: 11188–11193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Oliveira MF, Kycia SW, Gomez A, Kosar AJ, Bohle DS, et al. (2005) Structural and morphological characterization of hemozoin produced by Schistosoma mansoni and Rhodnius prolixus. FEBS Lett 579: 6010–6016. [DOI] [PubMed] [Google Scholar]
- 43. Trang DT, Huy NT, Uyen DT, Sasai M, Shiono T, et al. (2006) Inhibition assay of beta-hematin formation initiated by lecithin for screening new antimalarial drugs. Anal Biochem 349: 292–296. [DOI] [PubMed] [Google Scholar]
- 44.Xuan Trang DT, Huy NT, Uyen DT, Sasai M, Shiono T, et al.. (2005) Inhibition assay of beta-hematin formation initiated by lecithin for screening new antimalarial drugs. Anal Biochem. [DOI] [PubMed]
- 45. Huy NT, Maeda A, Uyen DT, Trang DT, Sasai M, et al. (2007) Alcohols induce beta-hematin formation via the dissociation of aggregated heme and reduction in interfacial tension of the solution. Acta Trop 101: 130–138. [DOI] [PubMed] [Google Scholar]
- 46.Cevc G (1993) Phospholipids Handbook: Marcel Dekker, New York. 939–956 p.
- 47. Nishino Y, Yasunaga T, Miyazawa A (2007) A genetically encoded metallothionein tag enabling efficient protein detection by electron microscopy. J Electron Microsc (Tokyo) 56: 93–101. [DOI] [PubMed] [Google Scholar]
- 48. Sato C, Hamada K, Ogura T, Miyazawa A, Iwasaki K, et al. (2004) Inositol 1,4,5-trisphosphate receptor contains multiple cavities and L-shaped ligand-binding domains. J Mol Biol 336: 155–164. [DOI] [PubMed] [Google Scholar]
- 49. Egan TJ, Ross DC, Adams PA (1994) Quinoline anti-malarial drugs inhibit spontaneous formation of beta-haematin (malaria pigment). FEBS Lett 352: 54–57. [DOI] [PubMed] [Google Scholar]
- 50. Huy NT, Uyen DT, Sasai M, Trang DT, Shiono T, et al. (2006) A simple and rapid colorimetric method to measure hemozoin crystal growth in vitro. Anal Biochem 354: 305–307. [DOI] [PubMed] [Google Scholar]
- 51. Noland GS, Briones N, Sullivan DJ Jr (2003) The shape and size of hemozoin crystals distinguishes diverse Plasmodium species. Mol Biochem Parasitol 130: 91–99. [DOI] [PubMed] [Google Scholar]
- 52. Orjih AU, Mathew TC, Cherian PT (2012) Erythrocyte membranes convert monomeric ferriprotoporphyrin IX to beta-hematin in acidic environment at malarial fever temperature. Exp Biol Med (Maywood) 237: 884–893. [DOI] [PubMed] [Google Scholar]
- 53.Pisciotta JM, Coppens I, Tripathi AK, Scholl PF, Shuman J, et al.. (2006) The role of neutral lipid nanospheres in Plasmodium falciparum heme crystallization. Biochem J. [DOI] [PMC free article] [PubMed]
- 54. Uyen DT, Huy NT, Trang DT, Nhien NT, Oida T, et al. (2008) Effects of amino acids on malarial heme crystallization. Biol Pharm Bull 31: 1483–1488. [DOI] [PubMed] [Google Scholar]
- 55. Stiebler R, Hoang AN, Egan TJ, Wright DW, Oliveira MF (2010) Increase on the initial soluble heme levels in acidic conditions is an important mechanism for spontaneous heme crystallization in vitro. PLoS One 5: e12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Light WR, 3rd, Olson JS (1990) The effects of lipid composition on the rate and extent of heme binding to membranes. J Biol Chem 265: 15632–15637. [PubMed] [Google Scholar]
- 57. Cannon JB, Kuo FS, Pasternack RF, Wong NM, Muller-Eberhard U (1984) Kinetics of the interaction of hemin liposomes with heme binding proteins. Biochemistry 23: 3715–3721. [DOI] [PubMed] [Google Scholar]
- 58. Tipping E, Ketterer B, Christodoulides L (1979) Interactions of small molecules with phospholipid bilayers. Binding to egg phosphatidylcholine of some organic anions (bromosulphophthalein, oestrone sulphate, haem and bilirubin) that bind to ligandin and aminoazo-dye-binding protein A. Biochem J 180: 327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]