Abstract
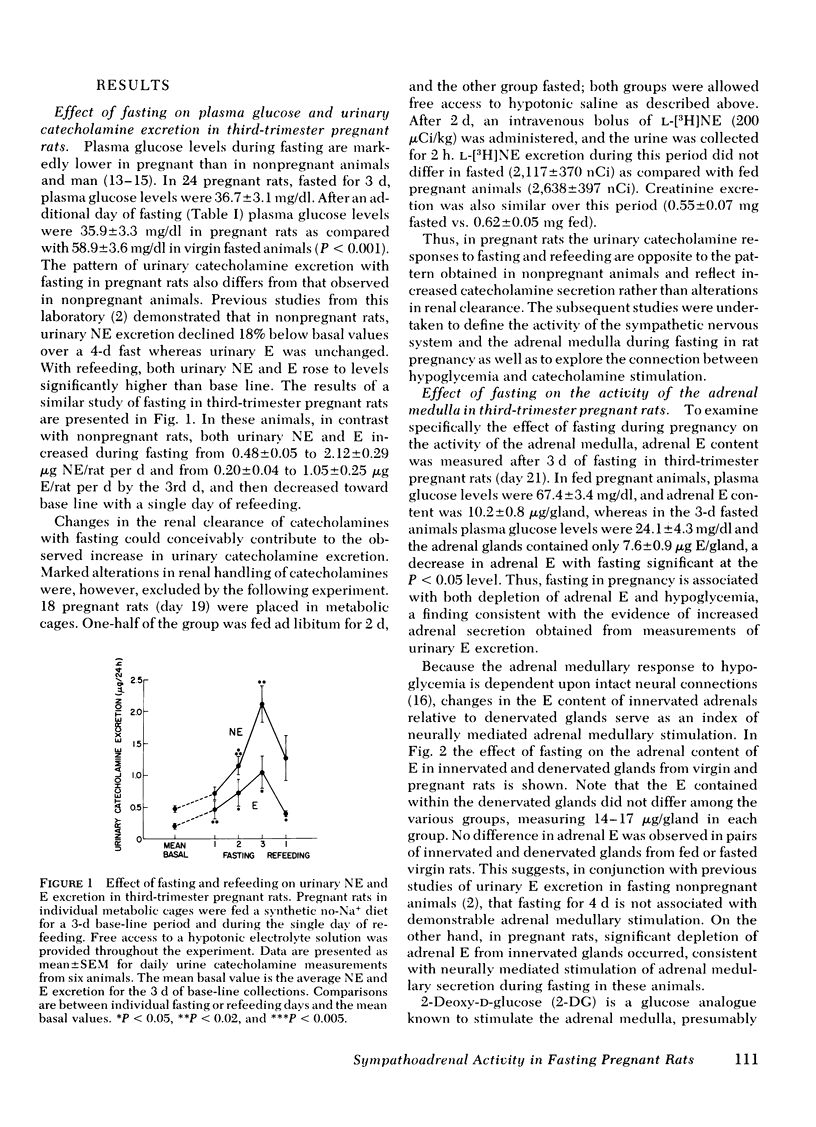
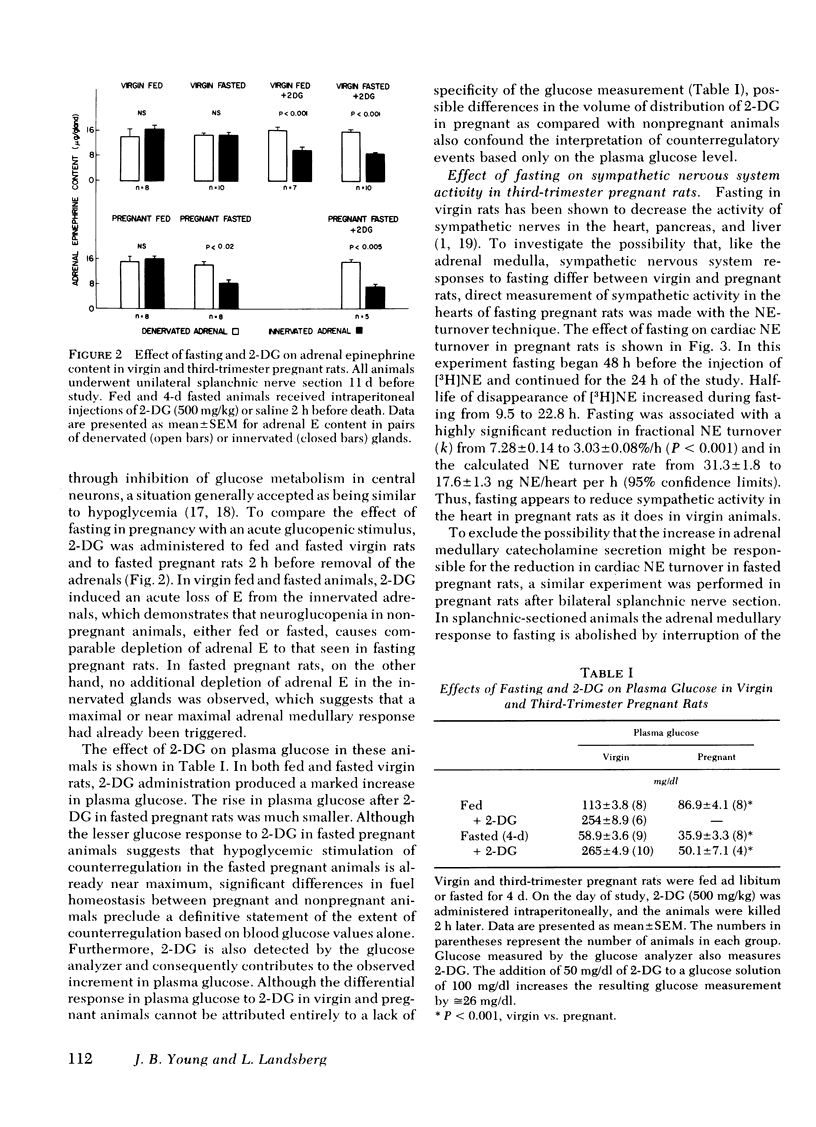
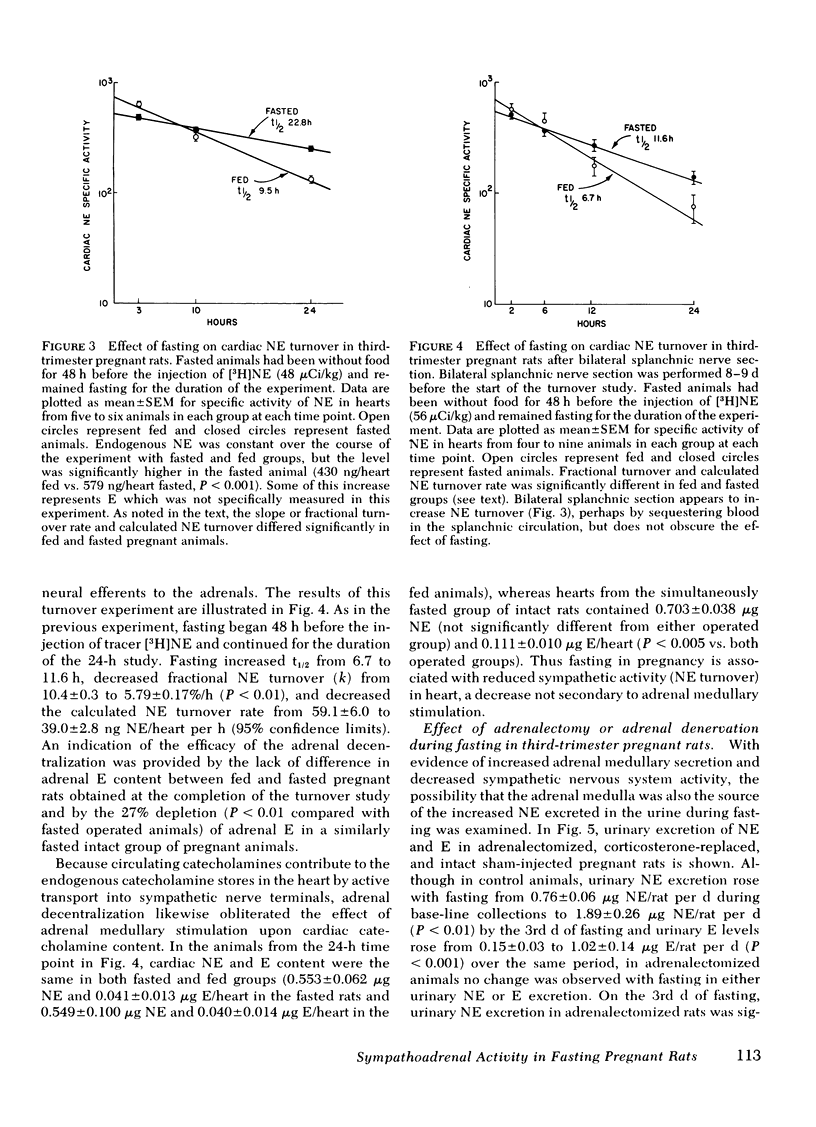
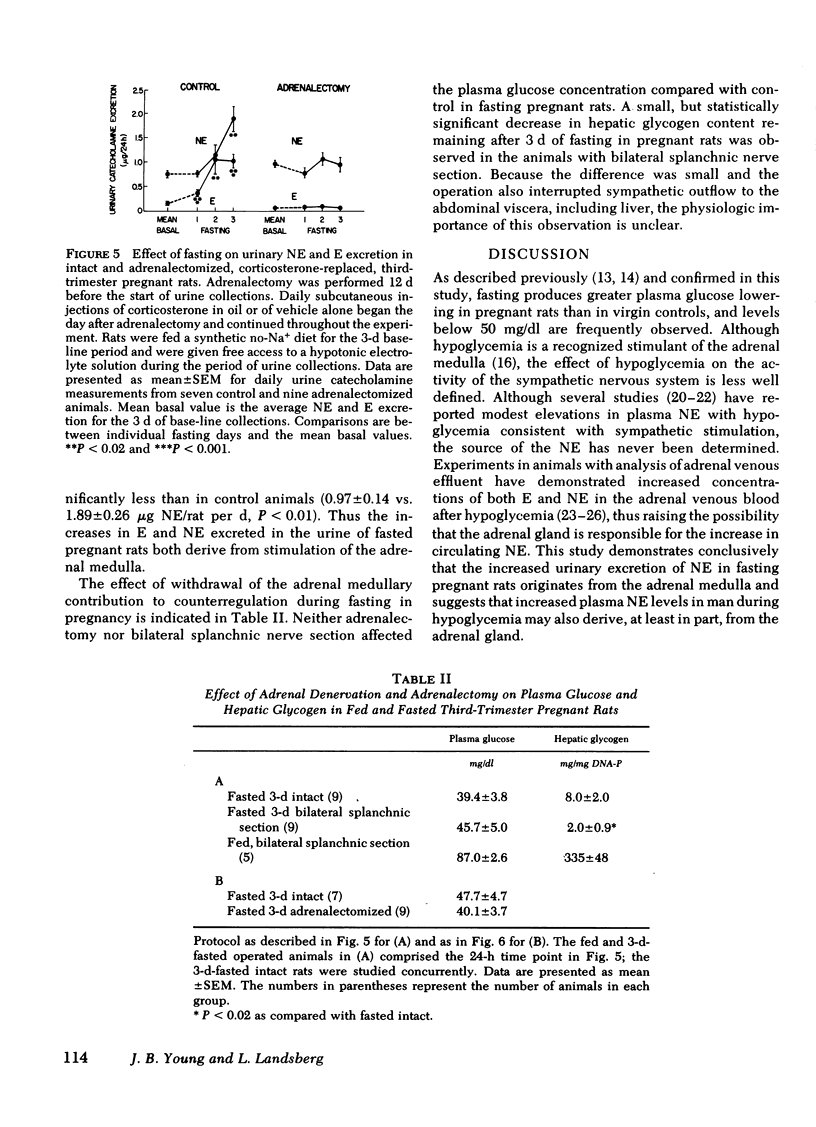
The pattern of urinary catecholamine excretion in fasting differs in pregnant and nonpregnant rats, which suggests that the sympathoadrenal response to fasting is altered by pregnancy. In fasting nonpregnant animals, urinary norepinephrine (NE) excretion decreases and epinephrine (E) excretion remains unchanged, whereas the excretion of both catecholamines rises significantly with refeeding. In contrast, fasting third-trimester pregnant rats exhibit a 420% increase in urinary E and a 345% increase in urinary NE, elevations which fall with refeeding. Specific evaluation of sympathoadrenal activity in fasting pregnant rats reveals stimulation of the adrenal medulla and suppression of sympathetic nerves. In fasting third-trimester rats the adrenal content of E is 37% lower in innervated adrenals as compared with contralateral denervated glands, which indicates the presence of neurally-mediated adrenal medullary activation. Adrenalectomy completely abolishes the fasting-induced rise in urinary E and NE in pregnant rats. Studies with 2-deoxy-D-glucose suggest that stimulation of the adrenal medulla results from hypoglycemia, which is present after 3 d of fasting in pregnant rats (plasma glucose 36.7 mg/dl). Sympathetic nervous system activity, as measured by [3H]NE turnover in the heart, decreases in fasting pregnant rats despite hypoglycemia, a response similar to that seen in fasting nonpregnant animals where plasma glucose is maintained above 50 mg/dl. The calculated NE turnover rate is 44% lower in 2-d fasted pregnant rats than in fed pregnant animals (17.6 ± 1.3 vs. 31.3 ± 1.8 ng NE/heart per h, respectively). Thus adrenal medullary and sympathetic nervous system responses in fasting pregnant rats appear to be dissociated, which suggests that diet-induced changes in sympathetic activity and stimulation of the adrenal medulla by hypoglycemia may be independently regulated.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANTON A. H., SAYRE D. F. A study of the factors affecting the aluminum oxide-trihydroxyindole procedure for the analysis of catecholamines. J Pharmacol Exp Ther. 1962 Dec;138:360–375. [PubMed] [Google Scholar]
- BROWN J. Effects of 2-deoxyglucose on carbohydrate metablism: review of the literature and studies in the rat. Metabolism. 1962 Oct;11:1098–1112. [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom S. R., Edwards A. V., Hardy R. N., Malinowska K. W., Silver M. Endocrine responses to insulin hypoglycaemia in the young calf. J Physiol. 1975 Jan;244(3):783–803. doi: 10.1113/jphysiol.1975.sp010826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRONE C. THE SECRETION OF ADRENAL MEDULLARY HORMONES DURING HYPOGLYCEMIA IN INTACT, DECEREBRATE AND SPINAL SHEEP. Acta Physiol Scand. 1965 Mar;63:213–224. doi: 10.1111/j.1748-1716.1965.tb04061.x. [DOI] [PubMed] [Google Scholar]
- Christensen N. J., Alberti K. G., Brandsborg O. Plasma catecholamines and blood substrate concentrations: studies in insulin induced hypoglycaemia and after adrenaline infusions. Eur J Clin Invest. 1975 Sep 12;5(5):415–423. doi: 10.1111/j.1365-2362.1975.tb00473.x. [DOI] [PubMed] [Google Scholar]
- Christensen N. J. Plasma norepinephrine and epinephrine in untreated diabetics, during fasting and after insulin administration. Diabetes. 1974 Jan;23(1):1–8. doi: 10.2337/diab.23.1.1. [DOI] [PubMed] [Google Scholar]
- Felig P., Lynch V. [Starvation in human pregnancy: hypoglycemia, hypoinsulinemia, and hyperketonemia]. Science. 1970 Nov 27;170(3961):990–992. doi: 10.1126/science.170.3961.990. [DOI] [PubMed] [Google Scholar]
- GOLDFIEN A., ZILELI M. S., DESPOINTES R. H., BETHUNE J. E. The effects of hypoglycemia on the adrenal secretion of epinephrine and norepinephrine in the dog. Endocrinology. 1958 Jun;62(6):749–757. doi: 10.1210/endo-62-6-749. [DOI] [PubMed] [Google Scholar]
- Garber A. J., Cryer P. E., Santiago J. V., Haymond M. W., Pagliara A. S., Kipnis D. M. The role of adrenergic mechanisms in the substrate and hormonal response to insulin-induced hypoglycemia in man. J Clin Invest. 1976 Jul;58(1):7–15. doi: 10.1172/JCI108460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen H. J., Christensen N. J. Intravenous insulin causing loss of intravascular water and albumin and increased adrenergic nervous activity in diabetics. Diabetes. 1977 Jun;26(6):551–557. doi: 10.2337/diab.26.6.551. [DOI] [PubMed] [Google Scholar]
- Herrera E. M., Knopp R. H., Freinkel N. Urinary excretion of epinephrine and norepinephrine during fasting in late pregnancy in the rat. Endocrinology. 1969 Feb;84(2):447–450. doi: 10.1210/endo-84-2-447. [DOI] [PubMed] [Google Scholar]
- Herrera E., Knopp R. H., Freinkel N. Carbohydrate metabolism in pregnancy. VI. Plasma fuels, insulin, liver composition, gluconeogenesis, and nitrogen metabolism during late gestation in the fed and fasted rat. J Clin Invest. 1969 Dec;48(12):2260–2272. doi: 10.1172/JCI106192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusaka M., Ui M. Activation of the Cori cycle by epinephrine. Am J Physiol. 1977 Feb;232(2):E145–E155. doi: 10.1152/ajpendo.1977.232.2.E145. [DOI] [PubMed] [Google Scholar]
- Landsberg L., Berardino M. B., Silva P. Metabolism of 3-H-L-dopa by the rat gut in vivo-evidence for glucuronide conjugation. Biochem Pharmacol. 1975 Jun 15;24(11-12):1167–1174. doi: 10.1016/0006-2952(75)90057-x. [DOI] [PubMed] [Google Scholar]
- Landsberg L., Young J. B. Fasting, feeding and regulation of the sympathetic nervous system. N Engl J Med. 1978 Jun 8;298(23):1295–1301. doi: 10.1056/NEJM197806082982306. [DOI] [PubMed] [Google Scholar]
- Neff N. H., Tozer T. N., Hammer W., Costa E., Brodie B. B. Application of steady-state kinetics to the uptake and decline of H3-NE in the rat heart. J Pharmacol Exp Ther. 1968 Mar;160(1):48–52. [PubMed] [Google Scholar]
- Niijima A. The effect of 2-deoxy-D-glucose and D-glucose on the efferent discharge rate of sympathetic nerves. J Physiol. 1975 Sep;251(1):231–243. doi: 10.1113/jphysiol.1975.sp011089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCOW R. O., CHERNICK S. S., BRINLEY M. S. HYPERLIPEMIA AND KETOSIS IN THE PREGNANT RAT. Am J Physiol. 1964 Apr;206:796–804. doi: 10.1152/ajplegacy.1964.206.4.796. [DOI] [PubMed] [Google Scholar]
- SILVER M. The output of adrenaline and noradrenaline from the adrenal medulla of the calf. J Physiol. 1960 Jun;152:14–29. doi: 10.1113/jphysiol.1960.sp006466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubin H. L., Djahanguiri B., Landsberg L. Noradrenaline concentration and turnover in different regions of the gastrointestinal tract of the rat: an approach to the evaluation of sympathetic activity in the gut. Gut. 1972 Oct;13(10):790–795. doi: 10.1136/gut.13.10.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITBY L. G., AXELROD J., WEIL-MALHERBE H. The fate of H3-norepinephrine in animals. J Pharmacol Exp Ther. 1961 May;132:193–201. [PubMed] [Google Scholar]
- Wegienka L. C., Grasso S. G., Forsham P. H. Estimation of adrenomedullary reserve by infusion of 2-deoxy-D-glucose. J Clin Endocrinol Metab. 1966 Jan;26(1):37–45. doi: 10.1210/jcem-26-1-37. [DOI] [PubMed] [Google Scholar]
- Young J. B., Landsberg L. Catecholamines and intermediary metabolism. Clin Endocrinol Metab. 1977 Nov;6(3):599–631. doi: 10.1016/s0300-595x(77)80073-x. [DOI] [PubMed] [Google Scholar]
- Young J. B., Landsberg L. Effect of diet and cold exposure on norepinephrine turnover in pancreas and liver. Am J Physiol. 1979 May;236(5):E524–E533. doi: 10.1152/ajpendo.1979.236.5.E524. [DOI] [PubMed] [Google Scholar]
- Young J. B., Landsberg L. Stimulation of the sympathetic nervous system during sucrose feeding. Nature. 1977 Oct 13;269(5629):615–617. doi: 10.1038/269615a0. [DOI] [PubMed] [Google Scholar]
- Young J. B., Landsberg L. Suppression of sympathetic nervous system during fasting. Science. 1977 Jun 24;196(4297):1473–1475. doi: 10.1126/science.867049. [DOI] [PubMed] [Google Scholar]
- von EULER U., LISHAJKO F. Improved technique for the fluorimetric estimation of catecholamines. Acta Physiol Scand. 1961 Apr;51:348–355. doi: 10.1111/j.1748-1716.1961.tb02128.x. [DOI] [PubMed] [Google Scholar]


