Abstract
The Atlantic salmon (Salmo salar) serum lectin (SSL) is a soluble C-type lectin that binds bacteria, including salmon pathogens. This lectin is a cysteine-rich oligomeric protein. Consequently, a Drosophilamelanogaster expression system was evaluated for use in expressing SSL. A cDNA encoding SSL was cloned into a vector designed to express it as a fusion protein with a hexahistidine tag, under the control of the Drosophila methallothionein promoter. The resulting construct was stably transfected into Drosophila S2 cells. After CdCl2 induction, transfected S2 cells secreted recombinant SSL into the cell culture medium. A cell line derived from stably transformed polyclonal cell populations expressing SSL was used for large-scale expression of SSL. Recombinant SSL was purified from the culture medium using a two-step purification scheme involving affinity binding to yeast cells and metal-affinity chromatography. Although yields of SSL were very low, correct folding and functionality of the recombinant SSL purified in this manner was demonstrated by its ability to bind to Aeromonas salmonicida. Therefore, Drosophila S2 cells may be an ideal system for the production of SSL if yields can be increased.
Keywords: Atlantic salmon, Salmo salar, C-type lectin, Drosophila, Protein expression, Protein purification, Innate immunity
Introduction
Lectins are proteins that bind specifically to carbohydrates and C-type lectins are the most diverse group of animal lectins with respect to protein structure, location, and binding specificities. C-type lectins require Ca2+ for carbohydrate binding and for the structural maintenance of the C-type lectin-like domain (CTLD). This superfamily includes proteins that function as carbohydrate-binding modules in various molecular recognition events such as immunity, cellular recognition, and development (Kilpatrick 2002; Sharon and Lis 2004).
Salmon serum lectin (SSL) is a soluble C-type lectin originally isolated from the serum of Atlantic salmon (Salmo salar) using mannose-affinity chromatography (Ewart et al. 1999). This lectin was found to bind salmon pathogens and to opsonise Aeromonas salmonicida (Ottinger et al. 1999). Analysis of SSL by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) under reducing and non-reducing conditions revealed an oligomeric structure composed of 17 kDa subunits held together by disulfide bonds (Ewart et al. 1999). Molecular analysis of SSL transcripts uncovered four distinct cDNA sequences and a fifth cDNA sequence was predicted based on the genomic sequence (Richards et al. 2003). SSL was classified as a long-form C-type lectin because its CTLD had an N-terminal extension containing two additional cysteines and there were no other associated domains. The cDNA sequences encode 173 amino acids each and show minor sequence microheterogeneity. The mature protein contains 8 cysteine residues. Protein sequence alignments between SSL and representative CTLDs revealed the presence of four highly conserved cysteines that typically form two disulfide bonds in all CTLDs and the presence of the N-terminal disulfide-bonded loop characteristic of the long-form CTLDs (Richards et al. 2003). A computer-generated model of the SSL-2 isoform subunit showed the three expected intramolecular disulfides and suggested that the two remaining cysteines are moderately exposed to the solvent. Both of these exposed cysteines were found to contribute to oligomerization of SSL (Hudson et al. 2011).
Protein-ligand interaction studies and three-dimensional structure determination require substantial amounts of pure, correctly folded and functional protein. Since SSL is encoded by a multigene-family, purification from Atlantic salmon serum is expected to result in a mixture of different lectin isoforms. Therefore, recombinant lectin expression would offer the possibility of producing milligram quantities of single-isoform SSL. However, SSL is a cystine-rich, oligomeric protein that has proven to be a challenging target for recombinant protein expression. Expression of soluble rSSL was achieved in Escherichia coli (Hudson et al. 2011), but the yield was very low and variable.
Eukaryotic expression systems have been effective for C-type lectins. Two studies achieved heterologous expression of type II antifreeze proteins that have C-type lectin folds and high sequence identity to SSL (Ewart et al. 1992; Ewart and Fletcher 1993; Richards et al. 2003) in Pichia pastoris (Li et al. 2001) and in Drosophila melanogaster S2 cells (Scotter et al. 2006). Specifically, active sea raven (Hemitripterus americanus) antifreeze protein (srAFP) was expressed at 95 mg L−1 of medium from S2 cells (Scotter et al. 2006). The reported success of the type II AFP expression in Drosophila cells prompted the investigation of this system as means to produce large amounts of pure functional SSL. Thus, the goals of the current study were to express rSSL in insect cells and to purify the protein from the cell medium.
Materials and methods
Construction of the plasmid pMT/Bip_SSL
The cDNA encoding SSL isoform 2 (GenBank Accession No. AY191314) was amplified by PCR using the sense primer SSL_BglII 5′-CGGGAGATCTACAGGAGCTAAGG-3′ and the antisense primer SSL_AgeI 5′-TGATGACCGGTGTTTTTCTGGATTTCACAG-3′. The primers were designed to introduce BglII and AgeI restriction sites (underlined), respectively. The amplicon was ligated into pCR4Blunt-TOPO (Invitrogen) to generate pCR4_SSL_Drosophila construct. The pCR4_SSL_Drosophila vector was digested with BglII and AgeI and the 482-bp SSL cDNA fragment obtained was cloned between the same sites of pMT/Bip/V5-His A (Invitrogen), and confirmed by sequencing using MT_Forward 5′-CATCTCAGTGCAACTAAA-3′ and BGH_Reverse 5′-TAGAAGGCACAGTCGAGG-3′ primers. The resulting expression vector, pMT/Bip_SSL, contains the sequences encoding the Drosophila N-terminal immunoglobulin-binding protein (BiP) signal sequence fused in-frame with SSL, and a C-terminal hexahistidine tag in tandem to produce an rSSL fusion protein.
Cell culture and transfection
Drosophila melanogaster S2 cells (Schneider 1972) (Invitrogen) were grown at 27 °C in DES medium (Invitrogen) supplemented with 10 % heat-inactivated fetal bovine serum (FBS, Sigma-Aldrich). The cells were split with supplemented medium at a ratio of 1:4 every 3–4 days. Transfection and stable cell line selection were performed as described (Scotter et al. 2006). For transient expression of rSSL, the cells were transfected with 19 μg pMT/BiP_SSL and for the selection of stable cell lines S2 cells were co-transfected with 19 μg pMT/BiP_SSL and 1 μg blasticidin resistance plasmid pCopBlast (Invitrogen) using a calcium phosphate transfection kit (Invitrogen) according to the manufacturer’s instructions.
Cell line selection
Stably transfected polyclonal cell populations were selected with blasticidin over a 4-week period, replacing the medium once per week by centrifuging the cells and resuspending them in an equal volume of fresh DES medium plus 10 % FBS and 16 μg mL−1 blasticidin (Invitrogen). To obtain clonal cell lines, the blasticidin-resistant cell population was diluted and mixed with non-transfected S2 cells at a cell density of 2.5 × 106 cells mL−1 to act as feeder cells, resulting in approximately 1–2 transfected cells per well. The cells were plated into 96-well tissue culture plates (Nunc) at 0.1 mL per well in FBS-enriched S2 medium containing 16 μg mL−1 blasticidin. At the end of the selection interval, wells containing growing clones were chosen and diluted twofold into parallel wells. One of the wells was induced with 500 μM CuSO4 to analyze SSL expression. Cells were harvested 96 h post-induction and rSSL in the supernatant was detected by western blotting.
Protein expression in Drosophila S2 cells
Clones expressing SSL were scaled up to 5 mL cultures and adapted to Ex-Cell 420 Insect Serum Free Media (Sigma-Aldrich) for protein production in the absence of serum. To characterize the optimal induction conditions, stably expressing S2 cells grown in serum free medium were induced with 3–100 μM CdCl2 or with 250–2,000 μM CoSO4. For large-scale protein expression, the cultures were scaled up through intermediate stages in spinner flasks for growth in 2.8 L Fernbach shaker flasks (800 mL per flask) at a constant temperature of 27 °C and 80 rpm in an INNOVA 4000 shaker incubator. Stably expressing S2 cells were seeded at a cell density of 5 × 106 cells mL−1 and induced with 3 μM CdCl2. The supernatant was harvested by centrifugation (2,000g, 10 min, RT) after 4 days of incubation during which a cell density of 4 × 107 cells mL−1 was obtained. The cells were resuspended in an equal volume of fresh medium for a second round of induction. The supernatant was cleared with a second centrifugation (12,000g for 20 min, 4 °C), and sodium azide and PMSF were each added to 1 mM final concentration. The supernatant was stored at −20 °C until use.
Purification of rSSL from insect cell medium
To the supernatant, 10 mM Tris–HCl pH 7.4, 10 mM CaCl2, 150 mM NaCl, 1 mM PMSF, and 1 μg mL−1 each pepstatin and leupeptin were added. The rSSL was purified from the supernatant by affinity binding to killed yeast (Saccharomyces cerevisiae) cells according to a published protocol (Tsutsui et al. 2007) with modifications. Briefly, 800 mL of supernatant containing rSSL was incubated for 1.5 h at 75 rpm at 16 °C with 10 mg of dried baker’s yeast (Fleischmann’s) cell pellet washed in TCS (10 mM Tris–HCl, 150 mM NaCl, 10 mM CaCl2, pH 7.5) buffer. The yeast cells were harvested by centrifugation at 14,000g for 12 min and washed twice in TCS buffer. Unbound medium was submitted to a second round of affinity binding to yeast cells to increase the yield of captured protein. Captured rSSL was eluted in 100 mL TBS plus mannose (10 mM Tris–HCl, 150 mM NaCl, 200 mM D-(+)-mannose, pH 7.4) and concentrated using a 3,000 Da MWCO centrifugal device (Amicon).
Concentrated rSSL was further purified by immobilized metal affinity chromatography (IMAC) using Ni2+-nitrilotriacetate (Ni–NTA) resin (Qiagen). The recombinant lectin solution was incubated with 2 mL of resin equilibrated in binding buffer (10 mM Tris–HCl, 300 mM NaCl, 10 mM imidazole, pH 7.5) on an inversion shaker for 24 h at 4 °C. The mixture was poured into an Econopac column (Bio-Rad) and unbound proteins were washed from the resin using the binding buffer. Weakly binding contaminating proteins were removed by increasing the imidazole concentration to 100 mM. The rSSL was eluted using a gradient of 200–500 mM imidazole. Fractions containing rSSL were pooled, concentrated as described above, and dialyzed into TCS buffer. Unbound insect cell medium and washes were concentrated tenfold by trichloroacetic acid (TCA) precipitation. The collected samples were analyzed by SDS-PAGE and western blotting.
SDS-PAGE and western blot analysis
Proteins were resolved by reducing SDS-PAGE (Laemmli 1970) on 15 % polyacrylamide gels. For detection of proteins in gels, Coomassie blue and silver staining was used. For western blotting, proteins were transferred to polyvinylidine difluoride (PVDF) membranes (GE Life Sciences) using standard methods (Towbin et al. 1979). After blocking for 1 h in TBS-T buffer (10 mM Tris–HCl, 150 mM NaCl, 0.5 % Tween-20, pH 7.5) with 5 % non-fat milk, the membrane was incubated with rabbit antiserum to SSL (anti-SSL) produced as previously described (E. Uribe et al., manuscript submitted) at a dilution of 1:3,000 in blocking buffer. Membranes were washed three times in TBS-T and probed with goat anti-rabbit antibody horseradish-peroxidase conjugate (1:6,000 v/v) (Sigma-Aldrich). For detection of the C-terminal hexahistidine tag (His6-tag) in recombinant SSL, the blots were incubated in a 1:2,000 dilution of the tetra-His antibody (Qiagen) in blocking buffer [phosphate-buffered saline (PBS), 0.05 % Tween, 5 % non-fat milk]. Three washing steps were done with PBS + 0.05 % Tween, followed by treatment with horseradish-peroxidase-conjugated anti-mouse antibody (Sigma-Aldrich) at a dilution of 1:3,000. The luminescent signal emitted by the enhanced chemiluminescence substrate (Plus-ECL, PerkinElmer) was detected with X-ray film. Standards used to estimate rSSL concentration in western blot analyses were 30–500 ng pure native SSL (E. Uribe et al., manuscript submitted) and 30–500 ng pure rSSL from E. coli (Hudson et al. 2011).
Oligomerization analysis
Two hundred mL of supernatant containing rSSL was concentrated by ultrafiltration to 15 mL using a 3,000 Da MWCO centrifugal device (Amicon). The concentrated supernatant was buffer exchanged into 10 mM Tris–HCl, 300 mM NaCl, 10 mM imidazole, pH 7.5 and purified by batch nickel affinity chromatography. The eluted rSSL was dialyzed into distilled and deionised water (ddH2O) and dried in a Speed-Vac (Eppendorf). The dried sample was dissolved in 30 μL ddH2O and the oligomeric organization of rSSL was analyzed by non-reducing SDS-PAGE on a 12.5 % polyacrylamide gel (NEXT GEL, Amresco) followed by western blotting on a PVDF membrane. Recombinant SSL expressed in E. coli as previously described (Hudson et al. 2011) was analyzed under the same conditions.
Bacterial binding assay
Aeromonas salmonicida subs. salmonicida strain A449 isolated from brown trout (Michel 1979) was obtained from the National Research Council of Canada (Halifax, NS). The bacteria were cultured in tryptic soy broth at 16 °C for 48–72 h. Recombinant lectin binding to A. salmonicida experiments were performed as described (Brooks et al. 2003; Lillie et al. 2006; Young et al. 2007), with minor changes. Briefly, 5 mL cultures of A. salmonicida were grown to an OD600nm of approximately 0.8. The cells were washed three times with 1 mL TCS buffer, fixed with 1 mL 0.5 % formaldehyde in TCS, washed three times with TCS, and harvested by low-speed centrifugation (3,000g, 5 min). A. salmonicida cells were resuspended in 0.5 mL purified rSSL, in TCS or in medium as a negative control. The cell suspensions were incubated with rotation for 1 h at RT and washed twice in TCS. Bound rSSL was eluted from the bacterial pellet by re-suspension and 1 h incubation in 0.5 mL TBS plus mannose. Unbound rSSL sample, pre-mannose wash, and mannose elution fractions were analyzed by SDS-PAGE and western blotting. Binding experiments were performed in duplicate.
Results
Expression of rSSL in S2 cells
Analysis of transient expression in Drosophila S2 cells of rSSL into the medium by western blotting using anti-SSL antibodies revealed a band of the expected molecular weight (~18 kDa; Fig. 1a). Recombinant SSL was detected in the supernatant of induced S2 cells, but not in the supernatant of non-induced cells. These transient transfection results showed rSSL to be present in the supernatant, indicating that SSL was successfully produced and secreted.
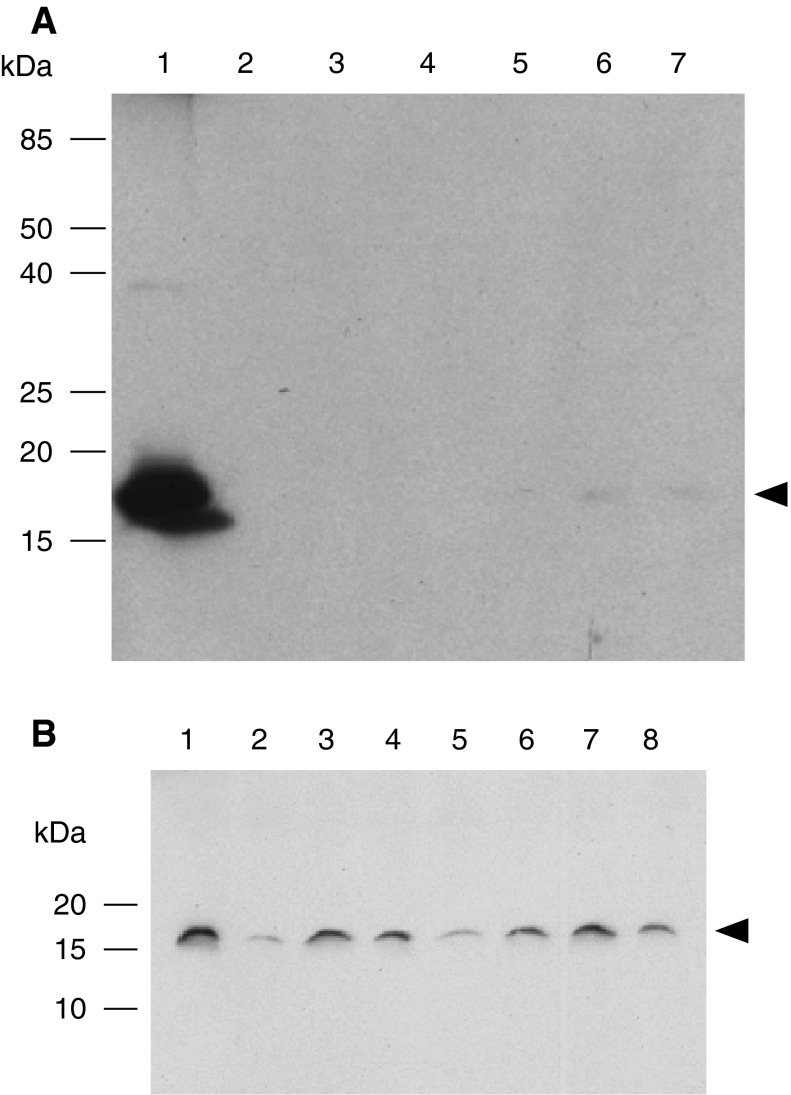
Fig. 1.
Western blot analysis of transient and stable expression of rSSL in Drosophila S2 cells. Cells grown in DES medium transfected with the plasmid pMT/BiP_SSL were induced with 500 μM CuSO4. Fifteen μL of supernatant was harvested after 4 days and screened for rSSL expression by western blotting using the tetra-His antibody after 15 % reducing SDS-PAGE. Transient expression is shown in A. Lanes are (1) 0.7 μg pure E. coli-produced rSSL, (2) fresh medium, (3) non-induced S2 cells, (4–7) supernatants at 1, 2, 3 and 4 days post-induction. Stable clonal cell lines are shown in B, with each lane showing a distinct line. In each panel, molecular mass marker sizes (kDa) are indicated and the arrow indicates the position of the rSSL protein
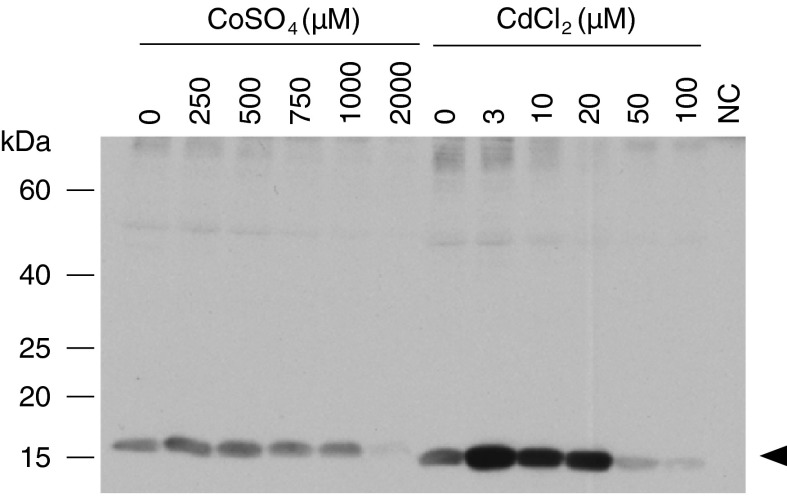
Stable cell lines were established to obtain a clonal cell line producing higher amounts of rSSL. A clone showing strong protein expression (Fig. 1b, lane 3) was chosen for large-scale rSSL production and it was adapted to serum free medium. To characterize the optimal induction conditions in serum free medium, transfected S2 cells were induced with various CdCl2 or CoSO4 concentrations. Maximal expression occurred with 3 μM CdCl2 (Fig. 2) when measured at 4 days post-induction. Large-scale expression of SSL in the stable cell line resulted in the accumulation of secreted rSSL in the supernatant, as detected by western blot analysis using anti-SSL antibodies (Fig. 3). Recombinant SSL expression was approximately 1.5 mg L−1 of culture medium, as indicated by the densitometry of western blot rSSL bands in comparison with standards of the same protein (data not shown).
Fig. 2.
Western blot analysis of optimization of inducer concentration for the expression of rSSL in stable transfected Drosophila S2 cells. Cells were grown in serum-free medium and induced with CdCl2 or CoSO4. Fifteen μL of supernatant was harvested after 4 days and screened for rSSL expression by western blotting using tetra-His antibody on 15 % reducing SDS-PAGE. Lanes are indicated with inducer concentrations and the lane NC contained negative fresh control medium. The arrow indicates the position of the rSSL protein
Fig. 3.
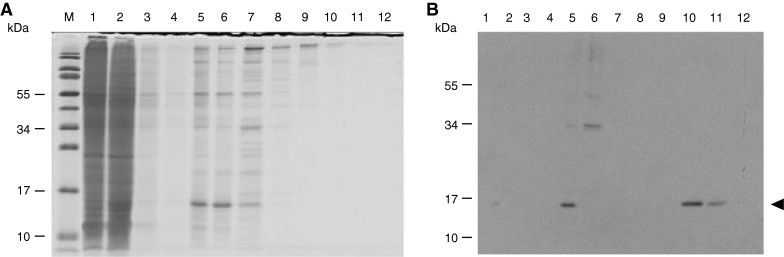
Purification of rSSL from Drosophila cell culture supernatant. Cells were grown in serum free medium induced with 3 μM CdCl2 and harvested after 4 days. The rSSL was purified by yeast affinity and IMAC and 15 % reducing SDS-PAGE followed by Coomassie staining (A) and western blotting using anti-SSL antibodies (B) were performed with 15 μL of fractions loaded in each lane. For A and B, lanes are (M) molecular mass marker, (1) 10 × concentrated culture supernatant, (2) 10 × concentrated yeast suspension, (3–4) 10 × concentrated TCS washes, (5) mannose eluate concentrated by ultrafiltration, (6) IMAC flow-through; (7–9) 15 μL imidazole washes; (10–11) 15 μL purified rSSL; (12) 15 μL 500 mM imidazole wash. The arrow indicates the position of the rSSL protein
Capture of rSSL from the supernatant
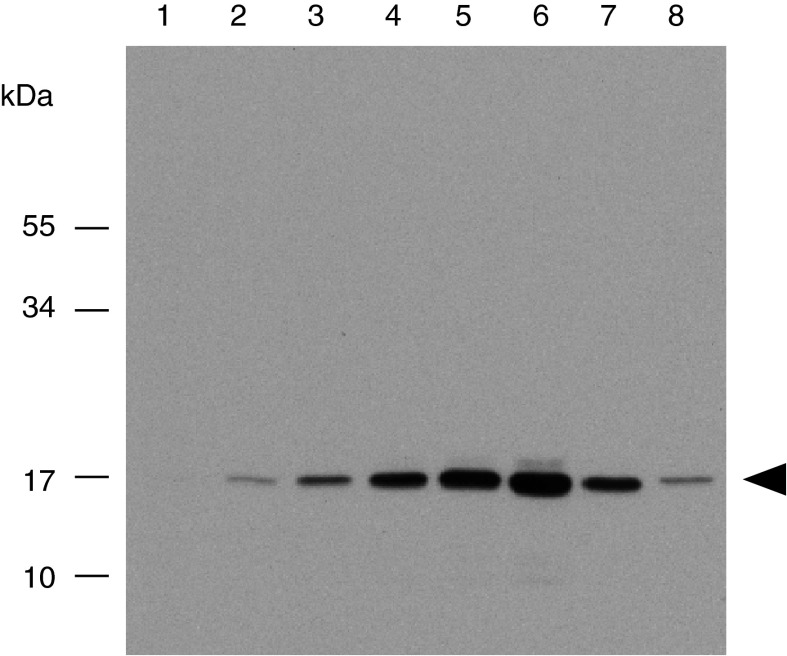
Recombinant lectin was isolated from the medium by affinity binding to yeast cells followed by IMAC. The recombinant lectin was not detected among the proteins eluted from the Ni2+ column detected by SDS-PAGE and silver staining, suggesting that it was a minor component; however, it was shown to be present by western blot analysis (Fig. 3). Initial batchwise yeast affinity purification revealed that rSSL bound to the yeast cells along with a higher molecular mass contaminant (~34 kDa). Consequently, the partially enriched supernatant was subjected to ion exchange chromatography to separate it from the contaminant. The yield of captured rSSL as estimated by the intensity of western blot in comparison with known standards was approximately 1.4 μg L−1 of culture (Fig. 4).
Fig. 4.
Determination of rSSL concentration in culture supernatant by western blot analysis. Supernatant was subject to 15 % reducing SDS-PAGE followed by western blotting using anti-SSL antibodies alongside pure native SSL, which was used as a standard. Lanes are (1) 6.25 ng SSL, (2) 31.3 ng SSL, (3) 62.5 ng SSL, (4) 188 ng SSL, (5) 313 ng SSL, (6) 500 ng SSL, (7) 125 ng SSL, (8) Drosophila—expressed rSSL representing 15 μL of supernatant. Molecular mass marker sizes (kDa) are indicated. The arrow indicates the position of the rSSL protein
Characterization of rSSL
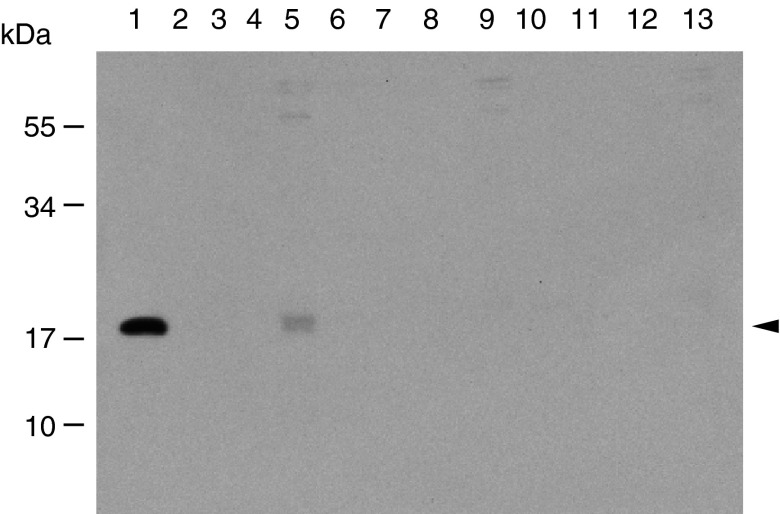
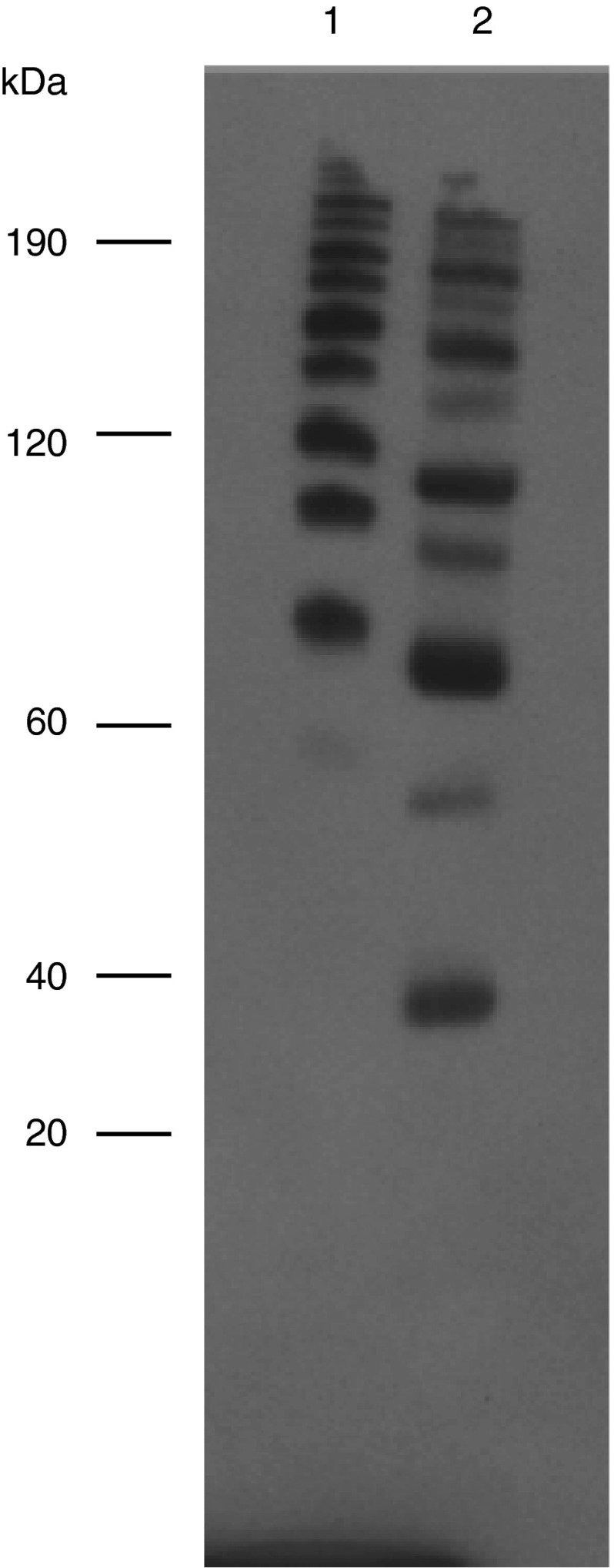
Although yeast binding was employed in purification of rSSL and expected to require functional binding to yeast surface carbohydrates, binding to a known natural ligand of SSL was evaluated as a more rigorous test of correct folding and normal functioning of the protein. Incubation of the rSSL with A. salmonicida and elution with mannose revealed effective and specific binding of this protein to the bacteria (Fig. 5). In addition, the disulfide-mediated oligomerization of the Drosophila rSSL was compared with the E. coli rSSL by SDS-PAGE under denaturing, non-reducing conditions and western blotting (Fig. 6). The two rSSL preparations exhibited a laddering pattern under non-reducing conditions suggesting that they have the capacity to oligomerize below 200 kDa. The observed pattern of rSSL from Drosophila contained two smaller oligomers corresponding to predicted dimers and trimers.
Fig. 5.
Interaction of rSSL with A. salmonicida. Purified rSSL, TCS buffer or fresh insect cell culture medium were incubated with A. salmonicida, bacteria were washed and then bound materials were eluted using mannose. Samples (15 μL per lane) were subject to 15 % reducing SDS-PAGE followed by western blotting using anti-SSL antibodies. Lanes are (1) 15 μL purified rSSL, (2) 15 μL unbound rSSL, (3–4) 15 μL rSSL washes, (5) 15 μL rSSL mannose eluate, (6) 15 μL unbound TCS buffer, (7–8) 15 μL TCS buffer washes, (9) 15 μL TCS buffer mannose eluate, (10) 15 μL unbound culture medium, (11–12) 15 μL culture medium washes, (13) 15 μL culture medium mannose eluate. The arrow indicates cross-reacting rSSL protein
Fig. 6.
Oligomerization of Drosophila rSSL. Bacterial and Drosophila cell-expressed rSSL were subject to SDS-PAGE under non-reducing conditions and then western blotting using the tetra-His antibody. Lanes are (1) 0.7 μg purified rSSL expressed in E. coli, (2) 15 μL of purified Drosophila rSSL. Molecular mass marker sizes (kDa) are indicated
Discussion
The Drosophila S2 insect cell line has been developed as a plasmid-based, inducible and non-lytic integration system for construction of stable transfected cell lines. It allows secretable expression in suspension cultures, and it is suitable for scale-up (Schneider 1972; Kirkpatrick et al. 1999). It is currently recognized as one of the most promising systems for heterologous protein expression (Sorensen 2010). The Drosophila S2 protein expression system has been successfully used to produce numerous complex recombinant proteins (Moraes et al. 2012; Cha et al. 2005; Torfs et al. 2000; Numao et al. 2003; Nilsen and Castellino 1999; Chaiken et al. 1995; Scotter et al. 2006; Southern et al. 1991; Culp et al. 1991; Johansson et al. 2007; Lim and Cha 2006; Perret et al. 2003; Yokomizo et al. 2007; Jorge et al. 2008). In particular, the excellent yield obtained for the homologous srAFP, suggested the potential for strong expression of SSL (Scotter et al. 2006).
To obtain large quantities of recombinant SSL, the lectin was expressed in stably transfected Drosophila S2 cells. Stable transfection also assures preservation of the cell line expressing the protein (Echalier 1997; Tan et al. 2000). These cells expressed and secreted rSSL to the medium and the identity of the resulting rSSL was confirmed by western blotting using antiserum against native SSL. Yeast affinity was employed as a purification strategy to capture rSSL from very large volumes. Besides allowing purification of the rSSL, yeast binding was also expected to allow the separation of functional rSSL from inactive forms. Binding to mannan-rich yeast cells followed by elution with mannose suggested that the rSSL isolated was folded and functional. It appears likely that this process would exclude improperly folded rSSL unless it were to bind to the yeast in a non-specific fashion and then to be displaced by competition with mannose-eluted proteins. As a known biological role of rSSL is to bind the A. salmonicida bacterium, bacterial cell interaction was subsequently used to confirm the functionality of the expressed lectin.
Although rSSL was functional, the yield was low. In the current study, rSSL was expressed in serum-free medium to substantially reduce the amount of contaminating proteins during purification. Two previous reports have shown that the medium composition influences heterologous protein expression in Drosophila S2 cells (Hu et al. 2004; Park et al. 2008). Recombinant anosim-1 was more abundant in the presence of serum than in serum-free culture (Hu et al. 2004). Similarly, the cultivation of stably transformed S2 cells using Hy®QSFX-insect MP medium approximately doubled the production of recombinant human ribonuclease/angiogenin inhibitor (Park et al. 2008). Therefore, expression of rSSL in S2 cells might benefit from the use of alternative media. Other possibilities, including manipulation of the temperature for example, could also enhance production. Low temperature appears to hold promise for enhancing protein expression yields in mammalian cells (Al-Fageeh et al. 2006; Wulhfard et al. 2008). Nonetheless, the production of rSSL as a secreted product in a eukaryotic expression system might remain difficult as a result of its function. The SSL is known to bind mannose, N-acetylglucosamine and related carbohydrates (Stratton et al. 2004; E. Uribe et al., manuscript submitted). Therefore, when expressed, the functional rSSL may bind to naturally occurring carbohydrates on the surfaces of the cells in culture, thereby causing agglutination or interfering with normal processes. This situation would effectively select for cells expressing the lowest levels of rSSL. It would therefore be interesting to determine whether the addition of specific carbohydrates to the medium during growth or other measures to impede potential cell recognition by the SSL might increase the proportion of cells with higher rSSL expression.
This is the first report on the expression of rSSL in a stably transformed insect cell system, which has resulted in correctly processed and functional rSSL. Purified rSSL from S2 cells is biologically active, as evident by its pathogen-binding activity. Optimization of rSSL expression with the established S2 cell lines for higher expression yields could make this a convenient source of rSSL for structural studies. It will also be interesting to determine whether Drosophila cells present a valuable option for the expression of other fish proteins.
Acknowledgments
We thank Sandra Sperker and Robert Richards for technical advice and we thank Doug Kuntz and Dr. Santosh Lall for review of the manuscript. We are grateful to Dr. Catherine Too for loan of the tissue culture flasks. This study was supported by an Izaak Walton Killam Predoctoral Scholarship (E. Uribe), an NSERC Discovery grant (K. V. Ewart) and by the NRC Institute for Marine Biosciences. This is NRC publication number 54062.
References
- Al-Fageeh MB, Marchant RJ, Carden MJ, Smales CM. The cold-shock response in cultured mammalian cells: harnessing the response for the improvement of recombinant protein production. Biotechnol Bioeng. 2006;93:829–835. doi: 10.1002/bit.20789. [DOI] [PubMed] [Google Scholar]
- Brooks AS, DeLay JP, Hayes MA. Purification and binding properties of porcine plasma ficolin that binds Actinobacillus pleuropneumoniae. Dev Comp Immunol. 2003;27:835–844. doi: 10.1016/S0145-305X(03)00057-0. [DOI] [PubMed] [Google Scholar]
- Cha HJ, Shin HS, Lim HJ, Cho HS, Dalal NN, Pham MQ, Bentley WE. Comparative production of human interleukin-2 fused with green fluorescent protein in several recombinant expression systems. Biochem Eng J. 2005;24:225–233. doi: 10.1016/j.bej.2005.03.002. [DOI] [Google Scholar]
- Chaiken I, Johanson K, Appelbaum E, Doyle M, Hensley P, Zhao B, Abdel-Meguid SS, Young P, Cook R, Carr S, Maticon R, Cusimano D, Dul E, Angelichio M, Brooks I, Winborne E, McDonnell P, Morton T, Bennett D. Binding interactions of human interleukin 5 with its receptor alpha subunit. Large scale production, structural, and functional studies of Drosophila-expressed recombinant proteins. J Biol Chem. 1995;270:9459–9471. doi: 10.1074/jbc.270.16.9459. [DOI] [PubMed] [Google Scholar]
- Culp JS, Johansen H, Hellmig B, Beck J, Matthews TJ, Delers A, Rosenberg M. Regulated expression allows high level production and secretion of HIV-1 gp120 envelope glycoprotein in Drosophila Schneider cells. Nat Biotechnol. 1991;9:173–177. doi: 10.1038/nbt0291-173. [DOI] [PubMed] [Google Scholar]
- Echalier G (1997) Drosophila continuous cell lines. In: Drosophila cells in culture. Academic Press, New York, pp 129–186
- Ewart KV, Fletcher GL. Herring antifreeze protein: primary structure and evidence for a C-type lectin evolutionary origin. Mol Mar Biol Biotechnol. 1993;2:20–27. [PubMed] [Google Scholar]
- Ewart KV, Rubinsky B, Fletcher GL. Structural and functional similarity between fish antifreeze proteins and calcium-dependent lectins. Biochem Biophys Res Commun. 1992;185:335–340. doi: 10.1016/S0006-291X(05)90005-3. [DOI] [PubMed] [Google Scholar]
- Ewart KV, Johnson SC, Ross NW. Identification of a pathogen-binding lectin in salmon serum. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1999;123:9–15. doi: 10.1016/S0742-8413(99)00002-X. [DOI] [PubMed] [Google Scholar]
- Hu Y, Gonzalez-Martinez D, Kim SH, Bouloux PM. Cross-talk of anosmin-1, the protein implicated in X-linked Kallmann’s syndrome, with heparan sulphate and urokinase-type plasminogen activator. Biochem J. 2004;384:495–505. doi: 10.1042/BJ20041078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson DM, Mattatall NR, Uribe E, Richards RC, Gong H, Ewart KV. Cystine-mediated oligomerization of the Atlantic salmon serum C-type lectin. BBA Proteins Proteom. 2011;1814:283–289. doi: 10.1016/j.bbapap.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Johansson DX, Drakenberg K, Hopmann KH, Schmidt A, Yari F, Hinkula J, Persson MAA. Efficient expression of recombinant human monoclonal antibodies in Drosophila S2 cells. J Immunol Methods. 2007;318:37–46. doi: 10.1016/j.jim.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Jorge S, Santos A, Spina A, Pereira C. Expression of the hepatitis B virus surface antigen in Drosophila S2 cells. Cytotechnology. 2008;57:51–59. doi: 10.1007/s10616-008-9154-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick DC. Animal lectins: a historical introduction and overview. BBA Biomembr. 2002;1572:187–197. doi: 10.1016/S0304-4165(02)00308-2. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick RB, Shatzman A, Joseph MF, James PH (1999) Drosophila S2 system for heterologous gene expression. In: Fernandez JN, Hoeffler JP (eds) Gene expression systems. Academic Press, San Diego, pp 289–330
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li Z, Xiong F, Lin Q, d’Anjou M, Daugulis AJ, Yang DS, Hew CL. Low-temperature increases the yield of biologically active herring antifreeze protein in Pichia pastoris. Protein Expr Purif. 2001;21:438–445. doi: 10.1006/prep.2001.1395. [DOI] [PubMed] [Google Scholar]
- Lillie BN, Hammermueller JD, MacInnes JI, Jacques M, Hayes MA. Porcine mannan-binding lectin a binds to Actinobacillus suis and Haemophilus parasuis. Dev Comp Immunol. 2006;30:954–965. doi: 10.1016/j.dci.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Lim HJ, Cha HJ. Observation and modeling of induction effect on human transferrin production from stably transfected Drosophila S2 cell culture. Enzym Microb Technol. 2006;39:208–214. doi: 10.1016/j.enzmictec.2005.10.021. [DOI] [Google Scholar]
- Michel C. Furunculosis of salmonids: vaccination attempts in rainbow trout (Salmo gairdneri) by formalin-killed germs. Ann Rech Vet. 1979;10:33–40. [PubMed] [Google Scholar]
- Moraes AM, Jorge SA, Astray RM, Suazo CA, Calderón Riquelme CE, Augusto EF, Tonso A, Pamboukian MM, Piccoli RA, Barral MF, Pereira CA. Drosophila melanogaster S2 cells for expression of heterologous genes: from gene cloning to bioprocess development. Biotechnol Adv. 2012;30:613–628. doi: 10.1016/j.biotechadv.2011.10.009. [DOI] [PubMed] [Google Scholar]
- Nilsen SL, Castellino FJ. Expression of human plasminogen in Drosophila Schneider S2 cells. Protein Expr Purif. 1999;16:136–143. doi: 10.1006/prep.1999.1045. [DOI] [PubMed] [Google Scholar]
- Numao S, Kuntz DA, Withers SG, Rose DR. Insights into the mechanism of Drosophila melanogaster Golgi alpha-mannosidase II through the structural analysis of covalent reaction intermediates. J Biol Chem. 2003;278:48074–48083. doi: 10.1074/jbc.M309249200. [DOI] [PubMed] [Google Scholar]
- Ottinger CA, Johnson SC, Ewart KV, Brown LL, Ross NW. Enhancement of anti-Aeromonas salmonicida activity in Atlantic salmon (Salmo salar) macrophages by a mannose-binding lectin. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1999;123:53–59. doi: 10.1016/S0742-8413(99)00009-2. [DOI] [PubMed] [Google Scholar]
- Park JH, Hwang IS, Kim KI, Lee JM, Park YM, Park CH, Chung IS. Functional expression of recombinant human ribonuclease/angiogenin inhibitor in stably transformed Drosophila melanogaster S2 cells. Cytotechnology. 2008;57:93–99. doi: 10.1007/s10616-008-9126-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perret BG, Wagner R, Lecat S, Brillet K, Rabut G, Bucher B, Pattus F. Expression of EGFP-amino-tagged human mu opioid receptor in Drosophila Schneider 2 cells: a potential expression system for large-scale production of G-protein coupled receptors. Protein Expr Purif. 2003;31:123–132. doi: 10.1016/S1046-5928(03)00140-2. [DOI] [PubMed] [Google Scholar]
- Richards RC, Hudson DM, Thibault P, Ewart KV. Cloning and characterization of the Atlantic salmon serum lectin, a long-form C-type lectin expressed in kidney. Biochim Biophys Acta. 2003;1621:110–115. doi: 10.1016/S0304-4165(03)00045-X. [DOI] [PubMed] [Google Scholar]
- Schneider I. Cell lines derived from late embryonic stages of Drosophila melanogaster. J Embryol Exp Morphol. 1972;27:353–365. [PubMed] [Google Scholar]
- Scotter AJ, Kuntz DA, Saul M, Graham LA, Davies PL, Rose DR. Expression and purification of sea raven type II antifreeze protein from Drosophila melanogaster S2 cells. Protein Expr Purif. 2006;47:374–383. doi: 10.1016/j.pep.2005.10.028. [DOI] [PubMed] [Google Scholar]
- Sharon N, Lis H. History of lectins: from hemagglutinins to biological recognition molecules. Glycobiology. 2004;14:53R–62R. doi: 10.1093/glycob/cwh122. [DOI] [PubMed] [Google Scholar]
- Sorensen HP. Towards universal systems for recombinant gene expression. Microb Cell Fact. 2010;9:27. doi: 10.1186/1475-2859-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern JA, Young DF, Heaney F, Baumgartner WK, Randall RE. Identification of an epitope on the P and V proteins of simian virus 5 that distinguishes between two isolates with different biological characteristics. J Gen Virol. 1991;72:1551–1557. doi: 10.1099/0022-1317-72-7-1551. [DOI] [PubMed] [Google Scholar]
- Stratton L, Wu S, Richards RC, Vanya Ewart K. Oligomerisation and carbohydrate binding in an Atlantic salmon serum C-type lectin consistent with non-self recognition. Fish Shellfish Immunol. 2004;17:315–323. doi: 10.1016/j.fsi.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Tan NS, Ng MLP, Yau YH, Chong PKW, Ho BOW, Ding JL. Definition of endotoxin binding sites in horseshoe crab Factor C recombinant sushi proteins and neutralization of endotoxin by sushi peptides. FASEB J. 2000;14:1801–1813. doi: 10.1096/fj.99-0866com. [DOI] [PubMed] [Google Scholar]
- Torfs H, Shariatmadari R, Guerrero F, Parmentier M, Poels J, Van Poyer W, Swinnen E, De Loof A, Akerman K, Broeck JV. Characterization of a receptor for insect tachykinin-like peptide agonists by functional expression in a stable Drosophila Schneider 2 cell line. J Neurochem. 2000;74:2182–2198. doi: 10.1046/j.1471-4159.2000.0742182.x. [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui S, Iwamoto K, Nakamura O, Watanabe T. Yeast-binding C-type lectin with opsonic activity from conger eel (Conger myriaster) skin mucus. Mol Immunol. 2007;44:691–702. doi: 10.1016/j.molimm.2006.04.023. [DOI] [PubMed] [Google Scholar]
- Wulhfard S, Tissot S, Bouchet S, Cevey J, De Jesus M, Hacker DL, Wurm FM. Mild hypothermia improves transient gene expression yields several fold in Chinese hamster ovary cells. Biotechnol Prog. 2008;24:458–465. doi: 10.1021/bp070286c. [DOI] [PubMed] [Google Scholar]
- Yokomizo A, Jorge S, Astray R, Fernandes I, Ribeiro O, Horton D, Tonso A, Tordo N, Pereira C. Rabies virus glycoprotein expression in Drosophila S2 cells. I. Functional recombinant protein in stable co-transfected cell line. Biotechnol J. 2007;2:102–109. doi: 10.1002/biot.200600211. [DOI] [PubMed] [Google Scholar]
- Young KM, Russell S, Smith M, Huber P, Ostland VE, Brooks AS, Anthony Hayes M, Lumsden JS. Bacterial-binding activity and plasma concentration of ladderlectin in rainbow trout (Oncorhynchus mykiss) Fish Shellfish Immunol. 2007;23:305–315. doi: 10.1016/j.fsi.2006.10.014. [DOI] [PubMed] [Google Scholar]