Abstract
Many studies underlined the great benefits of hydrolysates used as additives in animal free media on cell culture performances. However, to precisely define hydrolysate supplementation strategies, a deeper understanding of their effect on cell growth and protein production is required. In the present study, the effect of addition of one yeast extract (YE) and two yeast peptones (named YP.A and YP.B) in a chemically defined medium was first assessed on cell culture performances. Interestingly, specific effects were found depending on the degree of degradation of yeast hydrolysates. The YE at 1 g L−1 increased the maximal cell density by 70 %, while a mixture of YE (1 g L−1) and YP.A (4 g L−1) increased IgG production by 180 %. These conditions were then evaluated on the CHO cell kinetics all over cultures. Hydrolysates extended the cell growth phase in Erlenmeyer flask and increased the maximal growth rate in bioreactor up to 20 %. Cell growth stimulation induced by hydrolysates addition was linked with energetic metabolism improvement suggesting that they promote oxidative pathway. Furthermore, hydrolysates provided an additional source of substrate that supported cell growth despite glutamine limitation.
Keywords: Yeast extract, Yeast peptone, CHO cell culture, IgG recombinant protein, Bioreactor, Kinetic studies
Introduction
Currently, mammalian cells such as Chinese hamster ovary (CHO) cells are the most common expression systems to produce recombinant monoclonal antibodies (Butler 2005). Initially, mammalian cells were grown in culture media supplemented with bovine serum, but its high cost and lot-to-lot variability encouraged the development of substitutes such as purified proteins, recombinant proteins (Froud 1999) or hydrolysates from animal proteins (Schlaeger 1996). Nevertheless, the risk of contamination by viruses, mycoplasma or prions from animal supplement led to a strong demand for media formulations free from animal components (Grillberger et al. 2009). Then, the search for culture media that most closely replicated the benefits of animal-derived sera without their biosafety risks provoked a growing interest in the use of plant peptones (Heidemann et al. 2000; Burteau et al. 2003; Farges et al. 2008) or yeast extracts (YE) (Sung et al. 2004; Kim and Lee 2009).
Yeasts are classically produced with an oxidative process. Yeast hydrolysate is used as a generic name including yeast extracts (YE) and yeast peptones (YP). More precisely, YE correspond to the water-soluble fraction of the autolyzed yeast, whereas YP represent the soluble fraction of proteins hydrolysed by exogenous enzymes. Therefore, unlike plant peptones which depend on the vegetal source, climate, geography and protein extraction process, the YE and YP production can be better controlled to reach repeatable lot-to-lot composition. In fact, the influence of three different YE lots was already assessed on recombinant CHO cell culture without showing any variation of the cell culture performances (Sung et al. 2004). Interestingly, while YE and plant protein peptones were widely used, no study reported the influence of YP on mammalian cell culture. However, not only the source of hydrolysates (Sung et al. 2004; Kim and Lee 2009), but also the manufacturing process (Chun et al. 2007) may affect cell growth and protein production. Furthermore, hydrolysates mixtures, concentrations and times of addition are also operating conditions that potentially impact cell culture (Sung et al. 2004; Chun et al. 2007; Kim and Lee 2009).
The mechanisms of hydrolysates action on cells remain unclear. On one hand, some results suggested the presence of growth factor-like and anti-apoptotic peptides from plant peptones (Burteau et al. 2003; Farges et al. 2008; Franek and Katinger 2002) or YE (Sung et al. 2004), but without experimental validations. On the other hand, other studies concluded on nutritional efficiency of plant peptones as amino acid sources (Heidemann et al. 2000; Nyberg et al. 1999). Nevertheless, the addition of nutritional as well as “bioactive” compounds may improve the efficiency of energetic cell metabolism. For instance, it was shown in kidney brush border membrane that the uptake of di- and tri-peptides by specific transporters followed by intracellular hydrolysis, could represent an energetically more efficient mechanism than the uptake of free amino acids (Daniel et al. 1992). Furthermore, addition of mineral often present in hydrolysates could also improve oxidative metabolism as shown by the addition of copper (Luo et al. 2012). Metabolic flux analyses showed that a peptone obtained from an animal source promoted the entry of pyruvate to TCA cycle (Bonarius et al. 1996). Other studies underlined that peptones improved cell growth without increasing carbon source consumption and lactate production (Ballez et al. 2004; Chun et al. 2007), while ammonia production was slightly increased (Burteau et al. 2003). Moreover, some authors underlined the potential use of lactate as a source of carbon after glucose depletion, triggered by plant hydrolysates (Burteau et al. 2003). These observations suggested that peptones could influence the cell metabolism depending on the culture phase. Nevertheless, very few works used kinetic studies to evaluate the influence of hydrolysates on the cellular energetic metabolism.
In the present study, one YE and two YP were evaluated as medium additives for the production of a monoclonal antibody by CHO cells. The content of main biomolecules in yeast hydrolysates in was first characterized to highlight their different compositions. Then, the effect of various concentrations of each yeast hydrolysate was assessed on maximal cell and IgG concentrations. Moreover, the combination of yeast hydrolysates and the time of their addition in culture were investigated to evaluate some synergistic effects on culture performances. Finally, the influence of the chosen optimal conditions were analyzed on kinetics of cell growth, death, IgG production and metabolism of CHO cells cultivated in Erlenmeyer flask as well as in bioreactor. According to the culture phase, yeast hydrolysates were shown to exhibit different effects on cell culture behaviour.
Materials and methods
Yeast hydrolysates
The yeast extract (YE) and the yeast peptones A and B (YP.A and YP.B) were provided by Bio Springer (Maisons-Alfort, France). The extract corresponds to the water-soluble fraction of the autolyzed yeasts, whereas peptones represent the soluble fraction of yeast proteins hydrolyzed after exogeneous enzyme addition. The term hydrolysate is used as a generic name including YE and YP.
Cell culture
CHO-AMW cell line producing a recombinant IgG1 anti-human RhD antibody was provided by Dr. F. Wurm (EPFL-Lausanne) (Miescher et al. 2000). Cells were adapted to grow in suspension in PF-BDM, a protein free and chemically defined medium (Schneider 1989), consisting in a 5:5:1 (v:v:v) mixture of Iscove’s MDM (Eurobio, Courtaboeuf, France), Ham’s F12 (Eurobio), and NCTC 135 (Eurobio) media. PF-BDM medium was supplemented with 750 μM ethanolamine (Sigma, Saint-Quentin Fallavier, France), 500 μM ferric citrate (Sigma) with 1/1 Fe:citrate ratio, 0.1 % PF-68 (Sigma) and 4 mM glutamine (Eurobio). CHO-AMW cells were routinely cultivated at 37 °C in 125 mL Erlenmeyer flasks (Corning, Amsterdam, The Netherlands) with 25 mL working volume and orbitally shaken at a frequency of 110 rpm. The headspace of the flasks was initially purged with a mixture of air-CO2 (95 %/5 %). The cells were split every 3–4 days and seeded at a density of 0.2 × 106 cells mL−1.
For screening and kinetics studies, CHO-AMW cells were grown in complete PF-BDM with or without hydrolysate supplementation. They were cultivated in batch mode in 500 mL Erlenmeyer flasks (Corning) with 100 mL working volume (D = 0.1 m), orbitally shaken at a frequency of 110 rpm at 37 °C using an amplitude of 30 mm. The headspace of the flasks was purged once a day with a mixture of air-CO2 (95 %/5 %). Besides, kinetics studies of CHO-AMW were also performed in a sparged and mechanically stirred tank reactor (SGI, Toulouse, France) with a working volume of 1.4 L. Agitation was provided by a pitched-blade turbine (D = 0.07 m) at 60 rpm. Dissolved oxygen concentration, pH and temperature were regulated at 50 % of air saturation, 7.4 and 37 °C respectively. The pH was regulated with a solution of sodium hydroxide.
Cell counting and metabolite analyses
Samples were harvested once or twice a day from the Erlenmeyer flask or bioreactor. They were used for immediate cell counting, then centrifuged (1,500 rpm, 5 min) and stored at −20 °C for subsequent analyses. Cell density was evaluated by using a hemacytometer, and cell viability was measured by the trypan blue dye exclusion method with 10 % accuracy (Nielson et al. 1991). Apoptosis was quantified with the Guava® cytometer by the annexin V probe (Guava Technologies, Millipore, Billerica, MA, USA). Lysed cells were estimated through LDH released in cell culture supernatant with an enzymatic kit (LDH PAP, Ellitech, Salon-de-Provence, France). Briefly, intracellular LDH content of viable CHO cells were first measured as described by Goergen et al. (1993). Lysed cell densities were then calculated as the difference between total dead cell concentration, obtained from LDH assays and dead cell concentration from trypan blue staining.
Glucose and lactate concentrations were determined by using enzymatic kits (ELITech, Puteaux, France, Biomerieux, Marcy l Etoile, France, respectively) on a Vitalab Selectra E analyzer with 5 % accuracy. Glutamine was converted into glutamate with asparaginase (Sigma) and glutamate was measured using an enzymatic method (Roche, Meylan, France) with 10 % accuracy. Ammonia concentration was measured with a selective electrode (Orion from Thermo Scientific, Illkirch, France) with 10 % accuracy. IgG titer was determined by enzyme-linked immunosorbent assay (ELISA). Goat anti-human kappa light chain IgG (Invitrogen, Carlsbad, CA, USA) was used for coating well plates and the synthesized anti-human RhD IgG1 was detected with biotin-conjugated goat anti-human gamma chain IgG (Invitrogen). The recombinant human anti-RhD IgG1 produced by CHO-AMW cells was quantified from calibration prepared with a whole human IgG (Thermo Fisher Scientific), with 15% accuracy.
Specific rates of cell growth (μ), glucose and glutamine consumption (qGlc, qGln), and lactate and ammonia production (qLac, qNH4) were calculated as described by Petiot et al. (2010).
In Erlenmeyer flask, all conditions were repeated 3 times, whereas in bioreactor only one culture was performed by condition. Nevertheless, each culture, either in bioreactor or in Erlenmeyer flask, was performed in parallel with a control culture in Erlenmeyer flask. This allowed the validation of initial cell state, reference medium preparation and Erlenmeyer flask process parameters. Finally, control culture in Erlenmeyer flask was repeated 7 times. From these data, standard deviations of each parameter and condition were calculated and added in Tables 1 and 2. Furthermore, significant statistical differences between culture conditions performed in Erlenmeyer flasks were determined using the Student t test with p < 0.05.
Table 1.
Specific cell growth rates and metabolic yields of CHO cells cultivated in Erlenmeyer flask, with or without supplementation by yeast hydrolysate
| Culture phase | Erlenmeyer flasks | ||||
|---|---|---|---|---|---|
| Control | YE 1 g L−1 | YE 1 g L−1 + YP.A 4 g L−1 | |||
| μ (h−1) | 0–45 h | 0.039 ± 0.002 | 0.039 ± 0.002 | 0.037 ± 0.002 | |
| 45–80 h | 0.011 ± 0.001 | / | / | ||
| YlgG/cells (pg cell−1) | 0–90 h | 36 ± 4 | 25 ± 3 | 54 ± 6 | |
| Ycell/glc (cell pmol−1) | 0–50 h | 0.14 ± 0.02 | 0.15 ± 0.02 | 0.18 ± 0.03 | |
| 50–90 h | 0.45 ± 0.06 | 0.65 ± 0.09 | 0.61 ± 0.09 | ||
| Ylac/glc (mM mM−1) | 0–50 h | 1.78 ± 0.04 | 1.80 ± 0.04 | 1.79 ± 0.04 | |
| Ycell/gln (cell pmol−1) | 0–70 h | 0.47 ± 0.04 | 0.84 ± 0.06 | 0.62 ± 0.05 | |
| 70–90 h | 0.47 ± 0.04 | 3.0 ± 0.2 | 3.5 ± 0.3 | ||
| YNH4+/gln (mM mM−1) | 0–90 h | 0.90 ± 0.06 | 0.90 ± 0.06 | 0.90 ± 0.06 | |
Control culture was performed without yeast hydrolysate supplementation
Table 2.
Specific cell growth rates and metabolic yields of CHO cells cultivated in bioreactor with or without supplementation by yeast hydrolysate
| Culture phase | Bioreactor | |||
|---|---|---|---|---|
| Control | YE 1 g L−1 | YE 1 g L−1 + YP.A 4 g L−1 | ||
| μ (h−1) | 0–75 h | 0.024 ± 0.001 | 0.029 ± 0.001 | 0.027 ± 0.001 |
| YlgG/cells (pg cell−1) | 0–75 h | 34 ± 4 | 38 ± 5 | 65 ± 8 |
| Ycell/glc (cell pmol−1) | 0–75 h | 0.09 ± 0.01 | 0.13 ± 0.02 | 0.13 ± 0.02 |
| Ylac/glc (mM mM−1) | 0–75 h | 1.51 ± 0.03 | 1.40 ± 0.03 | 1.15 ± 0.03 |
| Ycell/gln (cell pmol−1) | 0–75 h | 0.24 ± 0.02 | 0.40 ± 0.03 | 0.45 ± 0.03 |
| YNH4+/gln (mM mM−1) | 0–75 h | 0.50 ± 0.04 | 0.70 ± 0.05 | 0.80 ± 0.06 |
Control culture was performed without yeast hydrolysate supplementation
Characterization of yeast hydrolysates
Molecular size distribution
The molecular size distribution of the peptides contained in yeast hydrolysates was set out by analytical size exclusion high performance liquid chromatography (SE-HPLC) using a Superdex peptide column coupled to an UV detector (Mosser et al. 2012).
Total and free amino acids
The total amino acid composition of freeze-dried samples was determined after peptide hydrolysis in HCl 6 N at 110 °C for 24 h. The solutions were then cooled at room temperature, adjusted to pH 4.5 with NaOH 4 N and filtered through a membrane of 0.22 μm pore size. Amino acids were derivatized with 9-fluoroenylmethyl chloroformate and o-phthalaldehyde and analyzed by reverse phase HPLC according to the conditions previously described (Mosser et al. 2012). The amino acid concentrations were calculated from calibration curves performed with an amino acid kit (Sigma-Aldrich Co., St. Louis, MO, USA).
Carbohydrates
The carbohydrate composition of freeze-dried samples was determined after polysaccharide hydrolysis in HCl 2 N at 104 °C for 4 h. The solutions were diluted 25 times in deionized water and filtered through a membrane of 0.22 μm pore size. Then, monosaccharides were analyzed by ion exchange HPLC according to the conditions previously described (Mosser et al. 2012). The concentration of mannose and glucose, which were the main monosaccharides in yeast polysaccharides, was calculated from calibration prepared with standard solutions (Sigma-Aldrich Co.).
Nucleic acids
The nucleic acid composition of freeze-dried samples was determined after hydrolysis in 60 % HClO4 at 95 °C for 70 min. The solutions were neutralized in NH4H2PO4 (2 M) and filtered through a membrane of 0.22 μm pore size. Then, the nucleobases (adenine, cytosine, uracile, guanine, thymine and hypoxanthine) were analyzed by reverse phase HPLC according to the conditions previously described (Mosser et al. 2012). The nucleobase concentrations were calculated from calibration performed with standards (Sigma-Aldrich Co.).
Results and discussion
Operating conditions for yeast hydrolysate supplementation to improve maximal cell and IgG levels
Composition of yeast hydrolysates
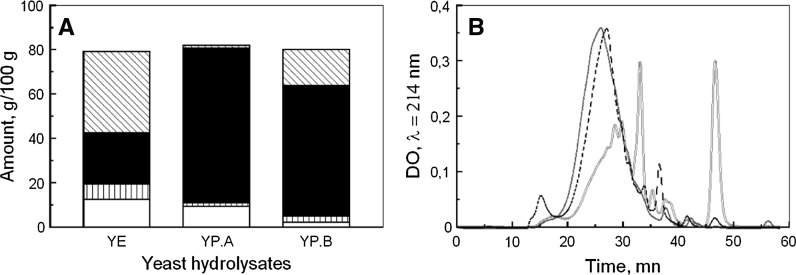
The three yeast hydrolysates were characterized by their composition in amino acids, peptides, carbohydrates and nucleic acids (Fig. 1A). YP.A and YP.B contained high amounts of total amino acids, either free amino acids or peptides, with 71 and 76 % of raw material mass, respectively, whereas total amino acid content of YE was only 60 %. Besides, YE contained 36 % of free amino acids, while YP.B and YP.A only 16 and 1 %, respectively. Furthermore, the molecular size distribution profiles of peptides underlined that YE peptides were shorter than those of peptones (Fig. 1B). Therefore, the protein degradation appeared higher in YE and gradually lower in YP.B and YP.A. On the other hand, similar quantities of carbohydrates were found in YE and YP.A with 10 and 9.5 %, respectively, but only 2 % in YP.B. Otherwise, YE exhibited a high level of nucleic acids (7 %) compared to YP.A and YP.B with only 1.5 and 3 %, respectively. Thus, the method of production led to a clear effect on the composition of the three yeast hydrolysates.
Fig. 1.
Composition of yeast hydrolysates: A carbohydrates (open bar), nucleic acids (vertical lines filled bar), peptides (bar with upper left to lower right fill) and free amino acids (filled bar) in yeast extract (YE), peptone A (YP.A) and peptone B (YP.B). B Molecular size distribution of peptides from YE (red colour solid line), YP.A (blue colour dotted line) and YP.B (dashed line)
Influence of composition and concentration of yeast hydrolysate
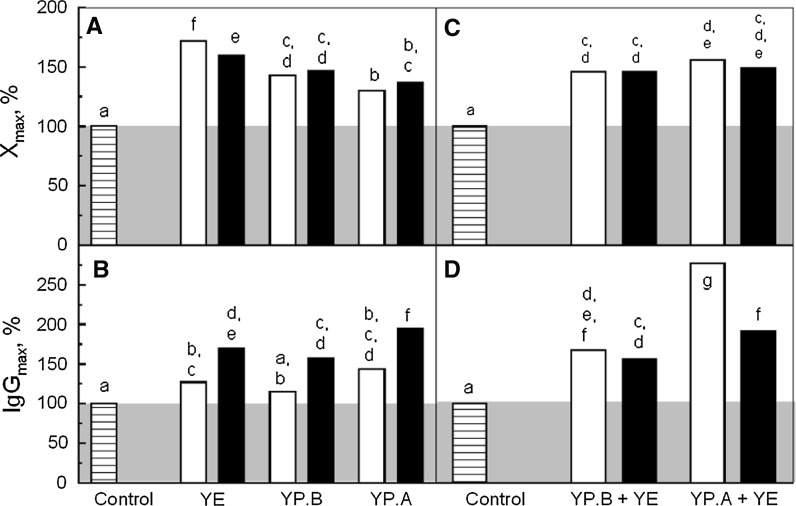
The yeast hydrolysates were used as additives in a chemically defined medium to improve CHO-AMW cell growth and IgG production. They were added at a concentration of 1 or 4 g L−1. The maximal viable cell (Xmax) and IgG (IgGmax) concentrations measured after 90 h of culture were then compared to the control culture performed without any supplementation (Fig. 2A, B).
Fig. 2.
Maximal cell concentration (A) and IgG production (B) of CHO-AMW cells cultivated in Erlenmeyer flask: without supplementation (horizontal lines filled bar), with supplementation of 1 g L−1 (open bar) or 4 g L−1 (filled bar) of YE, YP.A and YP.B. Maximal cell concentration (C) and IgG production (D) of CHO-AMW cells cultivated in Erlenmeyer flask: without supplementation (horizontal lines filled bar), with supplementation of mixture of 1 g L−1 YE and 4 g L−1 YP.A or YP.B at the seeding time (open bar) or after 40 h (filled bar). Control was performed without yeast hydrolysates supplementation. Maximal concentrations of cell and IgG were measured after 90 h of culture. The impact of yeast hydrolysates conditions of supplementation on maximal concentration of cell and IgG was first verified thanks to an analysis of variance (ANOVA). Possible significant differences between hydrolysates conditions of supplementation on the maximal concentration of cells and IgG were evaluated by least significant differences (LSD) multiple comparison tests with a confidence level of 95 %. 7 control cultures were conducted independently, while yeast hydrolysates supplemented cultures were repeated three times. The letters represent similar groups
The highest Xmax was obtained when YE was added at 1 g L−1 with an increase of 70 % compared with the control culture, while YP.B and YP.A only increased it by 50 and 30 %, respectively (Fig. 2A). These results underlined that the method of production of hydrolysates significantly impacted their influence on cell growth. Interestingly, these positive effects increased with the degree of protein degradation. Furthermore, no difference was observed between 1 and 4 g L−1 of YP.A and YP.B supplementations. Therefore, despite the higher levels of low molecular size molecules, provided by the increase of peptone concentration, the cell growth could not be improved to the same level as with YE supplementation. This suggested that YE contains specific compounds that improved cell growth better than YP.A and YP.B. In fact, the YE chromatogram showed an UV signal between 32 and 34.5 min that was not present in peptone chromatogram (Fig. 1). Therefore, YE may contain small peptides not present in peptones. On the other hand, similar values of Xmax were observed at 1 and 4 g L−1 of YE addition.
Alternatively, while IgGmax was not improved at 1 g L−1 by the addition of YE and YP.B, its level was significantly increased by the addition of hydrolysates at 4 g L−1, from 50 to 100 % compared to the control culture (Fig. 2B). The increase of medium osmolarity over 400 mOsm.kg−1 (Ju et al. 2009) and an additional amino acid level (Chua et al. 1994) have already shown a positive effect on protein production and could explain our results with hydrolysate supplementation. However, the addition of 4 g L−1 of hydrolysates only increased medium osmolarity from 300 to 320 mOsm kg−1 (data not shown). In fact, a low contribution of soy peptone at 2 g L−1 on the medium osmolarity was also reported (Michiels et al. 2011). Furthermore, the highest IgG concentration was obtained with YP.A that contained a very low concentration of free amino acids, suggesting a peptide specific effect on recombinant protein production. Thus, the effects of yeast hydrolysate supplementation on cell culture performances depend on their composition as well as their concentration. Furthermore, their effect on the cell growth and the IgG production appeared not to be correlated.
Yeast hydrolysate mixture and time of supplementation
As highest cell growth and IgG improvements were triggered by the supplementation of 1 g L−1 YE and 4 g L−1 peptones, respectively, some mixtures were assessed in order to evaluate potential synergistic effects. The peptones were added either at the beginning of the culture or after 40 h, while the YE was added at the beginning of the batch (Fig. 2C, D). The mixture of YE and YP.B did not increase Xmax, nor IgGmax compared to YP.B (4 g L−1) alone whatever the time of addition. The mixture of YE and YP.A increased Xmax by 20 % and IgGmax by 80 % compared to YP.A (4 g L−1) alone when added at the beginning of the culture. This increase in IgGmax was not observed when hydrolysates were added after 40 h, suggesting that YP.A exhibited a specific effect on IgG synthesis during the cell growth phase. Furthermore, the mixture of YE with both peptones led to a slight decrease of the cell growth by comparison with YE alone. Consequently, 1 g L−1 of YE and 1 g L−1 YE + 4 g L−1 YP.A added at the beginning of the culture were shown to be good conditions to improve cell growth and IgG production, respectively.
Effect of yeast hydrolysates on cell culture kinetics in stirred flask and bioreactor
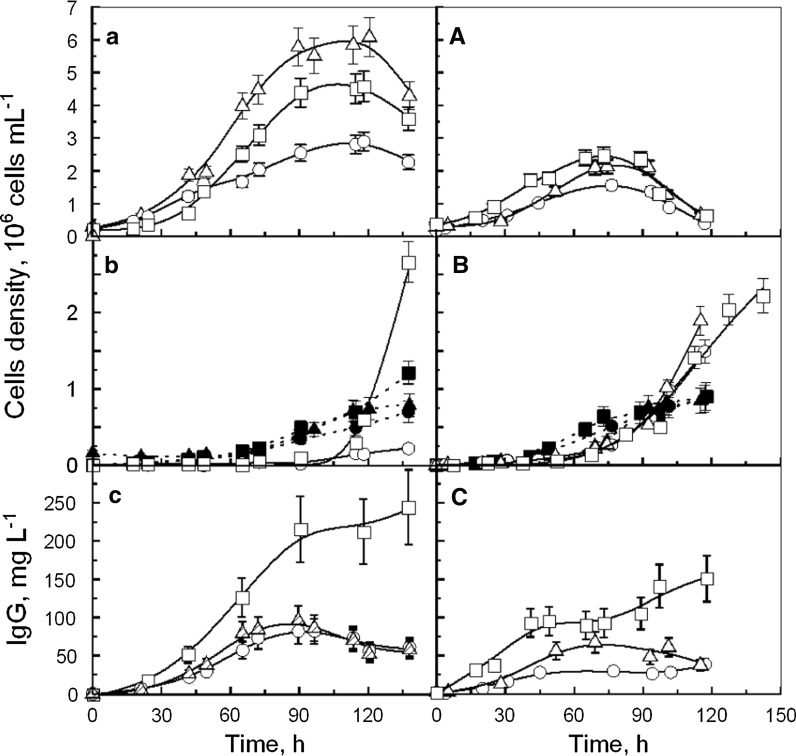
The two previous selected conditions (1 g L−1 of YE and 1 g L−1 YE + 4 g L−1 YP.A) were then used to study the kinetics of cell growth and IgG production. The cultures were performed either in Erlenmeyer flask or in bioreactor (Fig. 3).
Fig. 3.
Kinetics of CHO cells cultivated in Erlenmeyer flask (a–c) and in bioreactor (A–C): a, A viable cells; (b, B) Trypan blue dead cells (open symbols) and lysed cells (closed symbols); c, C IgG production, performed without supplementation (open circle), with 1 g L−1 YE (open triangle), or with 4 g L−1 YP.A + 1 g L−1 YE (open square)
Kinetics of CHO cell growth and death
Without hydrolysate supplementation, CHO cells cultivated in Erlenmeyer flask reached a maximal density (Xmax) of 2.9 × 106 cell mL−1 after 110 h of culture (Fig. 3a). Beside this, CHO cell cultures supplemented with YE or YE + YP.A displayed concentrations of 5.1 × 106 cell mL−1 and 4.7 × 106 cell mL−1, respectively, after only 90 h. During the first phase of culture (0–45 h) cells grew at a similar maximal specific rate of 0.04 h−1 whatever culture medium (Table 1). Several studies confirmed that maximal specific growth rates of CHO cells cultivated in Erlenmeyer flask were not enhanced by addition of YE or plant peptones (Sung et al. 2004; Burteau et al. 2003; Kim and Lee 2009). However, in the present study, the specific cell growth rate in control culture decreased from 0.04 h−1 to 0.01 h−1 during the phase from 45 to 80 h, while hydrolysates sustained its maximal value until 80 h of culture (Table 1).
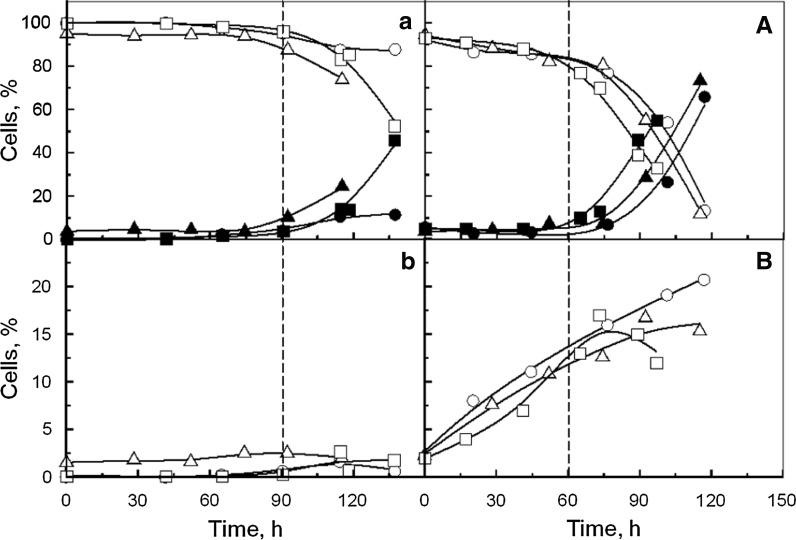
Cell death, measured by trypan blue staining, and lysis, evaluated by released LDH, occurred after 90 and 60 h of culture, respectively, whatever the culture medium (Fig. 3b). Furthermore, the analysis of early and late apoptotic cells, quantified by flow cytometry, underlined that hydrolysates did not prevent cells from apoptosis (Fig. 4a, b). Hence, the variations of the cell specific growth rate observed when hydrolysates were added, did not result from cell death prevention nor from anti-apoptotic effects, contrarily to the hypothesis proposed by other authors (Schlaeger 1996; Farges-Haddani et al. 2006; Franek and Katinger 2002; Franek 2004).
Fig. 4.
Kinetics of CHO cells cultivated in Erlenmeyer flask (a, b) and bioreactor (A, B): a, A viable cells (open symbols) and late apoptotic cells (closed symbols); b, B early apoptotic cells, performed without supplementation (open circle), with 1 g L−1 YE (open triangle), or with 4 g L−1 YP.A +1 g L−1 YE (open square)
In bioreactor, CHO cells cultivated without hydrolysates were able to grow at a maximal specific rate of 0.024 h−1 to reach a maximal density of 1.50 × 106 cell mL−1 after 75 h of culture (Fig. 3A, Table 2). Alternatively, cultures supplemented with YE or YE + YP.A displayed a maximal specific rate of 0.029 and 0.027 h−1 respectively to reach a maximal cell density of 2.20 × 106 cell mL−1 and 2.50 × 106 cell mL−1, respectively, after 75 h of culture. Therefore, hydrolysates both increased Xmax and cell specific growth rates in bioreactor cultures. However, these parameters were lower in the bioreactor than in the Erlenmeyer flask. Furthermore, higher proportions of dead and lysed cells were measured (Fig. 3B) and 10 % of late apoptotic cells were detected after only 24 h whatever the culture medium (Fig. 4B). The different hydrodynamic conditions, provoked by mechanical agitation in the bioreactor, may have induced cell death that could partially explain the change in cell behaviour between the two culture systems. The results also confirmed that yeast hydrolysates seemed not to provide any shear protecting or anti-apoptotic agents. Nevertheless, few differences of cell metabolism may also be assumed to explain these cell kinetics results.
Kinetics of IgG production
The results indicated that IgG production occurred during cell growth phase. In Erlenmeyer flask, the maximal IgG level was 80, 90 and 220 mg L−1 in control, YE and YE + YP.A culture medium, respectively, while it was 30, 60 and 120 mg L−1 in bioreactor (Fig. 3c, C). The ratio of IgG produced per cell was the same in both culture systems, in the medium without hydrolysate (≅ 35 pg cell−1) (Tables 1, 2). The addition of YE decreased this value down to 25 pg cell−1 in the Erlenmayer flask culture, without any effect in the bioreactor. On the contrary, the YP.A + YE supplementation in both culture systems increased the ratio up to 54 pg cell−1 (Erlenmeyer flask) and 65 pg cell−1 (bioreactor). These last results confirmed the positive effect of YP.A combined to YE on the IgG production. Furthermore, as no dead and no lysed cells were measured during cell growth phase, it can be assumed that YP.A increased the transcription level of IgG as previously observed by addition of YE in CHO cells culture to produce hTPO (Sung et al. 2004). Furthermore, the presence of YP.A seemed to induce a slight increase of IgG concentration during the cell death phase, whereas a slow decrease was observed without YP.A after the end of the growth phase. These results suggested an IgG degradation that can be due to protease or to sensitivity of physico-chemical parameters. In literature, the release of proteases during cell lysis was already shown to decrease the recombinant protein concentration due to proteolysis (Hansen et al. 1997). In the same time, the addition of a polypeptide (bacitracin A composed of 12 amino acids) in the culture medium was pointed out to prevent recombinant protein degradation by inhibiting protease activity. Other studies underlined that YE (Sung et al. 2004) and soy peptone (Michiels et al. 2011) did not protect recombinant protein from proteolysis. In our experiments, the presence of peptone YP.A was efficient to prevent the decrease of IgG concentration. Thus, it could be assumed that YP.A which is richer in polypeptides than YP.B and YE, may contain some protease inhibitors.
Effect of yeast hydrolysates on cell energetic metabolism
Glycolysis
In Erlenmeyer flask cultures, the glucose was consumed at a maximal specific rate (qGlc) of about 0.40 μmol h−1 10−6 cell whatever the culture medium (Fig. 5a). Lactate was produced at a maximal specific rate (qLac) of about 0.60 μmol h−1 10−6 cell and accumulated up to 20 mM. Thus, the lower cell growth rate observed in the absence of hydrolysates was not induced by lactate inhibition. During the first 45 h of culture, a high yield of lactate produced per glucose consumed (YLac/Glc) of 1.8 mol mol−1 was measured (Fig. 5b; Table 1) compared to values from 1.2 to 1.6 mol mol−1 commonly reported (Lao and Toth 1997; Farges-Haddani et al. 2006; Sung et al. 2004; Ahn and Antoniewicz 2011). Consequently, about 90 % of the glucose flow was directed toward lactate production instead of integrating the TCA cycle. During this culture phase, a low ratio between produced cells and consumed glucose (Ycell/glc) of 0.15 cell pmol−1 was observed whatever the medium (Fig. 5c; Table 1). However, from 45 h of culture, the lactate production rate decreased faster than the glucose uptake rate, resulting in lower YLac/Glc whatever the culture conditions. Furthermore, Ycell/glc was strongly increased to 0.40 and 0.60 cell p mol−1 in the control and in the hydrolysate supplemented cultures, respectively. It can thus be assumed that cell metabolism shifted from fermentative to oxidative pathway after 45 h of culture. In control culture, this metabolism shift was coupled to a decrease of the cell growth rate (from 0.04 to 0.01 h−1) (Table 1). In fact, the shift is often correlated with the end of the exponential cell growth phase (Burky et al. 2007; Wilkens et al. 2011). Interestingly, the addition of hydrolysates maintained the maximal cell specific growth rate (0.04 h−1), despite the metabolism shift. Accordingly, yeast hydrolysates seemed to provide molecules that improve the oxidative metabolism of CHO cells as already reported with meat peptone (Bonarius et al. 1996) or copper addition (Luo et al. 2012).
Fig. 5.
Kinetics of substrate consumption and metabolite production in Erlenmeyer flask (a, b and c) and bioreactor (A, B and C): a, A glucose consumption (open symbols) and lactate production (closed symbols); b, B correlation between produced lactate and consumed glucose; c, C correlation between produced cells and consumed glucose. Cultures performed without supplementation (open circle), with 1 g L−1 YE (open triangle), or with 4 g L−1 YP.A + 1 g L−1 YE (open square). Arrows represent metabolism shift
In bioreactor, whatever the culture conditions, glucose was consumed at a maximum specific rate around 0.30 μmol h−1 10−6 cell all over the cell growth phase (Fig. 5A). The depletion of glucose was correlated with the end of the cell growth phase, suggesting that it was a limiting substrate (Figs. 3A, 5A). In the control medium or in YE and YE + YP.A supplemented conditions, lactate accumulated up to 23, 22 and 21 mM, with a maximal specific production rate of 0.70, 0.60 and 0.50 μmol h−1 10−6 cell, respectively. As a consequence, the supplementation of YE and YE + YP.A led to a decrease of YLac/Glc, from 1.5 in the control medium, to 1.4 and 1.2 mol mol−1, respectively (Fig. 5B; Table 2). Furthermore, the hydrolysates addition induced an increase of Ycell/glc from 0.10 to 0.15 cell pmol−1 compared with the control culture (Fig. 5C). Therefore, hydrolysates containing amino acids and short peptides could contribute to supply the TCA cycle and support the oxidative metabolism.
On the other hand, in bioreactor cultures performed with pH control, hydrolysates improved the oxidative metabolism from the beginning of cell culture and no shift was observed during the culture. It can be noticed that the pH decrease has been already shown to decrease glucose uptake and to promote the cell oxidative metabolism (Yoon et al. 2005; Kuwae et al. 2005; Burky et al. 2007). The pH decrease occurring after 45 h in Erlenmeyer flask culture could have induced this metabolism shift. Indeed, during culture in Erlenmeyer flasks, the phenol red turned to yellow after two days, indicating a pH decrease from approximately 7.4 to 6.6. In this condition, yeast hydrolysates could maintain the cell growth phase by supplying oxidative metabolism. This may also partially explain the difference of maximal cell concentration observed between both culture systems.
Glutaminolysis
In Erlenmeyer flask cultures performed in control medium, glutamine was consumed at a maximal specific rate (qGln) of 0.06 μmol h−1 10−6 cell, while ammonia accumulated in the medium up to 5.0 mM (Fig. 6a) with a specific production rate (qNH4) below 0.10 μmol h−1 10−6 cell. Media supplemented with YE or YE + YP.A promoted an increase of qGln to 0.08 μmol h−1 10−6 cell and ammonia level reached higher values, 5.3–6.8 mM, respectively with qNH4 below 0.17 μmol h−1 106 cell. This increase of ammonia level in presence of protein hydrolysates has been previously described (Burteau et al. 2003). Therefore, the lower cell specific growth rate in the control medium could not be attributed to ammonia inhibition. This observation was consistent with a previous study that demonstrated that an ammonia concentration below 8 mM did not inhibit CHO cell growth (Hansen and Emborg 1994). The yield of produced ammonia by consumed glutamine (YNH4/Gln) was 0.9 mol mol−1 whatever the experimental condition. By contrast, the yield of cells produced by glutamine consumed (Ycell/gln) was increased by the presence of hydrolysates from 0.5 to 0.8 and 0.7 cell pmol−1 (Fig. 6b). Moreover, hydrolysates drastically increased Ycell/gln up to 3.8 cell pmol−1 after 75 h of culture, since glutamine concentration was becoming limiting in the medium (below 1 mM), suggesting that hydrolysates provided compounds that replaced glutamine to supply TCA cycle. This has already been shown with glutamine substitution by dipeptides containing glutamine (Christie and Butler 1994).
Fig. 6.
Kinetics of substrate consumption and metabolite production in Erlenmeyer flask (a, b) and bioreactor (A, B): a, A glutamine consumption (open symbols) and ammonia production (closed symbols); b, B Correlation between produced cells and consumed glutamine. Cultures performed without supplementation (open circle), with 1 g L−1 YE (open triangle), or with 4 g L−1 YP.A + 1 g L−1 YE (open square). Arrows underline the use of another source of substrate that partially replaced glutamine
Specific rates of glutamine were similar in bioreactor and in Erlenmeyer flask whatever the culture medium. In absence of hydrolysate, ammonia accumulated up to 3.9 mM (Fig. 6A), with a specific production rate below 0.10 μmol h−1 106 cells, and a YNH4/Gln of 0.5 mol mol−1 (Table 2). With supplementation by YE and YE + YP.A, ammonia increased up to 4.1 and 5.3 mM, respectively, with a specific production below 0.10 μmol h−1 10−6 cell and a YNH4/Gln of 0.7 and 0.8 mol mol−1, respectively. The higher quantity of nitrogenous source provided by hydrolysates may explain the increase of YNH4/Gln. Furthermore, hydrolysate supplementation increased Ycell/gln from 0.25 to 0.40 cell pmol−1 (Fig. 6B). These results underlined that hydrolysates may provide another nitrogen source than glutamine. However, Ycell/gln remained constant all along the cultures contrarily to the observation in Erlenmeyer flask cultures. This may be explained by the rapid exhaustion of glucose in bioreactor culture, while glutamine was first limiting in Erlenmeyer flask cultures.
Conclusion
The present work demonstrated that the most hydrolyzed yeast components, contained in YE, mainly promoted cell growth, while YP.A, richer in oligopeptides, greatly enhanced IgG productivity. In fact, the YE at 1 g L−1 increased maximal cell density by 70 %, while YP.A at 4 g L−1 increased IgG production by 100 %. A mixture of yeast hydrolysates allowed the synergy of their individual benefits leading to an increase of maximal IgG level by 180 %.
Furthermore, the kinetics of CHO cells cultivated in Erlenmeyer flask and in bioreactor led to a better knowledge of the hydrolysate effects. In the presence of hydrolysates, the cell growth phase was extended in Erlenmeyer flask, while the cell specific growth rate was improved in bioreactor. These effects on cell growth did not result from cell death prevention, but from the improvement of oxidative metabolism of CHO cells. Finally, hydrolysates provided an additional source of substrate that supported cell growth despite glutamine limitation.
Therefore, our results highlighted that hydrolysates efficiently supplied the TCA cycle that improved oxidative metabolism and CHO cell growth. Alternatively, the supplementation by YP.A significantly increased the specific rate of IgG production, without affecting the glucose and glutamine metabolism compared to the culture supplemented with YE alone, suggesting a specific effect of YP.A on IgG transcription. Furthermore, YP.A was suggested to protect IgG from proteolysis during cell death phase. Works are in progress to further analyse yeast hydrolysate molecules of interest by using a nanofiltration process. The characterization of these compounds will then allow a better understanding of the specific role of hydrolysates on cell culture performances.
Acknowledgments
The authors would like to acknowledge Dr. Florian Wurm (EPFL, Lausanne) for the supply of the recombinant CHO cell line.
References
- Ahn WS, Antoniewicz MR. Metabolic flux analysis of CHO cells at growth and non-growth phases using isotopic tracers and mass spectrometry. Metab Eng. 2011;13:598–609. doi: 10.1016/j.ymben.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Ballez JS, Mols J, Burteau C, Agathos SN, Schneider YJ. Plant protein hydrolysates support CHO-320 cells proliferation and recombinant IFN-γ production in suspension and inside microcarriers in protein-free media. Cytotechnology. 2004;44:103–114. doi: 10.1007/s10616-004-1099-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonarius HP, Hatzimanikatis V, Meeseters KP, de Gooijer CD, Schmid G, Tramper J. Metabolic flux analysis of hybridoma cells in different culture media using mass balances. Biotechnol Bioeng. 1996;50:299–318. doi: 10.1002/(SICI)1097-0290(19960505)50:3<299::AID-BIT9>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Burky JE, Wesson MC, Young A, Farnsworth S, Dionne B, Zhu Y, Hartman TE, Qu L, Zhou W, Sauer PW. Protein-free fed-batch culture of non-GS NS0 cell lines for production of recombinant antibodies. Biotechnol Bioeng. 2007;96:281–293. doi: 10.1002/bit.21060. [DOI] [PubMed] [Google Scholar]
- Burteau CC, Verhoeye FR, Mols JF, Ballez JS, Agathos SN, Schneider YJ. Fortification of a protein-free cell culture medium with plant peptones improves cultivation and productivity of an interferon-γ-producing CHO cell line. In Vitro Cell Dev Biol Anim. 2003;39:291–296. doi: 10.1290/1543-706X(2003)039<0291:FOAPCC>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Butler M. Animal cell cultures: recent achievements and perspectives in the production of biopharmaceuticals. Appl Microbiol Biotechnol. 2005;68:283–291. doi: 10.1007/s00253-005-1980-8. [DOI] [PubMed] [Google Scholar]
- Christie A, Butler M. Glutamine-based dipeptides are utilized in mammalian cell culture by extracellular hydrolysis catalyzed by a specific peptidase. J Biotechnol. 1994;37:277–290. doi: 10.1016/0168-1656(94)90134-1. [DOI] [PubMed] [Google Scholar]
- Chua FK, Yap MG, Oh SK. Hyper-stimulation of monoclonal antibody production by high osmolarity stress in eRDF medium. J Biotechnol. 1994;37:265–275. doi: 10.1016/0168-1656(94)90133-3. [DOI] [PubMed] [Google Scholar]
- Chun BH, Kim JH, Lee HJ, Chung N. Usability of size-excluded fractions of soy protein hydrolysates for growth and viability of Chinese hamster ovary cells in protein-free suspension culture. Bioresour Technol. 2007;98:1000–1005. doi: 10.1016/j.biortech.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Daniel H, Morse EL, Adibi SA. Determinants of substrate affinity for the oligopeptide/H+ symporter in the renal brush border membrane. J Biol Chem. 1992;267:9565–9573. [PubMed] [Google Scholar]
- Farges B, Chenu S, Marc A, Goergen JL. Kinetics of IFN-γ producing CHO cells and other industrially relevant cell lines in rapeseed-supplemented batch cultures. Process Biochem. 2008;43:945–953. doi: 10.1016/j.procbio.2008.04.025. [DOI] [Google Scholar]
- Farges-Haddani B, Tessier B, Chenu S, Chevalot I, Harscoat C, Marc I, Goergen JL, Marc A. Peptide fractions of rapeseed hydrolysates as an alternative to animal proteins in CHO cell culture media. Process Biochem. 2006;41:2297–2304. doi: 10.1016/j.procbio.2006.06.002. [DOI] [Google Scholar]
- Franek F. Gluten of spelt wheat (Triticum aestivum subspecies spelta) as a source of peptides promoting viability and product yield of mouse hybridoma cell cultures. J Agric Food Chem. 2004;52:4097–4100. doi: 10.1021/jf0400157. [DOI] [PubMed] [Google Scholar]
- Franek F, Katinger H. Specific Effects of Synthetic Oligopeptides on Cultured Animal Cells. Biotechnol Prog. 2002;18:155–158. doi: 10.1021/bp0101278. [DOI] [PubMed] [Google Scholar]
- Froud SJ. The development, benefits and disadvantages of serum-free media. Dev Biol Stand. 1999;99:157–166. [PubMed] [Google Scholar]
- Goergen JL, Marc A, Engasser JM. Determination of cell lysis and death kinetics in continuous hybridoma cultures from the measurement of lactate dehydrogenase release. Cytotechnology. 1993;11:189–195. doi: 10.1007/BF00749869. [DOI] [PubMed] [Google Scholar]
- Grillberger L, Kreil TR, Nasr S, Reiter M. Emerging trends in plasma-free manufacturing of recombinant protein therapeutics expressed in mammalian cells. Biotechnol J. 2009;4:186–201. doi: 10.1002/biot.200800241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen HA, Emborg C. Influence of ammonium on growth, metabolism, and productivity of a continuous suspension Chinese hamster ovary cell culture. Biotechnol Prog. 1994;10:121–124. doi: 10.1021/bp00025a014. [DOI] [PubMed] [Google Scholar]
- Hansen K, Kjalke M, Rasmussen PB, Kongerslev L, Ezban M. Proteolytic cleavage of recombinant two-chain factor VIII during cell culture production is mediated by protease(s) from lysed cells: the use of pulse labelling directly in production medium. Cytotechnology. 1997;24:227–234. doi: 10.1023/A:1007988713571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidemann R, Zhang C, Qi H, Larrick Rule J, Rozales C, Park S, Chuppa S, Ray M, Michaels J, Konstantinov K, Naveh D. The use of peptones as medium additives for the production of a recombinant therapeutic protein in high density perfusion cultures of mammalian cells. Cytotechnology. 2000;32:157–167. doi: 10.1023/A:1008196521213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju HK, Hwang SJ, Jeon CJ, Lee GM, Yoon SK. Use of NaCl prevents aggregation of recombinant COMP-Angiopoietin-1 in Chinese hamster ovary cells. J Biotechnol. 2009;143:145–150. doi: 10.1016/j.jbiotec.2009.06.017. [DOI] [PubMed] [Google Scholar]
- Kim SH, Lee GM. Development of serum-free medium supplemented with hydrolysates for the production of therapeutic antibodies in CHO cell cultures using design of experiments. Appl Microbiol Biotechnol. 2009;83:639–648. doi: 10.1007/s00253-009-1903-1. [DOI] [PubMed] [Google Scholar]
- Kuwae S, Ohda T, Tamashima H, Miki H, Kobayashi K. Development of a fed-batch culture process for enhanced production of recombinant human antithrombin by Chinese hamster ovary cells. J Biosci Bioeng. 2005;100:502–510. doi: 10.1263/jbb.100.502. [DOI] [PubMed] [Google Scholar]
- Lao MS, Toth D. Effects of ammonium and lactate on growth and metabolism of a recombinant Chinese hamster ovary cell culture. Biotechnol Prog. 1997;13:688–691. doi: 10.1021/bp9602360. [DOI] [PubMed] [Google Scholar]
- Luo J, Vijayasankaran N, Autsen J, Santuray R, Hudson T, Amanullah A, Li F. Comparative metabolite analysis to understand lactate metabolism shift in Chinese hamster ovary cell culture process. Biotechnol Bioeng. 2012;109:146–156. doi: 10.1002/bit.23291. [DOI] [PubMed] [Google Scholar]
- Michiels JF, Sart S, Schneider YJ, Agathos SN. Effects of a soy peptone on γ-IFN production steps in CHO-320 cells. Process Biochem. 2011;46:1759–1766. doi: 10.1016/j.procbio.2011.05.025. [DOI] [Google Scholar]
- Miescher S, Zahn-Zabal M, De Jesus M, Moudry R, Fisch I, Vogel M, Kobr M, Imboden MA, Kragten E, Bichler J, Mermod N, Stadler BM, Amstutz H, Wurm F. CHO expression of a novel human recombinant IgG1 anti-RhD antibody isolated by phage display. Br J Haematol. 2000;111:157–166. doi: 10.1046/j.1365-2141.2000.02322.x. [DOI] [PubMed] [Google Scholar]
- Mosser M, Kapel R, Aymes A, Bonnano LM, Olmos E, Chevalot I, Marc I, Marc A. Chromatographic fractionation of yeast extract: a strategy to identify physicochemical properties of compounds promoting CHO cell culture. Process Biochem. 2012;47:1178–1185. doi: 10.1016/j.procbio.2012.04.015. [DOI] [Google Scholar]
- Nielsen LK, Smyth GK, Greenfield PF. Haemocytometer cell count distributions: implications of non-poisson behaviour. Biotechnol Prog. 1991;7:560–563. doi: 10.1021/bp00012a600. [DOI] [Google Scholar]
- Nyberg GB, Balcarcel RR, Follstad BD, Stephanopoulos G, Wang DI. Metabolism of peptide amino acids by Chinese hamster ovary cells grown in a complex medium. Biotechnol Bioeng. 1999;62:324–335. doi: 10.1002/(SICI)1097-0290(19990205)62:3<324::AID-BIT9>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Petiot E, Guedon E, Blanchard F, Gény C, Pinton H, Marc A. Kinetic characterization of vero cell metabolism in a serum-free batch culture process. Biotechnol Bioeng. 2010;107:143–153. doi: 10.1002/bit.22783. [DOI] [PubMed] [Google Scholar]
- Schlaeger EJ. The protein hydrolysate, Primatone RL, is a cost-effective multiple growth promoter of mammalian cell culture in serum-containing and serum-free media and displays anti-apoptosis properties. J Immunol Methods. 1996;194:191–199. doi: 10.1016/0022-1759(96)00080-4. [DOI] [PubMed] [Google Scholar]
- Schneider YJ. Optimisation of hybridoma cell growth and monoclonal antibody secretion in a chemically defined, serum- and protein-free culture medium. J Immunol Methods. 1989;116:65–77. doi: 10.1016/0022-1759(89)90314-1. [DOI] [PubMed] [Google Scholar]
- Sung YH, Lim SW, Chung JY, Lee GM. Yeast hydrolysate as a low-cost additive to serum-free medium for the production of human thrombopoietin in suspension cultures of Chinese hamster ovary cells. Appl Microbiol Biotechnol. 2004;63:527–536. doi: 10.1007/s00253-003-1389-1. [DOI] [PubMed] [Google Scholar]
- Wilkens CA, Altamirano C, Gerdtzen ZP. Comparative metabolic analysis of lactate for CHO cells in glucose and galactose. Biotechnol Bioprocess Eng. 2011;16:714–724. doi: 10.1007/s12257-010-0409-0. [DOI] [Google Scholar]
- Yoon SK, Choi SL, Song JY, Lee GM. Effect of culture pH on erythropoietin production by Chinese hamster ovary cells grown in suspension at 32.5 and 37.0 degrees C. Biotechnol Bioeng. 2005;89:345–356. doi: 10.1002/bit.20353. [DOI] [PubMed] [Google Scholar]