Abstract
Photopolymerizable hydrogels offer great potential in cartilage tissue engineering due to their ability to conform to irregular defect shapes and be applied in a potentially minimally invasive manner. An important process requirement in the use of photopolymerizable hydrogels is the ability of the suspended cells to withstand low intensity ultraviolet light (UV) exposure (4–5 mW/cm2) and photoinitiator concentrations. For cartilage integration with underlying subchondral bone tissue, robust localized osteoblast activity is necessary. Yet, while it is known that osteoblasts do not respond well to UV light, limited work has been conducted to improve their survivability. In this study, we evaluated the cellular cytotoxicity of five different human cell sources at different UV exposure times, with and without a commercially used photoinitiator. We were able to confirm that human osteoblasts were the least tolerant to varying UV exposure times in comparison to bone marrow stem cell, periodontal ligament cell, smooth muscle and endothelial cell lineages. Moreover osteoblasts cultured at 39 °C did not deteriorate in terms of alkaline phosphatase expression or calcium deposition within the extracellular matrix (ECM), but did reduce cell proliferation. We believe however that the lower proliferation diminished osteoblast sensitivity to UV and the photoinitiator. In fact, the relative survivability of osteoblasts was found to be augmented by the combination of a biochemical factor and an elevated incubation temperature; specifically, the use of 50 mg/l of the anti-oxidant, ascorbic acid significantly (P < 0.05) increased the survivability of osteoblasts when cultured at 39 °C. We conclude that ascorbic acid at an incubation temperature of 39 °C can be included in in vitro protocols used to assess cartilage integration with bone ECM. Such inclusion will enhance conditions of the engineered tissue model system in recapitulating in vivo osteoblast activity.
Keywords: Cytotoxicity, Osteoblasts, Ascorbic acid, Photoinitiator, Photopolymerizable hydrogels
Introduction
Tissue engineering has been emerging as the potential approach to restoring and enhancing tissue or organ function in the health care industry (Nerem and Sambanis 1995; Nguyen and West 2002). In the musculoskeletal arena, photopolymerizable hydrogel approaches are thought to offer several advantages, namely that the geometry of defects to be filled with de novo tissue can be arbitrary, delivery may be carried out via injection (Elisseeff et al. 1999) thus permitting minimal invasiveness and finally, hydrogel materials are considerably less expensive than biodegradable fibrous scaffolds thus presenting a more cost effective procedure. In brief, a polymer mixture in monomer form is prepared, with a very low concentration of photoinitiator (0.02–0.05 % w/v) (Elisseeff et al. 2000); cells are suspended within the solution and the mixture is subsequently injected into the repair site. Next, the monomer solution is exposed to ultraviolet light (UV) at low intensity (4–5 mW/cm2) during which free radicals initiate polymerization (Williams et al. 2003). Despite their critical function, the highly mobile free radicals cause a number of adverse affects such as damage to extracellular proteins as well as cell apoptosis and necrosis events (Moan et al. 1989; Terakado et al. 1984; Atsumi et al. 1998).
Our long term goal is to provide better anchorage and fixation between tissue engineered cartilage and the underlying subchondral bone for the treatment of osteoarthritis, using a reliable engineered tissue model system for bone and cartilage. This system requires a reasonable degree of survivability of osteoblasts so that integration can occur. Previous experiments including our own experience suggests that osteoblasts ordinarily do not survive well under combined ultraviolet light and photoinitiator environments (Williams et al. 2005). This may explain the limited success that has been achieved thus far in co-relation of in vitro to in vivo results and the successful integration of engineered cartilage to bone in vitro (Wang et al. 2007).
Cartilage integration involves the following two challenges: first, the scaffold that is used initially must be harmonized in the joint; secondly, the ensuing developing tissue must anchor with the underlying bone to provide a strong fixation. We note that none of the existing techniques in cartilage tissue engineering have been able to restore a sustained articular surface because subchondral bone integration to de novo cartilage tissue via osteoblast activity at the interface has to date, not been sufficiently addressed (Buckwalter and Mankin 1998). Thus, the main objective of this study was to determine how the detrimental effects of photopolymerization parameters to human osteoblast survivability could be minimized while still permitting sufficient gelation. Primarily, these parameters were ultraviolet light (UV) exposure time and photoinitiator concentration. In addition, we included in this effort, four additional human cell sources as a means of comparison. Since our focus was targeted on osteoblasts which are traditionally known to be cultured at relatively lower temperatures (34 °C) (Bodine et al. 1996), we also examined the role of in vitro temperature incubation on osteoblast viability. Finally, we focused our efforts on the utility of the antioxidant, ascorbic acid on augmenting the survivability of the osteoblasts.
Materials and methods
Cell culture
Five different cell lines were culture expanded and comparatively evaluated as a function of varying UV intensities and photoinitiator concentrations:
Human bone marrow derived mesenchymal stem cells (BMSCs)
Human BMSCs are a well characterized population of adult stem cells that can differentiate into other cell lines to produce cartilage, bone, fat and muscle tissue. BMSCs (Science cell, Carlsbad, CA, USA) were seeded onto Poly d-lysine coated T-75 flasks (Fisher Scientific, Pittsburgh, PA, USA). The cells were cultured until passage 3 (P3) in low glucose medium [Dulbecco’s modified eagle medium, (DMEM), Invitrogen, Grand Island, NY, USA] supplemented with 10 % fetal bovine serum [(FBS), ATCC, Manassas, VA, USA] and 1 % penicillin–streptomycin (ATCC).
Human pulmonary artery endothelial cells (HPAEC)
HPAECs (Fisher Scientific) were seeded in T-75 flasks with proprietary endothelial cell growth medium (Fischer Scientific) and culture was expanded to P4.
Human pulmonary artery smooth muscle cells (HPASMC)
Human pulmonary artery smooth muscle cells (HPASMC) (Genlantis, San Diego, CA, USA) were cultured (up to P4) in T-75 flasks (Fisher Scientific) in proprietary smooth muscle cell growth medium (Genlantis).
Human osteoblasts (hFOB)
Human Osteoblasts (hFOB 1.19) (ATCC) were cultured (passage number was not provided by the depositor) in basal medium (Invitrogen) supplemented with 10 % FBS (ATCC), 1 % Penstrep (ATCC) and 0.3 mg/ml of an aminoglycoside antibiotic (Sigma Aldrich, St Louis, MO, USA).
Human periodontal ligament cells (PDLCs)
Human PDLCs (P3) were received as a gift from the University of Miami, FL (Dr. Herman Cheung). The cells were cultured in basal medium (up to P3) supplemented with 10 % FBS (ATCC), 1 % Penstrep (ATCC).
Effect of UV light at different exposure times
In order to study the effect of UV exposure times on the cells, a cell suspension (105 cells/ml) was prepared for each cell type; 200 μl was subsequently placed in each well of a 96 tissue culture well plate providing a final density of 20,000 cells/well. Cells (n = 4/group) were exposed to 4–5 mW/cm2 of long wave unfiltered UV light (365 nm) for time durations of 0, 5, 10, 15 and 20 min. These time intervals were chosen to provide an adequate dynamic range of exposure times. After exposing the wells to different time intervals, the plates were incubated at 37 °C and 5 % CO2 humidified environment for 2 days.
Effect of photoinitiator concentration on different cell lines
We used the commercially available photoinitiator, Irgacure 2959 (BASF Corporation, Florham Park, New Jersey, USA) given its extensive usage in tissue engineering research studies (Kim et al. 2009; Yang et al. 2005), and it’s relatively less toxic effect on cells. (Atsumi et al. 1998; Williams et al. 2005). Three concentrations were prepared: 0, 0.03 and 0.05 % (w/v ratios). Briefly, 50 and 100 mg of Irgacure 2959 were dissolved in 1 ml of 70 % ethanol solution. The contents were sterilized using 0.2 μm syringe filters and subsequently, 6 and 5 μl/ml of the solutions were added into 1 ml of cell solution respectively to give the eventual desired w/v ratios of 0.03 and 0.05 %. Cells were seeded in the 96 well plate as described in the UV exposure experiments (N = 4/group). The two different concentrations of photoinitiator were added in each of the respective groups and placed in an incubator for 2 days. Cells without photoinitiator exposure served as controls.
Combined effects of UV exposure times and photoinitiator concentration
The five different cell lines (BMSCs, HPAEC, HPASMC, hFOB 1.19, PDLCs) were exposed to combined Photoinitiator (0.05 % w/v ratio) and long wave UV light for different time durations of 0, 5, 10, 15 and 20 min. Well plates were housed in an incubator for 2 days to permit cell growth.
Effect of incubation temperature
We assessed the effect of incubator temperature on osteoblasts owing to the sensitivity of this cell type to this parameter. Osteoblasts were cultured in three 96 well plates. Wells in the 96 well plate were grouped into 4 wells and each group was treated with both UV light exposure of 10 min and photoinitiator concentration as 0.05 % w/v ratio. The wells without any treatment (n = 4) acted as controls. All samples were kept at 3 different incubation temperatures of 34°, 37° and 39 °C for 48 h and maintained at 5 % CO2 in a humidified environment.
Effect of temperature on the osteoblast phenotype
Alkaline phosphatase activity
The matrix maturation phase of osteoblasts is characterized by maximal expression of alkaline phosphatase (ALP) and once the mineralization is complete in the matrix, calcium deposits can be visualized in the extracellular matrix (ECM). In order to assess ALP activity (n = 3 samples/group), osteoblasts were plated using the same medium conditions as before [“Human bone marrow derived mesenchymal stem cells (BMSCs)” section] at 37 and 39 °C, via a colorimetric assay (Abcam, Cambridge, MA, USA). After 3 days of culture, 20,000 cells were taken for each sample and were lysed in 100 μl of assay buffer. Lysed cells were subsequently centrifuged at 13,000g for 3 min to remove the insoluble material. 30 μl of sample was taken in replicates of 3 for each sample in 96 well plates. Next, 50 μl of assay buffer was added to make a net volume of 80 μl in the wells. In addition, background samples were made by taking 30 μl of test samples, and adding 50 μl of assay buffer and 20 μl of stop solution. Thereafter, 50μ of 5 mM p-nitrophenyl phosphate solution was added to each well containing the test samples and background samples. The samples were covered to prevent exposure to light and the reaction was held at 25 °C for 1 h. Next the reaction was halted by the addition of 10 μl stop solution (Abcam) to each well (except to the background samples) and gently shaken. The optical density was measured per manufacturer instructions using a microplate reader (wavelength of 405 nm; model Synergy HT, Biotek Instruments, Winooski, VT, USA). For continuity in the text, ALP results are directly presented in the “Discussion” section.
Calcium distribution
As an indicator of the osteoblast maturation process during cell culture, sample calcium mineral distribution was evaluated using von-Kossa histological staining (IHC world, Woodstock, MD, USA). In brief, 20,000 cells/ml were grown on chamber slides (Fisher Scientific) and kept at the two different temperatures (37 and 39 °C) using the same medium conditions as before [“Human bone marrow derived mesenchymal stem cells (BMSCs)” section]. After 3 days, the slides were washed with phosphate buffered solution and were fixed with 10 % formalin (Fisher Scientific). Finally, the von-Kossa stain (IHC world) was applied by treating the slides with silver nitrate solution and exposing them to UV light for 1 h, which served to highlight spatial locations of calcium deposits in the specimens. For continuity in the text, histology on calcium distribution is directly presented in the “Discussion” section.
Effect of using anti-oxidants
In order to assess the benefits of anti-oxidant incorporation on osteoblast survival rate, we used 50 mg/ml of ascorbic acid in our cell culture system. Photoinitiator concentrations of 0.05 % w/v ratio were added into the selected wells. Ascorbic acid was added to the wells (n = 4) and exposed to long wave UV light for 10 min. Plates were incubated at three different temperatures of 34, 37 and 39 °C for 2 days.
Cytotoxicity evaluation using sulforhodamine B (SRB) assay
At the conclusion of each of the aforementioned experiments, a sulforhodamine B (SRB) assay (Sigma Aldrich) was performed to access cytotoxic effects of UV exposure times and photoinitiator concentrations on the different cells. The SRB assay is a measure of cell cytotoxicity as a function of proliferation rate of the surviving cells. The assay was performed similar to assays conducted previously (Tang and McGoron 2009). In brief, the assay is based on the measurement of viable cellular protein content in the cells colorimetrically (Vichai and Kirtikara 2006). Cells were first fixed to the bottom of a well plate with 10 % (wt/vol) trichloroacetic acid. After the cells were fixed, Sulforhodamine-B (SRB) dye was added for cell staining at a concentration of 0.4 % (wt/vol) which bound to viable cellular proteins. Next, 1 % (vol/vol) acetic acid was used to remove the unbound SRB dye. 10 mM Trizma-base was then used to extract the protein bound SRB. The optical density (OD) of the dye was measured (Pisco et al. 2006) at 565 nm wavelength using a microplate reader (Biotek, Winooski, VT, USA). Comparisons were made between control and treated samples. A higher OD value would translate to increased cell proliferation. Cell cytotoxicity was reported based on ‘‘Relative Survival’’ which was determined from the absorbance values (n = 4 samples/group) measured. For reporting purposes, a normalization process was carried out similar to previous studies (Williams et al. 2005). In brief, results were normalized such that the average absorbance of the control groups was equal to one; first, the average absorbance of the background wells that contained only the specific cell culture medium and the SRB dye were subtracted from each of the individual absorbance values. Next, normalization was performed by dividing each of the absorbance values by the average absorbances of the corresponding control group of cells, without any exposure to UV and photoinitiators, but otherwise grown under identical culture conditions.
Statistical analysis
Statistical analysis was performed using commercially available software (SPSS, IBM, version 20, Armonk, NY, USA). A one way ANOVA and post hoc Tukey test was used to determine statistical significance (P < 0.05) between groups in all experiments conducted. For continuity in the text, histology on calcium distribution is directly presented in the “Discussion” section.
Results
Effect of UV light at different exposure times
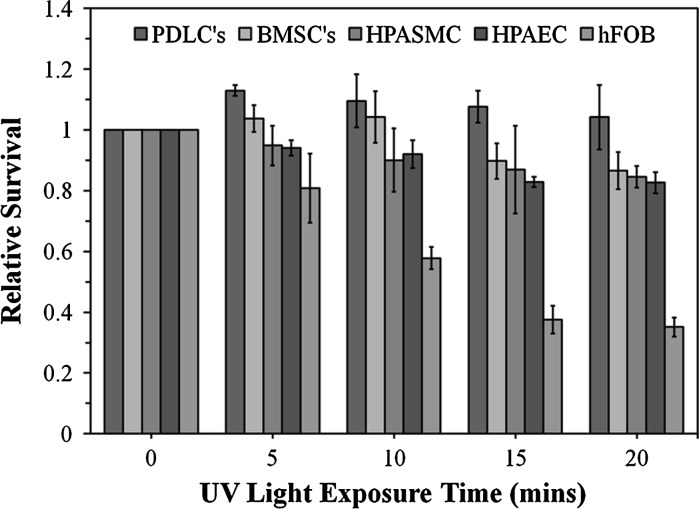
The human cell sources investigated showed altered viability after exposure to UV light (4–5 mW/cm2) (Fig. 1). The periodontal ligament cells were the most resistant to UV exposure. On the other hand, the Osteoblast survival rate considereably decreased, particularly after >10 min of UV exposure.
Fig. 1.
Cell toxicity results for human cells from different sources after different UV exposure times. Cells were incubated at 37 °C
Effect of photoinitiator concentration
Figure 2 demonstrates the survival rate of 5 different cell lines with different concentrations of photoinitiator (Irgacure 2959; 0, 0.03 and 0.05 %) and without UV exposure. In general, the effect of photoinitiator alone had little effect on the different cell types.
Fig. 2.
Cell toxicity results for human cells from different sources incubated at 37 °C for 48 h after exposure to different photoinitiator concentrations
Combined effect of UV exposure and photoinitiator
The effect of combined UV exposure and a photoinitiator concentration of 0.05 % (w/v) were assessed. This amount of photoinitiator concentration was chosen as it is very commonly used and reported in the literature (Kim et al. 2009; Yang et al. 2005; Burdick and Anseth 2002). The general trend was that of exaggerated decrease in survivability in all five cell types studied (Fig. 3) in comparison to UV exposure alone (Fig. 1). Our findings further suggested that osteoblasts were the least tolerant to the combined effect of UV light and photoinititator, ranging at ~17 % for 10 min of UV exposure and only 6 % at 20 min.
Fig. 3.
Cell toxicity results for human cells from different sources after exposure to different UV exposure times and concentrations and a fixed photoinitiator concentration of 0.05 % (w/v). Cells were incubated at 37 °C. Note that relative survivability of osteoblast was significantly reduced (P < 0.05) at all UV exposure times shown in comparison with the other cell types that were investigated
Effect of incubation temperature
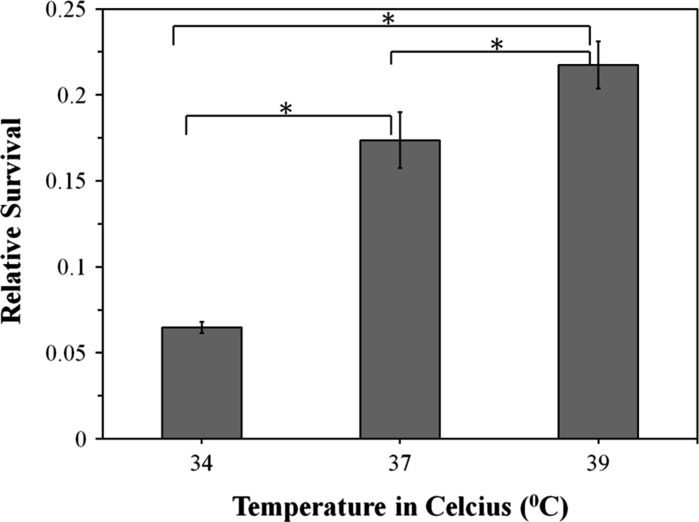
After obtaining baseline information on the variability of osteoblast viability on UV exposure times and photoinitiator concentrations in comparison to other human cell sources, we proceeded to focus on further improving osteoblast survivability within the framework of commonly utilized parameters in injectable photopolymerizable hydrogels. Namely these were UV exposure times of 10 min and a photoinitiator concentration of 0.05 % (w/v) (Yang et al. 2005; Burdick and Anseth 2002; Namba et al. 2009). We speculate that these numbers were chosen not only to ensure sufficient live cell numbers, but also to ensure sufficient gelation of the hydrogels. Human osteoblast survivability under these conditions was approximately 17 %. These experiments were conducted at 37 °C and we proceeded to augment it to 39 °C. At this higher temperature, a 5 % increase in osteoblast survivability was observed (Fig. 4).
Fig. 4.
Cell toxicity results for human osteoblasts as a function of incubator temperature. Cells were exposed for a total of 10 min under UV light and using a fixed photoinitiator concentration of 0.05 % (w/v). The “asterisk” indicates that the difference between the groups was statistically significant (P < 0.05). Consistent with previous Figs. (1–3) relative survival was based on comparisons to the normalized, average absorbances of the control group, i.e., without UV and photoinitiator
Effect of adding ascorbic acid
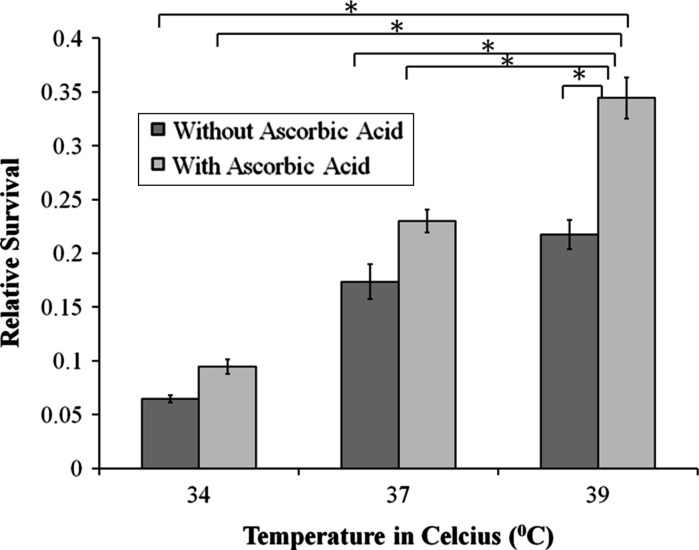
When, 50 mg/l of ascorbic acid was added to the cultured cells, incubated at 39 °C, we found that the osteoblast viability improved to ~34 %, an increase of 12 % in comparison to cultures grown at identical conditions but without the antioxidant (Fig. 5). The differences in normalized relative survivability (mean ± standard deviation) for osteoblast culture with and without ascorbic acid antioxidant at 39 °C incubation temperature was found to be significant (P < 0.05) (Table 1).
Fig. 5.
Survivability of osteoblasts with and without ascorbic acid. The “asterisk” indicates that the difference between the groups was statistically significant (P < 0.05). Consistent with previous Figs. (1–4) relative survival was based on comparisons to the normalized, average absorbances of the control group, i.e., without UV and photoinitiator
Table 1.
Osteoblast relative survivability with and without ascorbic acid antioxidant at 37 and 39 °C incubation temperatures
| Groups exposed to 0.05 % w/v photoinitiatior concentration and 10 min of UV exposure; (n = 4 samples/group) | Average | Standard deviation |
|---|---|---|
| 37 °C alone | 0.17 | 0.02 |
| 37 °C + ascorbic acid | 0.23 | 0.01 |
| 39 °C alone | 0.22 | 0.01 |
| 39 °C + ascorbic acid | 0.34 | 0.02 |
Discussion
In the past decade considerable efforts have focused on utilizing polymerizable scaffolds for tissue engineering (Mann et al. 2001; Paxton et al. 2009; Chan-Park et al. 2004; Beamish et al. 2010; Ramaswamy et al. 2006). In this context, the effects of UV exposure and photoinitiator concentrations on cell viability have always been a concern, but none more so than for osteoblasts which are extremely sensitive to ultraviolet light (Williams et al. 2005). Accordingly, we attempted to augment the survivability of human osteoblasts in these environments by exploring additional variables, namely, a higher incubation temperature and incorporation of the antioxidant, ascorbic acid. These changes may serve to improve photopolymerizable approaches in tissue engineering and outcomes related to bone-engineered tissue integration approaches (Ramaswamy et al. 2006, 2008). In this regard, on-going research efforts in our laboratories are specifically attempting to target an approach to more successfully integrate subchondral bone to tissue engineered cartilage constructs.
Osteoblasts were significantly reduced in survivability (P < 0.05) compared to all the other four human cell types (PDLCs, HPASMCs, HPAECs and BMSCs) investigated at all time durations of UV exposure (5, 10, 15 and 20 min) when in concomitant 0.05 % w/v photoinitiator environments. These results confirmed that human osteoblasts were considerably more prone to cell necrosis under combined UV light exposure and photo initiator concentrations. When osteoblasts were exposed to 10 min of UV exposure and a 0.05 % (w/v) concentration of photoinitiator at 34 °C, this lower temperature decreased cell viability. This was in contradiction to reports in the literature (Reinholz et al. 2000; Harris et al. 1995) where at 34 °C, rapid cell division was observed. We were then able to further ameliorate osteoblast survivability under photopolymerization environments (combined 10 min of UV exposure and 0.05 % (w/v) photoinitiator concentration) at an elevated temperature of 39 °C, suggesting thus, that for photopolymerization studies, osteoblasts require relatively higher, rather than reduced incubation temperatures. We proceeded to conduct statistical analysis on temperature effects of osteoblast survivability and noted that temperature alone at 39 °C augmented the cellular viability significantly (P < 0.05) in comparison to lower incubation temperatures (34 and 37 °C) (Fig. 4). This result illustrates the importance of this elevated temperature as a singular independent entity in promoting osteoblast viability. Meanwhile, anti-oxidation effects of ascorbic acid minimized free radical toxicity effects during UV polymerization and was found to be consistent with use of ascorbic acid elsewhere (Nojiri et al. 2011; Montecinos et al. 2007; Martinez et al. 2010). When ascorbic acid was combined with incubation temperatures at 39 °C, osteoblast survivability was further enhanced significantly (P < 0.05), (Fig. 5); [normalized average ± standard deviation (n = 4 samples/group) osteoblast survivability for: (a) 37 °C without ascorbic acid: 0.17 ± 0.02 (b) ascorbic acid at 37 °C: 0.23 ± 0.01, (c) elevated temperature of 39 °C without ascorbic acid: 0.22 ± 0.01, (d) ascorbic acid at 39 °C: 0.34 ± 0.02]. We speculate that the increased osteoblast survivability at the higher temperature of 39 °C can be attributed to a reduction in the proliferation rate of the cells. For example, Williams et al. (2005) showed that higher proliferation rates caused greater cell susceptibility to toxic components in photopolymerization environments i.e., (UV and photoinitiator) and thereby resulted in decreased cellular viability. Interestingly, use of either ascorbic acid or the 39 °C incubation temperature promoted roughly the same level of osteoblast survivability. However, we have for the first time demonstrated that the combined effects of ascorbic acid at a concentration of 50 mg/l and an elevated incubation of 39 °C significantly improves (P < 0.05) osteoblast survival versus using only one of either of these two parameters (Fig. 5).
Under these conditions, although the UV and photoinitator environmental conditions still considerably reduced human osteoblast survivability (owing to the innate sensitivity of osteoblasts to any changes in in vitro culture parameters) (Williams et al. 2005) in comparison to controls grown in more standard conditions, we note that we still were able to maintain ~34 % survivability; this is considerably greater than for other osteoblast groups in our study, as well as those that have been reported elsewhere (Williams et al. 2005). In addition, we note that average osteoblast-like cell densities in osteoinductive environmental conditions are in the order of roughly 240,000 cells/cm2 (Rajzer et al. 2010) whereas photopolymerizable tissue engineering studies use ~850,000 cells/cm2 (Ramaswamy et al. 2008, 2009). This amount is closer to the higher end of amounts typically used; the 66 % reduction (290,000 cells/cm2) nonetheless produces approximately the same number of osteoblast-like cell densities [240,000 cells/cm2, (Rajzer et al. 2010)] thought to be indicative for increased osteoblast activity. Thus, in spite of the greater sensitivity of osteoblasts to UV exposure time and photointiator concentrations, the addition of ascorbic acid during UV and photoinitiator exposure at an incubation temperature of 39 °C would retain a sufficient number of live osteoblast that inturn, would allow for an elevated level of cellular activity (e.g. such as osteoinduction). We noted however that an incubation temperature of 39 °C could potentially aggravate osteoblast ALP-activity and/or ECM-mineral distribution, and thus, we proceeded to look at alkaline phosphatase (ALP) expression and the presence of calcium deposits at 37 versus 39 °C. We determined that ALP expression (average ± standard deviation; n = 3 samples/group) was in the order of 2.26 ± 0.12 U/ml and 2.00 ± 0.06 U/ml for cells cultured at 37 versus 39 °C for 3 days. We believe that the relatively lower expression at 39 °C is due to the lower proliferation rate of the osteoblasts that we observed during the cell culture studies at this temperature. However the expression magnitudes at both temperatures are comparable to literature reports for ALP activity from osteoblasts that underwent similar (but not identical) cell culture, and which was found to be in the order of 2.23 ± 0.14 U/ml (Li et al. 2009). Overall therefore, we believe our findings for osteoblasts cultured at 39 °C while relatively lower than that at 37 °C, was due to the slower proliferation rate rather than a result of the cells being adversely affected.
We also assessed elevated temperature effects on the ability of the osteoblasts to synthesize calcium primarily because crystalline salts deposited in the matrix of bone are composed principally of calcium and phosphate, which are combined to form hydroxyapatite crystals and an indicator of healthy cellular activity. We confirmed that as observed during cell culture studies that at 39 °C, proliferation rate was affected and lower cell numbers were seen after 3 days of cell culture. However calcium mineral deposition was clearly visualized in regions surrounding cells in both the 37 and 39 °C conditions (Fig. 6), thereby providing preliminary evidence that the ability of osteoblasts to produce calcium is not affected at 39 °C.
Fig. 6.
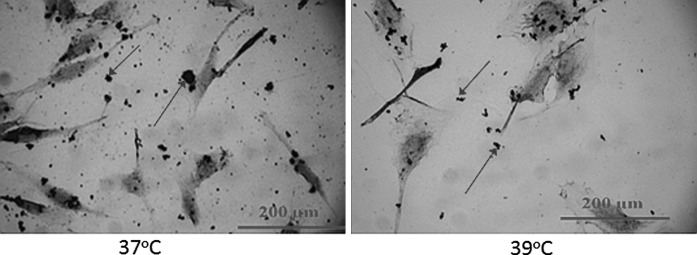
Von-Kossa staining conducted at 37 and 39 °C after 3 days of osteoblast culture. Osteoblasts appear as elongated structures while arrows point to examples of calcium deposited by the osteoblasts. Osteoblast proliferation rate was observed to be slower at 39 °C. On the other hand, this slower rate improved relative survivability of the cells, because high proliferation rates have shown to make cells more vulnerable to toxic effects (UV and photoinitiator concentrations) present in photopolymerized environments (Williams et al. 2005)
It is important to point out that the intended application of our findings is not in the clinic as cartilage tissue engineering is still an emerging field as indicated by several leading groups in the area (Prestwich 2011; Nöth et al. 2008; Elisseeff 2004); rather, these conditions can readily be used in in vitro engineered cartilage tissue model systems where experiments with injectable hydrogels are actively being conducted (Sukarto et al. 2012; Papadopoulos et al. 2011; Roberts et al. 2011; Zhang et al. 2011; Lee et al. 2008). The benefit of incorporating our findings is that controlled in vitro experiments to assess integration studies for engineered cartilage to bone extracellular matrix can be achieved without compromising osteoblast activity, a critical component to the integration process, prior to progressing to more variable in vivo models.
Conclusions
In conclusion, for studies involving cellular photopolymerization protocols, we have demonstrated that significant improvement (P < 0.05) of human osteoblasts in situ are possible by combined usage of an increased incubation temperature of 39 °C and the use of ascorbic acid at a concentration of 50 mg/l. We speculate that more improvements to osteoblast survivability may be possible by fine tuning the ascorbic acid concentration further and/or with the use of additional antioxidants such as uric acid and Beta-Carotene. A limitation of our approach was that we conducted our investigations in monolayer culture as opposed to 3-dimensional (3-D) matrices, such as hydrogels. However, we expect our results to represent a “worst case scenario” since the UV environmental conditions which severely diminish osteoblast viability were introduced by means of direct exposure, while in the case of tissue engineered 3-D constructs, cells remain suspended within a gel or scaffold material, thus offering some level of protection. We therefore recommend the implementation of the enhanced culture parameters found herein for cellular experiments that involve photopolymerizable materials and osteoblasts.
Acknowledgments
The authors gratefully acknowledge funding for this work which was provided through a RESEED award from the College of Engineering and Computing at Florida International University, Miami, FL. In addition, the authors thank Dr. Herman S. Cheung and Dr. Danny Pelaez, Department of Biomedical Engineering, University of Miami, Miami, FL, for providing us with an aliquot of human periodontal ligament derived cells.
Conflict of interest
The authors have nothing to disclose.
References
- Atsumi T, Murata J, Kamiyanagi I, Fujisawa S, Ueha T. Cytotoxicity of photosensitizers camphorquinone and 9-fluorenone with visible light irradiation on a human submandibular-duct cell line in vitro. Arch Oral Biol. 1998;43:73–78. doi: 10.1016/S0003-9969(97)00073-3. [DOI] [PubMed] [Google Scholar]
- Beamish JA, Zhu J, Kottke-Marchant K, Marchant RE. The effects of monoacrylated poly(ethylene glycol) on the properties of poly(ethylene glycol) diacrylate hydrogels used for tissue engineering. J Biomed Mater Res A. 2010;92:441–450. doi: 10.1002/jbm.a.32353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodine PV, Trailsmith M, Komm BS. Development and characterization of a conditionally transformed adult human osteoblastic cell line. J Bone Miner Res. 1996;11:806–819. doi: 10.1002/jbmr.5650110612. [DOI] [PubMed] [Google Scholar]
- Buckwalter AJ, Mankin J. Articular cartilage repair and transplantation. Arthritis Rheum. 1998;41:1331–1342. doi: 10.1002/1529-0131(199808)41:8<1331::AID-ART2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Burdick JA, Anseth KS. Photoencapsulation of osteoblasts in injectable RGD-modified PEG hydrogels for bone tissue engineering. Biomaterials. 2002;23:4315–4323. doi: 10.1016/S0142-9612(02)00176-X. [DOI] [PubMed] [Google Scholar]
- Chan-Park MB, Zhu AP, Shen JY, Fan AL. Novel photopolymerizable biodegradable triblock polymers for tissue engineering scaffolds: synthesis and characterization. Macromol Biosci. 2004;4:665–673. doi: 10.1002/mabi.200300139. [DOI] [PubMed] [Google Scholar]
- Elisseeff J. Injectable cartilage tissue engineering. Expert Opin Biol Ther. 2004;4:1849–1859. doi: 10.1517/14712598.4.12.1849. [DOI] [PubMed] [Google Scholar]
- Elisseeff J, Anseth K, Sims D, McIntosh W, Randolph M, Yaremchuk M, Langer R. Transdermal photopolymerization of poly(ethylene oxide)-based injectable hydrogels for tissue- engineered cartilage. Plast Reconstr Surg. 1999;104:1014–1022. doi: 10.1097/00006534-199909040-00017. [DOI] [PubMed] [Google Scholar]
- Elisseeff J, McIntosh W, Anseth K, Riley S, Ragan P, Langer R. Photoencapsulation of chondrocytes in poly(ethylene oxide)-based semi-interpenetrating networks. J Biomed Mater Res. 2000;51:164–171. doi: 10.1002/(SICI)1097-4636(200008)51:2<164::AID-JBM4>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Harris SA, Enger RJ, Riggs BL, Spelsberg TC. Development and characterization of a conditionally immortalized human fetal osteoblastic cell line. J Bone Miner Res. 1995;10:178–186. doi: 10.1002/jbmr.5650100203. [DOI] [PubMed] [Google Scholar]
- Kim J, Hefferan TE, Yaszemski MJ, Lu L. Potential of hydrogels based on poly(ethylene glycol) and sebacic acid as orthopedic tissue engineering scaffolds. Tissue Eng Part A. 2009;15(8):2299–2307. doi: 10.1089/ten.tea.2008.0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Yu C, Chansakul T, Hwang NS, Varghese S, Yu SM, Elisseeff JH. Enhanced chondrogenesis of mesenchymal stem cells in collagen mimetic peptide-mediated microenvironment. Tissue Eng Part A. 2008;14:1843–1851. doi: 10.1089/ten.tea.2007.0204. [DOI] [PubMed] [Google Scholar]
- Li SH, Guo DZ, Li B, Yin HB, Li JK, Xiang JM, Deng GZ. The stimulatory effect of insulin-like growth factor-1 on the proliferation, differentiation, and mineralisation of osteoblastic cells from Holstein cattle. Vet J. 2009;179:430–436. doi: 10.1016/j.tvjl.2007.10.026. [DOI] [PubMed] [Google Scholar]
- Mann BK, Gobin AS, Tsai AT, Schmedlen RH, West JL. Smooth muscle cell growth in photopolymerized hydrogels with cell adhesive and proteolytically degradable domainssynthetic ECM analogs for tissue engineering. Biomaterials. 2001;22:3045–3051. doi: 10.1016/S0142-9612(01)00051-5. [DOI] [PubMed] [Google Scholar]
- Martinez EC, Wang J, Gan SU, Singh R, Lee CN, Kofidis T. Ascorbic acid improves embryonic cardiomyoblast cell survival and promotes vascularization in potential myocardial grafts in vivo. Tissue Eng Part A. 2010;16:1349–1361. doi: 10.1089/ten.tea.2009.0399. [DOI] [PubMed] [Google Scholar]
- Moan J, Berg K, Kvam E, Western A, Malik Z, Rück A, Schneckenburger H. Intracellular localization of photosensitizers- Ciba Found Symp. 1989;146:95–107. doi: 10.1002/9780470513842.ch7. [DOI] [PubMed] [Google Scholar]
- Montecinos V, Guzmán P, Barra V, Villagrán M, Muñoz-Montesino C, Sotomayor K, Escobar E, Godoy A, Mardones L, Sotomayor P, Guzmán C, Vásquez O, Gallardo V, van Zundert B, Bono MR, Oñate SA, Bustamante M, Cárcamo JG, Rivas CI, Vera JC. Vitamin C is an essential antioxidant that enhances survival of oxidatively stressed human vascular endothelial cells in the presence of a vast molar excess of glutathione. J Biol Chem. 2007;282:15506–15515. doi: 10.1074/jbc.M608361200. [DOI] [PubMed] [Google Scholar]
- Namba RM, Cole AA, Bjugstad KB, Mahoney MJ. Development of porous PEG hydrogels that enable efficient, uniform cell-seeding and permit early neural process extension. Acta Biomater. 2009;5:1884–1897. doi: 10.1016/j.actbio.2009.01.036. [DOI] [PubMed] [Google Scholar]
- Nerem RM, Sambanis A. Tissue engineering: from biology to biological substitutes. Tissue Eng. 1995;1:3–13. doi: 10.1089/ten.1995.1.3. [DOI] [PubMed] [Google Scholar]
- Nguyen KT, West JL. Photopolymerizable hydrogels for tissue engineering applications. Biomaterials. 2002;23:4307–4314. doi: 10.1016/S0142-9612(02)00175-8. [DOI] [PubMed] [Google Scholar]
- Nojiri H, Saita Y, Morikawa D, Kobayashi K, Tsuda C, Miyazaki T, Saito M, Marumo K, Yonezawa I, Kaneko K, Shirasawa T, Shimizu T. Cytoplasmic superoxide causes bone fragility owing to low-turnover osteoporosis and impaired collagen cross-linking. J Bone Miner Res. 2011;26:2682–2694. doi: 10.1002/jbmr.489. [DOI] [PubMed] [Google Scholar]
- Nöth U, Steinert AF, Tuan RS. Technology insight: adult mesenchymal stem cells for osteoarthritis therapy. Nat Clin Pract Rheumatol. 2008;4:371–380. doi: 10.1038/ncprheum0816. [DOI] [PubMed] [Google Scholar]
- Papadopoulos A, Bichara DA, Zhao X, Ibusuki S, Randolph MA, Anseth KS, Yaremchuk MJ. Injectable and photopolymerizable tissue-engineered auricular cartilage using poly(ethylene glycol) dimethacrylate copolymer hydrogels. Tissue Eng Part A. 2011;17:161–169. doi: 10.1089/ten.tea.2010.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxton JZ, Donnelly K, Keatch RP, Baar K. Engineering the bone–ligament interface using polyethylene glycol diacrylate incorporated with hydroxyapatite. Tissue Eng Part A. 2009;15:1201–1209. doi: 10.1089/ten.tea.2008.0105. [DOI] [PubMed] [Google Scholar]
- Pisco L, Kordian M, Peseke K, Feist H, Michalik D, Estrada E, Carvalho J, Hamilton G, Rando D, Quincoces J. Synthesis of compounds with antiproliferative activity as analogues of prenylated natural products existing in Brazilian propolis. Eur J Med Chem. 2006;41:401–407. doi: 10.1016/j.ejmech.2005.10.020. [DOI] [PubMed] [Google Scholar]
- Prestwich GD. Hyaluronic acid-based clinical biomaterials derived for cell and molecule delivery in regenerative medicine. J Control Release. 2011;155:193–199. doi: 10.1016/j.jconrel.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajzer I, Menaszek E, Bacakova L, Rom M, Blazewicz M. In vitro and in vivo studies on biocompatibility of carbon fibres. J Mater Sci Mater Med. 2010;21:2611–2622. doi: 10.1007/s10856-010-4108-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswamy S, Wang DA, Fishbein KW, Elisseeff JH, Spencer RG. An analysis of the integration between articular cartilage and nondegradable hydrogel using magnetic resonance imaging. J Biomed Mater Res B Appl Biomater. 2006;77:144–148. doi: 10.1002/jbm.b.30404. [DOI] [PubMed] [Google Scholar]
- Ramaswamy S, Gurkan I, Sharma B, Cascio B, Fishbein KW, Spencer RG. Assessment of tissue repair in full thickness chondral defects in the rabbit using magnetic resonance imaging transverse relaxation measurements. J Biomed Mater Res B Appl Biomater. 2008;86:375–380. doi: 10.1002/jbm.b.31030. [DOI] [PubMed] [Google Scholar]
- Ramaswamy S, Uluer MC, Leen S, Bajaj P, Fishbein KW, Spencer RG. Noninvasive assessment of glycosaminoglycan production in injectable tissue-engineered cartilage constructs using magnetic resonance imaging. Tissue Eng Part C Methods. 2008;14:243–249. doi: 10.1089/ten.tec.2007.0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswamy S, Greco JB, Uluer MC, Zhang Z, Zhang Z, Fishbein KW, Spencer RG. Magnetic resonance imaging of chondrocytes labeled with superparamagnetic iron oxide nanoparticles in tissue-engineered cartilage. Tissue Eng Part A. 2009;15:3899–3910. doi: 10.1089/ten.tea.2008.0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinholz GG, Getz B, Pederson L, Sanders ES, Subramaniam M, Ingle JN, Spelsberg TC. Bisphosphonates directly regulate cell proliferation, differentiation, and gene expression in human osteoblasts. Cancer Res. 2000;60:6001–6007. [PubMed] [Google Scholar]
- Roberts JJ, Nicodemus GD, Greenwald EC, Bryant SJ. Degradation improves tissue formation in (un)loaded chondrocyte-laden hydrogels. Clin Orthop Relat Res. 2011;469:2725–2734. doi: 10.1007/s11999-011-1823-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukarto A, Yu C, Flynn LE, Amsden BG. Co-delivery of adipose-derived stem cells and growth factor-loaded microspheres in RGD-grafted n-methacrylate glycol chitosan gels for focal chondral repair. Biomacromolecules. 2012;13:2490–2502. doi: 10.1021/bm300733n. [DOI] [PubMed] [Google Scholar]
- Tang Y, McGoron AJ. Combined effects of laser-ICG photothermotherapy and doxorubicin chemotherapy on ovarian cancer cells. J Photochem Photobiol, B. 2009;97:138–144. doi: 10.1016/j.jphotobiol.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Terakado M, Yamazaki M, Tsujimoto Y, Kawashima T, Nagashima K, Ogawa J, Fujita Y, Sugiya H, Sakai T, Furuyama S. Lipid peroxidation as a possible cause of benzoyl peroxide toxicity in rabbit dental pulp–a microsomal lipid peroxidation in vitro. J Dent Res. 1984;63:901–905. doi: 10.1177/00220345840630061801. [DOI] [PubMed] [Google Scholar]
- Vichai V, Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoc. 2006;1:1112–1116. doi: 10.1038/nprot.2006.179. [DOI] [PubMed] [Google Scholar]
- Wang DA, Varghese S, Sharma B, Strehin I, Fermanian S, Gorham J, Fairbrother DH, Cascio B, Elisseeff JH. Multifunctional chondroitin sulphate for cartilage tissue–biomaterial integration. Nat Mater. 2007;6:385–392. doi: 10.1038/nmat1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CG, Kim TK, Taboas A, Malik A, Manson P, Elisseeff J. In vitro chondrogenesis of bone marrow-derived mesenchymal stem cells in a photopolymerizing hydrogel. Tissue Eng. 2003;9:679–688. doi: 10.1089/107632703768247377. [DOI] [PubMed] [Google Scholar]
- Williams CG, Malik AN, Kim TK, Manson PN, Elisseeff JH. Variable cytocompatibility of six cell lines with photoinitiators used for polymerizing hydrogels and cell encapsulation. Biomaterials. 2005;26:1211–1218. doi: 10.1016/j.biomaterials.2004.04.024. [DOI] [PubMed] [Google Scholar]
- Yang F, Williams CG, Wang DA, Lee H, Manson PN, Elisseeff J. The effect of incorporating RGD adhesive peptide in polyethylene glycol diacrylate hydrogel on osteogenesis of bone marrow stromal cells. Biomaterials. 2005;26:5991–5998. doi: 10.1016/j.biomaterials.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Zhang C, Sangaj N, Hwang Y, Phadke A, Chang CW, Varghese S. Oligo(trimethylene carbonate)-poly(ethylene glycol)-oligo(trimethylene carbonate) triblock-based hydrogels for cartilage tissue engineering. Acta Biomater. 2011;7:3362–3369. doi: 10.1016/j.actbio.2011.05.024. [DOI] [PubMed] [Google Scholar]