Abstract
The mouse retina constitutes an important research model for studies aiming to unravel the cellular and molecular mechanisms underlying ocular diseases. The accessibility of this tissue and its feasibility to directly obtain neurons from it has increased the number of studies culturing mouse retina, mainly retinal cell suspensions. However, to address many questions concerning retinal diseases and protein function, the organotypic structure must be maintained, so it becomes important to devise methods to transfect and culture whole retinas without disturbing their cellular structure. Moreover, the postmitotic stage of retinal neurons makes them reluctant to commonly used transfection techniques. For this purpose some published methods employ in vivo virus-based transfection techniques or biolistics, methods that present some constraints. Here we report for the first time a method to transfect P15-P20 whole murine retinas via nucleofection, where nucleic acids are directly delivered to the cell nuclei, allowing in vitro transfection of postmitotic cells. A detailed protocol for successful retina extraction, organotypic culture, nucleofection, histological procedures and imaging is described. In our hands the A-33 nucleofector program shows the highest transfection efficiency. Whole flat-mount retinas and cryosections from transfected retinas were imaged by epifluorescence and confocal microscopy, showing that not only cells located in the outermost retinal layers, but also those in inner retinal layers are transfected. In conclusion, we present a novel method to successfully transfect postnatal whole murine retina via nucleofection, showing that retina can be successfully nucleofected after some optimization steps.
Keywords: Retina, Mice, Nucleofection, Organotypic culture
Introduction
Transfection of nucleic acids into cells has become a central pillar of cell and molecular biology research. For example, transfection of small interfering and short hairpin RNAs constitutes a widely used technology to knock-down target proteins in live cells, unravelling the functions of such proteins (Dykxhoorn and Lieberman 2006; Sippl et al. 2011). The transfection of cells with tagged proteins allows the determination of their subcellular location (e.g. Su et al. 2012), and the expression of mutated forms of wild proteins help to determine the specific function of some domains (e.g. McDowall et al. 2010) or their binding to other proteins (e.g. Ning et al. 2012). The expression of recombinant proteins under the control of a cell-specific promoter allows the characterization of their role in a cell-specific manner (Montana et al. 2011; Cai et al. 2010), or the production of promising therapeutic proteins such as immunoglobulins (Inoue et al. 2010). Although the potential of transfection is enormous, transfection of many primary cells present many technical challenges.
Successful transfection and culture of tissues such as the retina without disturbing their organotypic structure is of outstanding interest, because transfected cells can be easily identified by their position in the retinal layers. Unfortunately, tissue transfection has shown to be more difficult than primary cell transfection, because cells located deep inside the tissue are not easily accessible. Moreover, some cells constituting these tissues (e.g. retinal neurons) are postmitotic cells, a fact that makes transfection even more difficult. Some of these constraints can be overridden by in vivo transfection using virus-based techniques, but these methods involve technical difficulties, along with high costs and biosafety concerns, making in vitro transfection techniques more attractive for certain studies such as electrophysiological recordings on transfected retinas (Koizumi et al. 2007). This underlines the importance of the development of in vitro techniques that allow the transfection of tissues containing post-mitotic cells.
Several transfection methods for neuronal cells exist, and they are usually grouped into four categories (Karra and Dahm 2010): electrical, chemical, physical and virus-based techniques. Electrical techniques include electroporation and nucleofection. In the case of electroporation the nucleic acid of interest enters the cytoplasm by cell exposure to electrical pulses; some authors successfully employed it for in vivo and in vitro transfection of newborn and embryonic mouse retinas (Matsuda and Cepko 2004; Donovan and Dyer 2006). Nucleofection is a modified form of electroporation developed by Amaxa Biosystems (Cologne, Germany), where cells are resuspended in a patented cell-specific buffer together with the nucleic acid of interest and electroporated under specific electrical parameters (see Methods for more information). This technique allows the delivery of nucleic acids directly into the cell nuclei, and therefore this method could be interesting for postmitotic neuron transfection, in opposition to other conventional transfection methods, where cellular division is required to achieve successful transfection. Thus, with this technique primary cells and hard-to-transfect cell lines can be transfected after identification of the proper buffer and the optimum electroporation parameters. Nucleofection is currently becoming widely used in neuroscience because some difficult-to-transfect primary cells, such as retinal ganglion cells (Leclere et al. 2005), neural progenitor cells (Dieterlen et al. 2009) or hippocampal neurons (Zeitelhofer et al. 2009) have been successfully transfected with this method. Among chemical techniques one of the most widely used is lipofection, where nucleic acids are complexed with lipids allowing the fusion of the complexes nucleic acid–lipid to the cell membrane, but this method does not work adequately in postmitotic cells. Biolistics (gene gun) is a promising physical transfection technique, where nanoparticles are coated with the nucleic acid of interest and shot onto the tissue, so the nucleic acid can transfect cells located inside the tissue (Moritoh et al. 2010). However, this technique lacks cell-type transfection specificity, so promoter-driven transfection could not be possible with biolistics; the only possibility could be optimizing the height of the gene gun and helium pressure to specifically shoot a very narrow cell layer, but this requires several optimization steps (Christianson and Lo 2011). Finally, virus-based techniques include the transduction of living cells with recombinant viruses, based on their infective capacity and their tropism, allowing in vivo transfection. Although having high rates of success, this technique is time-consuming, expensive and requires biosafety 2 level facilities.
Several authors have described protocols for in vitro transfection of whole murine retinas. Moritoh et al. (2010) successfully transfected adult murine retinas (>P35) by means of biolistics, but gene gun over the ganglionic side of the retina only allowed the transfection of cells situated on the retinal surface, and cells situated in the innermost regions of the retina were not transfected (Moritoh et al. 2010; Christianson and Lo 2011). Other studies (Matsuda and Cepko 2004; Montana et al. 2011) employed explant square wave electroporation in microchambers for transfection of embryonic and newborn explants of murine retinas, but these results showed either low numbers of transfected cells in adult murine retina (Matsuda and Cepko 2004) or that only photoreceptors were transfected (Montana et al. 2011).
In the present study we report a protocol for nucleofection and culture of P15-P20 whole murine retinas which yields transfected cells while maintaining the organotypic retinal structure. The difference with other in vitro published protocols is that in our case we achieve the transfection not only of surface cells but also of other cells located in the deep layers of the tissue, opening possibilities to further study the structure and function of retinal cells, with an inexpensive, easy, reproducible, and safe method. To our knowledge, this is the first reported protocol with these characteristics. The feasibility and reproducibility of nucleofection, together with its simplicity in terms of operation, make our protocol of great interest for in vitro retina research.
Materials and methods
C57BL/6 mice were purchased from Harlan Laboratories Models (Indianapolis, IN, USA). All animal protocols were performed according to the Directive 2010/63/EU on the protection of animals used for scientific purposes, and were approved by the University of Santiago de Compostela’s bioethics committee. C57BL/6 mice were housed in a specific pathogen free (SPF) unit in the animal facilities of the University of Santiago de Compostela.
Hank’s balanced solution (HBSS), phosphate buffered saline (PBS), foetal bovine serum (FBS), penicillin, streptomycin and Lipofectamine-2000 were purchased from Invitrogen (Life Technologies, New York, NY, USA). Ames medium (with l-glutamine, without sodium bicarbonate), paraformaldehyde and 4′,6-diamidino-2-phenylindole (DAPI) were purchased from Sigma–Aldrich (St. Louis, MO, USA). Ames medium was prepared following the manufacturer’s instructions and subsequently supplemented to reach final concentrations of 10 % FBS, 100 U/mL penicillin and 100 μg/mL streptomycin (complete Ames medium). Aqueous mounting medium (Mount Quick Aqueous) was purchased from Biooptica (Milano, Italy).
Retina extraction
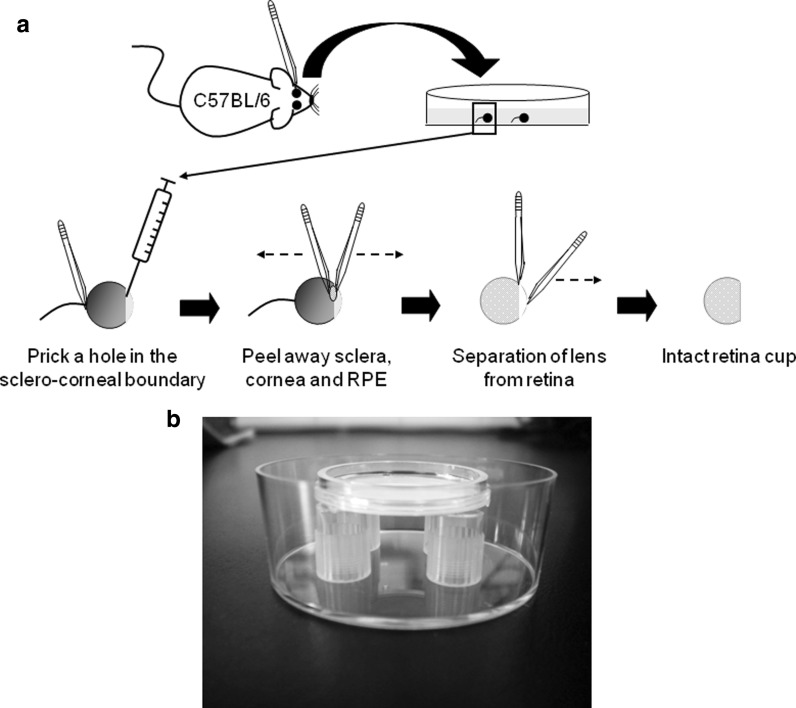
The dissection procedures described below do not need to be performed under a culture hood, but all solutions must be sterile. The retina extraction was made under a surgical microscope (model OM-5, Takagi: Manchester, UK) based on previously published protocols (Donovan and Dyer 2006) with some modifications. Mice on postnatal day 15 or 20 (P15-P20) were sacrificed by CO2 inhalation followed by cervical dislocation. The mouse head was cleaned with 70 % ethanol, and the eyes were extracted from the orbits by using small forceps. Eyes with the optic nerve still attached to them were transferred to a 50 mm Petri dish containing room temperature HBSS (Invitrogen, Life Technologies. New York, NY, USA). All the non-ocular tissue surrounding the eye was removed with fine forceps. A small hole was made in the sclero-corneal boundary with the tip of a 27-gauge needle while holding the optic nerve with a forceps. Sclera and cornea were grasped with two forceps by introducing one prong from each forceps through the hole. Sclera, retinal pigmented epithelium and cornea were peeled away taking care not to damage the retina. The optic nerve was also cut off. The lens was separated from the retina and the intact retinal cup was transferred to a new 50 mm Petri dish containing fresh HBSS (Fig. 1a).
Fig. 1.
a Diagram showing the procedures for retina extraction. C57BL/6 mice are sacrificed and eyes, maintaining the optic nerve, are removed and placed on a Petri dish containing HBSS. A small hole is pricked in the sclero-corneal junction with a needle while holding the eye by the optic nerve. Sclera and cornea are peeled away with two forceps, and the lens is separated from the retina. b Image showing the home-made stands inside a deep Petri dish, and the organotypic insert placed on the stands. When the retina is properly attached to the insert the culture medium is added to the dish until it reaches the filter by capillarity, the dish is capped and retinas are cultured at 37 °C and 5 % CO2
Nucleofection
For nucleofection we used the Amaxa Basic Nucleofector Kit for primary mammalian neurons, the supplied pmaxGFP vector and the Nucleofector II device, following the company′s technical support recommendations (Lonza. Cologne, Germany). For each transfection 2 or 4 μg of pmaxGFP vector were added to the nucleofector solution at room temperature reaching a final volume of 100 μL. One intact retinal cup was introduced into the supplied Amaxa certified cuvette with forceps, leaving the cup stuck to the cuvette wall. The DNA/solution mixture (100 μL) was carefully added over the retinal cup, softly pulling it with the tip of the p-100 micropipette until it reached the bottom of the cuvette. The retina and the DNA/solution mixture were incubated for 10 min at room temperature before nucleofection, the cuvette was introduced into the Nucleofector II device, the proper nucleofector program selected and the retinas electroporated under the program’s specific electric parameters. There are several available nucleofection programs in the device, all of them with unique electrical parameters that are patented by Amaxa. All buffer compositions are also under patent. Different nucleofector programs were tested: A-33, C-13, G-13, O-03, O-05, T-030, V-001, Y-005 and D-024. Immediately after transfection 500 μL of pre-warmed complete Ames medium were added to the cuvette.
Organotypic culture
After nucleofection the retinal cup was carefully taken out the cuvette and placed on a 50 mm Petri dish containing room temperature HBSS. Each retinal cup was flattened by making four incisions in the periphery with a scalpel under the surgical microscope. Then the flattened retina was transferred with forceps onto a Millicell Organotypic Culture Insert (Millipore: Darmstadt, Germany) with the ganglionar cell layer side up. A maximum of two retinas were cultured on the same insert. Gentle suction was softly applied to the insert just underneath the retina to ensure proper attachment of the tissue to the filter. All the procedures described below took place in a cell culture hood under aseptic procedures.
The retinal explants were cultured following a modified protocol from Moritoh et al. (2010). In our case home-made stands were made by cutting the base of p-1000 tips (1 cm high), and sterilizing them by autoclaving. Four of these stands were positioned in the periphery of a deep Petri dish (50 mm diameter, 20 mm height. Sterilin, ThermoFisher Scientific: Walthman, USA) and filled with complete Ames medium. The insert was placed on the stands, and complete Ames medium was slowly added to the dish until the liquid reached the bottom of the insert by capillarity (20 mL approximately). Retinas were incubated for 3 days at 37 °C and 5 % CO2 without disturbing the dish; during this time there was no need to change the medium or re-feed the dish. Figure 1b shows the arrangement of the insert on the home-made stands and inside the deep Petri dish. A maximum of two retinas were cultured in the same insert.
Histological procedures
All the following histological procedures took place without separating the retina from the filter, as we observed that the tissue is damaged during such manipulation; the presence of the filter does not interfere with the subsequent steps of inclusion, cryosectioning, staining and image acquisition (no blue, green or red fluorescence is emitted by the filter).
The insert containing the retina was washed twice with PBS: 2 mL were added under the insert and 1 mL was added on the top of it, taking care not to dispense the solution directly on the retina. After 1 min the PBS was discarded. Then the retina was fixed with 3 mL of freshly-prepared 4 % paraformaldehyde dispensed as described above for PBS, for 1 h at room temperature in the dark.
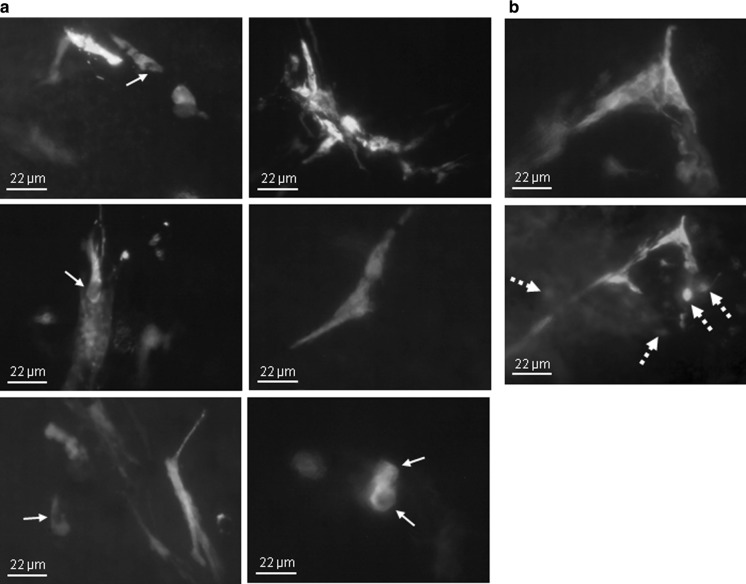
After two washes with PBS the retina was cryopreserved by incubation with 30 % sucrose in PBS for 1 h at room temperature. During the cryopreservation the whole mount retinas were imaged under the epifluorescence microscope (IX70 microscope, with a U-RFL-T power supply unit and a DP50 camera. Olympus: Tokyo, Japan) to identify the regions containing transfected cells (Fig. 3); images were analyzed with Viewfinder Lite and Studio Lite software (Olympus).
Fig. 3.
a Representative epifluorescence flat-mount images of nucleofected areas of whole murine retina (A-33 program was used). Some transfected cells have elongations spreading along the retina, while some other cells do not show elongations, indicating that different cell types have been transfected. Arrows indicate cell nuclei. b Two images taken from the same region of a nucleofected retina obtained by moving up and down the micrometer head of the microscope, showing that transfected cells are present in several retinal layers. Discontinuation arrows show transfected cells that are seen only when the micrometer head is moved
After washing twice with PBS the region of the insert containing the transfected regions of retina was cut off under the epifluorescence microscope with a scalpel and included in Tissue-Tek O.C.T. compound (Sakura Finetek Europe B.V., Leiden, Holland) on a cryomold, checking under the surgical microscope that the filter containing the retina was situated horizontally and in the centre of the cryomold. Preparations were frozen at −20 °C, and maintained at this temperature until sectioned at 30 μm thickness using a cryostat (CM 1850 UV model from Leica Microsystems GmbH: Wetzlar, Germany) and dried overnight. Then cryosections were stained for 1 h with 0.5 μg/mL of DAPI, washed, dried overnight and mounted with aqueous mounting medium.
Analysis of cryosections
Cryosections were first visualized under the epifluorescence microscope. DAPI staining allowed the identification of the retinal layers, and the maxGFP green fluorescence showed the transfected cells. The images were analyzed with the Viewfinder Lite and Studio Lite software. For a more detailed examination, maxGFP-positive cryosections were further visualized by confocal microscopy at the microscopy service of the University of Santiago de Compostela under a Leica TCS-SP2 confocal spectral microscope, with a 405 nm diode laser for DAPI and a 488 nm argon laser for maxGFP, employing the objective HCX PL APO CS 63 x/1.32 OIL. Confocal images were analyzed with the LAS AF Lite software.
Results and discussion
In this work we describe a method to transfect postnatal whole murine retina, allowing the transfection not only of the cells of the most superficial retinal layers, but also of those located deeply inside the tissue, preserving the possibility of performing promoter-driven transfection. In our case we observed transfected cells up to approximately 50 μm. As far as we know, no studies describing nucleofection in tissues have yet been published, mainly because this technique was originally developed and optimized for use with cell suspensions. It has been successfully used for the transfection of primary neural cell suspensions such as human neural progenitor cells (Dieterlen et al. 2009), adult rat retinal ganglional cells (Leclere et al. 2005) or hippocampal neurons (Zeitelhofer et al. 2009). Here we evaluated the feasibility of transfecting whole postnatal (P15-P20) murine retinas via nucleofection using the pmaxGFP plasmid, a vector that encodes the maxGFP green fluorescent protein, under the control of the cytomegalovirus (CMV) promoter.
The first step was to optimize the protocol for the extraction of retinal cups from P15-P20 C57BL/6 mice, because in the case of non-weaned pups (i.e. P21 or less) retinas were more fragile and usually broke up into several fragments when employing a standard protocol, so we needed to devise an alternative method to isolate these young retinas. Several methods to isolate rodent retinas have been described, such as those described by Skeie et al. (2011); Maminishkis and Miller (2010); Montana et al. (2011), or Donovan and Dyer (2006). In our hands, the latter showed to be the best in extracting intact retinal cups from P15-P20 mice, but with the slight modification of not cutting the optic nerve. As the eye is a sphere and tends to roll along the dish, holding it by the optic nerve while pricking the hole in the sclera-corneal junction made the dissection and isolation of the retina in these young animals easier (Fig. 1a), and retinas were always intact.
In combination with the extraction we also sought a convenient culture protocol. Recently, several authors published methods to successfully culture whole retinas by using organotypic tissue culture methods, in rabbit (Koizumi et al. 2007; Lye et al. 2007), rat (Moritoh et al. 2010) and mice (Moritoh et al. 2010; Montana et al. 2011). All of them underline the difficulties of setting organotypic culture from mouse retina, in comparison to rabbit, due to the elevated intra-retinal vascularity in rodent retina that impairs the survival of the tissue (Zhang. 1994). Nevertheless, Moritoh’s protocol (Moritoh et al. 2010) succeeded in using these methods in adult mice retinas (P49-P56), so we decided to evaluate this method for the in vitro culture of P15-P20 mice retinas. For this, we prepared home-made stands from p-1000 tips that proved to be very convenient, because they are inexpensive, easy to make, autoclavable, reusable and they are available in every laboratory (Fig. 1b). The establishment of their height as 1 cm tall allowed us to reduce the volume of medium to 20 mL. In contrast to the original method we did not need to shake the medium during the culture, and it was supplemented with bovine serum instead of horse serum. As Moritoh et al. (2010) showed, applying gentle suction just underneath the retina prior to culture proved to be critical, as the retinas that did not follow this step lost their organotypic structure at the end of the culture period (data not shown). Although effective transfection was seen after 48 h, we decided to incubate the retinas for a further 24-h period to ensure that all the transfected cells had enough time to express maxGFP. This time period could differ depending on the cell type and the protein to be expressed.
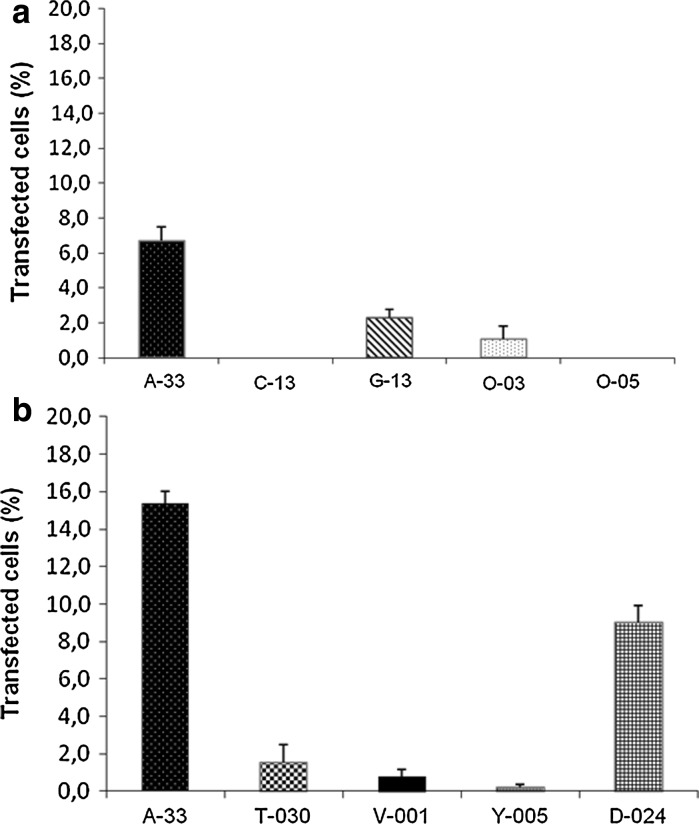
To transfect the retinas we first tested 5 different nucleofector programs (A-33, C-13, G-13, O-03 and O-05) employing 2 μg of pmaxGFP per retina, following the manufacturer’s instructions. As can be seen in Fig. 2a, the most efficient program was A-33, with an efficiency of 6.7 %. Given this low efficiency and following the manufacturer’s technical advice, the amount of DNA was doubled (4 μg per retina) to assess if a higher concentration of DNA could increase the efficiency of transfection, and stronger and longer protocols (T-030, V-001, Y-005 and D-024) were tested and compared with the best “weak” program, A-33. We observed that A-33 continued to be the best program for whole murine retinas, even when compared with the stronger and longer programs, and that the doubling in the DNA amount led to the increase of the efficiency (15.4 %). To a lesser extent program D-024 showed an acceptable efficiency (9.0 %). Three samples were nucleofected with each of the programs tested, with the exception of programs C-13, O-03 and Y-005, where only two samples were nucleofected.
Fig. 2.
a Transfection efficiency (percentage of transfected cells determined in flat-mount retinas) of several nucleofector programs using 2 μg of pmax-GFP vector. b Transfection efficiency when the DNA quantity is increased to 4 μg. Three samples were nucleofected with each of the programs tested, with the exception of programs C-13, O-03 and Y-005, where only two samples were nucleofected. Error bars show standard deviation
Leclere et al. (2005) found that both O-03 and G-13 programs successfully transfected retinal ganglion cells in suspension, being the G-13 program the one that showed the best performance. Since these authors did not use the program A-33 we cannot make comparisons with our results, but in our case both O-03 and G-13 programs showed poorer results compared to A-33 (Fig. 2a). Zeitelhofer et al. (2009) also employed the O-03 program for hippocampal neurons showing that, as in our experiments, this program shows poorer efficiency in comparison with other nucleofector programs (Fig. 2a). Dieterlen et al. (2009) used the A-33 program with neural progenitor cells, and although being the most efficient program A-33 showed worse long-term results than O-05. We failed to find transfected cells with the O-05 program in three independent experiments (Fig. 2a), but it must be taken into account that neural progenitor cell’s physiology differs from that of postmitotic neurons contained in postnatal retina. Following technical support recommendations we increased the quantity of DNA from 2 to 4 μg of pmax-GFP, causing the improvement of efficiency for A-33 program. In conclusion, it seems possible that the transfection efficiency of the programs is dependent on the postnatal time point, the cell type, the arrangement (suspension or whole tissue) of the cell sample and the quantity of DNA.
Figure 3a shows epifluorescence images of flat-mount whole retinas after the 3-day culture period and before freezing and cryosectioning. Most transfected cells appear as interconnected groups of cells, showing high numbers of elongations, while some other cells show no ramifications (low-right panel), indicating that different cell types are transfected via nucleofection. The nuclei of some cells can be clearly distinguished (arrows in Fig. 3a). Moreover, these transfected cells are present in several retinal layers, as can be noted by moving up and down the micrometer head of the microscope (Fig. 3b). This led us to believe that nucleofection allows the transfection not only of cells from the most superficial layers, but also of cells situated deep in the retina, e.g. horizontal cells, making this method of outstanding interest when trying to transfect different retinal cell types while maintaining the organotypic structure.
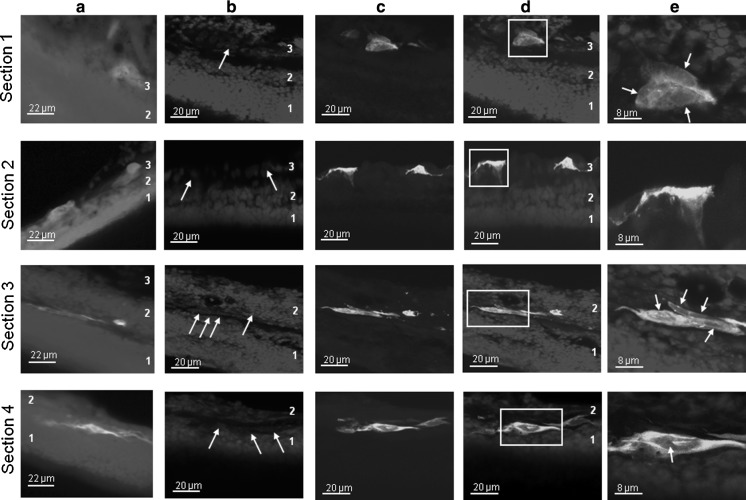
To determine the location of the transfected cells in the retina transfected retinal regions were cryosectioned, stained with DAPI to allow identification of nuclei and analyzed under epifluorescence and confocal microscopes. Figure 4a shows epifluorescence images of transfected cells from four representative sections (1, 2, 3, 4), and it can be noted that transfected cells are located not only in the retinal surface, but also in the inner regions of the retina (up to 50 μm). To further study the location and morphology of these transfected cells, the same sections were imaged by confocal microscopy (Figs. 4b–4e). Figure 4b shows DAPI fluorescence, allowing us to identify the retinal cell layers: the outer nuclear layer (1), the inner nuclear layer (2) and the ganglion cell layer (3). This confirms that the organotypic structure of retinas has been maintained throughout the process. When DAPI and GFP fluorescence are merged we can identify the green fluorescence areas as transfected cells (Fig. 4d); this is confirmed when further magnification is employed: in Fig. 4e some of the transfected cells are shown, and nuclei and cell ramifications are seen, confirming that cells with different morphology and up to the inner nuclear layer have been successfully transfected. It is interesting to note that some transfected cells usually appear as groups of 6-8 cells that seem to establish connections among them and emit prolongations along the outer plexiform layer.
Fig. 4.
Fluorescence images of transfected retina (4 representative cryosections. A-33 program was used) a Epifluorescence images of transversal cryosections from nucleofected retina, showing transfected cells. The blue fluorescence for section 1 appears saturated to favour visualizing the nuclei. b-e Same cryosections as in a but using confocal microscopy for imaging (Z-stacked images). b DAPI fluorescence, showing that the organotypic structure of the retinas has been maintained. Arrows indicate the nuclei of the transfected cells. c GFP fluorescence. d Merged image obtained by combining images b and c: transfected areas are identified as cells. e Cells boxed in d showing detailed cell morphologies. White arrows indicate nuclei. Note the tight contact between transfected cells in section 3. Numbers in images indicate retinal layers: 1, outer nuclear layer; 2, inner nuclear layer; 3, ganglion cell layer. In some images for sections 3 and 4 the ganglion cell layer is out of the microscope′s field of vision
As stated before, in vitro transfection techniques are frequently preferred to in vivo approaches for certain retinal studies like electrophysiological recordings (Koizumi et al. 2007); moreover, they are technically easier, quicker and safer. Among these in vitro techniques biolistics has been successfully employed to transfect murine retinas, but only cells located in the retinal surface where transfected (Moritoh et al. 2010). Moreover, this technique lacks cell-type transfection specificity, is expensive and can cause cell injury. Electroporation has shown to be the best approach for in vitro transfection of murine retinas, because cell-specific transfection is feasible and optimization is easier when compared to biolistics (Matsuda and Cepko 2004); however, these authors only where able to show efficient in vitro transfection on newborn mice (P0), and when older retinas where tested few positive cells were detected (Matsuda and Cepko 2004).
In our case we have been able to transfect cells located deeply inside the retina (up to 50 μm), improving the results obtained by biolistics, and have shown that transfection of retinas older than P0 (in this case P15-P20) is feasible by means of nucleofection. Thus, our method would be of interest for in vitro transfection of postnatal murine retinas, allowing the study of the physiology of cells located in the innermost regions of retina, cells that showed to be difficult to transfect by other commonly employed methods as biolistics or conventional electroporation. Retina nucleofection could be useful in the area of retina research to study the function of candidate genes of retinal diseases, the role of several transcription factors on retina function or for electrophysiology studies, while allowing the study of the innermost retinal cells (e.g. horizontal cells), among others.
In conclusion, we have developed a novel combined method to extract, culture and transfect postnatal (P15-P20) whole murine retina, showing that this method drives the transfection not only of cells situated on the surface of the retina but also of those located in the innermost regions. This method represents a good starting point in developing further improvements in retina nucleofection, and could open new possibilities to in vitro whole-retina research.
Acknowledgments
We are grateful to María Álvarez Bérmudez for her valuable support during this project and to Clara Álvarez for the use of the Nucleofector II Device. This work was supported by MCINN, Spain, (BFU-2010-14968), Xunta de Galicia, Spain, (10PXIB208126PR), NanoBioMed Consolider-INGENIO 2010 and FEDER.
Conflict of interest
All authors have no conflicts of interest.
References
- Cai X, Conley SM, Cheng T, Al-Ubaidi MR, Naash MI. A 350 bp region of the proximal promoter of Rds drives cell-type specific gene expression. Exp Eye Res. 2010;91:186–194. doi: 10.1016/j.exer.2010.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson MG, Lo DC. Development of a low-pressure microtargeting biolistic device for transfection of retinal explants. Mol Vis. 2011;17:2947–2955. [PMC free article] [PubMed] [Google Scholar]
- Dieterlen MT, Wegner F, Schwarz SC, Milosevic J, Schneider B, Busch M, Römuss U, Brandt A, Storch A, Schwarz J. Non-viral gene transfer by nucleofection allows stable gene expression in human neural progenitor cells. J Neurosci Methods. 2009;178:15–23. doi: 10.1016/j.jneumeth.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Donovan SL, Dyer MA. Preparation and square wave electroporation of retinal explant cultures. Nat Protoc. 2006;1:2710–2718. doi: 10.1038/nprot.2006.454. [DOI] [PubMed] [Google Scholar]
- Dykxhoorn DM, Lieberman J. Knocking down disease with siRNAs. Cell. 2006;126:231–235. doi: 10.1016/j.cell.2006.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue Y, Tsukamoto Y, Yamanaka M, Nakamura S, Inoue A, Nishino N, Kawahara H. Efficient production of recombinant IgG by metabolic control and co-expression with GLUT5 in a fructose-based medium. Cytotechnology. 2010;62:301–306. doi: 10.1007/s10616-010-9289-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karra D, Dahm R (2010) Transfection techniques for neuronal cells. J Neurosci 30:6171–6177 [DOI] [PMC free article] [PubMed]
- Koizumi A, Zeck G, Ben Y, Masland RH, Jakobs TC. Organotypic culture of physiologically functional adult mammalian retinas. PLoS ONE. 2007;2:e221. doi: 10.1371/journal.pone.0000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclere PG, Panjwani A, Docherty R, Berry M, Pizzey J, Tonge DA. Effective gene delivery to adult neurons by a modified form of electroporation. J Neurosci Methods. 2005;142:137–143. doi: 10.1016/j.jneumeth.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Lye MH, Jakobs TC, Masland RH, Koizumi A (2007) Organotypic culture of adult rabbit retina. J Vis Exp, e190, doi:10.3791/190 [DOI] [PMC free article] [PubMed]
- Maminishkis A, Miller SS. Experimental models for study of retinal pigment epithelial physiology and pathophysiology. J Vis Exp. 2010;45:2032. doi: 10.3791/2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda T, Cepko CL. Electroporation and RNA interference in the rodent retina in vivo and in vitro. Proc Natl Acad Sci USA. 2004;101:16–22. doi: 10.1073/pnas.2235688100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowall A, Svensson L, Stanley P, Patzak I, Chakravarty P, Howarth K, Sabnis H, Briones M, Hogg N. Two mutations in the KINDLIN3 gene of a new leukocyte adhesion deficiency III patient reveal distinct effects on leukocyte function in vitro. Blood. 2010;115:4834–4842. doi: 10.1182/blood-2009-08-238709. [DOI] [PubMed] [Google Scholar]
- Montana CL, Myers CA, Corbo JC. Quantifying the activity of cis-regulatory elements in the mouse retina by explant electroporation. J Vis Exp. 2011;52:2821. doi: 10.3791/2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritoh S, Tanaka KF, Jouhou H, Ikenaka K, Koizumi A. Organotypic tissue culture of adult rodent retina followed by particle-mediated acute gene transfer in vitro. PLoS ONE. 2010;5:e12917. doi: 10.1371/journal.pone.0012917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning X, Sun S, Zhang K, Liang J, Chuai Y, Li Y, Wang X. S100A6 protein negatively regulates CacyBP/SIP-mediated inhibition of gastric cancer cell proliferation and tumorigenesis. PLoS ONE. 2012;7:e30185. doi: 10.1371/journal.pone.0030185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sippl C, Bosserhoff AK, Fischer D, Tamm ER. Depletion of optineurin in RGC-5 cells derived from retinal neurons causes apoptosis and reduces the secretion of neurotrophins. Exp Eye Res. 2011;93:669–680. doi: 10.1016/j.exer.2011.08.011. [DOI] [PubMed] [Google Scholar]
- Skeie JM, Tsang SH, Mahajan VB. Evisceration of mouse vitreous and retina for proteomic analyses. J Vis Exp. 2011;50:2795. doi: 10.3791/2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Z, Li R, Song X, Liu G, Li Y, Chang X, Li C, Huang D. Identification of a novel isoform of DHRS4 protein with a nuclear localization signal. Gene. 2012;494:161–167. doi: 10.1016/j.gene.2011.12.033. [DOI] [PubMed] [Google Scholar]
- Zeitelhofer M, Karra D, Vessey JP, Jaskic E, Macchi P, Thomas S, Riefler J, Kiebler M, Dahm R. High-efficiency transfection of short hairpin RNAs-encoding plasmids into primary hippocampal neurons. J Neurosci Res. 2009;87:289–300. doi: 10.1002/jnr.21840. [DOI] [PubMed] [Google Scholar]
- Zhang HR. Scanning electron-microscopic study of corrosion casts on retinal and choroidal angioarchitecture in man and animals. Prog Ret Eye Res. 1994;13:243–270. doi: 10.1016/1350-9462(94)90012-4. [DOI] [Google Scholar]