Abstract
Valproic acid (VPA) as a broad-spectrum inhibitor of histone deacetylase, has been used in cancer therapy. Recently, the combination of VPA with other anticancer agents has been considered as a useful and necessary strategy to inhibit tumor growth and progression. The coumarin derivates from natural plants have been shown to be the promising natural anticancer agents. However, no literature is available on the anticancer effects of the combination of VPA and coumarin-3-carboxylic acid (HCCA). Here we show that this combination significantly increases inhibitory effects against the proliferation and migration in highly-metastatic lung cancer cells by inducing apoptosis and cell cycle arrest as well as regulating related protein expressions. Our results indicate that this combination of VPA with HCCA not only enhances the protein levels of Bax, cytosolic cytochrome c, caspase-3 and PARP-1 but also reduces the protein expressions of Bcl-2, cyclin D1 and NF-κB as well as inhibits the phosphorylation and expressions of Akt, EGFR, VEGFR2 and c-Met in the cancer cells. Our results suggest that the combination of VPA with HCCA suppresses the proliferation and migration of lung cancer cells via EGFR/VEGFR2/c-Met-Akt-NF-κB signaling pathways; this combination may have a wide therapeutic and/or adjuvant therapeutic application in the treatment of lung cancer.
Keywords: Valproic acid, Coumarin-3-carboxylic acid, Lung cancer cells, Proliferation and migration, EGFR/VEGFR2/c-Met-Akt-NF-κB pathways
Introduction
Lung adenocarcinoma is the leading cause of cancer-related deaths in the world (Jemal et al. 2008, 2011; Siegel et al. 2011, 2012). It is estimated that there will be more than 226,000 new cases and about 160,000 deaths from lung & bronchus cancer in the US in 2012 (Siegel et al. 2012). Despite recent advances in diagnosis and treatment, overall 5-year survival of lung cancer patients is only about 15 %. The patients with advanced disease have a median survival of approximately 10 months when treated with standard platinum-based therapy. Hence, there is an imminent need for better therapies and/or anticancer agents for lung cancer. Valproic acid (VPA, 2-propylpentanoic acid) is an established drug in the long-term therapy of epilepsy. During the past years, it has become evident that valproic acid is also associated with anticancer activity. Valproic acid not only suppressed some tumor growth and metastasis, but also induced tumor differentiation in vitro and in vivo (Blaheta and Cinatl 2002). Valproic acid inhibited the in vitro and in vivo cancer cell growth of melanoma (Landreville et al. 2012), urinary bladder cancer (Byun et al. 2009; Ozawa et al. 2010), breast cancer (Munster et al. 2009), and prostate cancer (Chou et al. 2011). Recently, the combination of valproic acid with other anticancer agents has been considered as a useful and necessary strategy to inhibit tumor growth and progression (Byun et al. 2009; Munster et al. 2009; Ozawa et al. 2010; Chou et al. 2011). The coumarin derivates from natural plants have been shown to be the promising natural anticancer agents. There are some reports showing that some coumarin derivates have enhanced effects with anticancer drugs against the cell growth of gastric cancer (Kim et al. 2012), bladder cancer (Rassouli et al. 2011), and leukemia (Lin et al. 2009). However, no literature is available on the anticancer effects of the combination of valproic acid and coumarin-3-carboxylic acid (HCCA) whose chemical structure is shown in Fig. 1a. In order to understand the effects of combination of VPA and HCCA on proliferation and migration of highly-metastatic lung cancer cells and its molecular mechanisms of action, we investigated the effects of combination of VPA and HCCA on apoptosis, cell cycle distribution, and migration as well as the related receptor-mediated signaling pathways in the cancer cells.
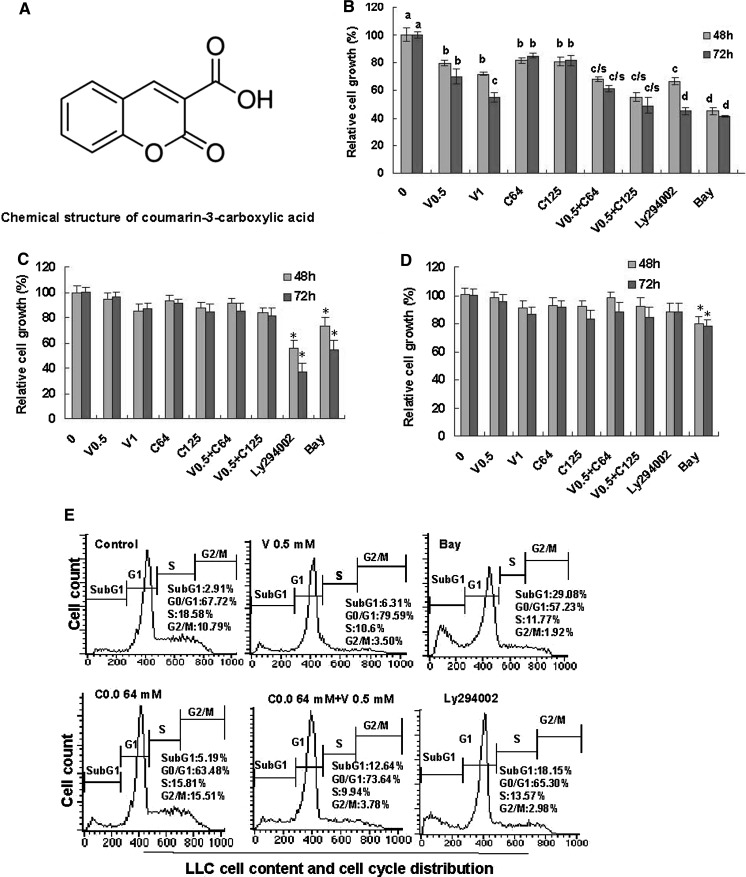
Fig. 1.
Effects of combination of sodium valproate (VPA) and coumarin-3-carboxylic acid (HCCA) on proliferation and induction of apoptosis and cell cycle arrest in LLC cells as well as the growth of NIH3T3 and EA.hy926 cells. a Chemical structure of HCCA. b Inhibition of the LLC cell growth by combination of VPA and HCCA. c Effect on NIH3T3 cell growth. d Effect on EA.hy926 cell growth. The cells were treated for 48 and 72 h with the indicated concentrations of VPA/V and HCCA/C (V0.5/0.5 mM, V1/1 mM, C64/0.064 mM, C125/0.125 mM), Ly (0.0125 mM), and Bay (0.0025 mM). The cells in control group (0) were treated with DMSO. The relative cell growth (%) was determined by the MTT assay. Ly294002 (Ly) and Bay are the inhibitors of PI3K/Akt and NF-κB, respectively. e Induction of apoptosis (cells in Sub-G1 phase) and cell cycle arrest in LLC cells by combination of VPA and HCCA was analyzed by flow cytometry. The cells were treated for 48 h with VPA (0.5 mM), HCCA (0.064 mM), Bay (0.0025 mM) and Ly294002 (0.0125 mM) as the positive control, and combination of VPA (0.5 mM) and HCCA (0.064 mM). The data are presented as the mean ± SD (Bar) (n = 6). The figures are the representative of 3 similar experiments performed. Values with different letters (a–d) differ significantly (P < 0.05). c/s represents the significant enhanced effects (P < 0.05) compared with VPA or HCCA alone. *P < 0.05
Materials and methods
Chemicals and antibodies
The primary antibodies to mouse Bcl-2, Bax, caspase-3, PARP-1, cytochrome c, cyclic D1, nuclear factor (NF-κB p-65), Akt, pAkt, and β-actin were purchased from Cell Signaling Technology Inc. (Beverley, MA, USA). The primary antibodies to mouse pVEGFR2, VEGFR2, pEGFR, EGFR, p-c-Met, and c-Met were purchased from Santa Cruz Technology Inc. (Santa Cruz, CA, USA). Fibronectin and Boyden chambers were purchased from BD Inc. (BD Biosciences, San Jose, CA, USA) and Corning Inc. (Corning, NY, USA), respectively. DMEM, penicillin, streptomycin, fetal bovine serum (FBS), trypsin/EDTA, propidium iodide, Ly294002 (Ly), Bay 11-7082 (Bay), 3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT), sodium valproate (VPA), coumarin-3-carboxylic acid (HCCA), and all other chemicals were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
Cell culture and in vitro proliferation assay
The highly-metastatic Lewis lung cancer (LLC) cell line, the normal mouse fibroblast NIH3T3, and the normal human umbilical endothelial vein cells (EA.hy926) were obtained from the American Type Culture Collection. The cell lines of LLC, NIH3T3 and EA.hy926 were cultured in DMEM containing 10 % heat-inactivated fetal bovine serum (FBS), glutamine (2 mM), penicillin (100 U/ml) and streptomycin (100 μg/ml) at 37 °C in a humidified incubator with 95 % air/5 % CO2 atmosphere. The in vitro assay was performed according to our published methods (Liu et al. 2009; Luan et al. 2010; Zhang et al. 2000; Jiang et al. 2010). Cells were plated in 96-well plates (Becton–Dickinson, Franklin Lakes, NJ, USA) at 2 × 103 per well and incubated overnight to allow attachment. The cells in control group were treated with DMSO [0.1 % (v/v), final concentration]. The cells were respectively incubated in DMEM medium supplemented with 10 % FBS containing different concentrations of sodium valproate (VPA, 0.5–1 mM), coumarin-3-carboxylic acid (HCCA, 0.064–0.125 mM), or in combination of VPA and HCCA, or Ly294002 (Ly, 0.0125 mM), Bay (0.0025 mM). Ly and Bay are the inhibitors of PI3K/Akt, and NF-κB, respectively. After 48 and 72 h of treatment, 20 μL of MTT (5 mg/ml) was added to each well of cells, and the plate was incubated for 3 h at 37 °C. One hundred and fifty microliters DMSO were added to each well to lyse the cells. Absorbance was measured at a wavelength of 570 nm and a reference wavelength of 630 nm using a Synergy H4 Hybrid Multi-Mode Microplate Reader (Bio-Tek, Instruments, Inc., Winooski, VT, USA). Absorbance values in each test group were normalized to the values of the control group treated with 0.1 % (v/v) DMSO to determine the relative cell growth (%) of LLC, NIH3T3 and EA.hy926 cell lines. Here the absorbance values are designated as the relative cell growth (%) of these cell lines. The relative cell growth (%) of these cell lines in the control group was designated as 100 %. Each experiment was performed in triplicate and repeated at least three times.
Flow cytometry for cell cycle analysis and apoptosis
These were done according to our published methods (Wang et al. 2009; Yu et al. 2009; Zhang et al. 2000, 2012a, b). In brief, LLC cells were treated for 48 h with sodium valproate (VPA, 0.5 mM), coumarin-3-carboxylic acid (HCCA, 0.064 mM), or in combination of VPA (0.5 mM) and HCCA (0.064 mM). The cells in control group were treated with DMSO [0.1 % (v/v), final concentration]. The treated cells were detached in PBS/2 mM EDTA, centrifuged at 300xg for 5 min, and then gently resuspended in 250 μL of hypotonic fluorochrome solution (PBS, 50 μg propidium iodide, 0.1 % sodium citrate, and 0.1 % Triton X-100) with RNase A (100 units/ml). The function of the fluorochrome solution is to stain cell nuclei. The DNA content was analyzed by flow cytometry FACS Vantage SE (Becton–Dickinson, San Jose, CA, USA). Twenty-thousand events were analyzed per sample and the cell cycle distribution and apoptosis were determined based on DNA content and the Sub-G1 cell population, respectively.
In vitro migration assay
Tumor cell migration was measured by examining cell migration through fibronectin-coated polycarbonate filters, using modified transwell chambers. In brief, LLC cells (5 × 104) were seeded onto the upper chamber in 200 μL of serum-free medium containing different concentrations of sodium valproate (VPA, 0.125–0.5 mM), coumarin- 3-carboxylic acid (HCCA, 0.008–0.016 mM), or in combination of VPA and HCCA; the lower compartment was filled with 0.66 mL of DMEM medium supplemented with 10 % of FBS (as a chemoattractant). The cells in control group were treated with DMSO (0.1 %, final concentration). After incubation for 6 h at 37 °C, the cells that migrated to the lower surface of the filter were fixed and stained using propidium iodide. The cells on the upper side of the filter were removed using a rubber scraper. The migrated cells on the underside of the filter were counted and recorded for images under a fluorescent microscope (Nikon, TE2000-U, Japan). Experiments were performed in triplicate.
Western blot analysis
This was performed according to our published methods with some modifications (Wang et al. 2009; Yu et al. 2009; Zhang et al. 2009, 2012a, b; Jiang et al. 2010). In brief, LLC cells were treated with different concentrations of sodium valproate (VPA, 0.5–1 mM), coumarin-3-carboxylic acid (HCCA, 0.064–0.125 mM), or in combination of VPA and HCCA, or Ly294002 (Ly, 0.0125 mM), Bay (0.0025 mM). The cells in control group were treated with DMSO [0.1 % (v/v), final concentration]. The treated cells were collected either at 30 min for detection of phosphorylation ratios of pVEGFR2/pVEGFR2, pEGFR/EGFR, p-c-Met/c-Met, and pAkt/Akt, or at 48 h for detection of protein expressions of Bcl-2, Bax, procaspase/caspase-3, PARP-1, cyclin D1, NF-κB (p65), and cytosolic cytochrome c. The cells were harvested and washed with PBS. Whole cellular proteins were extracted and cytosolic fractions were prepared following the procedure described by the manufacturer (Beyotime Institute of Biotechnology, Haimen, Jiangsu, China). The protein concentration of the extracts was determined using the Bradford method. Equal amounts of cell extracts were resolved by SDS-PAGE, transferred to nitrocellulose membranes, and probed with the primary antibodies to the detected proteins mentioned above, and then horseradish peroxidase-conjugated secondary antibodies, respectively. Anti-β-actin antibody was used as a loading control. Detection was done using an enhanced chemiluminescence system (GE Healthcare Life Sciences, Piscataway, NJ, USA).
Statistical analysis
The data are expressed as mean ± standard deviation (SD) and analyzed by the SPSS 13.0 software to evaluate the statistical difference. Statistical analysis was done using the ANOVA and Bonferroni test. Values between different treatment groups at different times were compared. The rate (%) of cell viability and mean protein levels are shown for each group. For all tests, P values <0.05 were considered statistically significant. All statistical tests were two-sided.
Results and discussion
Combination of sodium valproate (VPA) and coumarin-3-carboxylic acid (HCCA) enhances the proliferation inhibition and induction of apoptosis and cell cycle arrest in LLC cells
We first investigated the effects of combination of sodium valproate (VPA) and coumarin-3-carboxylic acid (HCCA) on the in vitro proliferation of highly-metastatic LLC. The MTT assay confirmed that the combination of HCCA and VPA significantly increased the inhibition of LLC cell growth after the cells were treated with VPA (0.5–1 mM) and HCCA (0.064–0.125 mM) for 48 and 72 h, respectively (Fig. 1b). In the same concentrations, the combination of HCCA and VPA did not show significant inhibition of the growth of the normal mouse fibroblast NIH3T3 (Fig. 1c) and the normal human umbilical endothelial vein cells (EA.hy926) (Fig. 1d). However, the inhibitor of PI3K/Akt (Ly294002, 0.0125 mM) and NF-κB (Bay, 0.0025 mM) significantly inhibited the growth of LLC (Fig. 1b) and NIH3T3 cells (Fig. 1c). The inhibitor of NF-κB (Bay, 0.0025 mM) rather than the inhibitor of PI3K/Akt (Ly294002, 0.0125 mM) exhibited significant inhibition of EA.hy926 cell growth (Fig. 1d). Furthermore, the FACS analysis showed that VPA (0.5 mM) and HCCA (0.064 mM) induced apoptosis and cell cycle arrest at G1 phase (in the case of VPA treatment) and G2 phase (in the case of HCCA treatment) in LLC cells, respectively, after the cells were treated for 48 h. The apoptotic ratio of LLC cells was 12.64 % in the treatment by combination of VPA and HCCA while the apoptotic ratios of LLC cells were 6.31 and 5.19 %, respectively, in the individual treatment by VPA and HCCA (Fig. 1e). The enhancement of the apoptotic ratio of LLC cells by combination of VPA and HCCA could greatly contribute to the additive effect of combination of VPA and HCCA on the proliferation inhibition of LLC cells, as compared with the influence of individual VPA and HCCA (Fig. 1b).
Combination of VPA and HCCA enhances the migration inhibition in LLC cells
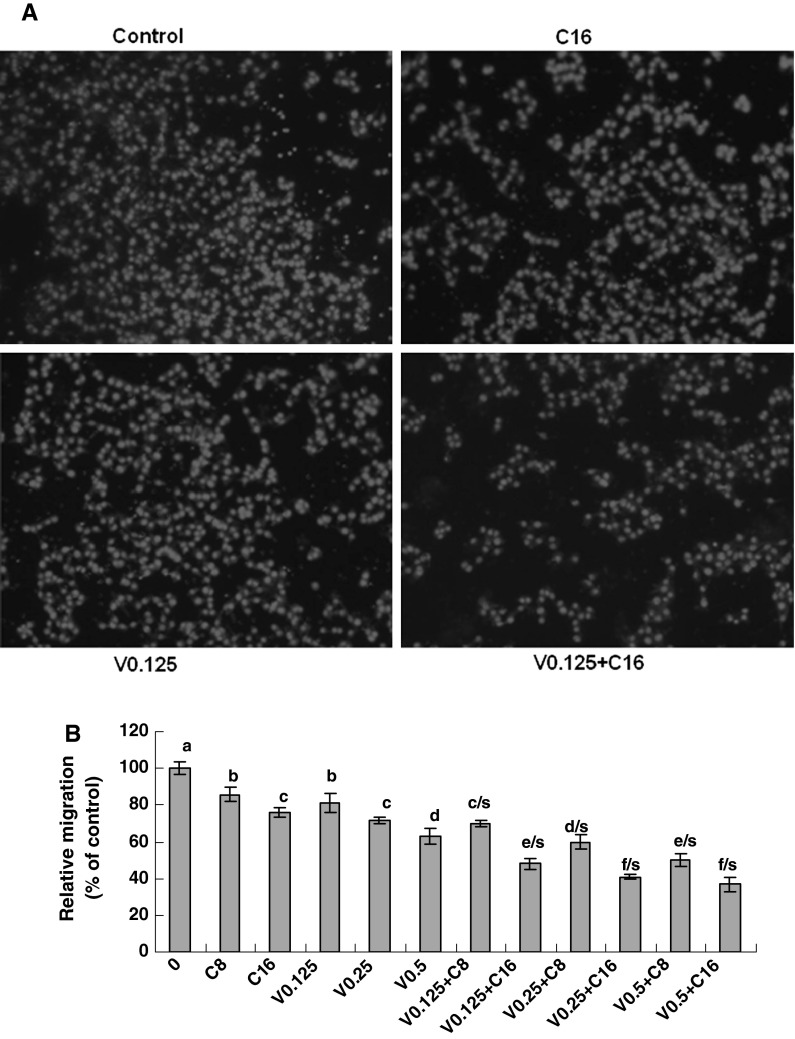
The presence of metastasis is the main cause of morbidity and mortality in millions of patients with cancer. During the complicated process of metastasis, the migration of cancer cells is the most important step. Clearly, an agent which could efficiently inhibit the growth and migration of cancer cells would be a hopeful candidate to suppress cancer progression and metastasis and thus could reduce mortality. Therefore, we examined the effects of combination of VPA and HCCA on the migration in LLC cells. The migration assay indicated that VPA (0.125–0.5 mM) and HCCA (0.008–0.016 mM) significantly and dose-dependently suppressed the migration of LLC cells after the cells were treated for 6 h with VPA and HCCA, which the combination of VPA and HCCA enhanced the migration inhibition in LLC cells (Fig. 2a, b). The relative migration rate was reduced from 70 to 37 % as the concentrations of VPA were increased from 0.125 to 0.5 mM in the presence of 0.008 and 0.016 mM of HCCA. However, the threshold concentration above which the inhibition of cell migration is not improved could be about 0.5 mM for VPA and 0.016 mM for HCCA. It seems that the increase in the concentrations of either VPA or HCCA in the threshold concentrations can not greatly enhance the suppression of LLC cell migration although the VPA concentrations in this study are less than those already reported for inhibition of cell migration in breast cancer (Li et al. 2012; Zhang et al. 2012a, b) and prostate cancer (Zhang et al. 2011; Wedel et al. 2011; Hudak et al. 2012) by VPA treatment (0.8–3.2 mM).
Fig. 2.
Inhibition of LLC cell migration by combination of VPA and HCCA. The effects of combination of VPA and HCCA on the migration of LLC cells were examined by the migration assay as described in the section of “Materials and methods”. The enhanced inhibition of LLC cell migration by combination of VPA and HCCA is shown in (a) and (b). a The photos (200X) show the propidium iodide-stained LLC cells that migrated through fibronectin-coated transwell chamber. b The cells were treated for 6 h with the indicated concentrations of VPA/V and HCCA/C (V0.125/0.125 mM, V0.25/0.25 mM, V0.5/0.5 mM, C8/0.008 mM, C16/0.016 mM). The control (0) received DMSO vehicle. The data are presented as the mean ± SD (Bar) (n = 6). The figure is the representative of 3 similar experiments performed. *P < 0.05. Values with different letters (a–f) differ significantly (P < 0.05); c/s, d/s, e/s and f/s represent the significant enhanced effects (P < 0.05) compared with VPA or HCCA alone
Combination of VPA and HCCA enhances the down-regulation of the protein levels of Bcl-2 and cyclin D1 and increased the up-regulation of the protein levels of Bax, cytosolic cytochrome c, caspase-3, and PARP-1 in LLC cells
To understand the molecular mechanisms of action of the combination of VPA and HCCA on the proliferation and induction of apoptosis and cell cycle arrest in LLC cells, we studied the effects of the combination of VPA and HCCA on the related protein expressions. Our results showed that VPA (0.5–1 mM) and HCCA (0.064–0.125 mM) significantly reduced the ratios of Bcl-2/Bax proteins (Fig. 3a) in LLC cells. The combination of VPA and HCCA enhanced the down-regulation of the expression of these proteins in LLC cells (Fig. 3a). Furthermore, VPA (0.5–1 mM) and HCCA (0.64–0.125 mM) up-regulated the expression of cytosolic cytochrome c (Fig. 3b), caspase-3 (Fig. 3c), and the cleavage of poly(ADP-ribose) polymerase-1 (PARP-1) (Fig. 3d) in LLC cells. The combination of VPA and HCCA also enhanced the up-regulation of the expression of these proteins in LLC cells (Fig. 3b, c, d). Moreover, VPA (0.5–1 mM) and HCCA (0.064–0.125 mM) significantly reduced the cyclin D1 protein expression (Fig. 3e) in LLC cells. The combination of VPA and HCCA enhanced the down-regulation of the expression of these proteins in LLC cells (Fig. 3e). In addition, the inhibitor of PI3K/Akt (Ly) showed the significant suppression of the expressions of the proteins Bcl-2, and cyclin D1 and increased the protein levels of Bax, cytosolic cytochrome c, caspase-3, and the cleavage of PARP-1 in LLC cells (Fig. 3).
Fig. 3.
Effects of combination of VPA and HCCA on the expression of the following proteins: Bcl-2/Bax (a), cytosolic cytochrome c (cyto c)(b), caspase-3/procaspase-3 (c), PARP-1 (d) and cyclin D1 (e) in LLC cells. The cells were treated for 48 h with the indicated concentrations of VPA/V and HCCA/C (V0.5/0.5 mM, V1/1 mM, C64/0.064 mM, C125/0.125 mM), and Ly (0.0125 mM). The protein expressions were analyzed by Western Blotting. The optical density (OD) of the band was normalized with respect to β-actin and was expressed as relative optical density (OD). The OD value of the band shown as mean ± SD is relative to that of the control (0, the DMSO vehicle) designated as 100 %. Ly is the inhibitor of PI3K/Akt. For one experiment, 3 assays were carried out and only one set of gels is shown. Values with different letters (a–f) differ significantly (P < 0.05). e/s represents the significant enhanced effects (P < 0.05) compared with VPA or HCCA alone
One of the main regulatory steps of apoptotic cell death is controlled by the ratio of antiapoptotic and proapoptotic members of the Bcl-2 family of proteins, which determines the susceptibility to apoptosis. Bax, a proapoptotic factor of the Bcl-2 family, is found in monomeric form in the cytosol or is loosely attached to the membranes under normal conditions. Following a death stimulus, cytosolic and monomeric Bax translocates to the mitochondria where it becomes an integral membrane protein and cross-linkable as a homodimer allowing for the release of factors from the mitochondria, such as cytochrome c, to propagate the apoptotic pathway (Oltvai et al. 1993). Bcl-2 and its related proteins control the release of cytochrome c from the mitochondria (Reed 1997). Hallmarks of the apoptotic process include the activation of cysteine proteases, which represent both initiators and executors of cell death. In the cytosol, cytochrome c activates caspase-9, which in turn activates the effector caspases such as caspase-3 and caspase-7 (Stennicke and Salvesen 2000). In the present study, we observed that the combination of VPA and HCCA significantly enhanced the down-regulation of the antiapoptotic Bcl-2 level and up-regulation of proapoptotic Bax level, leading to reduction of Bcl-2/Bax ratio (Fig. 3a). Furthermore, the combination of VPA and HCCA significantly enhanced the release of cytocrome c from the mitochondria (Fig. 3b) and subsequently increased caspase-3 activity (Fig. 3c). Caspase-3 is synthesized as a 35-kDa inactive precursor (procaspase-3), which is proteolytically cleaved to produce a mature enzyme composed of 17-kDa fragments. As shown in Fig. 3C, caspase-3 in cleaved form was enhanced with either the increase in the concentrations of VPA and HCCA, or the combination treatment of VPA and HCCA. In addition, we observed the up-regulation of cleavage of PARP-1, the substrate of caspase-3, in LLC cells treated with the combination of VPA and HCCA (Fig. 3d). Moreover, our results also indicated that the combination of VPA and HCCA significantly enhanced the down-regulation of cyclin D1 protein expression in LLC cells (Fig. 3e).
Taken together, all these results show that the combination of VPA and HCCA significantly enhanced the proliferation suppression of LLC cells and the induction of apoptosis and cell cycle arrest by reducing Bcl-2/Bax ratio and activating the mitochondrial and caspase-3 pathway as well as reducing the protein level of cyclin D1, the cell cycle regulator in LLC cells.
Combination of VPA and HCCA enhances the down-regulation of the receptor phosphorylation and protein expression of EGFR, VEGFR2, and c-Met in LLC cells
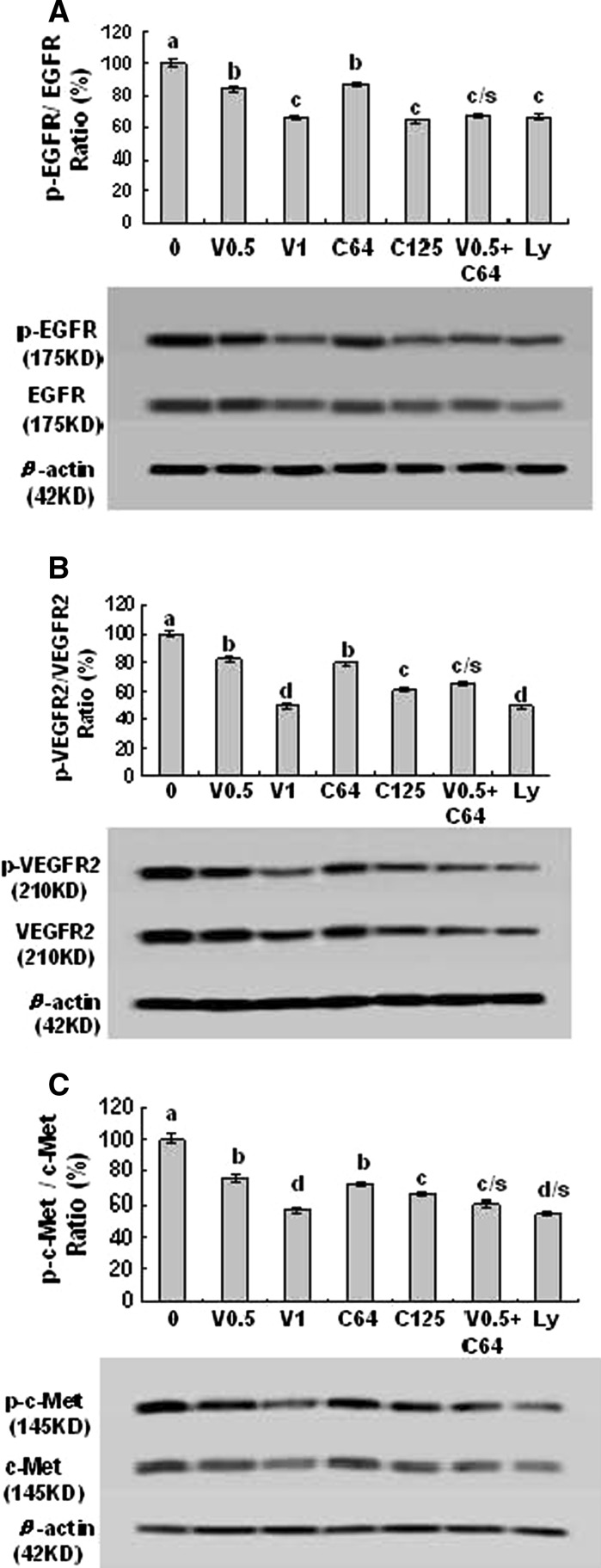
The activated receptors such as EGFR, VEGFR2, and c-Met play very important roles in the proliferation and migration of cancer cells including lung cancer cells (Morelli et al. 2006; Kim et al. 2008a, b; Puri and Salgia 2008; Naumov et al. 2009; Colon et al. 2011; Zhang et al. 2012a, b). In order to clarify the VPA- and HCCA-targeted receptors in LLC cells, we studied the effects of VPA and HCCA on the related receptor phosphorylation and expression in LLC cells. We have confirmed that the combination of VPA and HCCA significantly enhanced the inhibition of the phosphorylation and expression of EGFR (Fig. 4a), VEGFR2 (Fig. 4b), and c-Met (Fig. 4c) in LLC cells. In addition, the inhibitor of PI3K/Akt (Ly) also showed inhibitory effects on the phosphorylation and expression of these receptors (Fig. 4) in LLC cells. The down-regulation of the receptor phosphorylation and protein expression in LLC cells by the combination of VPA and HCCA could greatly contribute to the suppression of their downstream targets and the receptors-mediated PI3K/Akt as well as the related signaling pathways, and thus leads to the inhibition of the proliferation and migration in LLC cells.
Fig. 4.
Effects of combination of VPA and HCCA on receptor protein expressions and phosphorylation of pEGFR/EGFR (a), pVEGFR2/VEGFR2 (b), and p–c-Met/c-Met (c) in LLC cells. The cells were treated with the indicated concentrations of VPA/V and HCCA/C (V0.5/0.5 mM, V1/1 mM, C64/0.064 mM, C125/0.125 mM), and Ly (0.0125 mM). The protein expression and phosphorylation were analyzed by Western Blotting. The optical density (OD) of the band was normalized with respect to β-actin and is expressed as relative optical density (OD). The OD value of the bands shown as mean ± SD was relative to that of the control (0, the DMSO vehicle) designated as 100 %. Ly is the inhibitor of PI3K/Akt. For one experiment, 3 assays were carried out and only one set of gels is shown. Values with different letters (a–d) differ significantly (P < 0.05). c/s and d/s represent the significant enhanced effects (P < 0.05) compared with VPA or HCCA alone
Combination of VPA and HCCA enhances the down-regulation of the phosphorylation and/or protein expression of Akt and NF-κB in LLC cells
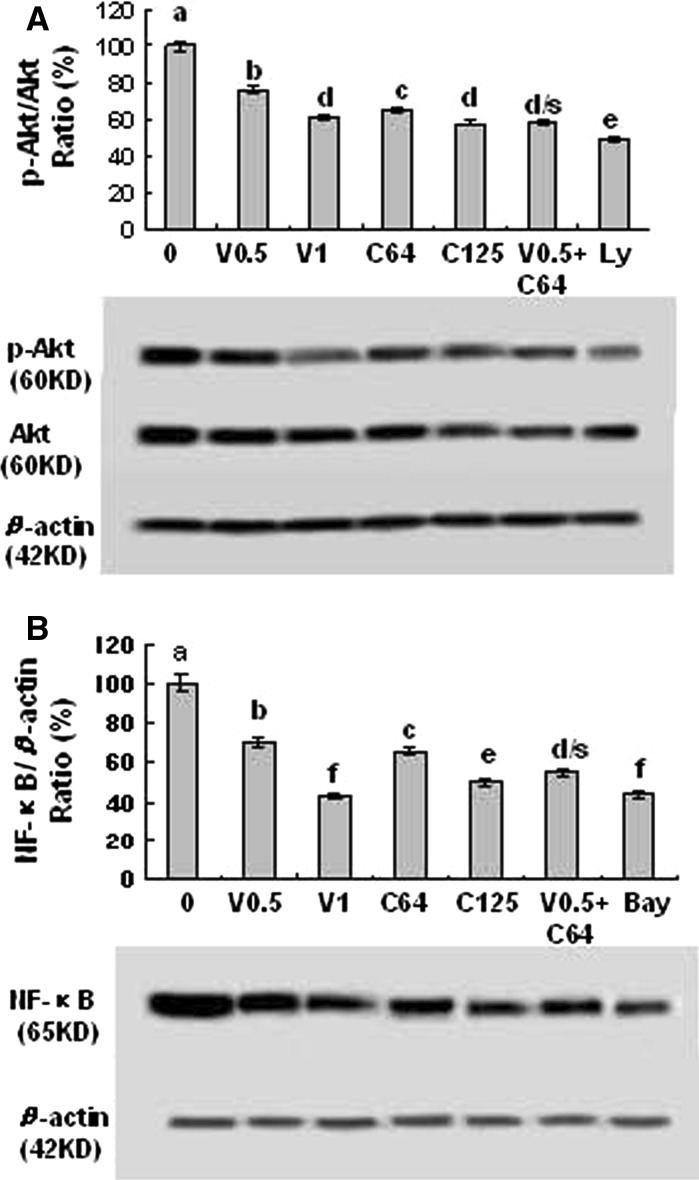
In order to further confirm the enhanced effects of the combination of VPA and HCCA on the receptor-mediated signaling pathways related to the proliferation and migration of LLC cells, we investigated the effects of the combination of VPA and HCCA on the suppression of the phosphorylation and/or expression of Akt and NF-κB in LLC cells. Our results indicated that the combination of VPA and HCCA significantly enhanced the suppression of the phosphorylation and expression of Akt (Fig. 5a) and the NF-κB (p65) expression (Fig. 5b) in LLC cells. In addition, the inhibitors of PI3K/Akt (Ly) and NF-κB (Bay) also displayed a significant suppression of the phosphorylation and protein expression of Akt and the NF-κB (p65) expression in LLC cells, respectively (Fig. 5a, b). Akt is a cytosolic signal transduction protein kinase that plays an important role in cell survival pathways (West et al. 2002). Induction of Akt activity is primarily dependent on the PI3K pathway. Akt can be activated by many forms of cellular stress, such as those observed under treatment with anticancer agents (West et al. 2002). Once activation, Akt controls cellular functions such as apoptosis, cell cycle, gene transcription, and protein synthesis through the phosphorylation of downstream substrates such as NF-κB (West et al. 2002). NF-κB is a nuclear transcription regulator with a specific motif for Bcl-2 transcription (Wang et al. 1996; Marsden et al. 2002). Activation of p-Akt and the NF-κB/Bcl-2 pathway leads to inhibition of chemotherapy-induced apoptosis, which results in treatment resistance (Wang et al. 1996). Our previous results confirmed that suppression of Akt and the NF-κB/Bcl-2 pathway by anticancer agents inhibited the growth and migration as well as induced the apoptosis and cell cycle arrest in human lung cancer A549 cells and breast cancer MDA-MB-231 cells (Wang et al. 2009, Liu et al. 2009, Yu et al. 2009; Zhang et al. 2012a, b). In the present study, we indicated that the combination of VPA and HCCA significantly enhanced the inhibition of the proliferation and migration of LLC cells and the PI3K/Akt and NF-κB pathway by suppressing the phosphorylation and expression of Akt and the NF-κB expression and activating the apoptotic pathway of Bcl-2/Bax-mitochondrial-caspase-3 in LLC cells.
Fig. 5.
Effects of combination of VPA and HCCA on the protein expression and phosphorylation of pAkt/Akt (a) and NF-κB (p65) protein expression (b) in LLC cells. The cells were treated with the indicated concentrations of VPA/V and HCCA/C (V0.5/0.5 mM, V1/1 mM, C64/0.064 mM, C125/0.125 mM), Ly (0.0125 mM), and Bay (0.0025 mM). The protein expressions and phosphorylation were analyzed by Western Blotting. The optical density (OD) of the band was normalized with respect to β-actin and is expressed as relative optical density (OD). The OD value of the bands shown as mean ± SD was relative to that of the control (0, the DMSO vehicle) designated as 100 %. Ly and Bay are the inhibitors of PI3K/Akt and NF-κB, respectively. For one experiment, 3 assays were carried out and only one set of gels is shown. Values with different letters (a–f) differ significantly (P < 0.05). d/s represents the significant enhanced effects (P < 0.05) compared with VPA or HCCA alone
There has been the cross-talk in EGFR and c-Met pathways in cancer cells which is related to cancer cell proliferation and migration (Puri and Salgia 2008). Suppression of EGFR, VEGFR, and c-Met led to the inhibition of cancer cell proliferation, migration and invasion (Morelli et al. 2006; Naumov et al. 2009; Wang et al. 2009; Colon et al. 2011; Zhang et al. 2012a, b). Here we have confirmed that the combination of VPA and HCCA significantly enhanced the inhibition of the EGFR, VEGFR2 and c-Met receptors and their downstream targets such as Akt and NF-κB in LLC cells, which could contribute to the inhibition of LLC cell proliferation and migration.
In summary, our present results have demonstrated that the combination of VPA and HCCA significantly enhanced the inhibition of LLC cell proliferation and migration, and induced apoptosis and cell cycle arrest by targeting the EGFR, VEGFR2, and c-Met receptors-mediated signaling pathways of Akt and NF-κB in LLC cells. The combination of VPA and HCCA did not show significant inhibition of the growth of the normal mouse fibroblast NIH3T3 and the normal human umbilical endothelial vein cells (EA.hy926). All these findings suggest that the combination of VPA and HCCA may have the wide therapeutic and/or adjuvant therapeutic application in the treatment of lung cancer.
Acknowledgments
This work is supported in part by grant (863) from the Ministry of Science and Technology of the People’s Republic of China to G.Z (2012AA020206), from the Ministry of Human Resources and Social Security of the People’s Republic of China to G.Z, Projects of Yantai University to GZ, Project from the National Natural Science Foundation of China to GZ (No. 30973553), and grants from the Department of Science and Technology of Shandong Province to GZ (ZR2012HM016; 2009GG10002087).
Conflict of interest
The authors declare no conflict of interest.
Abbreviations
- VPA
Valproic acid
- HCCA
Coumarin-3-carboxylic acid
- NF-κB
Nuclear factor κB
- VEGFR2
Vascular endothelial growth factor receptor-2
- EGFR
Epidermal growth factor receptor
- Ly
Ly294002
- Bay
Bay 11-7082
- PARP
Poly(ADP-ribose) polymerase
- Cyto C
Cytochrome c
- MTT
3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide
Footnotes
Xin Liu, Linlin Chen and Fujia Sun contributed equally to this work.
References
- Blaheta RA, Cinatl J., Jr Anti-tumor mechanisms of valproate: a novel role for an old drug. Med Res Rev. 2002;22:492–511. doi: 10.1002/med.10017. [DOI] [PubMed] [Google Scholar]
- Byun SS, Kim FJ, Khandrika L, Kumar B, Koul S, Wilson S, Koul HK. Differential effects of valproic acid on growth, proliferation and metastasis in HTB5 and HTB9 bladder cancer cell lines. Cancer Lett. 2009;281:196–202. doi: 10.1016/j.canlet.2009.02.045. [DOI] [PubMed] [Google Scholar]
- Chou YW, Chaturvedi NK, Ouyang S, Lin FF, Kaushik D, Wang J, Kim I, Lin MF. Histone deacetylase inhibitor valproic acid suppresses the growth and increases the androgen responsiveness of prostate cancer cells. Cancer Lett. 2011;311:177–186. doi: 10.1016/j.canlet.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colon J, Basha MR, Madero-Visbal R, Konduri S, Baker CH, Herrera LJ, Safe S, Sheikh-Hamad D, Abudayyeh A, Alvarado B, Abdelrahim M. Tolfenamic acid decreases c-Met expression through Sp proteins degradation and inhibits lung cancer cells growth and tumor formation in orthotopic mice. Invest New Drugs. 2011;29:41–51. doi: 10.1007/s10637-009-9331-8. [DOI] [PubMed] [Google Scholar]
- Hudak L, Tezeeh P, Wedel S, Makarević J, Juengel E, Tsaur I, Bartsch G, Wiesner C, Haferkamp A, Blaheta RA (2012) Low dosed interferon alpha augments the anti-tumor potential of histone deacetylase inhibition on prostate cancer cell growth and invasion. Prostate 72(16):1719–1735 [DOI] [PubMed]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Zhang Y, Luan J, Duan H, Yagasaki K, Zhang G. Effects of bufalin on the proliferation of human lung cancer cells and its molecular mechanisms of action. Cytotechnology. 2010;62:578–583. doi: 10.1007/s10616-010-9310-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Lee UJ, Kim MN, Lee EJ, Kim JY, Lee MY, Choung S, Kim YJ, Choi YC. MicroRNA miR-199a regulates the MET proto-oncogene and the downstream extracellular signal-regulated kinase 2 (ERK2) J Biol Chem. 2008;283:18158–18166. doi: 10.1074/jbc.M800186200. [DOI] [PubMed] [Google Scholar]
- Kim YT, Kim TY, Lee DS, Park SJ, Park JY, Seo SJ, Choi HS, Kang HJ, Hahn S, Kang CH, Sung SW, Kim JH. Molecular changes of epidermal growth factor receptor (EGFR) and KRAS and their impact on the clinical outcomes in surgically resected adenocarcinoma of the lung. Lung Cancer. 2008;59:111–118. doi: 10.1016/j.lungcan.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Kim NH, Kim SN, Oh JS, Lee S, Kim YK. Anti-mitotic potential of 7-diethylamino-3(2′-benzoxazolyl)-coumarin in 5-fluorouracil-resistant human gastric cancer cell line SNU620/5-FU. Biochem Biophys Res Commun. 2012;418:616–621. doi: 10.1016/j.bbrc.2012.01.049. [DOI] [PubMed] [Google Scholar]
- Landreville S, Agapova OA, Matatall KA, Kneass ZT, Onken MD, Lee RS, Bowcock AM, Harbour JW. Histone deacetylase inhibitors induce growth arrest and 16 differentiation in uveal melanoma. Clin Cancer Res. 2012;18:408–416. doi: 10.1158/1078-0432.CCR-11-0946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GF, Qian TL, Li GS, Yang CX, Qin M, Huang J, Sun M, Han YQ. Sodium valproate inhibits MDA-MB-231 breast cancer cell migration by upregulating NM23H1 expression. Genet Mol Res. 2012;11:77–86. doi: 10.4238/2012.January.13.1. [DOI] [PubMed] [Google Scholar]
- Lin TH, Lu FJ, Yin YF, Tseng TH. Enhancement of esculetin on arsenic trioxide-provoked apoptosis in human leukemia U937 cells. Chem Biol Interact. 2009;180:61–68. doi: 10.1016/j.cbi.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Liu Q, Duan H, Luan J, Yagasaki K, Zhang G. Effects of theanine on growth of human lung cancer and leukemia cells as well as migration and invasion of human lung cancer cells. Cytotechnology. 2009;59:211–217. doi: 10.1007/s10616-009-9223-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan J, Duan H, Liu Q, Yagasaki K, Zhang G. Inhibitory effects of norcantharidin against human lung cancer cell growth and migration. Cytotechnology. 2010;62:349–355. doi: 10.1007/s10616-009-9250-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden VS, O’Connor L, O’Reilly LA, Silke J, Metcalf D, Ekert PG, Huang DC, Cecconi F, Kuida K, Tomaselli KJ, Roy S, Nicholson DW, Vaux DL, Bouillet P, Adams JM, Strasser A. Apoptosis initiated by Bcl-2-regulated caspase activation independently of the cytochrome c/Apaf-1/caspase-9 apoptosome. Nature. 2002;419:634–637. doi: 10.1038/nature01101. [DOI] [PubMed] [Google Scholar]
- Morelli MP, Cascone T, Troiani T, Tuccillo C, Bianco R, Normanno N, Romano M, Veneziani BM, Fontanini G, Eckhardt SG, De Pacido S, Tortora G, Ciardiello F. Anti-tumor activity of the combination of cetuximab, an anti-EGFR blocking monoclonal antibody and ZD6474, an inhibitor of VEGFR and EGFR tyrosine kinases. Cell Physiol. 2006;208:344–353. doi: 10.1002/jcp.20666. [DOI] [PubMed] [Google Scholar]
- Munster P, Marchion D, Bicaku E, Lacevic M, Kim J, Centeno B, Daud A, Neuger A, Minton S, Sullivan D. Clinical and biological effects of valproic acid as a histone deacetylase inhibitor on tumor and surrogate tissues: phase I/II trial of valproic acid and epirubicin/FEC. Clin Cancer Res. 2009;15:2488–2496. doi: 10.1158/1078-0432.CCR-08-1930. [DOI] [PubMed] [Google Scholar]
- Naumov GN, Nilsson MB, Cascone T, Briggs A, Straume O, Akslen LA, Lifshits E, Byers LA, Xu L, Wu HK, Jänne P, Kobayashi S, Halmos B, Tenen D, Tang XM, Engelman J, Yeap B, Folkman J, Johnson BE, Heymach JV. Combined vascular endothelial growth factor receptor and epidermal growth factor receptor (EGFR) blockade inhibits tumor growth in xenograft models of EGFR inhibitor resistance. Clin Cancer Res. 2009;15:3484–3494. doi: 10.1158/1078-0432.CCR-08-2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-O. [DOI] [PubMed] [Google Scholar]
- Ozawa A, Tanji N, Kikugawa T, Sasaki T, Yanagihara Y, Miura N, Yokoyama M. Inhibition of bladder tumour growth by histone deacetylase inhibitor. BJU Int. 2010;105:1181–1186. doi: 10.1111/j.1464-410X.2009.08795.x. [DOI] [PubMed] [Google Scholar]
- Puri N, Salgia R. Synergism of EGFR and c-Met pathways, cross-talk and inhibition, in non-small cell lung cancer. J Carcinog. 2008;7:9. doi: 10.4103/1477-3163.44372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassouli FB, Matin MM, Iranshahi M, Bahrami AR, Behravan J, Mollazadeh S, Neshati V, Kalalinia F. Investigating the enhancement of cisplatin cytotoxicity on 5637 cells by combination with mogoltacin. Toxicol In Vitro. 2011;25:469–474. doi: 10.1016/j.tiv.2010.11.015. [DOI] [PubMed] [Google Scholar]
- Reed JC. Bcl-2 family proteins: regulators of apoptosis and chemoresistance inhematologic malignancies. Semin Hematol. 1997;34:S9–S19. [PubMed] [Google Scholar]
- Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- Stennicke HR, Salvesen GS. Caspases—controlling intracellular signals by protease zymogen activation. Biochim Biophys Acta. 2000;1477:299–306. doi: 10.1016/S0167-4838(99)00281-2. [DOI] [PubMed] [Google Scholar]
- Wang CY, Mayo MW, Baldwin AS., Jr TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-kappaB. Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- Wang S, Liu Q, Zhang Y, Liu K, Yu P, Liu K, Luan J, Duan H, Lu Z, Wang F, Wu E, Yagasaki K, Zhang G. Suppression of growth, migration and invasion of highly-metastatic human breast cancer cells by berbamine and its molecular mechanisms of action. Mol Cancer. 2009;8:81. doi: 10.1186/1476-4598-8-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedel S, Hudak L, Seibel JM, Makarević J, Juengel E, Tsaur I, Waaga-Gasser A, Haferkamp A, Blaheta RA. Molecular targeting of prostate cancer cells by a triple drug combination down-regulates integrin driven adhesion processes, delays cell cycle progression and interferes with the cdk-cyclin axis. BMC Cancer. 2011;11:375. doi: 10.1186/1471-2407-11-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West KA, Castillo SS, Dennis PA. Activation of the PI3K/Akt pathway and chemotherapeutic resistance. Drug Resist Updat. 2002;5:234–248. doi: 10.1016/S1368-7646(02)00120-6. [DOI] [PubMed] [Google Scholar]
- Yu P, Liu Q, Liu K, Yagasaki K, Wu E, Zhang G. Matrine suppresses breast cancer cell proliferation and invasion via VEGF-Akt-NF-kappaB signaling. Cytotechnology. 2009;59:219–229. doi: 10.1007/s10616-009-9225-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Miura Y, Yagasaki K. Induction of apoptosis and cell cycle arrest in cancer cells by in vivo metabolites of teas. Nutr Cancer. 2000;38:265–273. doi: 10.1207/S15327914NC382_16. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhang H, Yu P, Liu Q, Liu K, Duan H, Luan G, Yagasaki K, Zhang G. Effects of matrine against the growth of human lung cancer and hepatoma cells as well as lung cancer cell migration. Cytotechnology. 2009;59:191–200. doi: 10.1007/s10616-009-9211-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Wang G, Wang L, Song C, Wang X, Kang J. Valproic acid inhibits prostate cancer cell migration by up-regulating E-cadherin expression. Pharmazie. 2011;66:614–618. [PubMed] [Google Scholar]
- Zhang G, Wang Y, Zhang Y, Wan X, Li J, Liu K, Wang F, Liu Q, Yang C, Yu P, Huang Y, Wang S, Jiang P, Qu Z, Luan J, Duan H, Zhang L, Hou A, Jin S, Hsieh TC, Wu E. Anti-cancer activities of tea epigallocatechin-3-gallate in breast cancer patients under radiotherapy. Curr Mol Med. 2012;12:163–176. doi: 10.2174/156652412798889063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Wang G, Wang L, Song C, Leng Y, Wang X, Kang J. VPA inhibits breast cancer cell migration by specifically targeting HDAC2 and down-regulating Survivin. Mol Cell Biochem. 2012;361:39–45. doi: 10.1007/s11010-011-1085-x. [DOI] [PubMed] [Google Scholar]