Abstract
Flow cytometry is an advanced technology for efficient, rapid, specific and multi-parameter analysis of single cells in various basic research fields including cytobiology, immunology, genetic, hematology and other basic research. Beclin-1 protein is an important indicator in monitoring autophagic activity. However, quantitative flow cytometry had been rarely reported till now to be applied in the detection of Beclin-1 expression. The present study was aimed to establish a flow cytometric method for quantitative detection of Beclin-1 expression by employing the autophagy inhibitor 3-methyladenine as the control. A multi-parameter optimal method for Beclin-1 protein staining is as follows. 2 % bovine serum albumin in phosphate buffered saline was used for sample block. Concentration of primary antibody was 0.004 μg/μL. Samples were incubated at room temperature (25 °C) for 30 min. The prepared samples had better to be detected immediately or to be stored at 4 °C and detected within 6 h, otherwise the samples should be fixed in 1 % paraformaldehyde storing at 4 °C and detected within 3 d. Furthermore, we employed the immunohistochemistry to validate the method in vivo, the results confirmed flow cytometric method. The established flow cytometric analysis for Beclin-1 protein has the advantage of simpleness, speediness, sensitivity and reproducibility.
Keywords: Flow cytometry, Autophagy, Beclin-1, Methodology
Introduction
Autophagy is a common degradation pathway involving the lysosomal pathway, which exist in eukaryotic cells widely and as an important self-protective mechanism to maintain cell homeostasis. This self-eating process plays an important role in the tissue remodeling and damaged cell organelles removing. Beclin-1 is part of a class IIIPI3 K/Vps34 complex that induces autophagosome formation and regulates the moving of other autophagy related proteins into autophagosome. Autophagy is a rarely occurrence in normal conditions, so it is for Beclin-1, but in some pathological condition they are extremely active. Therefore autophagy occurrence can be confirmed through the expression detection of Beclin-1 (Maiuri et al. 2007).
Methods for analysis of autophagy include observation of autophagosome by electron microscopy, and detection of autophagy related proteins LC3, Beclin-1, etc., by western-blotting and immunohistochemistry (Kim et al. 2008; Koike et al. 2008; Hasui et al. 2011; Pickford et al. 2008). However, these methods that can be used for qualitative analysis are tedious and time-consuming and have limited sensitivity for quantitative detection. In contrast, flow cytometry is simple, rapid, highly sensitive, and can be used for quantitative detection using the same amount of cells. Multi-parameter can be analysis individually. It has been reported that flow cytometry could serve as a better detection method for the expression of LC3 protein (Shvets et al. 2008). However, up to now there are few reports on the quantitative detection of Beclin-1. In our previous studies, we found that Beclin-1 could be detected quantitatively by flow cytometry in the survey on the mechanism of ischemia–reperfusion-induced autophagy in rat brains and oxygen-glucose deprivation and reperfusion induced injury in PC12 cells (Liu et al. 2012; Mo et al. 2012).
Based on these finding, we established a method in this paper for quantitative detection of Beclin-1 protein in autophagy of PC12 cells induced by hypoxia/hypoglycemia and reoxygenation using flow cytometry. We investigated the effect of blocking reagent, concentration of primary antibody, incubation temperature, incubation time, sample preservation time and methods for expression of Beclin-1 protein, then we employed the immunohistochemistry to validate the method in vivo.
Materials and methods
Cell culture
PC12 cells, a cell line derived from a pheochromocytoma of the rat adrenal medulla, were used for a neuronal differentiation model system when treated with nerve growth factor (Greene and Tischler 1976). The cells (Culture Collection of Chinese Academy of Science, Shanghai, China) were seeded in 25 cm2 polystyrene flasks (Corning Costar Corp, Corning, NY, USA) with Dulbecco’s modifed Eagle’s medium (DMEM) (Gibco, USA) containing 4.5 g/L glucose, 5 % heat-inactivated foetal bovine serum (Gibco, Carlsbad, CA, USA) and 5 % horse serum (Gibco, USA). The cells were incubated in an atmosphere of 95 % air and 5 % CO2. Culture medium was replaced every 48 h.
Hypoxia/hypoglycemia and reoxygenation
PC12 cells were washed with phosphate buffered saline (PBS) for one time and incubated in Earle’s balanced salt solution (116 mM NaCl, 5.4 mM KCl, 0.8 mM MgSO4, l mM NaH2PO4, 0.9 mM CaCl2 and 10 mg/L phenol red) after growth for 48 h. Cells were incubated in a hypoxia chamber (Thermo Scientific, Hudson, NH, USA) with gas containing 95 % N2 and 5 % CO2 for 2 h. After hypoxia, the cells were transferred back to full culture medium with oxygen for 24 h. For the autophagy inhibited group, the cells were pretreated with 10 mM 3-methyladenine (3-MA) (Sigma, St. Louis, MO, USA) for 1 h before treatment with hypoxia/hypoglycemia followed by reoxygenation. Control cells were incubated in a regular cell culture incubator under normoxia conditions.
Application of 2 % bovine serum albumin in phosphate buffered saline (BSA/PBS) as blocking reagent to reduce non-specific binding
Cells were divided into two groups. For the blocked group, the cells (1 × 106) were treated with 2 % BSA/PBS at room temperature (25 °C) and collected 30 min later. Then 100 μL permeabilization ‘medium A’ and ‘medium B’ (Fix and Perm, Invitrogen, Carlsbad, CA, USA) were successively added and the cells were fixed for 15 min. Primary antibody (Mouse Anti-Rat Beclin-1 Monoclonal Antibody 0.004 μg/μL, Santa Cruz Biotechnology, Santa Cruz, CA, USA) and secondary antibody (PE-Conjugated Goat Anti-Mouse IgG1 Antibody 0.005 μg/μL, Multisciences (Hangzhou, China)) were added in succession and incubated in darkness at room temperature for 30 min. Cells were washed once with 2 % BSA/PBS before every agents were added. The expression of Beclin-1 was analyzed by flow cytometry (Beckman Coulter, Brea, CA, USA). For the non-blocked group, the cells were treated as the blocked group except that PBS took the place of 2 % BSA/PBS. Isotype controls were used as control (IgG antibody instead of primary antibody).
Screening analysis of the concentration of primary antibody
The cells were treated as described above for the blocking reagent analysis. Concentrations of primary antibody were 0.006, 0.004 and 0.002 μg/μL. The expression of Beclin-1 was analyzed by flow cytometry. Isotype controls were used as control.
Screening analysis of incubation temperature and time
The cells were treated as described above for the blocking reagent analysis. Incubation temperatures were room temperature (25 °C) and 37 °C. Incubation times were 15, 30 and 60 min. The expression of Beclin-1 was analyzed by flow cytometry. Isotype controls were used as control.
Screening analysis of sample preservation time and fixation methods
The prepared samples were analyzed immediately or stored at 4 °C and analyzed within 6 h or 24 h respectively. And the samples were fixed in 1 % paraformaldehyde stored at 4 °C and analyzed within 1, 3 or 7 d respectively.
Flow cytometric analysis
Standard fluorescent beads (Flow-Check) were used to align the optical path before samples were measured. The channel coefficient of variation (HCV value) should be equal to 2 %. Then parameters were selected, 1 × 104 cells were selected from the samples as 100 % total cell number and Beclin-1 positive expression was presented as a percentage of total cell number.
Transmission electron microscope for observation of autophagy
After normoxic or hypoxic treatment (hypoxia/hypoglycemia 2 h and reoxygenation 24 h), the cells were trypsinized and pelleted. The cells were suspended and fixed with 2 % glutaraldehyde in 0.1 M PBS (pH 7.4) at 4 °C for 2 h and postfixed with 1 % osmium tetroxide in 0.1 M PBS (pH 7.4) at 4 °C for 2 h. After washing twice with PBS, cells were subsequently washed as follows, with 50 % acetone for 10 min, with 70 % acetone for 10 min, twice with 80 % acetone for 10 min, twice with 90 % acetone for 10 min, twice with 100 % acetone for 10 min and were finally embedded in Epon 812 resin. The blocks were cut into ultrathin sections by a ultramicrotome and stained with uranyl acetate and lead citrate. The ultrastructure of the cells was observed under a transmission electron microscope (H-600, Hitachi, Tokyo, Japan).
Animals
Sprague–Dawley rats (Experimental Animal Center, Guangzhou University of Chinese Medicine, China), weighted 350–400 g, were housed at 25 °C room temperature with food and water and a 12 h light dark cycle. All animal studies were approved by the Experimental Animal Ethics Committee of Guangzhou University of Chinese Medicine.
Ischemia and reperfusion
Thirty Sprague–Dawley rats were randomly divided into three groups: the sham group, model group and 3-MA group. The treatment was as follows: model group, 2 mL/kg water intraperitoneally per day for 4 d; 3-MA group, 2 mL/kg of 3-MA (1.5 mg/mL) intraperitoneally per day for 4 d. The rats in the model and 3-MA groups underwent a middle cerebral artery occlusion (MCAO) (Longa et al. 1989). Briefly, the rats were anesthetized with intraperitoneal injection of 3 % chloral hydrate (350 mg/kg). Through a midline incision of the neck, the right common carotid artery, external carotid artery and internal carotid artery were exposed and ligated. A 40 mm length of monofilament nylon suture (Φ0.22–0.24 mm) was inserted from the right common carotid artery to the internal carotid artery through a small incision in the common carotid artery and then advanced to the Circle of Willis to occlude the origin of the right middle cerebral artery. The suture remained for 2 h and then removed. The rats in the sham group underwent the same surgical procedures except for the MCAO. After reperfusion 24 h, brains were harvested and hemi dissected, hemisphere was divided into two samples. One was for flow cytometry evaluation, while the other for immunohistochemistry evaluation.
Flow cytometric evaluation as the method
Samples were released by teasing through a steel mesh. Cell suspensions were filtered through a sterile nylon filter to remove the stroma and then the cells were washed twice with BSA/PBS. Cells were counted and adjusted to a density of 1 × 106 cells/mL. The cells were treated using the method as described above.
Immunohistochemistry
Beclin-1 expression was evaluated on 5 μm thick formalin-fixed, paraffin-embedded tissue sections by a standard immunohistochemistry protocol (Miracco et al. 2007). Briefly, sections were pretreated with 99 % formic acid for 5 min, and were deparaffinized and hydrated in a graded series of ethanol. Endogenous peroxidase was prevented by use of 3 % hydrogen peroxide in PBS at room temperature for 10 min. The slides were blocked with a protein blocking solution (VECTASTAIN Elite ABC kit, Vector Laboratories, Burlingame, CA, USA) for 30 min at room temperature, and then incubated with mouse anti-rat Beclin-1 antibody (1:50, Santa Cruz Biotechnology, USA) overnight at 4 °C. Then the slides were washed three times with PBS and incubated with PE-conjugated goat anti-mouse IgG1 antibody (1:100, Santa Cruz Biotechnology, USA) for 30 min at room temperature. After washing three times with PBS, the staining was performed by using 3, 3′-diaminobenzidine and the sections were counterstained with hematoxylin. Beclin-1 expression was identified by the brown-colored cytoplasmic staining. For each case and for each antibody, a negative control was obtained by replacing the specific antibody with non-immune serum immunoglobulins at the same concentration of the primary antibody. The sections were observed under a light microscope and five visual fields (×400) with rich positive substances were chosen. Then the integrated optical density (IOD) of the positive substances in the visual field (×400) was determined by using the Image-Pro Plus program 6 analysis software (Media Cybernetics, Silver Spring, MD, USA), and the mean value of the IOD of each visual field was calculated.
Statistical analysis
Measurement data are expressed as mean ± standard deviation (SD), and differences between groups were compared using Student’s t test or one way ANOVA followed by Student–Newman–Keuls test. The data were analyzed using SPSS 13.0 software, and P < 0.05 or < 0.01 were assessed as statistically significant.
Results
Effect of 3-MA and 2 % BSA/PBS on the analysis of Beclin-1 expression
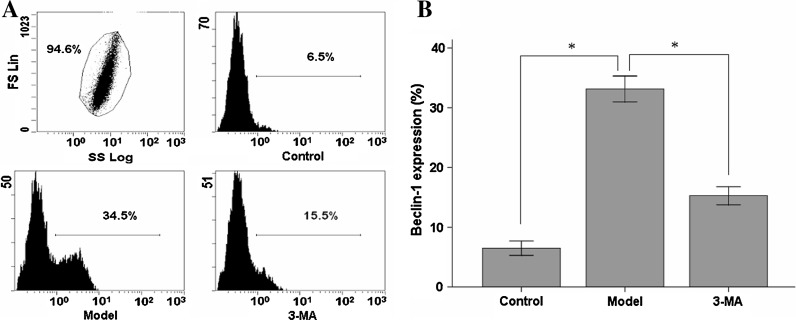
Beclin-1 expression was upregulated significantly in hypoxia/hypoglycemia and reoxygenation treated cells (33.2 ± 2.2 %) compared with the control group (6.5 ± 1.2 %). The autophagic inhibitor 3-MA reduced Beclin-1 expression significantly (15.3 ± 1.6 %) after treatment (P < 0.05, n = 10, Fig. 1).
Fig. 1.
Effect of 3-MA on the analysis of Beclin-1 expression. (a) 1 × 104 cells were selected from the samples as 100 % total cell number. Expressions of Beclin-1 were measured by flow cytometry in the different groups. (b) Quantitated results of Beclin-1 are represented as a percentage of positive expression. Data are expressed as Mean ± SD for 10 samples. * P < 0.05
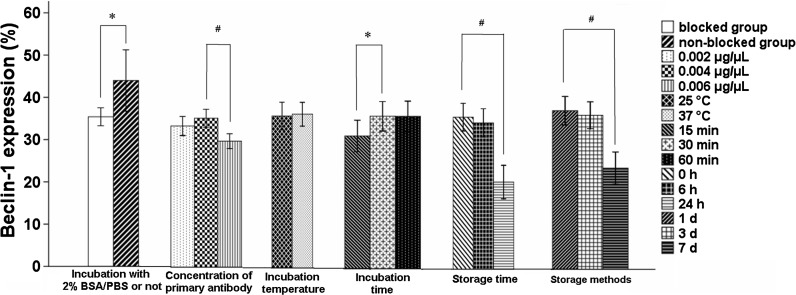
Using 2 % BSA/PBS during sample preparation could reduce non-specific binding significantly. There was a false positive rate of Beclin-1 expression in the group which did not used 2 % BSA/PBS. Beclin-1 expression was 35.4 ± 2.1 % or 43.9 ± 7.2 % in the blocked group or non-blocked group, respectively. There was a significant statistical difference between the two groups (P < 0.05, n = 10, Fig. 2).
Fig. 2.
Effect of 2 % BSA/PBS, the concentration of primary antibody (0.002, 0.004 and 0.006 μg/μL), incubation temperature (25 and 37 °C) and time (15, 30 and 60 min), storage time (0, 6 and 24 h) and fixation/storage methods (1, 3 and 7 d) on Beclin-1 expression. Data are expressed as Mean ± SD for 10 samples. * P < 0.05; #P < 0.01
Confirmation of the concentration of primary antibody, incubation temperature and time, preservation period and fixation/storage methods
There was no significant effect on Beclin-1 expression between 0.002 and 0.004 μg/μL of primary antibody, which was 33.5 ± 2.2 % and 35.4 ± 2.1 % in these two groups, respectively (P > 0.05, n = 10). However, 0.006 μg/μL of primary antibody reduced Beclin-1 expression significantly (29.9 ± 1.7 %) compared to the 0.002 or 0.004 μg/μL group (P < 0.01, n = 10, Fig. 2).
There was no significant statistical difference between Beclin-1 expressions of groups incubated at room temperature (25 °C) or at 37 °C, which was 36.2 ± 3.2 % or 36.7 ± 2.8 %, respectively (P > 0.05, n = 10, Fig. 2). In terms of incubation time, Beclin-1 expression did not show any statistical difference between incubation for 30 min (36.3 ± 3.5 %) and 60 min (36.3 ± 3.6 %) (P > 0.05, n = 10, Fig. 2). However, Beclin-1 expression in sample incubated for 15 min (31.6 ± 3.7 %) was decreased significantly compared to the groups incubated for 30 or 60 min (P < 0.05, n = 10, Fig. 2).
Samples were stored at 4 °C but not fixed with 1 % paraformaldehyde. There was no statistically significant difference of Beclin-1 expression between the group analyzed immediately and the group analyzed 6 h after sample preparation (36.3 ± 3.2, 34.9 ± 3.3 %, respectively) (P > 0.05, n = 10). Compared with the group analyzed immediately, Beclin-1 expression was dramatically decreased in the group analyzed 24 h after sample preparation (20.8 ± 3.9 %) (P < 0.01, n = 10, Fig. 2).
Samples were fixed with 1 % paraformaldehyde and stored at 4 °C. Beclin-1 expression reached 37.6 ± 3.3 % after storage for 1 d, and 36.5 ± 3.1 % after storage for 3 d, respectively. There was no statistically significant difference (P > 0.05, n = 10). Beclin-1 expression was reduced significantly after storaged 7 d (24.1 ± 3.8 %) compared to the group stored for 1 d (P < 0.01, n = 10, Fig. 2).
The characteristic ultrastructural morphology of autophagosome
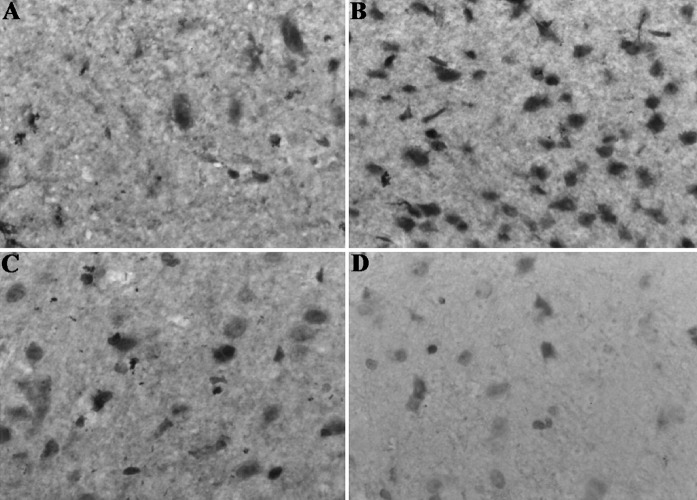
Ultrastructural morphology of cytoplasm was observed by transmission electron microscope. Autophagosomes could not be observed in normal cultured cells, but autophagosomes could be observed in the cells treated by hypoxia/hypoglycemia and reoxygenation. These observations were consistent with the results of flow cytometric evaluation (Fig. 3).
Fig. 3.
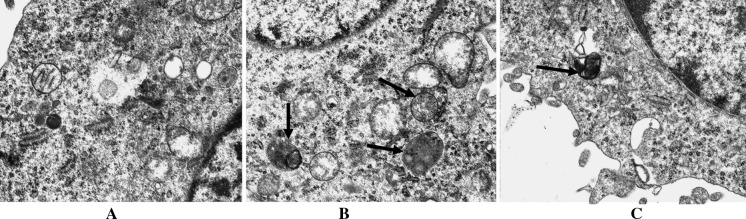
The characteristic ultrastructural morphology of autophagosome. Ultrastructural morphology of cytoplasm was observed by transmission electron microscope. (a) Normal morphology of cytoplasm was observed for the cytoplasm of the normal control group. (b) Autophagosomes can be observed obviously in the cytoplasm of cells treated by hypoxia/hypoglycemia and reoxygenation (×17,000). (c) Autophagosomes could be observed in the cells treated by 3-MA + hypoxia/hypoglycemia and reoxygenation (×20,000). (arrows indicate autophagosomes)
Representative immunohistochemical figures of Beclin-1 in rat
The staining was cytoplasmic. The model group showed strong cytoplasmic Beclin-1 expression (Fig. 4b), but the sham (Fig. 4a) and 3-MA (Fig. 4c) groups showed low Beclin-1 staining, Fig. 4d was the negative control sample.
Fig. 4.
Representative immunohistochemical figures of Beclin-1 in rat. A brown color represents positive staining of Beclin-1. Positive staining of Beclin-1 in sham group a (×400). Positive staining of Beclin-1 in model group b (×400). Positive staining of Beclin-1 in 3-MA group c (×400). No staining in negative control sample d (×400). (Color figure online)
The expression of Beclin-1 in rat was analyzed by flow cytometry
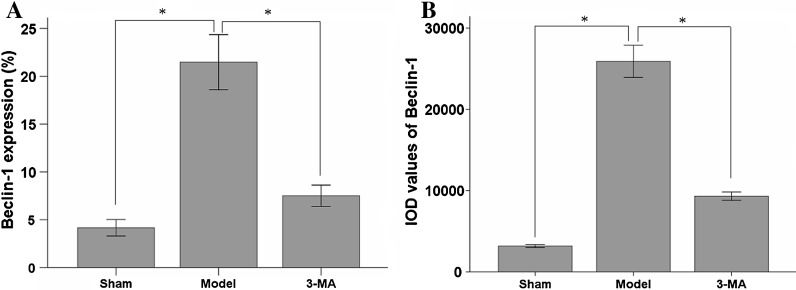
The expression of Beclin-1 was analyzed by flow cytometry, Beclin-1 expression of the ischemia and reperfusion treatment rat was upregulated significantly (21.4 ± 2.8 %) when being compared with that of sham group (4.1 ± 0.8 %). However, the autophagic inhibitor 3-MA reduced Beclin-1 expression significantly (7.5 ± 1.1 %) after ischemia and reperfusion treatment (P < 0.01, n = 10, Fig. 5a).
Fig. 5.
The expression of Beclin-1 was analyzed by flow cytometry and immunohistochemistry confirmed flow cytometry results of Beclin-1 expression in rat. (a) The expression of Beclin-1 was analyzed by flow cytometry in rat. (b) Immunohistochemistry confirmed flow cytometric results of Beclin-1 in rat. Data are expressed as Mean ± SD for 10 samples. *P < 0.01
Immunohistochemistry confirmed flow cytometric results of Beclin-1 in rat
In an immunohistochemical study, the IOD values of Beclin-1 proteins were significantly increased in the model group compared with the sham group (P < 0.01, n = 10). In the 3-MA treated group, the IOD values of Beclin-1 proteins were significantly decreased compared with the model group (P < 0.01, n = 10, Fig. 5b). The results showed that flow cytometry data are in accord with the immunohistochemistry data.
Discussion
Autophagic lysosomal pathway plays an important role in the regulaton of neuronal death (Rubinsztein et al. 2005; Rubinsztein 2006). Hypoxia/hypoglycemia and reoxygenation is one of the important incentives for autophagy activation (Kuma et al. 2004). Levels of autophagy are very low in normal conditions, but they are upregulated immediately when cell homeostasis changes such as aging or cells are disturbed by various stimuli including brain injury, oxygen-glucose deprivation and reperfusion (Levine 2007). Autophagy removes excessive or damaged intracellular organelles and components (Yoshimori 2004; Kurz et al. 2007), but excessive autophagy may also lead to cell death. Beclin-1 protein, the direct executor of autophagy that is involved in autophagosome formation, not only takes part in the autophagy process, but also receives other signals to regulate autophagy. Therefore the Beclin-l protein has become an indicator to monitor autophagic activity.
Flow cytometry is an advanced technology in efficient, rapid, specific, and multi-parameter analysis of single cells. It is an important means of detection and is widely used in cytobiology, immunology, genetics, hematology and other basic research fields (Craig and Foon 2008; Hoffman 2008; Ibrahim and van den Engh 2007). Cells should be fixed and penetrated so that fluorescent antinuclear antibodies can enter and label the antigens in the cells. Since Beclin-1 protein is located in the cytoplasm, flow cytometry can be used to detect the Beclin-1 expression. But, the appropriate conditions for antigen–antibody reaction need to be confirmed during the staining process, including concentration of antibody, incubation temperature and time. In this study, autophagy was induced by hypoxia/hypoglycemia and reoxygenation. We screened and analyzed the concentration of primary antibody, incubation temperature and time, sample storage time and fixation/storage methods and evaluated the effect of 2 % BSA/PBS and 3-MA on the analysis of Beclin-1 expression. The results indicated that the method for quantitative detection of Beclin-1 expression we established was simple, convenient and reliable, which will be better adapted for study of autophagy.
On the other hand, blocking solution can reduce false positive results caused by elevated levels of fluorescence-negative cells, through incubation of samples with blocking solution before adding the primary antibody. The reason is that blocking solution can interact with non-specific proteins of cell surface or cytoplasm. In this study, it was proven that 2 % BSA/PBS could serve as washing liquid and blocking solution. It reduced non-specific binding.
Moreover, antibody concentration is extremely important for the quantitative detection. When concentration is low, fluorescence intensity is proportional to the antibody concentration. But in contrast after the dye reaches a certain concentration, increased antibody concentration leads to a decrease in fluorescence signal. It is due to the phenomenon of fluorescence quenching and non-specific binding of antibodies. If the antibody concentration is too low, a relatively deficient amount of antibody leads to lower results because of a decrease in fluorescent molecules and signal. Therefore, antibody concentration should be optimized during dyeing in order to produce a maximum fluorescence quantum number. In this study, we chose three levels of the concentration (0.002, 0.004 and 0.006 μg/μL) according to the instructions. Beclin-1 expression did not show a significant difference between the groups 0.004 and 0.002 μg/μL of primary antibody (P > 0.05). But it was significantly decreased in group 0.006 μg/μL of primary antibody compared to 0.004 μg/μL of primary antibody (P < 0.01). Beclin-1 expression was a little higher in group 0.004 μg/μL of primary antibody than that in 0.002 μg/μL. Therefore, 0.004 μg/μL of primary antibody was the most appropriate concentration for detection of Beclin-1 protein.
Last but not least, incubation temperature and incubation time affects the fluorescence directly. Too high a temperature increases fluorescent molecule power and fluorescence quenching, while too low a temperature leads to insufficient antibody-antigen reaction and extends the incubation time. We considered that the cells had been fixed and permeated, so it has not been established a set of low-temperature tests for extending incubation time. The results showed that incubation time for 30 min at room temperature could achieve a better result and shorten the experimental time.
The samples after preparation often can not be analyzed immediately, so it needs a proper way to preserve the samples. We screened and analyzed the time and ways for preservation of the sample preparation, and came to a conclusion that it should be analyzed within 6 h after sample preparation, or analyzed within 3 d after fixing with 1 % paraformaldehyde at 4 °C.
Furthermore, we analyzed the expression of Beclin-1 by flow cytometry as method and employed immunohistochemistry with an image analysis software to validate the flow cytometric results of Beclin-1 in rat. In our previous studies, dissociated cell cultures derived from rat cerebral cortex were prepared and neurons were identified by immunohistochemistry of neuron specific enolase. We found that neurons almost accounted for 35 % of the total cultured cells, the other parts were glial cell and fibroblasts. In flow cytometry analysis, Beclin-1 expression in the rat treated with ischemia and reperfusion was significantly upregulated when being compared with that of the control group. However, the autophagic inhibitor 3-MA reduced Beclin-1 expression significantly after ischemia and reperfusion treatment (P < 0.01). In an immunohistochemical study, the IOD values of Beclin-1 proteins were significantly increased in the model group compared with the sham group (P < 0.01). In the 3-MA treated group, the IOD values of Beclin-1 proteins were significantly decreased compared with the model group (P < 0.01). The results showed that flow cytometry data are in accord with the immunohistochemistry data. The effect of autophagy inhibitor 3-MA on Beclin-1 proofed that the result of this study is valid. It is confirmed that the flow cytometric method for quantitative analysis of Beclin-1 expression is feasible.
Flow cytometry is adopted as an important quantitative analysis. Compared with immunohistochemistry, western blotting and transmission electron microscopy, flow cytometry has not only the advantageous analytical property with accuracy and quantitation, but also with simplicity and rapidity. Appropriate conditions for fluorescent labeling antibody are that the use of the primary antibody at a concentration of 0.004 μg/μL and incubation in darkness at room temperature for 30 min. Samples should be freshly handled and analyzed within 6 h after preparation, or analyzed within 3 d after fixing with 1 % paraformaldehyde at 4 °C. They should not be stored for a long time, otherwise the level of the amount of fluorescence and thus Beclin-1 expression will decrease.
Acknowledgments
This work was supported by the Guangdong Natural Science Foundation of China (No. 2003C34403). We would like to express our sincere thanks to the reviewers and editors for the constructive and positive comments.
Footnotes
Yuping He, Zhentao Mo, and Zhongfeng Xue contributed equally to this work.
References
- Craig FE, Foon KA. Flow cytometric immuno phenotyping for hematologic neoplasms. Blood. 2008;111:3941–3967. doi: 10.1182/blood-2007-11-120535. [DOI] [PubMed] [Google Scholar]
- Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976;73:2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasui K, Wang J, Jia X, Tanaka M, Nagai T, Matsuyama T, Eizuru Y. Enhanced autophagy and reduced expression of cathepsin D are related to autophagic cell death in epstein-barr virus-associated nasal natural killer/T-cell lymphomas: an immunohistochemical analysis of Beclin-1, LC3, mitochondria (AE-1), and cathepsin D in nasopharyngeal lymphomas. Acta Histochem Cytochem. 2011;44:119–131. doi: 10.1267/ahc.10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman RA. Flow cytometry: instrumentation, applications, future trends and limitations. Springer Ser Fluoresc. 2008;6:307–342. doi: 10.1007/4243_2008_037. [DOI] [Google Scholar]
- Ibrahim SF, van den Engh G. Flow cytometry and cell sorting. Adv Biochem Eng Biotechnol. 2007;106:19–39. doi: 10.1007/10_2007_073. [DOI] [PubMed] [Google Scholar]
- Kim JS, Nitta T, Mohuczy D, O’Malley KA, Moldawer LL, Dunn WA, Jr, Behrns KE. Impaired autophagy: a mechanism of mitochondrial dysfunction in anoxic rat hepatocytes. Hepatology. 2008;47:1725–1736. doi: 10.1002/hep.22187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike M, Shibata M, Tadakoshi M, Gotoh K, Komatsu M, Waguri S, Kawahara N, Kuida K, Nagata S, Kominami E, Tanaka K, Uchiyama Y. Inhibition of autophagy prevents hippocampal pyramidal neuron death after hypoxic-ischemic injury. Am J Pathol. 2008;172:454–469. doi: 10.2353/ajpath.2008.070876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, Yoshimori T, Ohsumi Y, Tokuhisa T, Mizushima N. The role of autophagy during the early neonatal starvation period. Nature. 2004;432:1032–1036. doi: 10.1038/nature03029. [DOI] [PubMed] [Google Scholar]
- Kurz T, Terman A, Brunk UT. Autophagy, ageing and apoptosis: the role of oxidative stress and lysosomal iron. Arch Biochem Biophys. 2007;462:220–230. doi: 10.1016/j.abb.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Levine B. Cell biology: autophagy and cancer. Nature. 2007;466:745–747. doi: 10.1038/446745a. [DOI] [PubMed] [Google Scholar]
- Liu L, Fang YQ, Xue ZF, He YP, Fang RM, Li L. Beta-asarone attenuates ischemia-reperfusion-induced autophagy in rat brains via modulating JNK, p-JNK, Bcl-2 and Beclin 1. Euro J Pharmacol. 2012;680:34–40. doi: 10.1016/j.ejphar.2012.01.016. [DOI] [PubMed] [Google Scholar]
- Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–89. doi: 10.1161/01.STR.20.1.84. [DOI] [PubMed] [Google Scholar]
- Maiuri MC, Le Toumelin G, Criollo A, Rain JC, Gautier F, Juin P, Tasdemir E, Pierron G, Troulinaki K, Tavernarakis N, Hickman JA, Geneste O, Kroemer G. Functional and physical interaction between bcl-x(l) and a bh3-like domain in beclin-1. EMBO J. 2007;26:2527–2539. doi: 10.1038/sj.emboj.7601689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miracco C, Cosci E, Oliveri G, Luzi P, Pacenti L, Monciatti I, Mannucci S, De Nisi MC, Toscano M, Malagnino V, Falzarano SM, Pirtoli L, Tosi P. Protein and mRNA expression of autophagy gene Beclin 1 in human brain tumors. Int J Oncol. 2007;30:429–436. [PubMed] [Google Scholar]
- Mo ZT, Fang YQ, He YP, Zhang S. Beta-asarone attenuates Beclin-1 dependent autophagy and protects PC12 cells against oxygen-glucose deprivation and reperfusion induced injury. Acta Pharmacol Sin. 2012;33:737–742. doi: 10.1038/aps.2012.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickford F, Masliah E, Britschgi M, Lucin K, Narasimhan R, Jaeger PA, Small S, Spencer B, Rockenstein E, Levine B, Wyss-Coray T. The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid β-accumulation in mice. J Clin Invest. 2008;118:2190–2199. doi: 10.1172/JCI33585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinsztein DC. The roles of intracellular protein-degradation pathways in neurodegeneration. Nature. 2006;443:780–786. doi: 10.1038/nature05291. [DOI] [PubMed] [Google Scholar]
- Rubinsztein DC, DiFiglia M, Heintz N, Nixon RA, Qin ZH, Ravikumar B, Stefanis L, Tolkovsky A. Autophagy and its possible roles in nervous system diseases, damage and repair. Autophagy. 2005;1:11–22. doi: 10.4161/auto.1.1.1513. [DOI] [PubMed] [Google Scholar]
- Shvets E, Fass E, Elazar Z. Utilizing flow cytometry to monitor autophagy in living mammalian cells. Autophagy. 2008;4:621–628. doi: 10.4161/auto.5939. [DOI] [PubMed] [Google Scholar]
- Yoshimori T. Autophagy: a regulated bulk degradation process inside cells. Biochem Biophys Res Commun. 2004;313:453–458. doi: 10.1016/j.bbrc.2003.07.023. [DOI] [PubMed] [Google Scholar]