Abstract
The cell growth and monoclonal antibody production of the 55-6 hybridoma cell co-cultured with the murine thymoma cell line EL-4 at different initial 55-6:EL-4 ratios were investigated. Both populations were seeded in co-culture without previous stimulation and therefore with low constitutive CD40 and CD40 ligand (CD154) expression levels, and in the absence of exogenous co-stimuli. Viable cell density and growth rate data seem to suggest a competition for nutrients, which is detrimental for both cells in terms of biomass production and also of growth rate for 55-6. Final concentrations of antibody and specific antibody production rates were affected by the initial 55-6:EL-4 ratio. The 4:1 ratio yielded the highest IgG2a concentration, whereas the highest specific antibody production rate was obtained at the 2:1 ratio. Changes mainly in CD154 and also in CD40 expression in co-cultures could suggest cross-talk between both populations. In conclusion, different types of interactions are probably present in this co-culture system: competition for nutrients, cognate interaction and/or autocrine or paracrine interactions that influence the proliferation of both cells and the hybridoma antibody secretion. We are hereby presenting a pre-scale-up process that could speed up the optimization of large-scale monoclonal antibodies production in bioreactors by emulating the in vivo cell–cell interaction between B and T cells without previous stimulation or the addition of co-stimulatory molecules.
Keywords: Hybridoma, Growth rate, Antibody, Co-culture, CD40, CD154
Introduction
Monoclonal antibodies (mAbs) currently account for a significant proportion of new drugs with diagnostic and therapeutic applications. In response to the increasing demand for large quantities of cost- and time-efficient mAbs, several methods have been established for optimization of in vitro mAb production. These methods include (a) the selection of highly productive, genetically stable clones (Meng et al. 2000; Soriano et al. 2002), (b) the extension of cell survival and the reduction of apoptosis (Figueroa et al. 2004; Figueroa et al. 2007), (c) the development of cell lines expressing recombinant mAbs (i.e., CHO, etc.) (Jones et al. 2003; Deer and Allison 2004), and (d) the use of stimulatory agents modulating the cell metabolism and, perhaps, also gene expression in favour of enhanced protein production and secretion (Franěk et al. 2003; Franěk and Fussenegger 2005).
In this regard, several in vitro models have been used to reproduce CD40 activation on B-cell and B-cell hybridomas by using soluble agonists or cellular ligands (Valle et al. 1989; Bergman et al. 1996; Tu et al. 2008; Wiesner et al. 2008). The effects observed in these in vitro models after CD40 activation (enhanced cell proliferation, cell survival and antibody production), make CD40 an ideal target for increasing the monoclonal antibody production by B-cell hybridomas, which are supposed to have a component of genes from normal B cells used for cell hybridization.
In previous studies (Martín-López et al. 2007a, b) we have shown that it is possible to activate in B-cell hybridomas the same metabolic routes that take place in B cells to enhance CD40 expression, cell proliferation and mAb production by the addition of lymphocyte mitogens, such as lipopolysaccharide (LPS) and antibodies anti mouse immunoglobulin G (IgG) antibodies. A relationship between cell cycle position, CD40 expression and mAb productivity has also been observed (Martín-López et al. 2007c, 2010).
In this study we have established a model of B-cell hybridoma stimulation to increase mAb production based on the in vitro interaction between hybridoma and T cells. This model involves the co-culture of the B-cell hybridoma line 55-6 with the murine T thymoma cell line EL-4 in the absence of exogenous co-stimuli. Unlike other methodologies for mAbs production, the production of mAbs by this method consists of a short process, which eliminates the time-consuming steps of transfection of the gene of interest into the cells, the selection of clones or the addition of stimulatory agents, which all contribute to increased cost of the final product.
Materials and methods
Cell lines and cell maintenance
The cell lines used were a mouse–mouse B-cell hybridoma designated 55-6 (ATCC: CRL-2156) and a T thymoma, designated EL-4 (ATCC: TIB-39). Hybridoma 55-6 produces IgG2a monoclonal antibodies to human immunodeficiency virus type 1 (HIV-1) glycoprotein 120 (gp120). The cells were grown in RPMI 1640 supplemented with 10 % fetal bovine serum (FBS), 2.1 mM l-glutamine, 100 U/mL penicillin–streptomycin, 0.125 μg/mL amphotericin B, hypoxanthine-thymidine (HT) media supplement (50X) Hybri-Max*Gamm, and incubated at 37 °C in a 5 % CO2 atmosphere. All chemicals were from Sigma-Aldich, Inc., St. Louis, MO, USA.
Co-cultures of 55-6 and EL-4 cells
Co-cultures were carried out in batch mode in 175 cm2 static T-flasks (Nunc, Roskilde, Denmark) with 200 mL of RPMI 1640, supplemented as described above, and were prepared by mixing 55-6 and EL-4 cells at different ratios. Four initial 55-6 to EL-4 ratios were used: 4:1, 3:1, 2:1 and 1:1. These ratios were obtained by varying the initial cell number of both populations while keeping a constant initial total cell density at 5 × 104 cells/mL. Separated cultures of both cell lines 55-6 and EL-4 with an initial cell density of 5 × 104 cells/mL were used as controls. Antibody anti-CD90.2, a pan T cell marker also known as anti-Thy-1.2, was used to distinguish EL-4 cells from 55-6 cells in cultures containing both cell types, as described in the next section.
Dual-platform protocol
The number of viable cells for each population in the co-culture was determined using a ‘dual-platform’ method, which derives the absolute viable cell count for each population from a percentage obtained by flow cytometry (by staining with a cell marker plus the exclusion of dead cells using propidium iodide (PI)), and an absolute total count obtained by manual cell counts on a hemocytometer. The relative EL-4 and 55-6 viable cell number in the co-culture was determined by the staining with antibodies anti-CD90.2 and PI. Only EL-4 cells showed a surface CD90.2 expression. Moreover, the percentage of EL-4CD90.2+ cells was almost 100 % of the EL-4 population. As CD90.2 is not expressed on B-cells, this approach is a robust method to distinguish both populations in the co-culture. The numbers of total and viable 55-6 and EL-4 cells in the control cultures were determined by counts using trypan blue (0.4 %) exclusion. Specific growth rates (μ) were calculated as described elsewhere (Acosta et al., 2007) using the following equation:
 |
where XT and XV are total and viable cell densities and t is time.
Flow cytometry analysis
The surface CD90.2, CD40 and CD40 ligand (CD154) expressions for both 55-6 and EL-4 cells, alone or in co-culture, were determined by direct immunofluorescence staining using a Coulter Epics XL-MCL flow cytometer (Beckman Coulter, Inc., Fullerton, CA, USA). For these analyses, 100 μL aliquots containing approximately 1 × 106 cells were incubated with 10 μL of mAbs fluorescein isothiocyanate (FITC) conjugated to defined antigens (eBioscience, San Jose, CA, USA) at 4 °C for 30 min. After two washing steps with 2 mL of PBS/BSA, the cells were analyzed by cytofluorometry. Dead cells were excluded both by PI staining shortly before cell analysis, and by gating alive cells in regards to their different forward and side scatter signals. In order to achieve that, data acquired until 10,000 events were collected from the live gate using forward/side scatter plots. The live gate was set to exclude events with low forward scatter signals (cell debris and dead cells). The data analysis was performed using FLOWJO software (Tree Star, Inc., Ashland, OR, USA).
Determination of antibody concentration
IgG2a concentration was measured by sandwich-type ELISA using goat anti-mouse IgG2a-coated 96-well plates. The bound antibody from culture supernatants was detected by incubation with peroxidase-conjugated goat anti-mouse IgG2a. All antibodies were obtained from Bethyl Laboratories, Inc. (Montgomery, TX, USA). Tetramethyl-benzidine (Pierce, Rockford, IL, USA) was used as substrate and the absorbance measured at 450 nm. Specific antibody production rates (qmAb) were calculated as described elsewhere (Acosta et al. 2007) using the following equation:
 |
where [mAb] is the concentration of IgG2a antibody.
Results
Cell growth
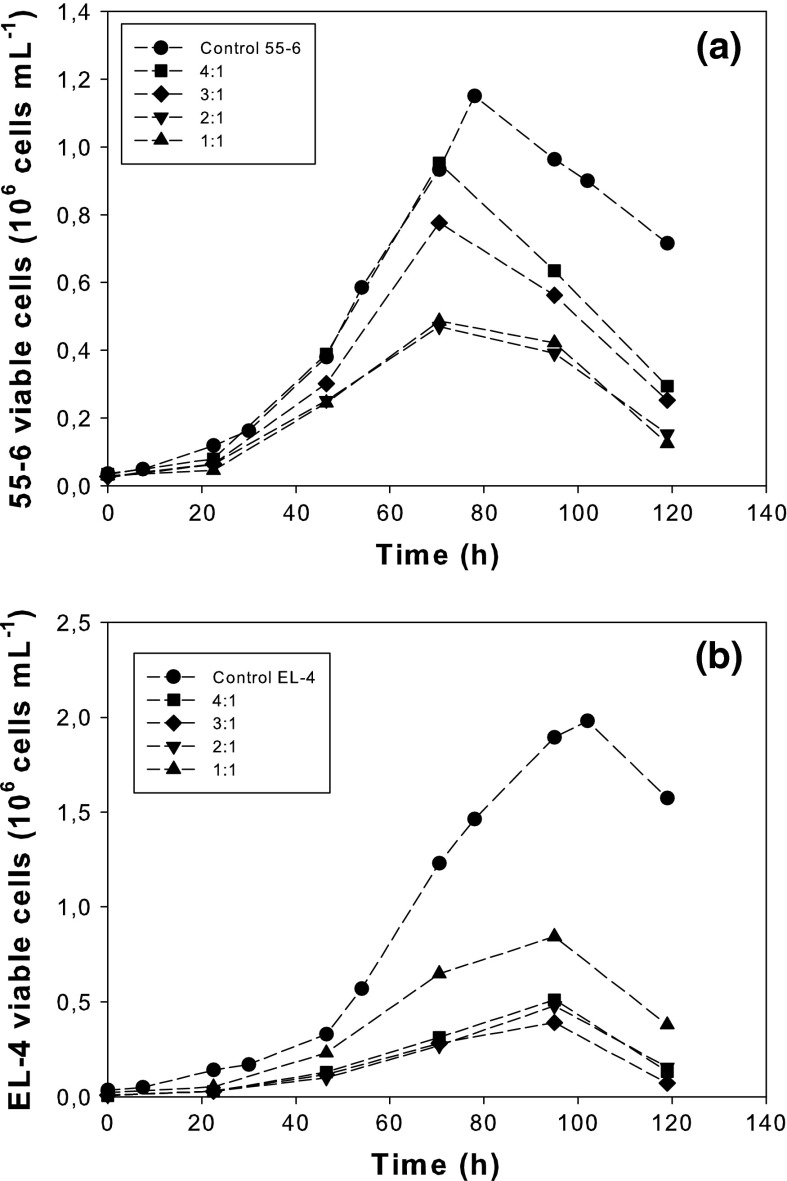
Figure 1 shows the progress of 55-6 and EL-4 viable cell concentrations in co-cultures at the different initial 55-6:EL-4 ratios. Figure 1a shows that for 55-6 control, the viable cell concentration increased continuously during the cultivation, reaching maximum values of about 1.15 × 106 cell/mL. After reaching the maximum, the concentration of viable cells declined immediately. A similar pattern was observed for 55-6 cells in co-cultures with peaks for viable cells between 70 and 80 h. Nonetheless, the maximum viable cell concentration was decreasing as the initial 55-6:EL-4 ratio was reduced. A lag phase was not observed at any 55-6:EL-4 ratio.
Fig. 1.
Viable cell concentration of 55-6 cells (a) and EL-4 cells (b). The data are from one experiment, which is representative of three independent experiments
Similar behaviour was observed for EL-4 cells (Fig. 1b). For the control of EL-4, the viable cell concentration increased continuously during the cultivation. Since EL-4 has a more efficient use of glucose and glutamine (data not shown), maximum cell concentration values of almost 2 × 106cell/mL were obtained at around 100 h. When EL-4 cells were in co-cultures, the maximum viable cell density decreased with the increase of the 55-6:EL-4 ratio.
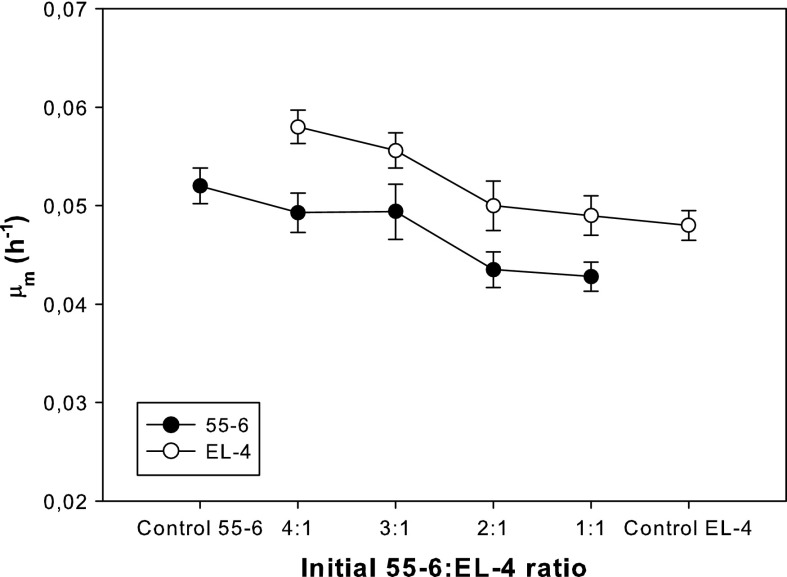
Figure 2 shows the effect of the initial 55-6:EL-4 ratio on the mean growth rate during the exponential growth phase, μm, of both 55-6 and EL-4 cell lines. Although no significant differences in μm were observed between the control cultures of 55-6 (0.052 h−1) and EL-4 (0.048 h−1), when they were co-cultured the μm of both cell lines was significantly affected. 55-6 cells were negatively affected by EL-4 cells, since their growth rate decreased as the initial 55-6:EL-4 ratio was reduced. For the 1:1 ratio, the μm (0.042 h−1) was 20 % lower than that of the 55-6 control culture. On the other hand, EL-4 cells were positively affected by 55-6 cells, since their growth rate increased when the initial 55-6:EL-4 ratio was increased. For the 4:1 ratio, the μm (0.058 h−1) was 20 % higher than that of the EL-4 control culture.
Fig. 2.
Mean specific growth rates during exponential growth phase (μm) for 55-6 and EL-4 cell lines as a function of the initial 55-6:EL-4 ratio. Values are shown as the average for triplicate cultures ± SD
Monoclonal antibody production
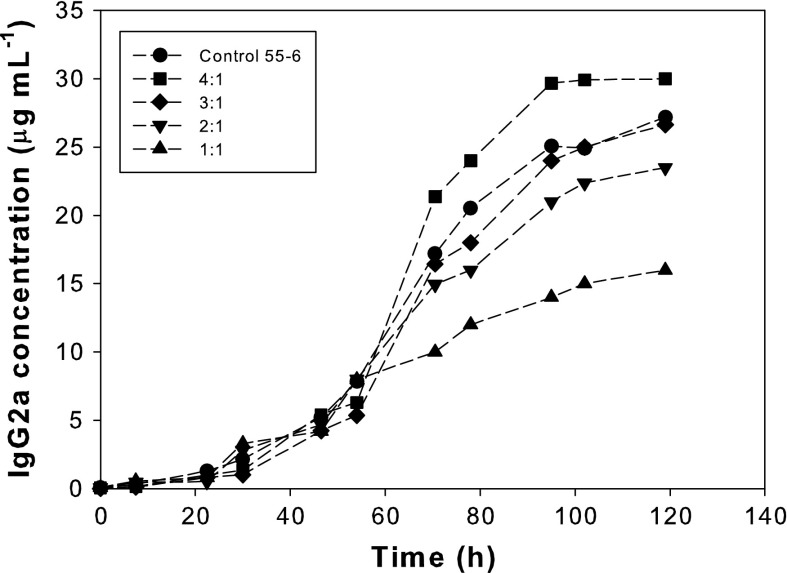
As shown in Fig. 3, the total IgG2a concentration increased with the culture time for all of the experiments. Final concentrations were affected by the initial 55-6:EL-4 ratio. The 4:1 ratio yielded the highest IgG2a concentration, even though the 55-6 control culture presented a higher initial cell density. For the rest of initial 55-6:EL-4 ratios, the final IgG2a concentration diminished as the initial ratio was reduced.
Fig. 3.
Time course of IgG2a concentration for the 55-6 control culture and co-cultures carried out at different initial 55-6:EL-4 ratios. The data are from one experiment, which is representative of three independent experiments
The effect of the co-culture on IgG2a productivity can be seen in Fig. 4, which shows the mean specific IgG2a productivity of the 55-6 cells during the exponential growth phase for the control and co-cultures. IgG2a productivity increased as the initial 55-6:EL-4 ratio was reduced from 4:1 to 2:1, reaching a maximum value of 1.1 × 10−6 μg/cell h, twice the value obtained in the control culture (0.54 × 10−6 μg/cell h). A further reduction to 1:1 produced a decrease in IgG2a productivity.
Fig. 4.
Mean specific IgG2a productivity during the exponential growth phase for the 55-6 control culture and co-cultures carried out at different initial 55-6:EL-4 ratios. Values are shown as the average for triplicate cultures ± SD
Regulation of surface CD40 and CD154 expression on 55-6/EL-4 cell interaction
In order to study the regulation of CD40 and CD154 expression on 55-6 and EL-4 cells, CD40 and CD154 were determined on the cell surface of 55-6 and EL-4 cells in control cultures and in co-cultures at a 2:1 ratio (in which the highest IGg2a productivity was reached).
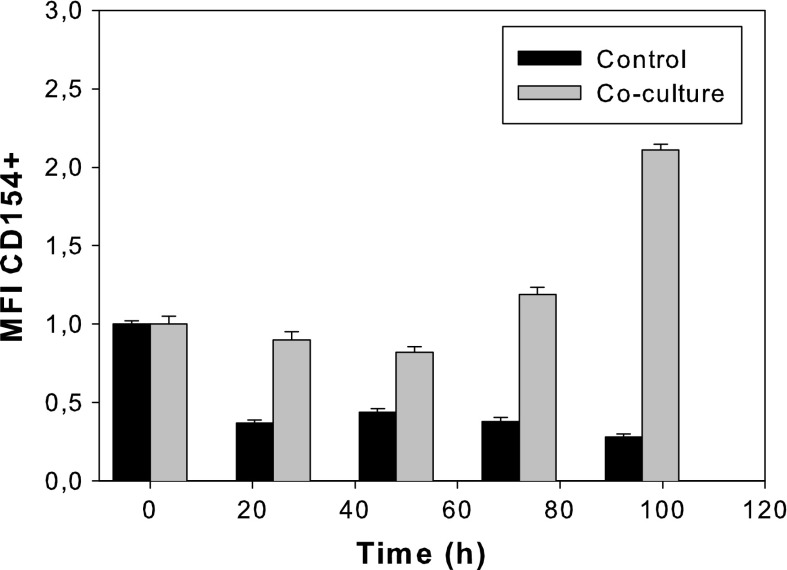
As evident from Fig. 5, the expression of CD40 in the 55-6 control culture was low. No significant changes were observed in the co-culture, in which a similar or slightly reduced CD40 expression compared to that of the control culture was obtained. Figure 6 indicates that the CD154 expression by EL-4 cells increased over 100 % in the presence of 55-6 cells in a time-dependent manner, compared to the CD154 expression by EL-4 control cells.
Fig. 5.
CD40 + MFI relative to the 55-6 control culture at time 0 h for the control culture and co-culture with an initial 55-6:EL-4 ratio of 2:1. Values are shown as the average for triplicate cultures ± SD
Fig. 6.
CD154 + MFI relative to the EL-4 control culture at time 0 h for the control culture and co-culture with an initial 55-6:EL-4 ratio of 2:1. Values are shown as the average for triplicate cultures ± SD
Discussion
In this study we have analyzed the proliferation and IgG2a production of a B-cell hybridoma, 55-6, co-cultured with the EL-4 thymoma cell line. Data of viable cell numbers and growth rates shown in Figs. 1 and 2 suggest a competition for nutrients that is detrimental to both cells in terms of biomass production, since the maximum viable cell concentrations in co-cultures are always lower than the maximum viable cell concentrations in the control cultures; and also in terms of growth rate for 55-6 cell line, since its growth rate in co-cultures is always lower than the growth rate of the control culture. Nonetheless, Fig. 2 also suggests another type of interaction between both cell lines, which counteracts the negative effect of competition and leads to an increase in the growth rate of EL-4 cells. This will be further discussed below.
Clearly, the bidirectional mixed cell culture system established in this study did not arrest the proliferation of any of the two cells. Traditionally, most mixed cell cultures are unidirectional, involving the cell cycle arrest of one population, known as feeder cells. Because feeder cells need to provide active signals to the target cells, it is important to maintain them viable and in a metabolically active state, allowing the continued expression of specific ligands or cytokines. The most commonly used techniques to inactivate the feeder cells in this manner are the exposure of cells to γ-irradiation or to mitomycin C (Kasakura and Lowenstein 1967). Although these procedures are powerful and an efficient means to support cell growth, in some cases they have the disadvantage of altering cell surface antigen expression, loss of viability, loss of function, biochemical changes and alterations in biophysical structure and subcellular components of the cells (Kasakura and Lowenstein 1967; Malinowski et al. 1992). The bidirectional, in vivo, cell–cell interaction system used in this study avoids these disadvantages. However, bidirectional systems require the optimization of the culture conditions and the initial ratio of two populations, in order to avoid domination of one of the two populations in co-culture. Furthermore, consequences of metabolic regulation in these processes could be unpredictable, like in other complex systems in which mixed cultures of cells or microorganisms are involved, and potentially including metabolic shifts and transient behaviour.
In previous works we have reported that the same metabolic routes that take place in B cells can be triggered in the B-cell hybridoma line 55-6, in order to increase cell proliferation and mAb production using B-cell mitogens (Martín-López et al. 2007a, b). Furthermore, the results in Figs. 3 and 4 clearly show that hybridoma cell-T cell interaction can also be used to increase mAb production. In terms of antibody concentration, the highest concentration of IgG2a was obtained in the co-culture with the 4:1 ratio, thus making this condition the most ideal for use in batch bioreactors. When continuous perfusion bioreactors are used, specific antibody productivity per cell becomes an important economic variable (Cohen et al. 1992), thus co-cultures with a 2:1 ratio would be a candidate for perfusion bioreactor configuration.
The data on antibody production show that, as pointed out above, another type of interaction apart from competition exists between 55-6 and EL-4. This interaction may include cognate interaction and/or interaction between T-cell-derived cytokines and hybridoma cells. Although many cell interactions or triggering molecules exist and may well contribute to T-cell-dependent hybridoma-cell stimulation, CD40:CD154 interaction plays a critical role (Bergman et al. 1996). We therefore decided to analyze CD40 and CD154 expression in 55-6 and EL-4 cells, respectively, in the control cultures and in the co-culture with the highest specific production of antibody (initial 55-6:EL-4 ratio 2:1).
The low expression of CD40 in 55-6 cells (Fig. 5) is in line with other results reported in literature (Tone et al. 2001, 2002). The signal-transducing CD40 isoform is constitutively expressed in most non-stimulated cells at low levels (Tone et al. 2001). It is thought that the CD40 gene expression occurs in a range of processes that operate at three levels of gene expression: transcriptional, post-transcriptional and post-translational regulation (Tone et al. 2002). Post-transcriptional regulation and post-translational regulation have become involved in the form of alternative splicing to reduce the amount of CD40 functional isoform, and block signalling through this receptor (Tone et al. 2001, 2002).
The observed increase in CD154 expression in a time-dependent manner by EL-4 cells (Fig. 6) is in accordance with the CD154 expression found in mouse CD4 T cells, which demonstratedly occurs in two phases (Lee et al. 2002). The early phase of CD154 expression occurs within hours of the T-cell activation and is regulated independently of the local cytokine milieu (Lee et al. 2002). In this phase, the CD154 expression diminishes rapidly and is undetectable within 12–24 h after activation. However, the second phase of CD154 expression occurs between 24 and 72 h and is highly dependent on cytokines (Skov et al. 2000; Lee et al. 2002; Snyder et al. 2007) and co-stimulatory molecules (Jenkins and Johnson 1993; Kaminski et al. 2009), which can sustain CD154 expression for several days.
These changes in CD40 and mainly in CD154 expression on the surface of 55-6 and EL-4 cells could suggest a cross-talk between both populations. The CD40 down-regulation may be due to an increase of other CD40 isoforms by a mechanism of post-transcriptional regulation, which reduces and blocks the signal-transducing CD40 isoform. CD154 up-regulation induced by the presence of 55-6 cells probably corresponds to the second phase of CD154 expression sustained by cytokines and co-stimulatory molecules released to the medium during 55-6/EL-4 cell interaction.
In conclusion, the results observed for proliferation of both cell lines, antibody production by 55-6, and CD40-CD154 expression suggest a complex relationship between the two cells, where all types of interactions described above are probably present: competition for nutrients, cognate interaction and/or autocrine or paracrine interactions that influence the proliferation of both cells and the hybridoma antibody secretion.
The production of mAb in mammalian cells consists of a long process that involves pre- and scale-up processes for the optimization of large-scale mAb production in bioreactors. The existing pre-scale-up processes, such as the transfection of the gene of interest into the cells, the selection of the most appropriate clone, and the adaptation to different culture conditions, are very time-consuming and therefore responsible for the delays observed in mAb becoming commercially available. We herewith present an efficient pre-scale-up process, which could speed up the optimization of large-scale mAb production in bioreactors by emulating the in vivo cell–cell interaction between B and T cells without previous stimulation or the addition of co-stimulatory molecules. The next step will be the scaling of the process to stirred bioreactors and the optimization of the operational conditions (agitation rate, oxygen saturation, pH, and so on). Nonetheless, more work is needed to explore the applicability of this type of mixed cultures to other hybridomas and stimulatory cells, including activated normal T cells expressing CD154.
Acknowledgments
The authors acknowledge and appreciate the financial support received from Junta de Andalucía-Spain (P07-CVI-03193) and Ministerio de Ciencia e Innovación-Spain (BIO2008-06505).
References
- Acosta A, Sánchez A, García F, Contreras A, Molina E. Analysis of kinetic, stoichiometry and regulation of glucose and glutamine metabolism in hybridoma batch cultures using logistic equations. Cytotechnology. 2007;54:189–200. doi: 10.1007/s10616-007-9089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman MC, Attrep JF, Grammer AC, Lipsky PE. Ligation of CD40 influences the function of human Ig-secreting B cell hybridomas both positively and negatively. J Immunol. 1996;156:3118–3132. [PubMed] [Google Scholar]
- Cohen DC, Mered M, Simmons R, Dadson AC, Figueroa C, Rice CW. Systems for large-scale continuous perfusion of mammalian cells grown in suspension. In: Ladis MR, Bose A, editors. Harnesing biotechnology for the 21st century. Washington: American Chemical Society; 1992. pp. 211–214. [Google Scholar]
- Deer JR, Allison DS. High-level expression of proteins in mammalian cells using transcription regulatory sequences from the Chinese hamster EF-1α gene. Biotechnol Prog. 2004;20:880–889. doi: 10.1021/bp034383r. [DOI] [PubMed] [Google Scholar]
- Figueroa B, Jr, Chen S, Oyler GA, Hardwick JM, Betenbaugh MJ. Aven and Bcl-xL enhance protection against apoptosis for mammalian cells exposed to various culture conditions. Biotechnol Bioeng. 2004;85:589–600. doi: 10.1002/bit.10913. [DOI] [PubMed] [Google Scholar]
- Figueroa B, Jr, Ailor E, Osborne D, Hardwick JM, Reff M, Betenbaugh MJ. Enhanced cell culture performance using inducible anti-apoptotic genes E1B–19 K and Aven in the production of a monoclonal antibody with Chinese Hamster Ovary cells. Biotechnol Bioeng. 2007;97:877–892. doi: 10.1002/bit.21222. [DOI] [PubMed] [Google Scholar]
- Franěk F, Fussenegger M. Survival factor-like activity of small peptides in hybridoma and CHO cells cultures. Biotechnol Prog. 2005;21:96–98. doi: 10.1021/bp0400184. [DOI] [PubMed] [Google Scholar]
- Franěk F, Eckschlager T, Katinger H. Enhancement of monoclonal antibody production by lysine-containing peptides. Biotechnol Prog. 2003;19:169–174. doi: 10.1021/bp020077m. [DOI] [PubMed] [Google Scholar]
- Jenkins MK, Johnson JG. Molecules involved in T-cell costimulation. Curr Opin Immunol. 1993;5:361–367. doi: 10.1016/0952-7915(93)90054-V. [DOI] [PubMed] [Google Scholar]
- Jones D, Kroos N, Anema R, Van Montfort B, Vooys A, Van Der Kraats S, Van Der Helm E, Smits S, Schouten J, Brouwer K, Lagerwerf F, Van Berkel P, Opstelten DJ, Logtenberg T, Bout A. High-level expression of recombinant IgG in the human cell line PER.C6. Biotechnol Prog. 2003;19:163–168. doi: 10.1021/bp025574h. [DOI] [PubMed] [Google Scholar]
- Kaminski DA, Lee BO, Eaton SM, Haynes L, Randall TD. CD28 and inducible costimulator (ICOS) signalling can sustain CD154 expression on activated T cells. Immunology. 2009;127:373–385. doi: 10.1111/j.1365-2567.2008.02991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasakura S, Lowenstein L. Irradiated and preserved leukocytes in mixed leukocyte cultures. Proc Soc Exp Biol Med. 1967;125:355–360. doi: 10.3181/00379727-125-32090. [DOI] [PubMed] [Google Scholar]
- Lee BO, Haynes L, Eaton SM, Swain SL, Randall TD. The biological outcome of CD40 signaling is dependent on the duration of CD40 ligand expression: reciprocal regulation by interleukin (IL)-4 and IL-12. J Exp Med. 2002;196:693–704. doi: 10.1084/jem.20020845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinowski K, Pullis C, Raisbeck AP, Rapaport FT. Modulation of human lymphocyte marker expression by γ irradiation and mitomycin C. Cell Immunol. 1992;143:368–377. doi: 10.1016/0008-8749(92)90033-L. [DOI] [PubMed] [Google Scholar]
- Martín-López A, García-Camacho F, Belarbi EH, Martínez-Escobar S, Contreras-Gómez A, Molina-Grima E. Induction of CD40 expression and enhancement of monoclonal antibody production on murine B cell hybridomas by cross-linking of IgG receptors. Biotechnol Prog. 2007;23:452–457. doi: 10.1021/bp060292t. [DOI] [PubMed] [Google Scholar]
- Martín-López A, García-Camacho F, Contreras-Gómez A, Molina-Grima E. Enhanced monoclonal antibody production in hybridoma cells by LPS and anti-mIgG. Biotechnol Prog. 2007;23:1447–1453. doi: 10.1021/bp070191a. [DOI] [PubMed] [Google Scholar]
- Martín-López A, García-Camacho F, Contreras-Gómez A, Molina-Grima E. Effects of synchronization on CD40 expression and antibody production in hybridoma cells stimulated with anti-mIgG. Biotechnol Prog. 2007;23:958–963. doi: 10.1021/bp070087t. [DOI] [PubMed] [Google Scholar]
- Martín-López A, Sánchez-Mirón A, García-Camacho F, Contreras-Gómez A, Molina-Grima E. Effects of hydroxyurea on monoclonal antibody production induced by anti-mIgG and LPS stimulation on murine B cell hybridomas. Cytotechnology. 2010;62:205–215. doi: 10.1007/s10616-010-9278-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng YG, Liang J, Wong WL, Chisholm V. Green fluorescent protein as a second selectable marker for selection of high producing clones from transfected CHO cells. Gene. 2000;242:201–207. doi: 10.1016/S0378-1119(99)00524-7. [DOI] [PubMed] [Google Scholar]
- Skov S, Bonyhadi M, Ødum N, Ledbetter JA. IL-2 and IL-15 regulate CD154 expression on activated CD4 T cells. J Immunol. 2000;164:3500–3505. doi: 10.4049/jimmunol.164.7.3500. [DOI] [PubMed] [Google Scholar]
- Snyder JT, Shen J, Azmi H, Hou J, Fowler DH, Ragheb JA. Direct inhibition of CD40L expression can contribute to the clinical efficacy of daclizumab independently of its effects on cell division and Th1/Th2 cytokine production. Blood. 2007;109:5399–5406. doi: 10.1182/blood-2006-12-062943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano E, Borth N, Katinger H, Mattanovich D. Optimization of recombinant protein expression level in Escherichia coli by flow cytometry and cell sorting. Biotechnol Bioeng. 2002;80:93–99. doi: 10.1002/bit.10353. [DOI] [PubMed] [Google Scholar]
- Tone M, Tone Y, Fairchild PJ, Wykes M, Waldmann H. Regulation of CD40 function by its isoforms generated through alternative splicing. Proc Natl Acad Sci USA. 2001;98:1751–1756. doi: 10.1073/pnas.98.4.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tone M, Tone Y, Babik JM, Lin CY, Waldmann H. The role of Sp1 and NF-κB in regulating CD40 gene expression. J Biol Chem. 2002;277:8890–8897. doi: 10.1074/jbc.M109889200. [DOI] [PubMed] [Google Scholar]
- Tu W, Lau YL, Zheng J, Liu Y, Chan PL, Mao H, Dionis K, Schneider P, Lewis DB. Efficient generation of human alloantigen-specific CD4+ regulatory T cells from naive precursors by CD40-activated B cells. Blood. 2008;112:2554–2562. doi: 10.1182/blood-2008-04-152041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle A, Zuber CE, Defrance T, Djossou O, De Rie M, Banchereau J. Activation of human B lymphocytes through CD40 and interleukin 4. Eur J Immunol. 1989;19:1463–1467. doi: 10.1002/eji.1830190818. [DOI] [PubMed] [Google Scholar]
- Wiesner M, Zentz C, Mayr C, Wimmer R, Hammerschmidt W, Zeidler R, Moosmann A (2008) Conditional immortalization of human B cells by CD40 ligation. PLoS One 3:e1464 [DOI] [PMC free article] [PubMed]