Abstract
The number and quality of endothelial progenitor cells (EPCs) are damaged to varying degrees in patients at risk for developing atherosclerosis. The improvement of the quantity and functions of EPCs can enhance repair of injured endothelial monolayer resulting in inhibiting atherosclerosis. The purpose of this study was to investigate the effect of pinocembrin (PIN), a major flavonoid in propolis on the differentiation and biological functions of EPCs and the potential mechanisms of these effects. Flow cytometry analysis revealed that PIN treatment increased the number of CD34+, CD133+, FLK-1+, CD133+/FLK-1+ and CD34+/FLK-1+ mononuclear cells (MNCs) in the peripheral blood of apoE−/− mice compared to untreated control mice. In vitro PIN treatment significantly increased the number of CD34+, CD133+, FLK-1+ and CD133+/FLK-1+ MNCs derived from SD bone marrow compared to untreated controls by 42.1, 84.6, 165.9 and 23.1 %, respectively. Additionally, PIN can improve biological functions of EPCs, such as proliferation, migration, adhesion, and in vitro tube formation and NO release. All of these improvements were inhibited by LY294002, while L-NAME only inhibited the PIN-induced increase in EPC proliferation and adhesion. We conclude that PIN can both promote the differentiation of EPCs in vitro and ex vivo and improve the biological functions of EPCs. The PI3K-eNOS-NO signaling pathway may be involved in the PIN-induced increase in the proliferation and adhesion of EPCs.
Keywords: Pinocembrin, Endothelial progenitor cells, Mononuclear cells, CD133, FLK-1
Introduction
Impaired vascular endothelium is one of the first signs of atherosclerosis development (Ross 1993). Repair of the endothelium by neighboring endothelial cells and the mobilization of endothelial progenitor cells (EPCs) from bone marrow or other organs is important for the prevention of atherosclerosis progression (Fujiyama et al. 2003). In patients at risk for developing atherosclerosis the number and quality of EPCs are damaged to varying degrees so that the physiological equilibrium between endothelial damage and regeneration is disturbed. This leads to endothelial damage, enhanced endothelial permeability to monocytes and lipids and allows vascular smooth muscle cells to migrate to the intima and proliferate excessively, resulting in the formation of neointimal lesions. So improvement of quantity and functions of EPCs, such as proliferation, migration, adhesion, NO production and tube formation in vitro, can enhance repairment of injured endothelial monolayer, which can inhibit initiation and progression of atherosclerosis (Fujiyama et al. 2003).
Propolis is a natural substance derived from buds and exudates of certain trees and plants in the beehives. Pinocembrin (PIN, 5,7-dihydroxyflavanone), one of the major flavonoids in propolis, exhibits many pharmacological activities, including anti-inflammatory, antioxidant, anti-thrombotic, antimicrobial, anti-allergic, hepatoprotective, anti-viral, cancer chemopreventive, anti-asthmatic and endothelium-relaxation effects (Sala et al. 2003; Santos et al. 1998; Hwang et al. 2003; Pepeljnjak et al. 1985). A recent study found that low-doses of PIN provide acute neurovascular protection in a glutamate injury model partly through the inhibition of p53 expression and cytochrome c release and by changing the Bax/Bcl2 ratio (Gao et al. 2008). High doses of PIN induce mitochondrial apoptosis in a variety of cancer cells but are nontoxic to human umbilical cord endothelial cells (Kumar et al. 2007). Our previous study found that PIN treatment significantly reduced atherosclerotic plaque area, plasma lipid levels, and increased the levels of plasma NO in apoE−/− mice fed a high-fat diet for 14 weeks. In an intima damage model, mobilization of EPCs can repair damaged intima, suppress smooth muscle cell activity in the tunica media, and inhibit neointima formation. We hypothesized that the ability of PIN to inhibit the progression of atherosclerosis may be due to increases in the number and function of EPCs, leading to an increased ability to restore damaged intima. The present study finds that PIN stimulates the differentiation of bone marrow-derived MNCs into EPCs in vivo and in vitro and improves the biological functions of EPCs partly via PI3K-eNOS-NO pathway in vitro.
Materials and methods
Animals
Eight-week-old apoE−/− mice were purchased from the institute of Laboratory Animal Science, Chinese Academy of Medical Sciences and randomly separated into 3 groups (high-fat diet, high-fat diet with low dose PIN at 10 mg/kg/d, or high-fat diet with high-dose PIN at 20 mg/kg/d), 12 mice each group. PIN from propolis dissolved in honey was intragastrically administered for 14 weeks, and high-fat group was intragastrically administered with the same dosage of honey. All animal procedures conformed to the Guide for the Care and Use of Laboratory Animals published by the Taishan Medical University. The protocol was approved by the Committee on the Ethics of Animal Experiments of Taishan Medical University (Permit Number: 2011-001). All surgery was performed under chloral hydrate anesthesia, and every effort was made to minimize suffering.
Isolation and in vitro culture of rat bone marrow-derived EPCs
4–8 weeks old SD rats were sacrificed by cervical dislocation after anesthetization with intraperitoneal injection of chloral hydrate (Tianjin, China) at a dose of 380 mg/kg. Rat bone marrow-derived MNCs were isolated by density gradient centrifugation with Ficoll, as previously described (Yin et al. 2008). MNCs were seeded on fibronectin-coated plates at a density of 106 cells per cm2 in EGM-2MV (Lonza, Verviers/B, Endothelial cell basal medium-2, plus FBS, VEGF, R3-IGF-1, rhEGF, rhFGF-B, GA-1000, hydrocortisone and ascorbic acid). After 3–4 days, non-adherent cells were removed, and fresh medium was added.
Immunocytochemistry
DiI-ac-LDL uptake and FITC-UEA binding assays were performed as previously described (Yang et al. 2011). MNCs were cultured for 10 days, incubated with 2.5 mg/L DiI-ac-LDL (Molecular Probes, Eugene, OR, USA) for 2 h at 37 °C, and then fixed for 5 min with 1–2 % paraformaldehyde. The fixed cells were washed with PBS, incubated with 10 mg/L of FITC-UEA (Sigma, St. Louis, MO, USA) for 1 h at 37 °C, and analyzed using a fluorescence microscope.
After fixation in 2 % paraformaldehyde for 10 min, the differentiated MNCs were blocked with goat serum at room temperature for 30 min, incubated with anti-rat FLK-1 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and vWF (Boster Corp., Wuhan, China) for 1 h at 37 °C, washed with PBS, and then incubated with secondary antibodies conjugated with Cy3 or FITC (Santa Cruz Biotechnology) for 30 min at 37 °C. The slides were imaged using a fluorescence microscope.
Measuring the biological functions of EPCs
Cell proliferation
EPC proliferative capacity was measured using the 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay as previously described (Yin et al. 2008). After serum starvation for 24 h, cells were treated with PIN (1.25–200 mg/L, Sigma) dissolved in DMSO or pretreated with PI3-kinase inhibitor (LY294002) or NOS inhibitor (L-NAME) before treatment with PIN. After 24 h, 20 μL of MTT (5 g/L) (Sigma) was added to each well, and the cells were cultured for an additional 4 h at 37 °C. The culture medium was removed, and 150 μL of dimethyl sulfoxide (DMSO, Gibco - Life Technologies, Carlsbad, CA, USA) was added to each well. Cell proliferation was photometrically determined at a wavelength of 490 nm.
Cell adhesion
EPCs were treated with PIN (1.25–25 mg/L), LY294002 (20 μM), L-NAME (100 μM) or the same dosage of DMSO as control group, and digested with 0.25 % trypsin. Control and treated EPCs (105 cells/ml) were then seeded on 96-well plates for 30 min. Dishes were vigorously washed three times with PBS, and the number of adherent cells was calculated as the average of five fields counted by independent blinded investigators under a microscope (magnified 10×).
Cell migration
The ability of EPCs to migrate was measured using a 8 μm pore 24-well Cell Migration Assay kit (BD Biosciences, San Jose, CA, USA). EPCs pretreated with LY294002 or L-NAME for 1 h, were treated with PIN (10–20 mg/L) and digested with 0.25 % trypsin. The same number of cells in M199 was placed in the upper compartment of the transwell chamber. The lower compartment was filled with EGM-2MV. After 24 h, the upper cells were removed, and the lower cells were fixed and stained with DAPI and counted under a fluorescence microscope (magnified 10×).
Formation of tubes in vitro
An in vitro angiogenesis assay was performed as previously described (Loomans et al. 2006). EPCs pretreated with LY294002 or L-NAME for 1 h, were treated with PIN (10–20 mg/L) and digested with 0.25 % trypsin. Equal numbers of EPCs were seeded on matrigel. After 18 h, the formation of tubes in the different groups was detected under a microscope (magnified 4×). The average of the total length of complete tubes formed by cells was compared by using computer software, Image-Pro Plus.
Concentration of NO in the media
The detection of NO released from EPCs was conducted with a NO assay kit (Nanjing Jiancheng Institute of Biological Engineering, China). 100 μl of supernatant from cultured EPCs was used to detect NO levels in the different groups at 550 nm. Total protein in EPCs was extracted with RIPA lysis buffer, and quantitated by the BCA method. The NO-releasing ability of EPCs was calculated as the ratio of NO concentration (μM) to total protein (μg).
Western blotting
Total protein was extracted from EPCs with RIPA lysis buffer and quantitated using the BCA method. Protein (30 μg) was electrophoresed on a 10 % denaturing polyacrylamide gel and transferred to PVDF membrane (Millipore, Billerica, MA, USA). The membrane was incubated overnight with the following primary antibodies: rabbit anti-rat p-AKT (CST), rabbit anti-rat AKT (CST), goat anti-rat p-eNOS (Santa Cruz), and rabbit anti-rat eNOS (Santa Cruz). The membrane was then exposed to the corresponding secondary antibodies conjugated to horseradish peroxidase (Santa Cruz) and detected using the Phototope-HRP Western Detection Kit (Thermo Fisher Scientific, Waltham, MA, USA).
FCM detection of EPC surface markers
MNC surface markers from cells isolated from the peripheral blood of apoE−/− mice were detected by labeling whole blood samples with monoclonal antibody against mouse FITC-CD34 (BD), monoclonal antibody against mouse PE-FLK-1 (BD), monoclonal antibody against mouse APC-CD133 (BD) and monoclonal antibody against mouse FITC-CD45 (BD). A gating strategy and isotype-identical antibodies were employed to exclude debris and nonspecific fluorescent signals.
EPCs were treated with 10 mg/L PIN (Sigma) for 24 h. Adherent cells were digested into a single-cell suspension, blocked for 30 min and incubated with FITC-CD34 (BD), rabbit anti-rat CD133 (Abcam, Cambridge, CA, USA), and mouse anti-rat FLK-1 (Santa Cruz) separately for 30 min at 4 °C. After two PBS washes, the cells were incubated with FITC-goat anti-rabbit secondary antibody (BD) and APC-labeled goat anti-mouse secondary antibody (BD) for 30 min at 4 °C, washed twice with PBS, and fixed with 4 % paraformaldehyde. Isotype-identical antibodies, FITC-goat anti-rabbit secondary antibody and APC-labeled goat anti-mouse secondary antibody were used to exclude false positives. EPC surface markers were then analyzed by flow cytometry.
Statistical analysis
Statistical analysis was performed using the software program SPSS11.5. All data are presented as mean ± SEM. Intergroup comparisons were performed using a paired Student’s t test or ANOVA. Probability values less than 0.05 were interpreted as being statistically significant.
Results
Pinocembrin affects EPC markers in MNCs derived from the peripheral blood of apoE−/− mice and in EPCs derived from rat bone marrow
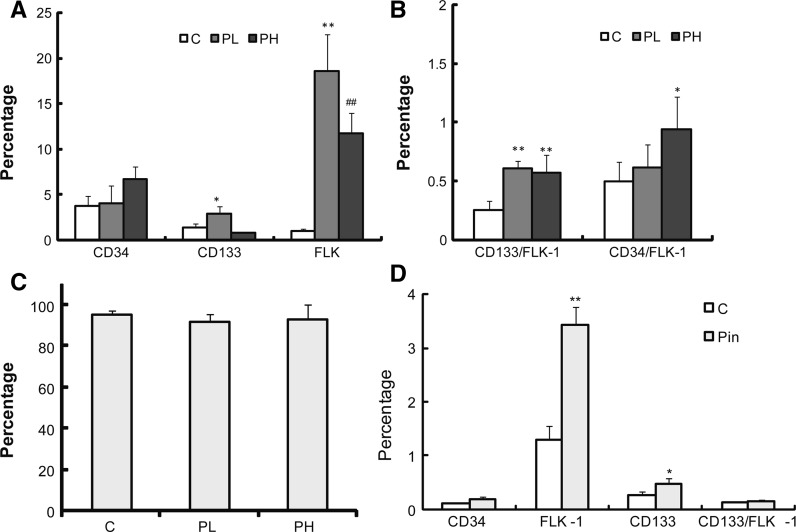
Eight-week-old apoE−/− mice were randomly separated into 3 groups: high-fat diet (C), high-fat diet with low dose PIN (10 mg/kg/d) (PL), and high-fat diet with high-dose PIN (20 mg/kg/d) group (PH). PIN was intragastrically administered for 14 weeks. The number of CD34+, CD133+, FLK-1+, CD133+/FLK-1+ and CD34+/FLK-1+ MNCs in the peripheral blood of apoE−/− mice treated with PIN was significantly increased compared to the high-fat diet control group (Fig. 1a, b), while there was no obvious change for CD45+ MNCs in PIN groups and the high-fat diet control group (Fig. 1c).
Fig. 1.
Pinocembrin (Pin) increases the number of EPC in the peripheral blood of apoE−/− mice and in Pin-treated MNCs derived from rat bone marrow. a The percentage of cells staining positive for the EPC markers CD133, CD34 and FLK-1 in peripheral blood-derived MNCs from apoE−/− mice administered with high-fat diet (c), high-fat diet with low dose PIN at 10 mg/kg/d (PL) and high-fat diet with high-dose PIN at 20 mg/kg/d (PH). Data are mean ± SEM of six to eight mice per group from three independent experiments. *p < 0.05; **p < 0.01 versus control. ##p < 0.01 versus PL. b The percentage of cells staining positive for the EPC markers CD133/FLK-1, CD34/FLK-1 in peripheral blood-derived MNCs from three groups of apoE−/− mice (C, PL and PH). Data are mean ± SEM of six to eight mice per group from three independent experiments. *p < 0.05; **p < 0.01 versus control. c The percentage of MNCs staining positive for the EPC marker CD45 in peripheral blood-derived MNCs is no significantly different in the three groups of apoE−/− mice (C, PL and PH). Data are mean ± SEM of 6–8 mice per group from three independent experiments. d The percentage of rat bone marrow-derived EPCs induced by Pin staining positive for the EPC markers CD133, CD34, FLK-1 and CD133/FLK-1 (n = 3 for each group). Pin significantly increases the expression of FLK-1 and CD133. Data are mean ± SEM from at least three independent experiments. *p < 0.05; **p < 0.01 versus control
Similar results were obtained in vitro. Rat bone marrow-derived MNCs cultured for 21 days which differentiated into late EPCs, were treated with PIN (10 mg/L) for 24 h and then analyzed by flow cytometry. Compared with the control group, the number of CD34+, CD133+, FLK-1+ and CD133+/FLK-1+ MNCs treated with PIN was increased by 42.1, 84.6, 165.9 and 23.1 %, respectively (Fig. 1d).
Identification of EPCs from rat bone marrow in EGM-2MV
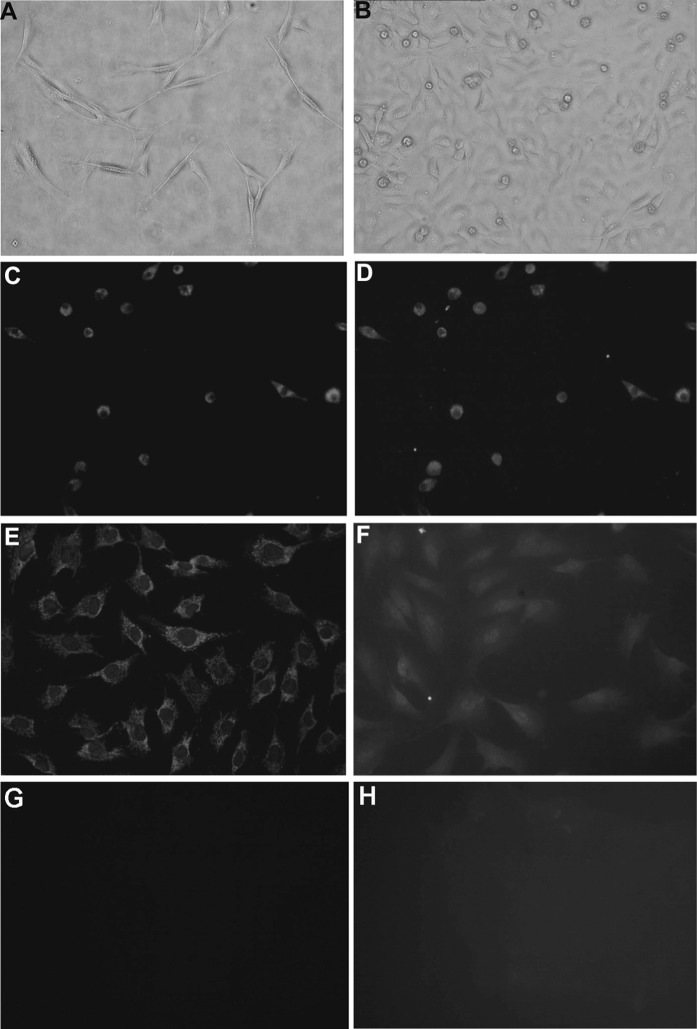
Rat bone marrow MNCs differentiated into EPCs following treatment with EGM-2MV for 11 days. The differentiated EPCs exhibited spindle or cobblestone-like morphology (Fig. 2a, b), absorbed ac-LDL and bound lectin (Fig. 2c, d). EPCs were also induced to differentiate into mature ECs with the expression of FLK-1 and vWF (Fig. 2e, f).
Fig. 2.
Identification of EPCs derived from rat bone marrow. a EPCs exhibiting spindle morphology cultured for 7 days in EGM-2MV medium (×10). b EPCs exhibiting a cobblestone-like morphology cultured for 7 days in EGM-2MV medium (×10). c Absorption of DiI-ac-LDL by EPCs cultured for 11 days (×10). d Binding of FITC-UEA-1 by EPCs cultured for 11 days (×10). e Expression of FLK-1 in EPCs cultured for 21 days (×20). f Expression of vWF in EPCs cultured for 21 days (×20). g Negative control of FLK-1. h Negative control of vWF. Above all EPCs identifications were repeated in more than three independent experiments, and almost all of the EPCs could take up DiI-ac-LDL, bind FITC-UEA-1 and express FLK-1 and vWF in every time of experiment
Pinocembrin affects the biological functions of EPCs
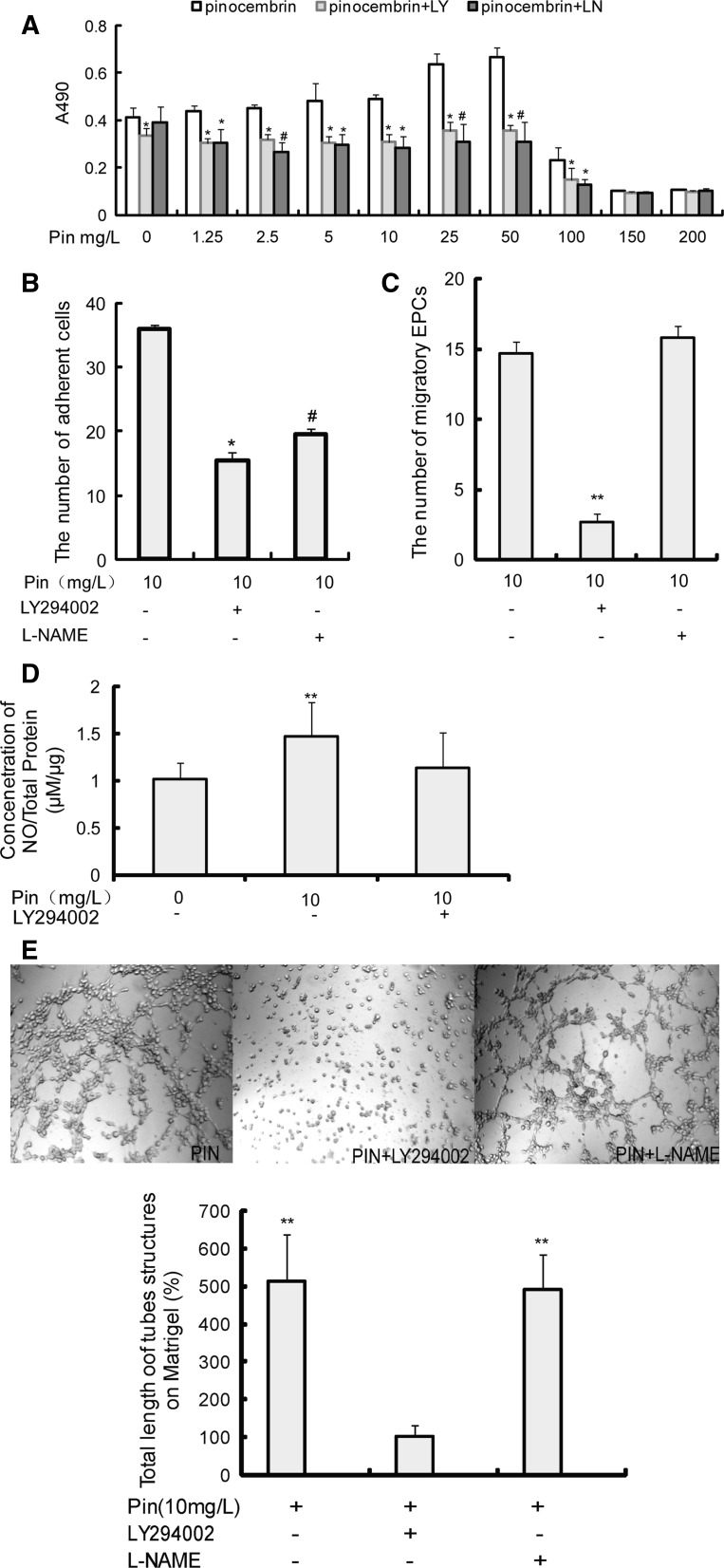
At doses lower than 50 mg/L, PIN significantly improved the proliferative ability of EPCs (Fig. 3a); however, at doses higher than 50 mg/L, PIN inhibited EPC proliferation, which might be due to the fact that PIN at higher doses promote EPCs apoptosis (Kumar et al. 2007). Similar dose-dependent effects of PIN on the migratory, adhesion and tube-forming abilities of EPCs were observed (Fig. 3b–d). In summary, PIN enhances some EPC biological functions in a dose-dependent manner.
Fig. 3.
Pin treatment affects the biological functions of EPCs. a The effects of different concentrations of Pin on the proliferative capacity of EPC. Pin (5–50 mg/L) significantly increases the ability of EPC to proliferation, while Pin (≥100 mg/L) significantly inhibits the ability of EPC to proliferate. Data are presented as mean ± SEM (n = 6) from at least three independent experiments. **p < 0.01 versus Pin (0 mg/L). b Pin treatment affects the migration of EPCs. Data are presented as mean ± SEM (n = 5) from at least three independent experiments. **p < 0.01 versus Pin (0 mg/L). ##p < 0.01 versus Pin (10 mg/L). c The number of adherent cells increased with increasing concentrations of Pin. Data are mean ± SEM (n = 3) from at least three independent experiments. *p < 0.05 versus Pin (0 mg/L). d Pin-induced EPC in vitro tube formation, and the total length of tubules (% of EPCs induced by 0 mg/L Pin) was compared in each group. Data are mean ± SEM (n = 3) from at least three independent experiments. **p < 0.01 versus Pin (0 mg/L)
Pinocembrin stimulates protein kinase B (PKB/Akt), phosphorylates eNOS at serine residue 1177 and promotes NO release from EPCs
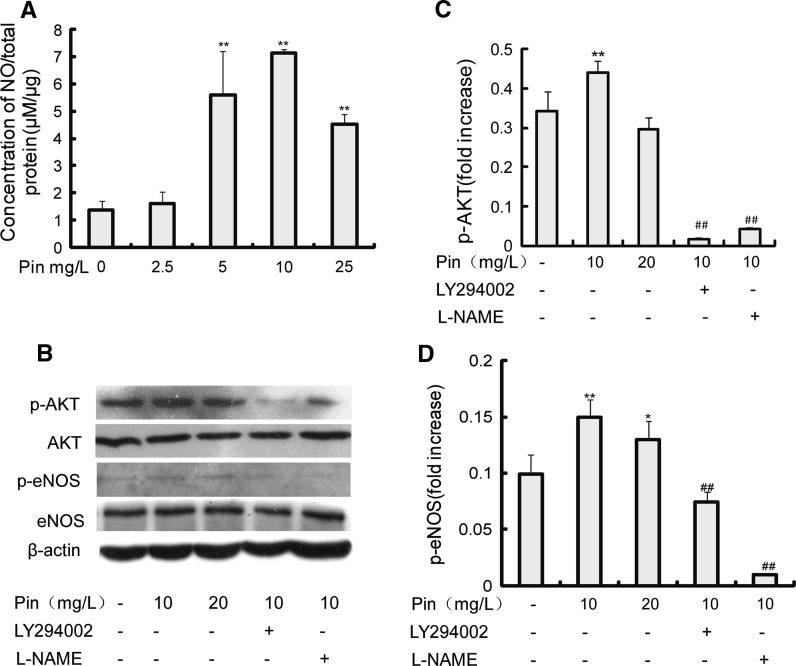
PIN treatment increased NO release from EPCs (Fig. 4a). The expression patterns of some proteins in the PI3K-eNOS-NO signaling pathway were identified. Expression of p-Akt (Fig. 4b, c) and p-eNOS (Fig. 4b, d) was increased in EPCs treated with PIN compared with EPCs cultured in basic medium (BM, M199 with 10 % FBS).
Fig. 4.
Pin increases the expression of p-AKT and p-eNOS, and stimulates NO release from EPCs. a The effect of Pin treatment on the concentration of NO released from rat bone marrow-derived EPCs. Data are mean ± SEM (n = 4) from at least three independent experiments. **p < 0.01 versus Pin (0 mg/L). b Western blot showing the expression levels of p-AKT, AKT, p-eNOS, eNOS and β-actin in EPCs. Western blot results are from three independent experiments. c, d, The density ratio of p-AKT and p-eNOS to β-actin. Administration of Pin significantly upregulate the expression level of p-AKT and p-eNOS, both PI3-kinase inhibitor (LY294002) and NOS inhibitor (L-NAME) significantly inhibit Pin-induced activation of p-eNOS. Results shown are mean ± SEM from three independent experiments. *p < 0.05; **p < 0.01 versus Pin (0 mg/L). ##p < 0.01 versus Pin (10 mg/L)
LY294002 and L-NAME partly inhibit pinocembrin-induced biological functions of EPCs
The ability of PIN to enhance EPC proliferation (Fig. 5a), adhesion (Fig. 5b), migration (Fig. 5c), and in vitro tube formation (Fig. 5e) was significantly inhibited by the PI3-kinase inhibitor LY294002 (20 μM). L-NAME (100 μM), a NOS inhibitor, partly inhibited the effect of PIN on EPC proliferation (Fig. 5a) and adhesion (Fig. 5b) but had no significant effect on the ability of PIN to increase EPC migration (Fig. 5c) or in vitro tube formation (Fig. 5e). When LY294002 was added to cells prior to the addition of PIN, the concentration of NO released from EPCs was not increased (Fig. 5d), and p-Akt (Fig. 4b, c) and p-eNOS (Fig. 4b, d) expression decreased.
Fig. 5.
Pin-induced upregulation of biological functions of EPCs are partially inhibited by LY294002 and L-NAME. a LY294002 (20 μM) and L-NAME (100 μM) block the Pin-induced increase in the proliferative capacity of EPCs. Data are mean ± SEM (n = 6) from three independent experiments. *p < 0.05 versus Pin, #p < 0.05 versus Pin + LY. b LY294002 (20 μM) and L-NAME (100 μM) block the Pin-induced increase in the attachment ability of EPCs. Data are mean ± SEM (n = 3) from three independent experiments. *p < 0.05 versus Pin (10 mg/L). #p < 0.05 versus Pin + LY. c LY294002 (20 μM), but not L-NAME (100 μM), blocks Pin-induced increase in EPC migratory capacity. Data are mean ± SEM (n = 5) from three independent experiments. **p < 0.01 versus Pin (10 mg/L). d LY294002 blocked the Pin-induced increase in EPC NO release. Data are mean ± SEM (n = 4) from three independent experiments. **p < 0.01 versus Pin (0 mg/L). e LY294002, but not L-NAME, blocks the Pin-induced increase in the in vitro tube formation of EPCs. The total length of tubules (% of EPCs in Pin + LY group) was compared. Data are mean ± SEM (n = 3) from three independent experiments. **p < 0.01 versus Pin + LY
Discussion
Classical risk factors for atherosclerosis, such as smoking, hypercholesterolemia, diabetes, and hypertension, have been shown to decrease the number and function of EPCs, leading to the impairment of the endogenous repair system in the endothelial monolayer (Vasa et al. 2001; Chen et al. 2004). Therefore, the ability to restore the number and function of EPCs would provide novel therapies for the treatment of atherosclerotic and ischemic diseases. The number and function of EPCs has been shown to be increased by certain drugs such as aspirin, calcium antagonists, and PDE5 inhibitors (Chen et al. 2006; Sugiura et al. 2008; Dussault et al. 2009). Although the effect of PIN on EPCs has not been studied previously, Du suggests that PIN induces the relaxation of rat aortic rings through endothelium-dependent and -independent pathways (Zhu et al. 2007) and that it exerts an acute neurovascular protective effect against permanent focal cerebral ischemia (Guang and Du 2006). Our group members have found that PIN can significantly reduce atherosclerotic plaque area. In the present study we find that PIN treatment in apoE−/− mice increases EPC numbers in peripheral blood. To further characterize the effect of PIN on EPC differentiation, we isolated SD rat bone marrow MNCs and observed that PIN treatment stimulated the differentiation of MNCs into EPCs. We suggest that the decrease in atherosclerotic plaque area in PIN-treated groups is possibly associated with the differentiation of MNCs into EPCs. The signaling pathways involved in PIN-induced EPC differentiation in vivo and in vitro will be investigated in future studies.
At present, no specific antigen is used to identify EPCs in peripheral blood (Schatteman et al. 2007). CD34 and CD133 have generally been used as markers of progenitor and hematopoietic stem cells (Murohara 2001). CD34 is expressed at low levels in mature endothelial cells (Fadini et al. 2005), while CD133 is absent from mature endothelial and monocytic cells (Salven et al. 2003). FLK-1 is usually considered an endothelial cell marker. Thus, CD34+/FLK-1+ or FLK-1+/CD133+ cells generally have been recognized as EPCs. Huang et al. (2010) has defined circulating EPCs as FLK-1+/CD133+, CD34+/CD133+, CD34+/FLK-1+. Urbich and Dimmeler propose that CD133+/FLK-1+ cells are immature progenitor cells, whereas CD34+/FLK-1+ cells may represent cells shed from the vessel wall (Urbich and Dimmeler 2004). In our study, we utilized CD34+/FLK-1+ and FLK-1+/CD133+ as markers of circulating EPCs, and found that PIN treatment significantly increased the number of circulating CD34+, FLK-1+, CD133+, CD34+/FLK-1+ and FLK-1+/CD133+ cells in peripheral blood. Additionally we found that there was no obvious change for CD45+ MNCs in PIN groups and the high-fat diet control group.
Some drugs, such as the platelet inhibitor aspirin, the HMG-CoA reductase-inhibitor statins, and the calcium antagonist nifedipine, can increase EPC numbers, and enhance the proliferation, migration, adhesion and vasculogenic capacity of EPCs through the PI3K/AKT/eNOS pathway. A PPARγ agonist increased circulating and cultured EPC levels and promoted EPC hypoxia-directed migration in a PI3K, but not eNOS, -dependent manner (Gensch et al. 2007). The ability of telmisartan, an angiotensin II receptor blocker, to improve the migration, apoptosis, and colony-forming and neovascularization abilities of EPCs, depends on the PI3K/AKT and PPARγ pathways but does not upregulate eNOS expression (Honda et al. 2009). In this study, we found that PIN significantly increased the proliferation, migration, adhesion and vasculogenic capacity of EPCs, and increased p-eNOS expression and NO release levels. LY294002, a PI3K inhibitor, inhibited the PIN-induced increase in EPC proliferation, migration, adhesion, NO release levels and the formation of tubes in vitro. Yin reported that inhibition of NO can attenuate the adhesion-promoting effects of AngII on EPCs (Yin et al. 2008), and Huang et al. showed that EPC proliferation is dependent on the PI3K/AKT/eNOS pathway (Huang et al. 2010). In the present study, we found that L-NAME inhibited the ability of PIN to increase EPC proliferation and adhesion, but it did not significantly inhibit the effect of PIN on EPC migration and in vitro tube formation. It is possible that PI3K affects EPC migration and tube formation through downstream signaling pathways not dependent on the PI3K/AKT/eNOS pathway. This study found that LY294002, a PI3K inhibitor, blocked PIN-induced eNOS phosphorylation at serine residue 1177, which indicates that the effect of PIN on eNOS activity is dependent on PI3K/AKT. These findings are consistent with the results from Dimmeler et al. showing that VEGF actives PI3K/AKT to phosphorylate eNOS at serine residue 1177 (Dimmeler et al. 1999).
Many signaling pathways, such as P38 MAPK (Tang et al. 2011), Notch/Jagged1 (Ii et al. 2010), and RhoA/Rock (Xu et al. 2009), influence the differentiation and biological functions of EPCs. Whether PIN-induced regulation of EPCs involves other signaling pathways remains to be investigated.
In the initiation of atherosclerosis, injured endothelial monolayer was repaired with circulating EPCs and bone marrow-derived EPCs to inhibit the atherosclerosis progression. The level of circulating EPCs can predict atherosclerotic disease progression and future cardiovascular events (Werner et al. 2005). Therefore improving the level and function of circulating EPCs by pharmacological strategies may provide new insights into clinical therapeutic potential. In this study, PIN can stimulate the differentiation of MNCs into EPCs both in vivo and in vitro, and improve the biological functions of bone marrow-derived EPCs in a PI3K-dependent manner. PIN-induced effects on EPC proliferation and adhesion involves the PI3K/AKT/eNOS pathway. These findings may explain the inhibitory effect of PIN on atherosclerosis in ApoE−/− mice, and further provide a new pharmacological strategy to inhibite of atherosclerosis progression through enhancing the level and function of EPCs.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No. 30971098), the Province Natural Science Foundation of Shandong (No. Z2008C03), the Province Natural Science Foundation of Shandong (No. ZR2012HL18), Taishan Scholars Project Funding from Shandong Province and Natural Science Foundation of Taishan Medical University (2011ZR004). Science and technology plan projects of education department of Shandong (No. J07YD03).
Abbreviations
- MNCs
Mononuclear cells
- EPCs
Endothelial progenitor cells
- eNOS
Endothelial nitric oxide synthase
- FCM
Flow cytometry
- ac-LDL
Acetylation-low-density lipoprotein
References
- Chen JZ, Zhang FR, Tao QM, Wang XX, Zhu JH. Number and activity of endothelial progenitor cells from peripheral blood in patients with hypercholesterolaemia. Clin Sci (Lond) 2004;107:273–280. doi: 10.1042/CS20030389. [DOI] [PubMed] [Google Scholar]
- Chen TG, Chen JZ, Xie XD. Effects of aspirin on number, activity and inducible nitric oxide synthase of endothelial progenitor cells from peripheral blood. Acta Pharmacol Sin. 2006;27:430–436. doi: 10.1111/j.1745-7254.2006.00298.x. [DOI] [PubMed] [Google Scholar]
- Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- Dussault S, Maingrette F, Menard C, Michaud SE, Haddad P, Groleau J, Turgeon J, Perez G, Rivard A. Sildenafil increases endothelial progenitor cell function and improves ischemia-induced neovascularization in hypercholesterolemic apolipoprotein E-deficient mice. Hypertension. 2009;54:1043–1049. doi: 10.1161/HYPERTENSIONAHA.109.139451. [DOI] [PubMed] [Google Scholar]
- Fadini GP, Agostini C, Avogaro A. Endothelial progenitor cells and vascular biology in diabetes mellitus: current knowledge and future perspectives. Curr Diabetes Rev. 2005;1:41–58. doi: 10.2174/1573399052952640. [DOI] [PubMed] [Google Scholar]
- Fujiyama S, Amano K, Uehira K, Yoshida M, Nishiwaki Y, Jin D, Takai S, Miyazaki M, Egashira K, Imada T, Iwasaka T, Matsubara H. Bone marrow monocyte lineage cells adhere on injured endothelium in a monocyte chemoattractant protein-1-dependent manner and accelerate reendothelialization as endothelial progenitor cells. Circ Res. 2003;93:980–989. doi: 10.1161/01.RES.0000099245.08637.CE. [DOI] [PubMed] [Google Scholar]
- Gao M, Zhang WC, Liu QS, Hu JJ, Liu GT, Du GH. Pinocembrin prevents glutamate-induced apoptosis in SH-SY5Y neuronal cells via decrease of bax/bcl-2 ratio. Eur J Pharmacol. 2008;591:73–79. doi: 10.1016/j.ejphar.2008.06.071. [DOI] [PubMed] [Google Scholar]
- Gensch C, Clever YP, Werner C, Hanhoun M, Bohm M, Laufs U. The PPAR-gamma agonist pioglitazone increases neoangiogenesis and prevents apoptosis of endothelial progenitor cells. Atherosclerosis. 2007;192:67–74. doi: 10.1016/j.atherosclerosis.2006.06.026. [DOI] [PubMed] [Google Scholar]
- Guang HM, Du GH. Protections of pinocembrin on brain mitochondria contribute to cognitive improvement in chronic cerebral hypoperfused rats. Eur J Pharmacol. 2006;542:77–83. doi: 10.1016/j.ejphar.2006.04.054. [DOI] [PubMed] [Google Scholar]
- Honda A, Matsuura K, Fukushima N, Tsurumi Y, Kasanuki H, Hagiwara N. Telmisartan induces proliferation of human endothelial progenitor cells via PPARgamma-dependent PI3K/Akt pathway. Atherosclerosis. 2009;205:376–384. doi: 10.1016/j.atherosclerosis.2008.12.036. [DOI] [PubMed] [Google Scholar]
- Huang PH, Chen YH, Tsai HY, Chen JS, Wu TC, Lin FY, Sata M, Chen JW, Lin SJ. Intake of red wine increases the number and functional capacity of circulating endothelial progenitor cells by enhancing nitric oxide bioavailability. Arterioscler Thromb Vasc Biol. 2010;30:869–877. doi: 10.1161/ATVBAHA.109.200618. [DOI] [PubMed] [Google Scholar]
- Hwang EI, Kaneko M, Ohnishi Y, Horinouchi S. Production of plant-specific flavanones by Escherichia coli containing an artificial gene cluster. Appl Environ Microbiol. 2003;69:2699–2706. doi: 10.1128/AEM.69.5.2699-2706.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ii M, Takeshita K, Ibusuki K, Luedemann C, Wecker A, Eaton E, Thorne T, Asahara T, Liao JK, Losordo DW. Notch signaling regulates endothelial progenitor cell activity during recovery from arterial injury in hypercholesterolemic mice. Circulation. 2010;121:1104–1112. doi: 10.1161/CIRCULATIONAHA.105.553917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar MA, Nair M, Hema PS, Mohan J, Santhoshkumar TR. Pinocembrin triggers Bax-dependent mitochondrial apoptosis in colon cancer cells. Mol Carcinog. 2007;46:231–241. doi: 10.1002/mc.20272. [DOI] [PubMed] [Google Scholar]
- Loomans CJ, Wan H, de Crom R, van Haperen R, de Boer HC, Leenen PJ, Drexhage HA, Rabelink TJ, van Zonneveld AJ, Staal FJ. Angiogenic murine endothelial progenitor cells are derived from a myeloid bone marrow fraction and can be identified by endothelial NO synthase expression. Arterioscler Thromb Vasc Biol. 2006;26:1760–1767. doi: 10.1161/01.ATV.0000229243.49320.c9. [DOI] [PubMed] [Google Scholar]
- Murohara T. Therapeutic vasculogenesis using human cord blood-derived endothelial progenitors. Trends Cardiovasc Med. 2001;11:303–307. doi: 10.1016/S1050-1738(01)00128-1. [DOI] [PubMed] [Google Scholar]
- Pepeljnjak S, Jalsenjak I, Maysinger D. Flavonoid content in propolis extracts and growth inhibition of Bacillus subtilis. Pharmazie. 1985;40:122–123. [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Sala A, Recio MC, Schinella GR, Manez S, Giner RM, Cerda-Nicolas M, Rosi JL. Assessment of the anti-inflammatory activity and free radical scavenger activity of tiliroside. Eur J Pharmacol. 2003;461:53–61. doi: 10.1016/S0014-2999(02)02953-9. [DOI] [PubMed] [Google Scholar]
- Salven P, Mustjoki S, Alitalo R, Alitalo K, Rafii S. VEGFR-3 and CD133 identify a population of CD34+ lymphatic/vascular endothelial precursor cells. Blood. 2003;101:168–172. doi: 10.1182/blood-2002-03-0755. [DOI] [PubMed] [Google Scholar]
- Santos AC, Uyemura SA, Lopes JL, Bazon JN, Mingatto FE, Curti C. Effect of naturally occurring flavonoids on lipid peroxidation and membrane permeability transition in mitochondria. Free Radic Biol Med. 1998;24:1455–1461. doi: 10.1016/S0891-5849(98)00003-3. [DOI] [PubMed] [Google Scholar]
- Schatteman GC, Dunnwald M, Jiao C. Biology of bone marrow-derived endothelial cell precursors. Am J Physiol Heart Circ Physiol. 2007;292:H1–H18. doi: 10.1152/ajpheart.00662.2006. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Kondo T, Kureishi-Bando Y, Numaguchi Y, Yoshida O, Dohi Y, Kimura G, Ueda R, Rabelink TJ, Murohara T. Nifedipine improves endothelial function: role of endothelial progenitor cells. Hypertension. 2008;52:491–498. doi: 10.1161/HYPERTENSIONAHA.108.111914. [DOI] [PubMed] [Google Scholar]
- Tang Y, Huang B, Sun L, Peng X, Chen X, Zou X. Ginkgolide B promotes proliferation and functional activities of bone marrow-derived endothelial progenitor cells: involvement of Akt/eNOS and MAPK/p38 signaling pathways. Eur Cell Mater. 2011;21:459–469. doi: 10.22203/ecm.v021a34. [DOI] [PubMed] [Google Scholar]
- Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95:343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1–E7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, Bohm M, Nickenig G. Circulating endothelial progenitor cells and cardiovascular. N Engl J Med. 2005;353:999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- Xu S, Jiang H, Wu B, Yang J, Chen S. Urotensin II induces migration of endothelial progenitor cells via activation of the RhoA/Rho kinase pathway. Tohoku J Exp Med. 2009;219:283–288. doi: 10.1620/tjem.219.283. [DOI] [PubMed] [Google Scholar]
- Yang N, Li D, Jiao P, Chen B, Yao S, Sang H, Yang M, Han J, Zhang Y, Qin S. The characteristics of endothelial progenitor cells derived from mononuclear cells of rat bone marrow in different culture conditions. Cytotechnology. 2011;63:217–226. doi: 10.1007/s10616-010-9329-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin T, Ma X, Zhao L, Cheng K, Wang H. Angiotensin II promotes NO production, inhibits apoptosis and enhances adhesion potential of bone marrow-derived endothelial progenitor cells. Cell Res. 2008;18:792–799. doi: 10.1038/cr.2008.69. [DOI] [PubMed] [Google Scholar]
- Zhu XM, Fang LH, Li YJ, Du GH. Endothelium-dependent and -independent relaxation induced by pinocembrin in rat aortic rings. Vascul Pharmacol. 2007;46:160–165. doi: 10.1016/j.vph.2006.09.003. [DOI] [PubMed] [Google Scholar]