Abstract
B cells have been implicated both with pathogenic as well as protective capabilities in induction and regulation of autoimmune diseases. Rheumatoid arthritis (RA) is an autoimmune disease that occurs more often in women than men. A significant role of B cells as antibody producing and antigen-presenting cells has been demonstrated in RA. Predisposition to RA is associated with the presence of certain HLA class II alleles that share sequences with DRB1*0401. To determine the role of HLA genes and B cells in vivo, we have generated transgenic mice carrying HLA genes, DRB1*0401 and DQ8, known to be associated with susceptibility to RA. Humanized mice can be induced to develop arthritis that mimics human disease in clinical, histopathological and sex bias. Effect of hormones on immune cells and their function has been described in humans and mice and has been suggested to be the major reason for female bias of autoimmune diseases. An immune response to an antigen requires presentation by HLA molecules thus suggesting a critical role of MHC in combination with sex hormones in susceptibility to develop rheumatoid arthritis. Based on our observations, we hypothesize that modulation of B cells by estrogen, presentation of modified antigens by DR4 and production of antigen-specific B cell modulating cytokines leads to autoreactivity in females. These data suggest that considering patient’s sex may be crucial in selecting the optimal treatment strategy. Humanized mice expressing RA susceptible and resistant haplotype provide a means to investigate mechanism sex-bias of arthritis and future strategies for therapy.
Keywords: B regulatory cells, antigen presentation, MHC polymorphism, HLA transgenic mice, Rheumatoid arthritis
INTRODUCTION
B cells play a central role in the adaptive immune response to antigens. Historically, the major function of B cells for antibody production by plasma cells and generation of B memory cells for enhanced recall response to an antigen has been well recognized. Their role as antibody producing cells in autoimmune diseases like systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA) is well accepted. However, besides producing antibodies, studies in animal models have shed light on the importance of B cells as antigen presenting cells and influencing immune response via cytokine production and regulating lymphoid tissue architecture. Recent advances in B-cell mediated immune responses have brought forth an important role of B cells in immune regulation and protection from diseases. Thus B cells have both pathogenic and immunoregulatory functions in the induction and maintenance of autoimmune diseases. B cells undergo many developmental checkpoints under normal conditions from immature to mature stage to prevent the development of autoimmunity. Healthy humans do have self-reactive B cells which escape negative selection and are silent but may become activated under certain conditions and in genetically predisposed individuals, a break in tolerance may lead to production of autoantibodies and autoimmunity. Autoimmune diseases like SLE and RA occur more frequently in women than men. Many studies have shown a hormonal regulation of B cells and their functions. However, the mechanism of action by which B cells contribute to a sex-bias disease is still poorly understood and remains an active area of research.
Peripheral B cell maturation and checkpoints for autoimmunity
B cells are generated throughout life in the bone marrow (BM). Upon completing B cell maturation, immature naïve B cells have two choices; either they stay in the marrow and further mature [1, 2] or they exit the marrow, enter the blood and travel to the secondary lymphoid organs. Peripheral B maturation is BCR and antigen dependent. Conventional B cells, or B2 cells, reside in the spleen, lymph nodes, and blood and are of two major types, follicular (FO) or marginal zone (MZ) B cells. MZ B cells respond to T-independent antigen whereas FO B cells require T cell help, undergo affinity maturation, and are the primary source of long lived plasma cells and memory B cells.
Peripheral B cell maturation proceeds through a series of developmental intermediates including transitional (T) subsets (T1-3), FO subsets (type I and II), MZ precursors and MZ B cells[3]. A variety of studies in genetically manipulated mouse models support the notion that BCR signal strength regulates the FO versus MZ fate decision [3]). However, FO versus MZ cell fate bias has been observed in mature B cells under conditions of lymphopenia or impaired B cell development, and is believed to reflect enhanced homeostatic proliferation [4, 5]. Recirculating FO B cells can adopt a MZ phenotype upon transfer into lymphopenic hosts[6]. The production of naïve B lymphocytes in BM is severely suppressed during pregnancy or after experimental elevation of estrogen, while mature recirculating B cells are unaffected[7, 8]. Together these findings provide evidence that mature B cells are sensitive and responsive to physiologic cues.
Effective humoral immune responses are contingent on the ability to generate a diverse and highly specific antibody repertoire. To limit the generation and/or survival of autoreactive B cells, the B cell maturation pathway has multiple tolerance checkpoints (Figure1). Central tolerance mechanisms limit the exit of naïve B cells with self-reactive BCRs from BM. Central tolerance, although highly effective, is restricted to antigens expressed and/or encountered in BM. Therefore, small numbers of self-reactive B cells escape central tolerance as transitional immature B cells. These cells are removed by a second tolerance checkpoint in the periphery, recognition of self-antigen in the absence of co-stimulatory signals provided by T cells. However, self-reactive peripheral B cells do escape tolerance checkpoints[9, 10]. Defects in BCR signaling can affect receptor editing and counterselect for autoreactive B cells. Heightened BAFF levels can promote the survival and differentiation of self-reactive anergic B cells. A high proportion of mature naïve self reactive B cells have been found in patients deficient in CD40L or MHC Class II patients with Bare Lymphocyte Syndrome, revealing that CD40/CD40L interactions together with antigen presentation impact naïve peripheral B cell tolerance. Finally, sex differences in susceptibility to autoimmune disease and modulation of disease pathophysiology during pregnancy or after hormone administration are well established[11]. The decrease in production of naïve B cells in BM could alter the repertoire of antibody specificities and it is unknown whether the process of affinity maturation is altered by changes in systemic levels of sex steroids.
Figure 1.

Checkpoints for B cell tolerance during maturation.
B cells in Rheumatoid arthritis
Rheumatoid arthritis is a chronic inflammatory joint disease of unknown etiology. It occurs more often in women than men suggesting a role of sex hormones in pathogenesis. Recent studies suggest that compared to men, women tend to be younger at disease onset, have higher disease activity and disability, and lower rates of remission—yet a similar progression of erosive disease [12-15]. However, not all studies agree; one has suggested that after accounting for disease duration, sex has minimal effect on disease severity [16]. It has been suggested that bias in the definition or measurement of disease characteristics or differences in pain perception or muscular strength might, in part, account for the reported differences in disease characteristics among women and men [13, 14]. However, the variance among the published studies in the relationships of sex with disease phenotype has not been adequately explained. In attempting to understand the sources of this variance, we must consider that while immune regulation by sex hormones clearly is polarized between the sexes, estrogens and androgens to varying degrees have biochemical activity in both sexes.
Predisposition to rheumatoid arthritis has been linked to environmental factors and presence of certain Major Histocompatibility Complex (MHC) class II HLA-DRB1 locus [17]. Among the HLA-DR4 genes, DRB1*0401 (Dw4), DRB1*0404/0408 (Dw14), and DRB1*0405 (Dw15) alleles confer genetic predisposition to RA while DRB1*0402 (Dw10) does not [18]. The DQB1*0301 (DQ7) and DQB1*0302 (DQ8) genes linked with DRB1*04 (DR4) allele occur with increased frequency in some RA patients [19, 20]. Thus, the HLA class II contribution to RA predisposition may be the result of the interaction between HLA-DQ and HLA-DR molecules on both haplotypes carried by an individual. A strong correlation of RA with the class II alleles could be due to their ability to present the antigenic peptides to T cells. Published data is consistent with an involvement of T cells; specially CD4+ T cells, in pathogenesis of RA [21, 22]. However, in synovial tissue of RA patients, both T and B cell infiltrates are observed [23, 24]. B cells have been touted as the main culprits in pathogenesis of certain autoimmune diseases, like rheumatoid arthritis and systemic lupus erythematosus, mainly due to their contribution via antibody production. For decades rheumatoid factor (RF) and more recently anti-citrullinated peptide antibodies (ACPAs) produced by autoreactive B cells have been used as diagnostic markers for RA. Studies have shown that onset of RA is preceded by the presence of autoantibodies suggesting 1) a break in tolerance occurs much before the actual clinical phenotype and 2) B cells have a significant role in events leading up to development of disease (Figure 2).
Figure 2.
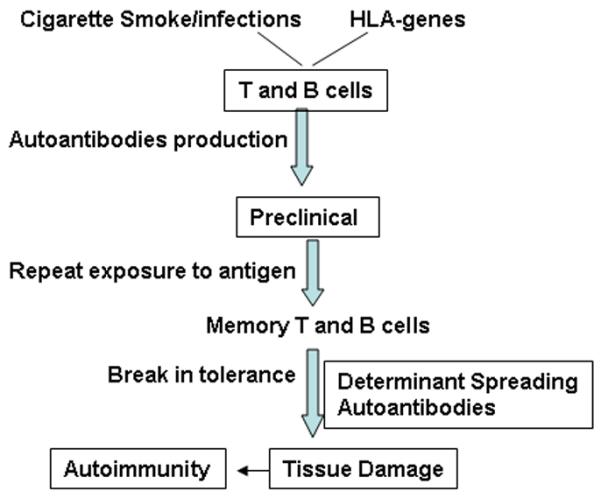
Genetic (HLA) and environmental (Cigarette smoke, infections) factors predispose an individual to develop autoimmunity. The onset of clinical symptoms of autoimmune disease are preceded by several years of activation of T and B cells leading to production of autoantibodies and ultimate a break in tolerance, tissue damage and epitope spreading of the organ-specific autoantigen.
B cells could contribute towards pathogenesis by a) producing autoantibodies directed to self-antigen such as rheumatoid factor and anti-citrullinated antibodies to various synovium-derived antigens like type II collagen, fibrinogen and filaggrin among others b) presenting antigen and activation of T cells and 3) by producing inflammatory cytokines like TNF-α [25-28]. Enhanced function of B cells resulting in higher acute phase response and increased levels of rheumatoid factor IgA has been associated with the presence of shared epitope in RA patients [29]. Microarray studies on peripheral B cells from RA patients showed that pathways controlling proliferation, activation and apoptosis of B cells are defective in these patients [30]. Serum levels of IL-5 that induces B cell proliferation and IL-10, known to induce differentiation, enhancement of class II expression and inhibition of apoptosis, were found to be increased in RA patients compared to controls [30-32]. Chemokines involved in B cell trafficking, inflammatory pathways and formation of ectopic lymphoid tissue are present with differential expression on B cells of RA patients compared to healthy controls [33]. There are indications that B cell activity is enhanced in RA and their elimination by anti-CD20 antibodies from the synovial tissue can provoke a disruption of T cell activity and production of monokines [34, 35]. Serum levels of B cell activating factor (BAFF) has been shown to regulate maturation and survival of autoreactive peripheral B cells [36, 37]. Stimulation via TLR3 has been shown to stimulate production of BAFF in synovial fibroblasts from RA patients and leads to Ig class switching to IgG/IgA in B cells [38]. Patients with RA have elevated BAFF serum levels that can result in survival of autoreactive B cell clones in them [39]. Therefore it is not surprising that depletion of B cells via anti-CD20 antibody treatment provides an attractive therapeutic option for RA. Although originally developed for the treatment of B cell lymphoma, B cell depletion therapy has been shown to be effective for many autoimmune diseases like RA, multiple sclerosis and SLE thus underpinning the role of B cells in pathogenesis of autoimmunity. It is difficult to study the kinetics of B cell activation and their differentiation into autoreactive cells and also it is not known which B cell function is most critical for autoimmunity. Animal models of disease have provided a great avenue to advance our knowledge and understand how B cells are involved in autoimmunity.
Experimental Collagen-induced arthritis and role of B cells
A critical role of B cells has been demonstrated in several models of autoimmune diseases that include lupus, arthritis and diabetes [40-43]. Collagen induced arthritis (CIA) represents an experimental autoimmune arthritis that is dependent on the MHC-restricted immune response to type II collagen, a tissue restricted organ protein. The immune reactivity to type II collagen varies between different mouse strains, thus providing a model to examine how the development of an immune response to foreign/self antigen may progress to an autoimmune response and subsequently to disease. Type II collagen constitutes 80-90% of total collagen content of hyaline cartilage found in joints. Collagen specific T cells have also been found in the synovial membrane of patients with rheumatoid arthritis [44-46]. Circulating antibodies to type II collagen have been demonstrated in the sera and synovial fluids of RA patients [47, 48]. Ours and other data have shown CIA to be a T and B cell dependent disease similar to that of RA [49, 50]. The B cell response in CIA has been shown to be directed to epitopes located at position 358-369 of CII [51]. Crystal structure of an arthritogenic anti-collagen immune complex has recently been resolved and suggests that pathogenic autoantibodies bind a conserved sequence on CII [52]. In the experimental model of arthritis, transfer of autoantibodies can induce transient and mild arthritis, presumably via formation of immune complexes that induces proinflammatory cytokines like TNFα, IL-1β and IL-8 [53, 54]. There is overwhelming evidence that B cells can present antigens to CD4+ T cells, although the role of B cells in presenting antigenic peptides to naïve CD4+ T cells has been somewhat controversial [55, 56]. Mouse studies using B-less mice have shown an important role of B cells as antigen presenting cell (APC) to autoreactive T cells [57, 58]. Depletion of B cells has been shown to reduce autoreactive T cells, suggesting a direct role of B cells in pathogenesis[59]. Recent studies suggest that B cells can act as APCs by internalizing antigen through BCR or by formation of immune complexes and their internalization through FcgR expressed on professional APCs [60, 61]. Using Balb/C mice in the proteoglycan-induced arthritis model, depletion of CD20 cells have been shown to enhance T regulatory activity thus suppressing the disease and B cell deficient Balb/c mice were resistant to proteoglycan-induced arthritis [62, 63] suggesting that B cells are required as antigen presenting cells for inducing arthritis.
The most direct example of the role of B cells in pathogenesis of arthritis comes from the KRN mouse model of spontaneous arthritis [64]. The molecular target of both T and B cells in this model is an enzyme of the glycolytic pathway, glycosyl-phosphatidyl inositol (GPI). This model showed that even though dendritic cells (DCs) are potent stimulators of T cells, TLR ligation in B cells enhances their ability to stimulate T cells and production of TNFα, suggesting a possible way by which polyclonal B cell activation contributes to disease severity. [65]. The tumor necrosis factor (TNF) cytokine superfamily plays a central role in immune regulation. B cell activating factor (BAFF) is a TNF family cytokine that binds BAFF-R on B cells and promotes activation and survival of B cells in vitro and in vivo [66, 67]. Arthritis in DBA/1 mice was found to be associated with increased expression of BAFF on dendritic cells [68]. Further, CII-immunized DBA/1 mice that were treated with recombinant BAFF showed an early onset of arthritis with a severe disease, while neutralization of BAFF by administering soluble TACI-Fc to arthritic mice inhibited proliferation of T and B cells, production of anti-collagen antibodies and arthritis progression [68-70]. Silencing of the BAFF gene locally suppresses IL-17 cytokine and ameliorates CIA in DBA/1 mice [71]. These studies show that B cells contribute to pathogenesis of collagen-induced arthritis in many different ways.
B cells as effectors in humanized model of collagen-induced arthritis
The advent of class II knock-out mice expressing human HLA-DR and HLA-DQ transgenes has significantly advanced our understanding of the role of individual HLA class II molecules in various clinical conditions. Transgenic mice expressing HLA-DQA1*0301/DQB1*0302 (DQ8) but lacking endogenous class II molecules (Aβo) have been helpful in determining how HLA alleles contribute to pathogenesis. Transgenic mice develop normally and can positively select various CD3 cells and B cells similar to that of a wild type CD57/BL6 mouse [72, 73]. To determine if accessory molecules function normally in these transgenic mice for proper folding of the HLA molecules and generation of immune response via T and B cells, DQ8 mice lacking invariant chain, DQ8.Ii−/− were generated [74]. Both strains positively selected CD8 and B220+ cells although DQ8.Ii−/− mice exhibited much lower numbers of B cells with reduced expression of DQ8 and no CD4 T cells compared to DQ8.Ii+/+ mice. Immunization of DQ8 and DQ8.Ii−/− mice with CII elicited a vigorous CD4 mediated, DQ8-restricted CII-specific cellular and humoral response which progressed to a severe form of CIA in around 70% of DQ8 mice [49, 74-76]. The arthritic paws showed cellular infiltration, marked synovitis consisting of synovial cell hyperplasia and erosion of articular cartilage. DQ8.Ii−/− mice did not develop measurable anti-CII antibodies and very low T cell response to CII and developed CIA with an incidence of 20% suggesting an important role of invariant chain for proper functioning of HLA molecules and cell selection process in transgenic mice. Studies using DQA1*0103/DQB1*0601(DQ6).Aβo mice confirmed that positive selection of cells by MHC molecules is an important phenomenon for susceptibility to or protection from arthritis [77]. HLA-DQ6 mice do not develop CIA and produce lower levels of antibodies than DQ8 mice even though the total number of T and B cells are similar to that of DQ8 mice (Figure 3), thus suggesting that positive and negative selection of T cells and B cells by HLA class II molecules might form an important basis for the presence of autoreactive cells in genetically predisposed individuals.
Figure 3.
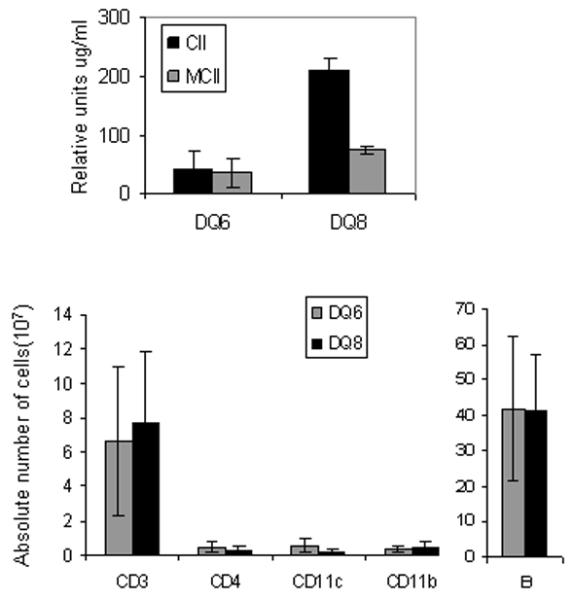
HLA-DQ8 mice are susceptible to collagen-induced arthritis and HLA-DQ6 mice are resistant. DQ8 mice produce higher amounts of anti-type II collagen antibodies against chick type II collagen (CII) and mouse CII (MCII) compared to DQ6 mice upon immunization with CII. Naïve DQ8 and DQ6 mice do not differ in absolute numbers of splenic antigen presenting cells, CD11c and B cells.
The role of B cells has been extensively studied in mouse models of lupus. In one of the lupus mouse model, activation of autoreactive B cells via endogenous Toll like receptor ligands rather than T cells has been suggested as the first step towards autoreactivity [78]. A critical role of B cells in antigen presentation along with autoantibody production has been described in both the MRL-lpr/lpr mouse model and proteoglycan induced arthritis model [42, 79, 80]. Using an Ig transgenic mouse model, B cells have been implicated in driving T cell autoimmunity prior to autoantigen presentation by DCs [58]. Requirement of B cells to initiate systemic immune response that can lead to development of CIA in DQ8 mice was studied by using DQ8 mice lacking mature B cells, DQ8.umt mice. DQ8.umt mice did not produce any autoantibodies or develop CIA suggesting, that B cells are required for induction of arthritis. Also, DQ8.umt mice generated low cellular immune response to known DQ8-restricted CII-derived peptides even though the professional APCs were present with much higher numbers than in DQ8 mice. In vivo substitution of DQ8.μmt mice with primed B cells from parent DQ8 mice restored T cell response and production of IFN-γ when challenged in vitro, confirming that B cells can contribute towards pathogenesis by presenting antigen to autoreactive T cells. For adaptive immune response, antigen presentation requires CD4 T cells and costimulatory molecules like CD28. Using DQ8 transgenic mice lacking CD4 or CD8 molecules, CD4 cells were shown to be essential for the initiation of CIA. While all the 3 strains of mice, DQ8, DQ8.CD4−/− and DQ8.CD8−/−, showed similar B cell numbers, DQ8.CD8−/− mice produced higher levels of anti-CII antibodies to self and priming CII compared to other strains. All arthritic mice produced IgM and IgG rheumatoid factors, but DQ8.CD8−/− mice also produced anti-nuclear antibodies, known to be present in lupus patients and around 30-40% of RA patients. These data suggested that CD8 T cells may have a regulatory effect, specially on activation of B cells. Upon activation, CD8 T cells produce IFNγ that can inhibit production of IL-4 and thus down regulate proliferation of B cells. Thus in RA patients, dysregulation or a defect in CD8 T cells may be the first step towards B cell activation and proliferation in certain conditions.
Role of B cells in sex-bias of arthritis
Rheumatoid arthritis affects approximately 1% of the population and occurs two to three times more often in women than in men with about 70% of patients being women. Most of the models of experimental arthritis including DQ8 mice do not develop the sex-biased arthritis that is observed in humans. A quantitative trait loci on chromosome 11 has been shown to affect incidence and severity of arthritis and anti CII antibodies in female mice in a mouse model of arthritis [81]. Although, there are not many models where arthritis is observed with a sex-bias similar to that in humans, an antigen-induced arthritis model using methylated bovine serum albumin showed severe arthritis in old females compared to young female and male mice even though antibodies and T cell response were similar in all groups [82]. Genes on the X chromosome have been shown to affect B cell populations and CIA in a rat model [83]. Enumeration of resident leukocytes has shown that the numbers of leukocytes occupying the naive peritoneal and pleural cavities is higher in female than in male mice [84], and contribute to increase in immune response in females. In humans, DR4 is associated with susceptibility to RA in most ethnic populations. Mice carrying DR4/IE transgene are susceptible to CIA though no sex bias was observed [85]. To determine the role of DR4 and B cells in arthritis, we generated DRB1*0401 and DRB1*0401/DQ8.AE−/− mice lacking all endogenous class II mice, DR4.AE−/− and DR4/DQ8.AE−/− mice. Both of these strains develop sex-biased CIA with predominantly females being affected, female to male 3:1, a ratio similar to that in RA. In humans, DR4 and DQ8 occur in linkage, making it difficult to define the role of each gene. Since DQ8 mice develop arthritis with a similar incidence in male and female mice, data in DR4 and DR4/DQ8 transgenic mice suggested a role of DR4 in rendering susceptibility to develop arthritis in female mice. Female DR4 transgenic mice had more absolute numbers of CD4+ T cells and B cells than males. In humans, a similar phenomenon has been shown with women having more absolute number of CD4 cells and expressing more potent T and B cell responses to antigens relative to men, which may contribute to autoimmunity [86]. Arthritic mice produced both IgG and IgM rheumatoid factor (RF) as well as anti-citrullinated peptide antibodies (ACPAs), with female mice producing much higher levels of autoantibodies than males. Recent data in RA patients has suggested ACPAs to be a more sensitive diagnostic marker than RF for RA. Female DR4 mice showed a higher expression level of DR4 on B cells compared to male B cells. Similar to transgenic mice, RA patients also showed higher expression level of DR4 on peripheral blood mononuclear cells in women compared to men [87]. Since the DR4 transgenic mice do not carry any endogenous class II molecules, autoimmune response is generated by a DR4-restricted antigen presentation. This led to the speculation that DR-restricted antigen presentation by B cells may contribute to autoimmune response. Presentation of an autoantigen by B cells may induce activation of T cells leading to epitope spreading and a break in tolerance.
In humans and animal models, estrogen has been shown to modulate antigen presentation by DCs and leads to differential HLA-restricted presentation of CII by DCs and macrophages [88]. Evidence for differential cytokine production by T cells in response to antigen presentation by B cells and macrophages has also been demonstrated [89]. The first study describing sexual dimorphism in HLA-restricted antigen presentation by B cells was shown using HLA-DR4 transgenic mice [87]. HLA-DR4 restricted presentation of an immunodominant CII peptide by B cells generate a much more robust response in female mice compared to males, while DCs present the same peptide robustly in males [87]. Further, a sex–specific cytokine profile was generated by the DR4 restricted CII peptide presented by B cells. Female mice produced more B cell modulating cytokines, with IL-13 being the most distinguishing cytokine between sexes, while male mice produced Th1/TH2 cytokines. In RA patients, high levels of IL-13 has been correlated with ACPA positivity [90]. On the other hand, DQ8-restricted CII peptide was presented robustly by B cells as well as DCs in males compared to female mice and led to production of a sex-specific cytokine profile. This data suggest that not only the type of antigen presenting cells, but HLA-DR and DQ restricted presentation may also show sexual dimorphism. The results from the humanized HLA-DR4 and DR4/DQ8 transgenic mice imply that mechanistic differences in B cell- and dendritic cell (DC)-mediated antigen presentation and HLA-DR4-restricted immune responses may underlie the sex-based differences in disease phenotype. If the hypothesis is true, then it may be beneficial to target therapies to these distinct cellular mechanisms in women versus men.
Recent investigations have shown that ACPAs precede arthritis development, making citrullinated proteins strong candidates for driving the autoimmune response in this disease [91]. Citrullination of a protein requires the posttranslational conversion of peptidyl arginine to peptidyl citrulline. Female sex hormone modulates expression of and can increase production of the peptidylarginine deiminase (PAD) enzymes required for citrullination of proteins [92]. Presence of DRB1 shared epitope is considered a primary risk factor for production of ACPAs in RA [93]. However, DR4-restricted CII peptide shown to be immunodominant in DR4 transgenic mice [85], does not have any arginine. Interestingly, immunization with a peptide non-responsive in native state and recall in vitro with citrullinated peptide generated a higher response in female DR4 mice compared to males confirming 1) previous studies that citrullinated peptides bind *0401 molecules with higher affinity than native peptides[94] and 2) suggesting a way that DR4 and B cells can contribute to sex-bias in autoimmunity.
Impact of sex hormones on B cell functions and sex bias of arthritis
Increased prevalence of autoimmune diseases in women, sex-differences in immune system and remission of symptoms of disease during pregnancy have suggested a role of female sex hormones in the pathogenesis of arthritis, although data in humans and animal models of RA has been controversial [95]. Fluctuations in disease activity during pregnancy have been mainly explained due to hormonal changes favoring a Th2 response. However, one study found no correlation between disease course and autoantibodies levels in pregnant RA patients, suggesting role of B cells other than antibody production may be involved in pregnancy-induced remission [96]. Estrogen has been shown to enhance immunoglobulin production in PBMCs and women have been known to produce elevated levels of antibodies normally as well as in autoimmune diseases [86, 97, 98]. A central role of estradiol has been shown in modulating antigen presentation, enhancing B cell responses by increasing survival of autoreactive B cells and production of proinflammatory cytokines such as IL-1, IL-6 and TNFα [86, 99-101]. Estrogen has been shown to impair negative selection of high affinity autoreactive B cells. Male RA patients have higher levels of estradiol compared with healthy controls [102]. Animals with CIA show decreased disease activity during pregnancy [103] and estrogen treatment in non-pregnant CIA mice leads to an increase in IgG1 antibodies which may explain remission during pregnancy [104]. In mice, estrogen has been shown to inhibit autoreactive B cell apoptosis thus increasing their survival and contributing to disease severity mainly via engagement of estrogen receptor alpha [105, 106]. Castration and ovariectomy in mice showed an increase in circulating B cells [107]. However, ovariectomized mice treated with estradiol showed decreased B lymphocytes and milder CIA, suggesting a down regulatory effect of estrogen on B lymphopoiesis [108]. Contrasting reports in mouse models of arthritis have been reported where a protective role of estrogen and an inflammatory role of male hormones has been suggested based on castration and exogenous supply of estradiol [103]. Since none of the models showed a sex-bias in CIA, even though these studies contributed a wealth of information, the mechanism of sex-bias still remained unknown.
Since DR4 mice developed arthritis predominantly in females, we tested how estrogen will modulate DR-restricted antigen presentation. Male DR4 mice implanted with slow release estradiol tablets showed an increase in expression levels of DR4 in splenic cells, in particular on B cells, with a significant higher antigen-specific response in treated versus untreated mice. Also, incidence of CIA doubled in castrated mice compared to non-castrated mice, suggesting a protective effect of androgen. A higher antigen presentation has been shown by splenic cells and epithelial cells during proestrous when estrogen is high [86, 109]. These data suggest that sex-bias in arthritis requires modulation of APC function by estrogen, which leads to differential antigen presentation by DR4 molecule resulting in a cytokine milieu of Th1/Th2/Th17 that decides the final outcome.
Since estrogen can increase expression of PAD enzyme, one can speculate that the increased expression of PAD enzyme during estrous cycle could cause an increase in citrullination of peptides, not only in the uterus, but other tissues including synovial tissue. This might result in the citrullination of multiple synovial specific autoantigens and their presentation by DRB1*0401 molecule leading to activation of autoreactive CD4 T cells and autoimmunity. It is intriguing to contemplate that the explanation could be as simple as the difference in posttranslational modification of arginine in both sexes which may dictate the final antigen-specific immune response. Though the hypothesis needs to be proven, it can be further speculated that in males there is less citrullination of the arginine residue due to lower levels of PAD enzymes, making them available for other modifications. This would suggest that posttranslational modifications of the arginine in an antigen may be a critical factor while association of RA with HLA alleles is by virtue of antigen presentation by specific HLA molecule and APC.
B cells as regulators in experimental arthritis
There is ample evidence indicating that B cells are essential for regulating immune responses and maintaining a healthy state by producing antibodies and also as APCs that allow development of memory CD4+ T cells [110]. While professional APCs can uptake non-specific antigens, selective uptake of antigens by B cells is highly efficient in the generation of antigen-specific immune response. In addition to the functions noted above, B cells also produce cytokines, IL-4, IL-6, IL-10 and TNF-α, which have regulatory effects on DCs and the differentiation of T cells, suggesting a regulatory function of B cells [111, 112]. Recent studies have highlighted the regulatory role of B cells in various autoimmune models [113-115]. B cells producing IL-10 have been shown to prevent CIA in DBA/1 mice[116]. The phenotype of these regulatory B cells was shown to be T2-MZ precursor cells and they needed to be stimulated via CD40 for regulatory function [117]. Apoptotic cells have been shown to induce B regulatory cells that secrete IL-10 and inhibit CIA [118]. More than one type of B regulatory cell has been described. Another type of B regulatory cell that represents only about 1-2 % of splenocytes, expresses CD19+CD5+CD1dhi and produces IL-10, known as B10 cells, has been described [119]. Adoptive transfer of B regulatory cells has been shown to prevent autoimmunity in various experimental models including arthritis [113]. However, none of these studies showed a sex difference in the presence of B regulatory cells and whether these B regulatory cells contribute to resistance to develop CIA in genetically predisposed mice. Studies using DR4 transgenic mice described an increased production of IL-10 by male mice in response to presentation of DR4-restricted CII peptide by B cells. Male DR4 mice were shown to have higher numbers of B regulatory, CD19+CD5+CD1dhi cells, and T regulatory cells, CD4+FOXP3+, compared to female mice. These data do not directly prove a role of B regulatory cells in resistance to develop arthritis in male mice and does not describe at what stage of arthritis B regulatory cells may be expanded. However this data does suggest that serious consideration should be given to sex-dependent treatment option for patients.
B cell directed Immunotherapy in RA
The various ways B cells can contribute to pathogenesis and severity of autoimmunity has made them a perfect target for therapy, specially in a disease like RA where autoantibodies precede the actual clinical phenotype. B cell depletion with a monoclonal anti-CD20 therapeutic antibody is effective and approved for the treatment of moderate to severe RA with an unsatisfactory response to a TNF inhibitor [120-122]. Anti-CD20 depletion therapy eliminates all of B cell functions although the function that is critical in determining its efficacy is still unknown. B cell depletion in RA patients has shown a positive clinical response with a reduction in B cells and improvement in ACR response and reduction in Ig levels [123]. Since CD20 is not expressed by antibody-secreting plasma cells, antibody secreting cells still exist although the source for plasma cells is eliminated. CD20 is expressed on B cells from pre-B stage in the bone marrow to the mature B cell stage, these lymphocyte subsets are eliminated by anti-CD20 treatment as demonstrated by several clinical trails involving autoimmune diseases. The Phase III Randomized Evaluation of Long-Term Efficacy of Rituximab in RA (REFLEX) trial demonstrated efficacy of RTX in patients with a history of non response to tumor necrosis factor inhibitors [124]. However, in another trial with CD20 depletion in RF-negative RA patients, no difference was observed as compared to placebo-treated patients [121]. Treatment with anti-CD20 antibody has been shown to deplete B cells in synovium although no change in B cell survival factor expression was observed [125]. A more recent addition to treatment of RA is a fully human monoclonal antibody against B lymphocyte stimulator (a growth and survival factor for B cells). In a double-blind, placebo-controlled, phase II trial, it was well tolerated and had a significant beneficial effect on the ACR 20 response. However, the exact mechanism by which depletion of B cells works is still unknown. In CIA model in DBA/1 mice, treatment with anti-CD20 antibodies delayed the onset of arthritis[126]. In other models of arthritis, B cell depletion suppressed arthritis development by inhibiting autoreactive T cell responses and increasing T regulatory cells [59, 62, 127]. Based on our data in DR4 transgenic mice, we speculate that B cell depletion therapy, on average, might be more effective in women as compared to men. To our knowledge, the published trials of rituximab therapy in RA do not distinguish the treatment efficacy by sex, perhaps due to the smaller number of male participants and hence low power to detect sex-based differences. In addition to B cells, DCs are central in inducing immunity and in mediating immune tolerance in humans. Evidence supports a significant role for DCs in the initiation, maintenance, and progression of RA [128]. If the hypothesis based on the HLA-DR4 transgenic mice data is correct, then therapeutic strategies that target DCs might be relatively more important for men than women. Indeed one study reported that male sex predicted a higher probability of achieving DAS28 remission with anti-TNF therapy, which is potentially consistent with observations in transgenic mice, as TNF is an important mediator of myeloid cells such as DCs [15]. Emerging therapies that exploit the capacity of tolerogenic DCs or that target the proinflammatory products of DCs might be particularly useful in managing men with RA. Observations in DR4 transgenic mice shed light on how B cells behave in sex dependent manner and persuade us to look into considering patient’s sex in selecting the optimal treatment strategy.
Concluding Remarks
An initiating autoantigen for rheumatoid arthritis is not known. Association of HLA class II molecules and genetic studies strongly indicate a role of class II-restricted responses in pathogenesis although such a mechanism is not yet defined. Rheumatoid arthritis occurs two to three times more often in women than men, however the mechanisms underlying the sex bias on the phenotype of RA are unknown. B cells play a critical role in pathogenesis of RA as is obvious with efficacy of anti-CD20 antibody treatment. Females have higher levels of estrogen and thus have high PAD enzymes resulting in increase in citrullination of peptides that can be presented by RA susceptible DR alleles. Estrogen modulates B cell function in many ways thus enhancing MHC-restricted presentation of synovial antigens. Difference in the presence of B regulatory cells and antigen presenting ability of B cells in sex-specific manner has not been explored. We propose that B cells are hyperactive in females and antigen presentation via B cells in the context of specific HLA molecules generates sex-specific immune response determining the outcome of clinical phenotype (Figure 4).
Figure 4.

DRB1*0401-restricted antigen presentation by B cells regulates sex bias of arthritis. Estrogen modulates B cell functions; increasing their survival, antigen presentation and autoantibody production. Males have higher numbers of B regulatory cells that may expand during immune response and produce IL-10 thus inhibiting T cell proliferation and limiting autoreactivity and autoantibody production. Antigen presentation via B cells in females leads to production of B cell modulating cytokines that increase their survival and proliferation thus enhancing autoreactive immune response. This suggests that males need to have a higher genetic load to develop autoimmunity.
Defining the mechanism of pathogenesis in experimental models will help define sex-bias of disease. Humanized mice expressing HLA genes provide one way to study the role of individual genes for investigating genetic, environmental and pathogenic aspects of an autoimmune disease in a biologically relevant situation in order to address questions about optimizing treatment based on patient’s sex.
Acknowledgements
We thank Marshall Behrens and Michele Smart for their help with the arthritis model in transgenic mice. Studies are supported by NIH grants AI 75262, AR60077 and AR30752
References
- [1].Cariappa A, Chase C, Liu H, Russell P, Pillai S. Naive recirculating B cells mature simultaneously in the spleen and bone marrow. Blood. 2007;109:2339–45. doi: 10.1182/blood-2006-05-021089. [DOI] [PubMed] [Google Scholar]
- [2].Lindsley RC, Thomas M, Srivastava B, Allman D. Generation of peripheral B cells occurs via two spatially and temporally distinct pathways. Blood. 2007;109:2521–8. doi: 10.1182/blood-2006-04-018085. [DOI] [PubMed] [Google Scholar]
- [3].Allman D, Pillai S. Peripheral B cell subsets. Current opinion in immunology. 2008;20:149–57. doi: 10.1016/j.coi.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Carvalho TL, Mota-Santos T, Cumano A, Demengeot J, Vieira P. Arrested B lymphopoiesis and persistence of activated B cells in adult interleukin 7(−/)− mice. The Journal of experimental medicine. 2001;194:1141–50. doi: 10.1084/jem.194.8.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hao Z, Rajewsky K. Homeostasis of peripheral B cells in the absence of B cell influx from the bone marrow. The Journal of experimental medicine. 2001;194:1151–64. doi: 10.1084/jem.194.8.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Agenes F, Freitas AA. Transfer of small resting B cells into immunodeficient hosts results in the selection of a self-renewing activated B cell population. The Journal of experimental medicine. 1999;189:319–30. doi: 10.1084/jem.189.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Medina KL, Kincade PW. Pregnancy-related steroids are potential negative regulators of B lymphopoiesis. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:5382–6. doi: 10.1073/pnas.91.12.5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Medina KL, Smithson G, Kincade PW. Suppression of B lymphopoiesis during normal pregnancy. The Journal of experimental medicine. 1993;178:1507–15. doi: 10.1084/jem.178.5.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Pillai S, Mattoo H, Cariappa A. B cells and autoimmunity. Current opinion in immunology. 23:721–31. doi: 10.1016/j.coi.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Meffre E, Wardemann H. B-cell tolerance checkpoints in health and autoimmunity. Current opinion in immunology. 2008;20:632–8. doi: 10.1016/j.coi.2008.09.001. [DOI] [PubMed] [Google Scholar]
- [11].Mountz J. Animal models of systemic lupus erythematosus and Sjogren’s syndrome. Current opinion in rheumatology. 1990;2:740–8. doi: 10.1097/00002281-199002050-00010. [DOI] [PubMed] [Google Scholar]
- [12].Tengstrand B, Ahlmen M, Hafstrom I. The influence of sex on rheumatoid arthritis: a prospective study of onset and outcome after 2 years. J Rheumatol. 2004;31:214–22. [PubMed] [Google Scholar]
- [13].Sokka T, Toloza S, Cutolo M, Kautiainen H, Makinen H, Gogus F, et al. Women, men, and rheumatoid arthritis: analyses of disease activity, disease characteristics, and treatments in the QUEST-RA study. Arthritis Res Ther. 2009;11:R7. doi: 10.1186/ar2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ahlmen M, Svensson B, Albertsson K, Forslind K, Hafstrom I. Influence of gender on assessments of disease activity and function in early rheumatoid arthritis in relation to radiographic joint damage. Ann Rheum Dis. 2010;69:230–3. doi: 10.1136/ard.2008.102244. [DOI] [PubMed] [Google Scholar]
- [15].Atzeni F, Antivalle M, Pallavicini FB, Caporali R, Bazzani C, Gorla R, et al. Predicting response to anti-TNF treatment in rheumatoid arthritis patients. Autoimmun Rev. 2009;8:431–7. doi: 10.1016/j.autrev.2009.01.005. [DOI] [PubMed] [Google Scholar]
- [16].Gossec L, Baro-Riba J, Bozonnat MC, Daures JP, Sany J, Eliaou JF, et al. Influence of sex on disease severity in patients with rheumatoid arthritis. J Rheumatol. 2005;32:1448–51. [PubMed] [Google Scholar]
- [17].Stastny P. Association of the B-cell alloantigen DRw4 with rheumatoid arthritis. The New England journal of medicine. 1978;298:869–71. doi: 10.1056/NEJM197804202981602. [DOI] [PubMed] [Google Scholar]
- [18].Winchester R, Dwyer E, Rose S. The genetic basis of rheumatoid arthritis. The shared epitope hypothesis. Rheumatic diseases clinics of North America. 1992;18:761–83. [PubMed] [Google Scholar]
- [19].Stephens HA, Sakkas LI, Vaughan RW, Teitsson I, Welsh KI, Panayi GS. HLA-DQw7 is a disease severity marker in patients with rheumatoid arthritis. Immunogenetics. 1989;30:119–22. doi: 10.1007/BF02421540. [DOI] [PubMed] [Google Scholar]
- [20].Taneja V, Mehra NK, Chandershekaran AN, Ahuja RK, Singh YN, Malaviya AN. HLA-DR4-DQw8, but not DR4-DQw7 haplotypes occur in Indian patients with rheumatoid arthritis. Rheumatology international. 1992;11:251–5. doi: 10.1007/BF00301502. [DOI] [PubMed] [Google Scholar]
- [21].Gonzalez-Quintial R, Baccala R, Pope RM, Theofilopoulos AN. Identification of clonally expanded T cells in rheumatoid arthritis using a sequence enrichment nuclease assay. The Journal of clinical investigation. 1996;97:1335–43. doi: 10.1172/JCI118550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Strober S, Holoshitz J. Mechanisms of immune injury in rheumatoid arthritis: evidence for the involvement of T cells and heat-shock protein. Immunological reviews. 1990;118:233–55. doi: 10.1111/j.1600-065x.1990.tb00818.x. [DOI] [PubMed] [Google Scholar]
- [23].Kim HJ, Krenn V, Steinhauser G, Berek C. Plasma cell development in synovial germinal centers in patients with rheumatoid and reactive arthritis. J Immunol. 1999;162:3053–62. [PubMed] [Google Scholar]
- [24].Van Boxel JA, Paget SA. Predominantly T-cell infiltrate in rheumatoid synovial membranes. The New England journal of medicine. 1975;293:517–20. doi: 10.1056/NEJM197509112931101. [DOI] [PubMed] [Google Scholar]
- [25].Silverman GJ, Carson DA. Roles of B cells in rheumatoid arthritis. Arthritis research & therapy. 2003;5(Suppl 4):S1–6. doi: 10.1186/ar1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bazzoni F, Beutler B. The tumor necrosis factor ligand and receptor families. The New England journal of medicine. 1996;334:1717–25. doi: 10.1056/NEJM199606273342607. [DOI] [PubMed] [Google Scholar]
- [27].Panayi GS. B cells: a fundamental role in the pathogenesis of rheumatoid arthritis? Rheumatology (Oxford, England) 2005;44(Suppl 2):ii3–ii7. doi: 10.1093/rheumatology/keh616. [DOI] [PubMed] [Google Scholar]
- [28].Vidard L, Kovacsovics-Bankowski M, Kraeft SK, Chen LB, Benacerraf B, Rock KL. Analysis of MHC class II presentation of particulate antigens of B lymphocytes. J Immunol. 1996;156:2809–18. [PubMed] [Google Scholar]
- [29].Wagner U, Kaltenhauser S, Pierer M, Wilke B, Arnold S, Hantzschel H. B lymphocytopenia in rheumatoid arthritis is associated with the DRB1 shared epitope and increased acute phase response. Arthritis research. 2002;4:R1. doi: 10.1186/ar420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Szodoray P, Alex P, Frank MB, Turner M, Turner S, Knowlton N, et al. A genome-scale assessment of peripheral blood B-cell molecular homeostasis in patients with rheumatoid arthritis. Rheumatology (Oxford, England) 2006;45:1466–76. doi: 10.1093/rheumatology/kel095. [DOI] [PubMed] [Google Scholar]
- [31].Huston MM, Moore JP, Mettes HJ, Tavana G, Huston DP. Human B cells express IL-5 receptor messenger ribonucleic acid and respond to IL-5 with enhanced IgM production after mitogenic stimulation with Moraxella catarrhalis. J Immunol. 1996;156:1392–401. [PubMed] [Google Scholar]
- [32].Mosmann T. Complexity or coherence? Cytokine secretion by B cells. Nature immunology. 2000;1:465–6. doi: 10.1038/82707. [DOI] [PubMed] [Google Scholar]
- [33].Henneken M, Dorner T, Burmester GR, Berek C. Differential expression of chemokine receptors on peripheral blood B cells from patients with rheumatoid arthritis and systemic lupus erythematosus. Arthritis research & therapy. 2005;7:R1001–13. doi: 10.1186/ar1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Dorner T, Burmester GR. The role of B cells in rheumatoid arthritis: mechanisms and therapeutic targets. Current opinion in rheumatology. 2003;15:246–52. doi: 10.1097/00002281-200305000-00011. [DOI] [PubMed] [Google Scholar]
- [35].Takemura S, Klimiuk PA, Braun A, Goronzy JJ, Weyand CM. T cell activation in rheumatoid synovium is B cell dependent. J Immunol. 2001;167:4710–8. doi: 10.4049/jimmunol.167.8.4710. [DOI] [PubMed] [Google Scholar]
- [36].Lesley R, Xu Y, Kalled SL, Hess DM, Schwab SR, Shu HB, et al. Reduced competitiveness of autoantigen-engaged B cells due to increased dependence on BAFF. Immunity. 2004;20:441–53. doi: 10.1016/s1074-7613(04)00079-2. [DOI] [PubMed] [Google Scholar]
- [37].Thien M, Phan TG, Gardam S, Amesbury M, Basten A, Mackay F, et al. Excess BAFF rescues self-reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity. 2004;20:785–98. doi: 10.1016/j.immuni.2004.05.010. [DOI] [PubMed] [Google Scholar]
- [38].Bombardieri M, Kam NW, Brentano F, Choi K, Filer A, Kyburz D, et al. A BAFF/APRIL-dependent TLR3-stimulated pathway enhances the capacity of rheumatoid synovial fibroblasts to induce AID expression and Ig class-switching in B cells. Annals of the rheumatic diseases. 70:1857–65. doi: 10.1136/ard.2011.150219. [DOI] [PubMed] [Google Scholar]
- [39].Groom J, Kalled SL, Cutler AH, Olson C, Woodcock SA, Schneider P, et al. Association of BAFF/BLyS overexpression and altered B cell differentiation with Sjogren’s syndrome. The Journal of clinical investigation. 2002;109:59–68. doi: 10.1172/JCI14121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Serreze DV, Fleming SA, Chapman HD, Richard SD, Leiter EH, Tisch RM. B lymphocytes are critical antigen-presenting cells for the initiation of T cell-mediated autoimmune diabetes in nonobese diabetic mice. J Immunol. 1998;161:3912–8. [PubMed] [Google Scholar]
- [41].Matsumoto I, Maccioni M, Lee DM, Maurice M, Simmons B, Brenner M, et al. How antibodies to a ubiquitous cytoplasmic enzyme may provoke joint-specific autoimmune disease. Nature immunology. 2002;3:360–5. doi: 10.1038/ni772. [DOI] [PubMed] [Google Scholar]
- [42].Chan OT, Madaio MP, Shlomchik MJ. The central and multiple roles of B cells in lupus pathogenesis. Immunological reviews. 1999;169:107–21. doi: 10.1111/j.1600-065x.1999.tb01310.x. [DOI] [PubMed] [Google Scholar]
- [43].Svensson L, Jirholt J, Holmdahl R, Jansson L. B cell-deficient mice do not develop type II collagen-induced arthritis (CIA) Clinical and experimental immunology. 1998;111:521–6. doi: 10.1046/j.1365-2249.1998.00529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].He X, Kang AH, Stuart JM. Accumulation of T cells reactive to type II collagen in synovial fluid of patients with rheumatoid arthritis. The Journal of rheumatology. 2000;27:589–93. [PubMed] [Google Scholar]
- [45].Andriopoulos NA, Mestecky J, Miller EJ, Bradley EL. Antibodies to native and denatured collagens in sera of patients with rheumatoid arthritis. Arthritis and rheumatism. 1976;19:613–7. doi: 10.1002/art.1780190314. [DOI] [PubMed] [Google Scholar]
- [46].Clague RB, Shaw MJ, Holt PJ. Incidence of serum antibodies to native type I and type II collagens in patients with inflammatory arthritis. Annals of the rheumatic diseases. 1980;39:201–6. doi: 10.1136/ard.39.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Londei M, Savill CM, Verhoef A, Brennan F, Leech ZA, Duance V, et al. Persistence of collagen type II-specific T-cell clones in the synovial membrane of a patient with rheumatoid arthritis. Proc Natl Acad Sci U S A. 1989;86:636–40. doi: 10.1073/pnas.86.2.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Tarkowski A, Klareskog L, Carlsten H, Herberts P, Koopman WJ. Secretion of antibodies to types I and II collagen by synovial tissue cells in patients with rheumatoid arthritis. Arthritis and rheumatism. 1989;32:1087–92. doi: 10.1002/anr.1780320906. [DOI] [PubMed] [Google Scholar]
- [49].Taneja V, David CS. Role of HLA class II genes in susceptibility/resistance to inflammatory arthritis: studies with humanized mice. Immunological reviews. 233:62–78. doi: 10.1111/j.0105-2896.2009.00858.x. [DOI] [PubMed] [Google Scholar]
- [50].Brand DD, Kang AH, Rosloniec EF. Immunopathogenesis of collagen arthritis. Springer seminars in immunopathology. 2003;25:3–18. doi: 10.1007/s00281-003-0127-1. [DOI] [PubMed] [Google Scholar]
- [51].Bajtner E, Nandakumar KS, Engstrom A, Holmdahl R. Chronic development of collagen-induced arthritis is associated with arthritogenic antibodies against specific epitopes on type II collagen. Arthritis research & therapy. 2005;7:R1148–57. doi: 10.1186/ar1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Dobritzsch D, Lindh I, Uysal H, Nandakumar KS, Burkhardt H, Schneider G, et al. Crystal structure of an arthritogenic anticollagen immune complex. Arthritis and rheumatism. 63:3740–8. doi: 10.1002/art.30611. [DOI] [PubMed] [Google Scholar]
- [53].Wooley PH, Luthra HS, Singh SK, Huse AR, Stuart JM, David CS. Passive transfer of arthritis to mice by injection of human anti-type II collagen antibody. Mayo Clinic proceedings. 1984;59:737–43. doi: 10.1016/s0025-6196(12)65583-9. [DOI] [PubMed] [Google Scholar]
- [54].Mullazehi M, Mathsson L, Lampa J, Ronnelid J. High anti-collagen type-II antibody levels and induction of proinflammatory cytokines by anti-collagen antibody-containing immune complexes in vitro characterise a distinct rheumatoid arthritis phenotype associated with acute inflammation at the time of disease onset. Annals of the rheumatic diseases. 2007;66:537–41. doi: 10.1136/ard.2006.064782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Constant S, Schweitzer N, West J, Ranney P, Bottomly K. B lymphocytes can be competent antigen-presenting cells for priming CD4+ T cells to protein antigens in vivo. J Immunol. 1995;155:3734–41. [PubMed] [Google Scholar]
- [56].Kurt-Jones EA, Liano D, HayGlass KA, Benacerraf B, Sy MS, Abbas AK. The role of antigen-presenting B cells in T cell priming in vivo. Studies of B cell-deficient mice. J Immunol. 1988;140:3773–8. [PubMed] [Google Scholar]
- [57].Shlomchik MJ, Craft JE, Mamula MJ. From T to B and back again: positive feedback in systemic autoimmune disease. Nature reviews. 2001;1:147–53. doi: 10.1038/35100573. [DOI] [PubMed] [Google Scholar]
- [58].Yan J, Harvey BP, Gee RJ, Shlomchik MJ, Mamula MJ. B cells drive early T cell autoimmunity in vivo prior to dendritic cell-mediated autoantigen presentation. J Immunol. 2006;177:4481–7. doi: 10.4049/jimmunol.177.7.4481. [DOI] [PubMed] [Google Scholar]
- [59].Frey O, Bruns L, Morawietz L, Dunussi-Joannopoulos K, Kamradt T. B cell depletion reduces the number of autoreactive T helper cells and prevents glucose-6-phosphate isomerase-induced arthritis. PloS one. 6:e24718. doi: 10.1371/journal.pone.0024718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Amigorena S, Lankar D, Briken V, Gapin L, Viguier M, Bonnerot C. Type II and III receptors for immunoglobulin G (IgG) control the presentation of different T cell epitopes from single IgG-complexed antigens. J Exp Med. 1998;187:505–15. doi: 10.1084/jem.187.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Hamano Y, Arase H, Saisho H, Saito T. Immune complex and Fc receptor-mediated augmentation of antigen presentation for in vivo Th cell responses. J Immunol. 2000;164:6113–9. doi: 10.4049/jimmunol.164.12.6113. [DOI] [PubMed] [Google Scholar]
- [62].Hamel KM, Cao Y, Ashaye S, Wang Y, Dunn R, Kehry MR, et al. cell depletion enhances T regulatory cell activity essential in the suppression of arthritis. J Immunol. 187:4900–6. doi: 10.4049/jimmunol.1101844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Hamel K, Doodes P, Cao Y, Wang Y, Martinson J, Dunn R, et al. Suppression of proteoglycan-induced arthritis by anti-CD20 B Cell depletion therapy is mediated by reduction in autoantibodies and CD4+ T cell reactivity. J Immunol. 2008;180:4994–5003. doi: 10.4049/jimmunol.180.7.4994. [DOI] [PubMed] [Google Scholar]
- [64].Kyburz D, Corr M. The KRN mouse model of inflammatory arthritis. Springer seminars in immunopathology. 2003;25:79–90. doi: 10.1007/s00281-003-0131-5. [DOI] [PubMed] [Google Scholar]
- [65].Shih FF, Racz J, Allen PM. Differential MHC class II presentation of a pathogenic autoantigen during health and disease. J Immunol. 2006;176:3438–48. doi: 10.4049/jimmunol.176.6.3438. [DOI] [PubMed] [Google Scholar]
- [66].Mackay F, Woodcock SA, Lawton P, Ambrose C, Baetscher M, Schneider P, et al. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med. 1999;190:1697–710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Schneider P, MacKay F, Steiner V, Hofmann K, Bodmer JL, Holler N, et al. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J Exp Med. 1999;189:1747–56. doi: 10.1084/jem.189.11.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Zhang M, Ko KH, Lam QL, Lo CK, Srivastava G, Zheng B, et al. Expression and function of TNF family member B cell-activating factor in the development of autoimmune arthritis. Int Immunol. 2005;17:1081–92. doi: 10.1093/intimm/dxh287. [DOI] [PubMed] [Google Scholar]
- [69].Gross JA, Dillon SR, Mudri S, Johnston J, Littau A, Roque R, et al. TACI-Ig neutralizes molecules critical for B cell development and autoimmune disease. impaired B cell maturation in mice lacking BLyS. Immunity. 2001;15:289–302. doi: 10.1016/s1074-7613(01)00183-2. [DOI] [PubMed] [Google Scholar]
- [70].Liu Y, Zhang L, Wu Y, Tong T, Zhao W, Li P, et al. Therapeutic effects of TACI-Ig on collagen-induced arthritis by regulating T and B lymphocytes function in DBA/1 mice. European journal of pharmacology. 654:304–14. doi: 10.1016/j.ejphar.2011.01.002. [DOI] [PubMed] [Google Scholar]
- [71].Lai Kwan Lam Q, King Hung Ko O, Zheng BJ, Lu L. Local BAFF gene silencing suppresses Th17-cell generation and ameliorates autoimmune arthritis. Proc Natl Acad Sci U S A. 2008;105:14993–8. doi: 10.1073/pnas.0806044105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Nabozny GH, Baisch JM, Cheng S, Cosgrove D, Griffiths MM, Luthra HS, et al. HLA-DQ8 transgenic mice are highly susceptible to collagen-induced arthritis: a novel model for human polyarthritis. J Exp Med. 1996;183:27–37. doi: 10.1084/jem.183.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Taneja V, Hansen J, Smart M, Griffiths M, Luthra H, David CS. Expression of the H2-E molecule mediates protection to collagen-induced arthritis in HLA-DQ8 transgenic mice: role of cytokines. Int Immunol. 1997;9:1213–9. doi: 10.1093/intimm/9.8.1213. [DOI] [PubMed] [Google Scholar]
- [74].Behrens M, Smart M, Luckey D, Luthra H, Taneja V. To B or not to B: role of B cells in pathogenesis of arthritis in HLA transgenic mice. Journal of autoimmunity. 37:95–103. doi: 10.1016/j.jaut.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Taneja V, Taneja N, Behrens M, Griffiths MM, Luthra HS, David CS. Requirement for CD28 may not be absolute for collagen-induced arthritis: study with HLA-DQ8 transgenic mice. J Immunol. 2005;174:1118–25. doi: 10.4049/jimmunol.174.2.1118. [DOI] [PubMed] [Google Scholar]
- [76].Taneja V, Taneja N, Paisansinsup T, Behrens M, Griffiths M, Luthra H, et al. CD4 and CD8 T cells in susceptibility/protection to collagen-induced arthritis in HLA-DQ8-transgenic mice: implications for rheumatoid arthritis. J Immunol. 2002;168:5867–75. doi: 10.4049/jimmunol.168.11.5867. [DOI] [PubMed] [Google Scholar]
- [77].Bradley DS, Nabozny GH, Cheng S, Zhou P, Griffiths MM, Luthra HS, et al. HLA-DQB1 polymorphism determines incidence, onset, and severity of collagen-induced arthritis in transgenic mice. Implications in human rheumatoid arthritis. The Journal of clinical investigation. 1997;100:2227–34. doi: 10.1172/JCI119760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Herlands RA, Christensen SR, Sweet RA, Hershberg U, Shlomchik MJ. T cell-independent and toll-like receptor-dependent antigen-driven activation of autoreactive B cells. Immunity. 2008;29:249–60. doi: 10.1016/j.immuni.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Chan OT, Hannum LG, Haberman AM, Madaio MP, Shlomchik MJ. A novel mouse with B cells but lacking serum antibody reveals an antibody-independent role for B cells in murine lupus. J Exp Med. 1999;189:1639–48. doi: 10.1084/jem.189.10.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Mamula MJ, Fatenejad S, Craft J. B cells process and present lupus autoantigens that initiate autoimmune T cell responses. J Immunol. 1994;152:1453–61. [PubMed] [Google Scholar]
- [81].Liljander M, Andersson A, Holmdahl R, Mattsson R. Increased susceptibility to collagen-induced arthritis in female mice carrying congenic Cia40/Pregq2 fragments. Arthritis research & therapy. 2008;10:R88. doi: 10.1186/ar2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].van Beuningen HM, van den Berg WB, Schalkwijk J, Arntz OJ, van de Putte LB. Age- and sex-related differences in antigen-induced arthritis in C57Bl/10 mice. Arthritis and rheumatism. 1989;32:789–94. doi: 10.1002/anr.1780320620. [DOI] [PubMed] [Google Scholar]
- [83].Jansson L, Holmdahl R. Genes on the X chromosome affect development of collagen-induced arthritis in mice. Clinical and experimental immunology. 1993;94:459–65. doi: 10.1111/j.1365-2249.1993.tb08218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Scotland RS, Stables MJ, Madalli S, Watson P, Gilroy DW. Sex differences in resident immune cell phenotype underlie more efficient acute inflammatory responses in female mice. Blood. 118:5918–27. doi: 10.1182/blood-2011-03-340281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Rosloniec EF, Brand DD, Myers LK, Esaki Y, Whittington KB, Zaller DM, et al. Induction of autoimmune arthritis in HLA-DR4 (DRB1*0401) transgenic mice by immunization with human and bovine type II collagen. J Immunol. 1998;160:2573–8. [PubMed] [Google Scholar]
- [86].Whitacre CC, Reingold SC, O’Looney PA. A gender gap in autoimmunity. Science (New York, NY. 1999;283:1277–8. doi: 10.1126/science.283.5406.1277. [DOI] [PubMed] [Google Scholar]
- [87].Behrens M, Trejo T, Luthra H, Griffiths M, David CS, Taneja V. Mechanism by which HLA-DR4 regulates sex-bias of arthritis in humanized mice. Journal of autoimmunity. 35:1–9. doi: 10.1016/j.jaut.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Tsark EC, Wang W, Teng YC, Arkfeld D, Dodge GR, Kovats S. Differential MHC class II-mediated presentation of rheumatoid arthritis autoantigens by human dendritic cells and macrophages. J Immunol. 2002;169:6625–33. doi: 10.4049/jimmunol.169.11.6625. [DOI] [PubMed] [Google Scholar]
- [89].Duncan DD, Swain SL. Role of antigen-presenting cells in the polarized development of helper T cell subsets: evidence for differential cytokine production by Th0 cells in response to antigen presentation by B cells and macrophages. European journal of immunology. 1994;24:2506–14. doi: 10.1002/eji.1830241037. [DOI] [PubMed] [Google Scholar]
- [90].Minty A, Chalon P, Derocq JM, Dumont X, Guillemot JC, Kaghad M, et al. Interleukin-13 is a new human lymphokine regulating inflammatory and immune responses. Nature. 1993;362:248–50. doi: 10.1038/362248a0. [DOI] [PubMed] [Google Scholar]
- [91].Kinloch AJ, Lundberg KE, Moyes D, Venables PJ. Pathogenic role of antibodies to citrullinated proteins in rheumatoid arthritis. Expert review of clinical immunology. 2006;2:365–75. doi: 10.1586/1744666X.2.3.365. [DOI] [PubMed] [Google Scholar]
- [92].Senshu T, Akiyama K, Nagata S, Watanabe K, Hikichi K. Peptidylarginine deiminase in rat pituitary: sex difference, estrous cycle-related changes, and estrogen dependence. Endocrinology. 1989;124:2666–70. doi: 10.1210/endo-124-6-2666. [DOI] [PubMed] [Google Scholar]
- [93].van der Helm-van Mil AH, Verpoort KN, Breedveld FC, Huizinga TW, Toes RE, de Vries RR. The HLA-DRB1 shared epitope alleles are primarily a risk factor for anti-cyclic citrullinated peptide antibodies and are not an independent risk factor for development of rheumatoid arthritis. Arthritis and rheumatism. 2006;54:1117–21. doi: 10.1002/art.21739. [DOI] [PubMed] [Google Scholar]
- [94].Hill JA, Southwood S, Sette A, Jevnikar AM, Bell DA, Cairns E. Cutting edge: the conversion of arginine to citrulline allows for a high-affinity peptide interaction with the rheumatoid arthritis-associated HLA-DRB1*0401 MHC class II molecule. J Immunol. 2003;171:538–41. doi: 10.4049/jimmunol.171.2.538. [DOI] [PubMed] [Google Scholar]
- [95].Cutolo M. Sex and rheumatoid arthritis: mouse model versus human disease. Arthritis and rheumatism. 2007;56:1–3. doi: 10.1002/art.22322. [DOI] [PubMed] [Google Scholar]
- [96].de Man YA, Bakker-Jonges LE, Goorbergh CM, Tillemans SP, Hooijkaas H, Hazes JM, et al. Women with rheumatoid arthritis negative for anti-cyclic citrullinated peptide and rheumatoid factor are more likely to improve during pregnancy, whereas in autoantibody-positive women autoantibody levels are not influenced by pregnancy. Annals of the rheumatic diseases. 69:420–3. doi: 10.1136/ard.2008.104331. [DOI] [PubMed] [Google Scholar]
- [97].Kanda N, Tamaki K. Estrogen enhances immunoglobulin production by human PBMCs. The Journal of allergy and clinical immunology. 1999;103:282–8. doi: 10.1016/s0091-6749(99)70503-8. [DOI] [PubMed] [Google Scholar]
- [98].Rowley MJ, Mackay IR. Measurement of antibody-producing capacity in man. I. The normal response to flagellin from Salmonella adelaide. Clinical and experimental immunology. 1969;5:407–18. [PMC free article] [PubMed] [Google Scholar]
- [99].Grimaldi CM, Cleary J, Dagtas AS, Moussai D, Diamond B. Estrogen alters thresholds for B cell apoptosis and activation. The Journal of clinical investigation. 2002;109:1625–33. doi: 10.1172/JCI14873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Cutolo M, Capellino S, Sulli A, Serioli B, Secchi ME, Villaggio B, et al. Estrogens and autoimmune diseases. Annals of the New York Academy of Sciences. 2006;1089:538–47. doi: 10.1196/annals.1386.043. [DOI] [PubMed] [Google Scholar]
- [101].Grimaldi CM, Jeganathan V, Diamond B. Hormonal regulation of B cell development: 17 beta-estradiol impairs negative selection of high-affinity DNA-reactive B cells at more than one developmental checkpoint. J Immunol. 2006;176:2703–10. doi: 10.4049/jimmunol.176.5.2703. [DOI] [PubMed] [Google Scholar]
- [102].Tengstrand B, Carlstrom K, Fellander-Tsai L, Hafstrom I. Abnormal levels of serum dehydroepiandrosterone, estrone, and estradiol in men with rheumatoid arthritis: high correlation between serum estradiol and current degree of inflammation. The Journal of rheumatology. 2003;30:2338–43. [PubMed] [Google Scholar]
- [103].Jansson L, Holmdahl R. Estrogen-mediated immunosuppression in autoimmune diseases. Inflamm Res. 1998;47:290–301. doi: 10.1007/s000110050332. [DOI] [PubMed] [Google Scholar]
- [104].Nalbandian G, Kovats S. Understanding sex biases in immunity: effects of estrogen on the differentiation and function of antigen-presenting cells. Immunologic research. 2005;31:91–106. doi: 10.1385/IR:31:2:091. [DOI] [PubMed] [Google Scholar]
- [105].Hill L, Jeganathan V, Chinnasamy P, Grimaldi C, Diamond B. Differential roles of estrogen receptors alpha and beta in control of B-cell maturation and selection. Molecular medicine (Cambridge, Mass. 17:211–20. doi: 10.2119/molmed.2010.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Medina KL, Strasser A, Kincade PW. Estrogen influences the differentiation, proliferation, and survival of early B-lineage precursors. Blood. 2000;95:2059–67. [PubMed] [Google Scholar]
- [107].Ellis TM, Moser MT, Le PT, Flanigan RC, Kwon ED. Alterations in peripheral B cells and B cell progenitors following androgen ablation in mice. Int Immunol. 2001;13:553–8. doi: 10.1093/intimm/13.4.553. [DOI] [PubMed] [Google Scholar]
- [108].Engdahl C, Jochems C, Windahl SH, Borjesson AE, Ohlsson C, Carlsten H, et al. Amelioration of collagen-induced arthritis and immune-associated bone loss through signaling via estrogen receptor alpha, and not estrogen receptor beta or G protein-coupled receptor 30. Arthritis and rheumatism. 62:524–33. doi: 10.1002/art.25055. [DOI] [PubMed] [Google Scholar]
- [109].Wira CR, Fahey JV, Abrahams VM, Rossoll RM. Influence of stage of the reproductive cycle and estradiol on thymus cell antigen presentation. The Journal of steroid biochemistry and molecular biology. 2003;84:79–87. doi: 10.1016/s0960-0760(03)00002-5. [DOI] [PubMed] [Google Scholar]
- [110].Linton PJ, Harbertson J, Bradley LM. A critical role for B cells in the development of memory CD4 cells. J Immunol. 2000;165:5558–65. doi: 10.4049/jimmunol.165.10.5558. [DOI] [PubMed] [Google Scholar]
- [111].Harris DP, Haynes L, Sayles PC, Duso DK, Eaton SM, Lepak NM, et al. Reciprocal regulation of polarized cytokine production by effector B and T cells. Nature immunology. 2000;1:475–82. doi: 10.1038/82717. [DOI] [PubMed] [Google Scholar]
- [112].Lund FE, Garvy BA, Randall TD, Harris DP. Regulatory roles for cytokine-producing B cells in infection and autoimmune disease. Current directions in autoimmunity. 2005;8:25–54. doi: 10.1159/000082086. [DOI] [PubMed] [Google Scholar]
- [113].Bouaziz JD, Yanaba K, Tedder TF. Regulatory B cells as inhibitors of immune responses and inflammation. Immunological reviews. 2008;224:201–14. doi: 10.1111/j.1600-065X.2008.00661.x. [DOI] [PubMed] [Google Scholar]
- [114].Mauri C, Blair PA. Regulatory B cells in autoimmunity: developments and controversies. Nat Rev Rheumatol. 6:636–43. doi: 10.1038/nrrheum.2010.140. [DOI] [PubMed] [Google Scholar]
- [115].Fillatreau S, Gray D, Anderton SM. Not always the bad guys: B cells as regulators of autoimmune pathology. Nature reviews. 2008;8:391–7. doi: 10.1038/nri2315. [DOI] [PubMed] [Google Scholar]
- [116].Mauri C, Gray D, Mushtaq N, Londei M. Prevention of arthritis by interleukin 10-producing B cells. J Exp Med. 2003;197:489–501. doi: 10.1084/jem.20021293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Evans JG, Chavez-Rueda KA, Eddaoudi A, Meyer-Bahlburg A, Rawlings DJ, Ehrenstein MR, et al. Novel suppressive function of transitional 2 B cells in experimental arthritis. J Immunol. 2007;178:7868–78. doi: 10.4049/jimmunol.178.12.7868. [DOI] [PubMed] [Google Scholar]
- [118].Gray M, Miles K, Salter D, Gray D, Savill J. Apoptotic cells protect mice from autoimmune inflammation by the induction of regulatory B cells. Proc Natl Acad Sci U S A. 2007;104:14080–5. doi: 10.1073/pnas.0700326104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28:639–50. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- [120].Cohen SB, Emery P, Greenwald MW, Dougados M, Furie RA, Genovese MC, et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: Results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 2006;54:2793–806. doi: 10.1002/art.22025. [DOI] [PubMed] [Google Scholar]
- [121].Emery P, Fleischmann R, Filipowicz-Sosnowska A, Schechtman J, Szczepanski L, Kavanaugh A, et al. The efficacy and safety of rituximab in patients with active rheumatoid arthritis despite methotrexate treatment: results of a phase IIB randomized, double-blind, placebo-controlled, dose-ranging trial. Arthritis and rheumatism. 2006;54:1390–400. doi: 10.1002/art.21778. [DOI] [PubMed] [Google Scholar]
- [122].Edwards JC, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close DR, et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med. 2004;350:2572–81. doi: 10.1056/NEJMoa032534. [DOI] [PubMed] [Google Scholar]
- [123].Engel P, Gomez-Puerta JA, Ramos-Casals M, Lozano F, Bosch X. Therapeutic targeting of B cells for rheumatic autoimmune diseases. Pharmacological reviews. 63:127–56. doi: 10.1124/pr.109.002006. [DOI] [PubMed] [Google Scholar]
- [124].Cohen SB. Updates from B Cell Trials: Efficacy. The Journal of rheumatology. 2006;77:12–7. [PubMed] [Google Scholar]
- [125].Kavanaugh A, Rosengren S, Lee SJ, Hammaker D, Firestein GS, Kalunian K, et al. Assessment of rituximab’s immunomodulatory synovial effects (ARISE trial). 1: clinical and synovial biomarker results. Annals of the rheumatic diseases. 2008;67:402–8. doi: 10.1136/ard.2007.074229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Yanaba K, Hamaguchi Y, Venturi GM, Steeber DA, St Clair EW, Tedder TF. B cell depletion delays collagen-induced arthritis in mice: arthritis induction requires synergy between humoral and cell-mediated immunity. J Immunol. 2007;179:1369–80. doi: 10.4049/jimmunol.179.2.1369. [DOI] [PubMed] [Google Scholar]
- [127].Huang H, Benoist C, Mathis D. Rituximab specifically depletes short-lived autoreactive plasma cells in a mouse model of inflammatory arthritis. Proc Natl Acad Sci U S A. 107:4658–63. doi: 10.1073/pnas.1001074107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Khan S, Greenberg JD, Bhardwaj N. Dendritic cells as targets for therapy in rheumatoid arthritis. Nature reviews. 2009;5:566–71. doi: 10.1038/nrrheum.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]


