Abstract
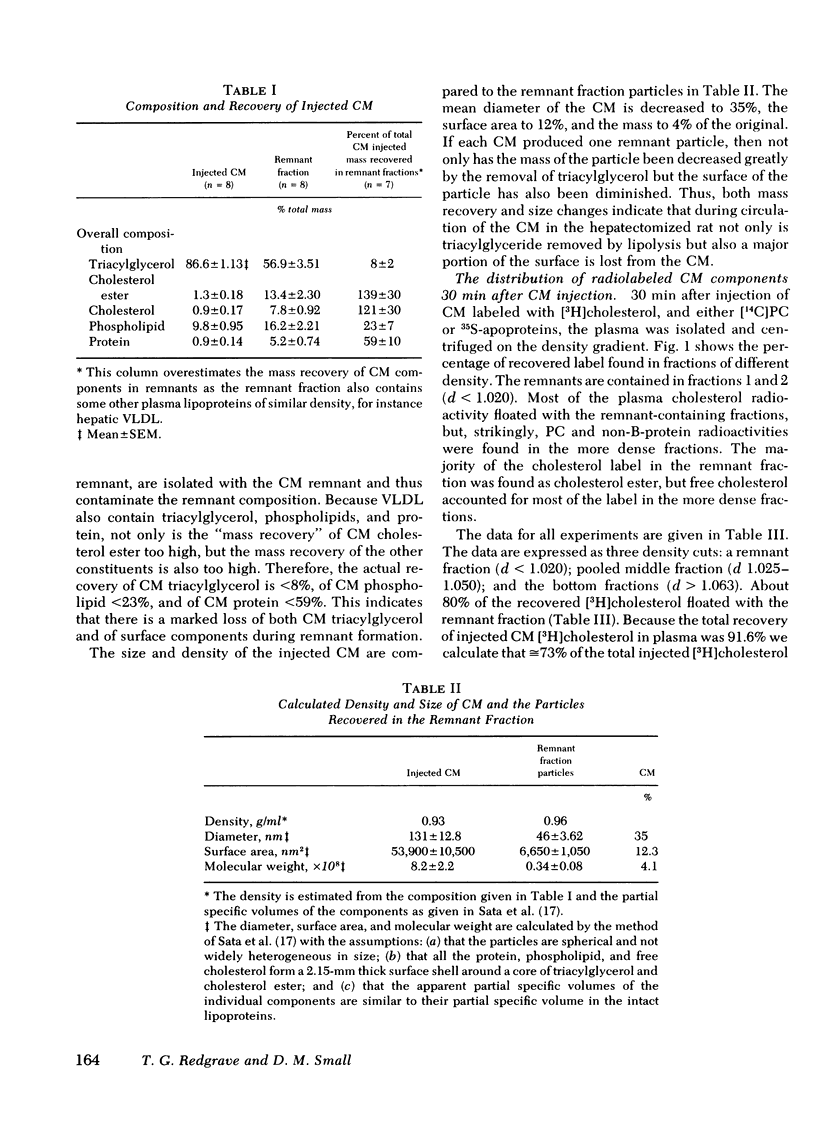
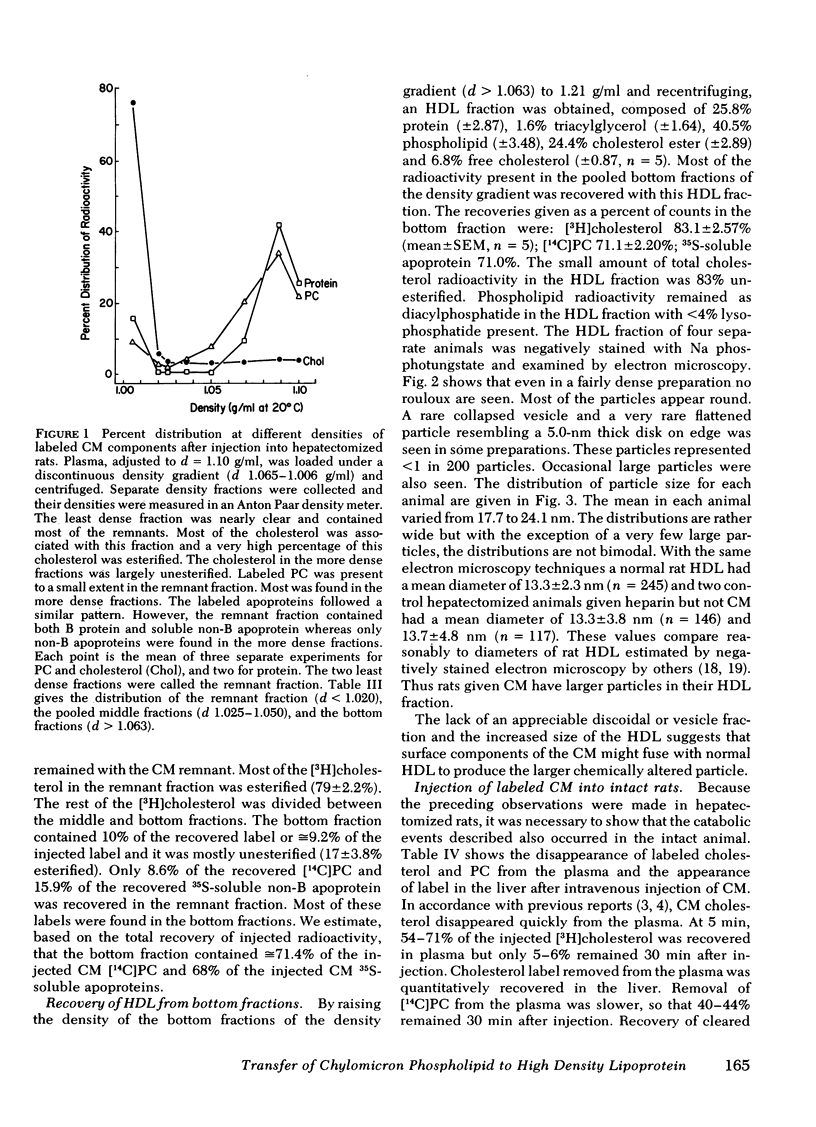
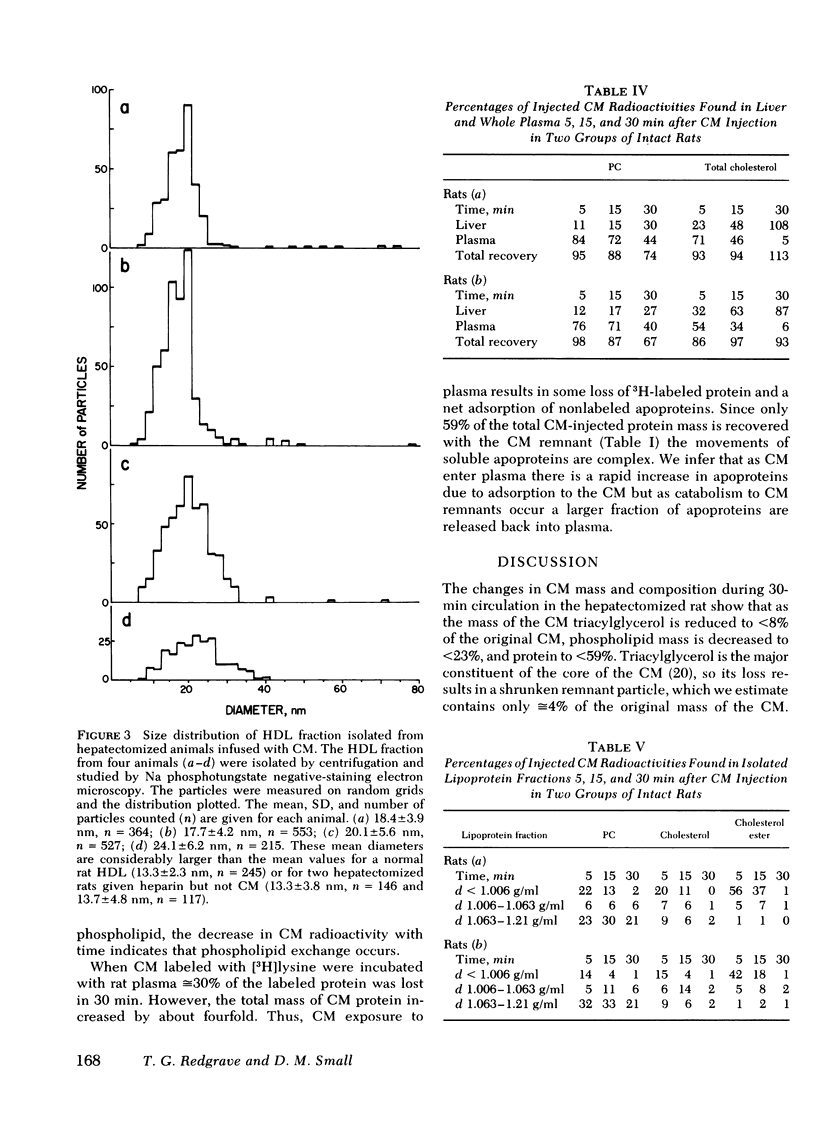
Small chylomicrons (CM) labeled with cholesterol, cholesterol ester, phospholipid, and, in some cases, protein, were used to study the fate of these constituents as the CM are catabolized in the circulations of the hepatectomized and intact rat. In the hepatectomized animal after ½ h, CM are greatly reduced in volume, surface area, and diameter. During this period, the CM lost >92% of the mass of their triacylglycerol, >77% of the mass of their phospholipid, and >39% of their protein. Compared to the injected CM, the chemically altered particles, called CM “remnants,” have a reduction in volume of 96% and in surface area of 88%. The labeled cholesterol esters remain with the CM remnants but, strikingly, a major fraction of the labeled phospholipids and labeled soluble apoproteins leave the CM and are found in the high density lipoprotein (HDL) fraction.
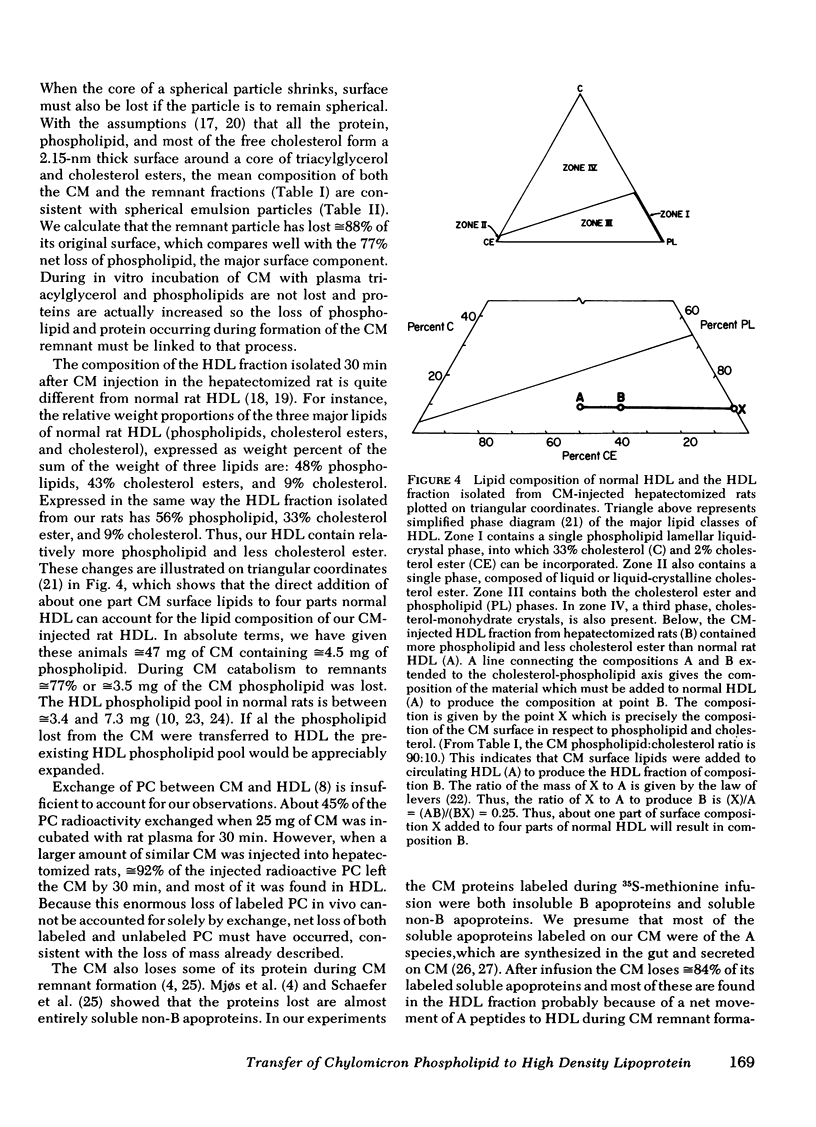
The chemical composition of this HDL fraction contains relatively more phospholipid and less cholesterol ester than normal rat HDL. Because of the difference in composition of HDL between normal rats and those given CM, we estimate that the HDL phospholipid pool increased by ≅25% by the infusion of ≅ 4-5 mg of CM phospholipid. Approximately 5 mg of phospholipid is secreted on CM by a fed rat in 1 h.
The findings in hepatectomized rats indicate that a major fraction of the phospholipid and a minor fraction of the protein (soluble non-B apoproteins) of newly secreted CM are transferred from the CM to the HDL fraction during remnant formation. The same process probably occurs in intact rats except that the remnant particles are rapidly removed from the plasma by the liver and a smaller fraction of the surface of the CM enters the HDL fraction.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blum C. B., Levy R. I., Eisenberg S., Hall M., 3rd, Goebel R. H., Berman M. High density lipoprotein metabolism in man. J Clin Invest. 1977 Oct;60(4):795–807. doi: 10.1172/JCI108833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOLE V. P., HAMLIN J. T., 3rd Particulate fat in lymph and blood. Physiol Rev. 1962 Oct;42:674–701. doi: 10.1152/physrev.1962.42.4.674. [DOI] [PubMed] [Google Scholar]
- De Pury G. G., Collins F. D. Composition and concentration of lipoproteins in the serum of normal rats and rats deficient in essential fatty acids. Lipids. 1972 Apr;7(4):225–228. doi: 10.1007/BF02533217. [DOI] [PubMed] [Google Scholar]
- Downing D. T. Photodensitometry in the thin-layer chromatographic analysis of neutral lipids. J Chromatogr. 1968 Nov 5;38(1):91–99. doi: 10.1016/0021-9673(68)85011-3. [DOI] [PubMed] [Google Scholar]
- Eisenberg S., Schurr D. Phospholipid removal during degradation of rat plasma very low density lipoprotein in vitro. J Lipid Res. 1976 Nov;17(6):578–587. [PubMed] [Google Scholar]
- Forte T., Norum K. R., Glomset J. A., Nichols A. V. Plasma lipoproteins in familial lecithin: cholesterol acyltransferase deficiency: structure of low and high density lipoproteins as revealed by elctron microscopy. J Clin Invest. 1971 May;50(5):1141–1148. doi: 10.1172/JCI106586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman R. M., Green P. H., Lees R. S., Tall A. Apoprotein A-I synthesis in normal intestinal mucosa and in Tangier disease. N Engl J Med. 1978 Dec 28;299(26):1424–1427. doi: 10.1056/NEJM197812282992602. [DOI] [PubMed] [Google Scholar]
- Glickman R. M., Green P. H. The intestine as a source of apolipoprotein A1. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2569–2573. doi: 10.1073/pnas.74.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green P. H., Tall A. R., Glickman R. M. Rat intestine secretes discoid high density lipoprotein. J Clin Invest. 1978 Feb;61(2):528–534. doi: 10.1172/JCI108963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAVEL R. J. Early effects of fat ingestion on lipids and lipoproteins of serum in man. J Clin Invest. 1957 Jun;36(6 Pt 1):848–854. doi: 10.1172/JCI103491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton R. L., Williams M. C., Fielding C. J., Havel R. J. Discoidal bilayer structure of nascent high density lipoproteins from perfused rat liver. J Clin Invest. 1976 Sep;58(3):667–680. doi: 10.1172/JCI108513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havel R. J., Kane J. P., Kashyap M. L. Interchange of apolipoproteins between chylomicrons and high density lipoproteins during alimentary lipemia in man. J Clin Invest. 1973 Jan;52(1):32–38. doi: 10.1172/JCI107171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane J. P. A rapid electrophoretic technique for identification of subunit species of apoproteins in serum lipoproteins. Anal Biochem. 1973 Jun;53(2):350–364. doi: 10.1016/0003-2697(73)90081-x. [DOI] [PubMed] [Google Scholar]
- Katz S. S., Shipley G. G., Small D. M. Physical chemistry of the lipids of human atherosclerotic lesions. Demonstration of a lesion intermediate between fatty streaks and advanced plaques. J Clin Invest. 1976 Jul;58(1):200–211. doi: 10.1172/JCI108450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MINARI O., ZILVERSMIT D. B. BEHAVIOR OF DOG LYMPH CHYLOMICRON LIPID CONSTITUENTS DURING INCUBATION WITH SERUM. J Lipid Res. 1963 Oct;4:424–436. [PubMed] [Google Scholar]
- Mahley R. W., Holcombe K. S. Alterations of the plasma lipoproteins and apoproteins following cholesterol feeding in the rat. J Lipid Res. 1977 May;18(3):314–324. [PubMed] [Google Scholar]
- Mjos O. D., Faergeman O., Hamilton R. L., Havel R. J. Characterization of remnants produced during the metabolism of triglyceride-rich lipoproteins of blood plasma and intestinal lymph in the rat. J Clin Invest. 1975 Sep;56(3):603–615. doi: 10.1172/JCI108130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland J. B., Bertram P. D., Sabesin S. M. Identification of nascent high density lipoproteins containing arginine-rich protein in human plasma. Biochem Biophys Res Commun. 1978 Jan 13;80(1):81–88. doi: 10.1016/0006-291x(78)91107-5. [DOI] [PubMed] [Google Scholar]
- Redgrave T. G. Formation of cholesteryl ester-rich particulate lipid during metabolism of chylomicrons. J Clin Invest. 1970 Mar;49(3):465–471. doi: 10.1172/JCI106255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redgrave T. G., Martin G. Effects of chronic ethanol consumption on the catabolism of chylomicron triacylglycerol and cholesteryl ester in the rat. Atherosclerosis. 1977 Sep;28(1):69–80. doi: 10.1016/0021-9150(77)90200-3. [DOI] [PubMed] [Google Scholar]
- Sata T., Havel R. J., Jones A. L. Characterization of subfractions of triglyceride-rich lipoproteins separated by gel chromatography from blood plasma of normolipemic and hyperlipemic humans. J Lipid Res. 1972 Nov;13(6):757–768. [PubMed] [Google Scholar]
- Schaefer E. J., Jenkins L. L., Brewer H. B., Jr Human chylomicron apolipoprotein metabolism. Biochem Biophys Res Commun. 1978 Jan 30;80(2):405–412. doi: 10.1016/0006-291x(78)90691-5. [DOI] [PubMed] [Google Scholar]
- Sears B., Hutton W. C., Thompson T. E. Effects of paramagnetic shift reagents on the 13C nuclear magnetic resonance spectra of egg phosphatidylcholine enriched with 13C in the N-methyl carbons. Biochemistry. 1976 Apr 20;15(8):1635–1639. doi: 10.1021/bi00653a007. [DOI] [PubMed] [Google Scholar]
- Small D. M. Cellular mechanisms for lipid deposition in atherosclerosis (first of two parts). N Engl J Med. 1977 Oct 20;297(16):873–877. doi: 10.1056/NEJM197710202971608. [DOI] [PubMed] [Google Scholar]
- Small D. M., Shipley G. G. Physical-chemical basis of lipid deposition in atherosclerosis. Science. 1974 Jul 19;185(4147):222–229. doi: 10.1126/science.185.4147.222. [DOI] [PubMed] [Google Scholar]
- Stokke K. T., Norum K. R. Determination of lecithin: cholesterol acyltransfer in human blood plasma. Scand J Clin Lab Invest. 1971 Feb;27(1):21–27. doi: 10.3109/00365517109080184. [DOI] [PubMed] [Google Scholar]
- Windmueller H. G., Herbert P. N., Levy R. I. Biosynthesis of lymph and plasma lipoprotein apoproteins by isolated perfused rat liver and intestine. J Lipid Res. 1973 Mar;14(2):215–223. [PubMed] [Google Scholar]
- Zilversmit D. B. The surface coat of chylomicrons: lipid chemistry. J Lipid Res. 1968 Mar;9(2):180–186. [PubMed] [Google Scholar]