Abstract
Human γδ T cells expressing the Vδ3 TCR comprise a minor lymphocyte subset in blood but are enriched in liver and in patients with some chronic viral infections and leukemias. We analysed the frequencies, phenotypes, restriction elements and functions of fresh and expanded peripheral blood Vδ3 T cells. Vδ3 T cells accounted for ~0.2% of circulating T cells, included CD4+, CD8+ and CD4−CD8− subsets, and variably expressed CD56, CD161, HLA-DR and NKG2D, but not NKG2A nor NKG2C. Vδ3 T cells were sorted and expanded by mitogen stimulation in the presence of IL-2. Expanded Vδ3 T cells recognised CD1d, but not CD1a, CD1b nor CD1c. Upon activation, they killed CD1d+ target cells, released Th1, Th2 and Th17 cytokines and induced maturation of dendritic cells into APCs. Thus, Vδ3 T cells are glycolipid-reactive T cells with distinct antigen specificities but functional similarities to natural killer T cells.
Introduction
In addition to conventional MHC-restricted T cells, a number of innate T cell populations that recognise non-peptide antigens in an MHC-unrestricted manner have been described in mice and humans. Invariant natural killer T (iNKT4) cells express a TCR composed of an invariant α-chain (Vα24Jα18 in humans and Vα14Jα18 in mice) that pairs with a limited number of β-chains and recognize glycolipid antigens presented by the MHC class I-like molecule CD1d (1, 2). Mucosal associated invariant T (MAIT4) cells express an invariant Vα7.2-Jα33 TCR in humans (Vα19-Jα33 in mice) and recognise microbial vitamin B metabolites presented by MR1 (3). Recently, a second CD1d-restricted T cell population bearing invariant Vα10-Jα50 TCR α-chains with a distinct glycolipid antigen specificity was described in mice (4). The most abundant innate T cells in humans are γδ T cells, of which there are two major subsets. Vγ9Vδ2 T cells recognise pyrophosphate intermediates of isoprenoid synthesis in certain bacteria (5) and Vδ1 T cells can be activated by CD1c, CD1d or the stress-inducible molecule MICA/B expressed by virus-infected and tumor cells (6, 7, 8).
Innate T cells can respond to ligand stimulation by rapidly and potently killing target cells, by releasing cytokines that polarise adaptive immune responses and by transactivating NK cells, dendritic cells (DC4) and B cells (1–3, 5, 9–12). iNKT cells can prevent disease in animal models (1, 2). Human iNKT cells and Vγ9Vδ2 T cells display potent anti-tumor activity in vitro and are currently being tested as adjuvants for cellular immunotherapies in clinical trials for cancer (13, 14).
The majority of non-Vδ1 and non-Vγ9Vδ2 γδ T cells in humans express the Vδ3 TCR chain. The ligand specificities of Vδ3 T cells are unknown, but these cells are reported to be expanded in peripheral blood of renal and stem cell transplant recipients with cytomegalovirus activation (15–17), in patients with HIV infection (18) and B cell chronic lymphocytic leukemia (19) and in healthy livers (20). Here we have enumerated and phenotyped Vδ3 T cells from human peripheral blood and developed a method for their expansion ex vivo. We show that Vδ3 T cells include cells that recognise CD1d and respond by killing CD1d+ target cells, releasing Th1, Th2 and Th17 cytokines and promoting maturation of DC into APCs. Thus, Vδ3 T cells include CD1d-restricted T cells with functional similarities but distinct antigen specificities to those of iNKT cells, properties which place Vδ3 T cells as candidate targets for therapeutic immunomodulation.
Materials and Methods
Enumeration and phenotyping of Vδ3 T cells
PBMCs were isolated from healthy donors. Vδ3 T cells were enumerated and phenotyped by staining PBMC with an anti-Vδ3 TCR mAb (clone P11.5B; Beckman Coulter) and mAbs specific for CD3, CD3, CD4, CD8, CD25, CD28, CD56, CD69, CD161, HLA-DR, NKG2A, NKG2C, NKG2D and the Vα24Jα18 TCR expressed by iNKT cells. Cells were acquired using a CyanTM flow cytometer (Beckman Coulter) and analysed using Flow Jo (Treestar, USA) using fluorescence-minus-one controls.
Generation of Vδ3 T cell and iNKT cell lines
Vδ3 T cells were enriched from PBMC by sorting of Vδ3+CD3+ cells using a MoFlo™ XDP Cell Sorter (Beckman Coulter). 1,000 Vδ3 T cells were cultured in RPMI medium containing 0.05 mM L-glutamine, 10% v/v HyClone FBS, 0.02M HEPES buffer, 100 U/ml penicillin and 100 μg/ml streptomycin and 2.5 μg/ml amphotericin B Fungizone. Cells were stimulated with 1 μg/ml PHA-P and 250 U/ml IL-2 in the presence of excess (2×105) irradiated allogeneic PBMC prepared from two donors. After 24 and 48 hours, medium was replaced with fresh medium containing 250 U/ml IL-2. Vδ3 cells were expanded for 2–4 weeks in the presence of IL-2. Purity and phenotype of Vδ3 T cell lines were assessed by flow cytometry. iNKT cell lines were generated and characterised as described previously (21).
Analysis of CD1 recognition and effector function of Vδ3 T cell lines
Expanded Vδ3 T cells or iNKT cells were co-cultured with equal numbers of mock-transfected HeLa cells or HeLa cell expressing transfected CD1a, CD1b, CD1c or CD1d in the absence or presence of the iNKT cell agonist glycolipid α-galactosylceramide (α-GC4; 100 ng/ml), PMA (1 ng/ml) and blocking mAbs against CD1d, Vδ3 (clones 42.1 and P11.5B) or isotype control Ab (10 μg/ml). As positive controls, cells were stimulated with 10 ng/ml PMA and 1 μg/ml ionomycin. Vδ3 T cell activation was measured by flow cytometric analysis of cell-surface expression of the marker of cytolytic degranulation, CD107a, and intracellular production of cytokines as described (21). Levels of cytokines released into cell supernatants were measured by ELISA.
Analysis of adjuvant effects of Vδ3 T cell lines for DC
Monocyte-derived DC were generated by culturing magnetic bead-enriched CD14+ monocytes for 6 days with GM-CSF and IL-4 (11). Immature DC were then cultured with equal numbers of expanded Vδ3 T cells or 1 μg/ml LPS in the absence or presence of blocking mAbs against CD1d, Vδ3, CD40, CD40L or isotype control Ab (10 µg/ml). The expression by the DC of molecules commonly found on APCs (CD40, CD54, CD80, CD83, CCR7 and HLA-DR) was analysed after 3 days by flow cytometry. Cytokine release (IL-10 and IL-12) was measured by ELISA. The capacity of DC, cultured for 3 days in the absence or presence of Vδ3 T cells or LPS, to drive proliferation of naïve alloreactive T cells was determined by labelling the T cells with Cell Trace Violet (Invitrogen) and analysis of dye dilution after 6 days by flow cytometry.
Statistical analysis
Groups were compared using Mann Whitney U or Student t-test as appropriate. P valuses <0.05 were considered statistically significant.
Results and Discussion
Frequency and phenotype of fresh Vδ3 T cells
Flow cytometric analysis of CD3 and Vδ3 TCR expression by PBMC from 20 healthy donors revealed that Vδ3 T cells account for 0.2±0.3% (mean±SEM) of peripheral T cells (Fig. 1A). This compares with ~0.05% for human iNKT cells (2, 21), ~3% for Vγ9Vδ2 T cells (5, 11) and up to 10% for MAIT cells (3) in blood. Phenotypic analysis showed that fresh Vδ3 T cells can express CD4 or CD8, but the majority (69±19%) were double-negative (DN4) for CD4 and CD8 (Fig. 1B). Interestingly, CD4+, CD8+ and DN cell subsets are also found within iNKT cells, Vγ9Vδ2 T cells and MAIT cells and have distinct functional activities (3, 11, 21). Like other innate T cells, most fresh Vδ3 T cells expressed the NK cell-associated receptors NKG2D, CD56 and CD161 but not NKG2A nor NKG2C. Most displayed phenotypes of resting T cells, being CD28+ and CD25−CD69−, while HLA-DR was variably expressed (Fig. 1C). Therefore, human Vδ3 T cells are similar to other innate T cell populations in that they display phenotypic heterogeneity with regard to their expression of NK cell-associated receptors and CD4 and CD8.
Fig. 1. Frequencies and phenotypes of human Vδ3 T cells.
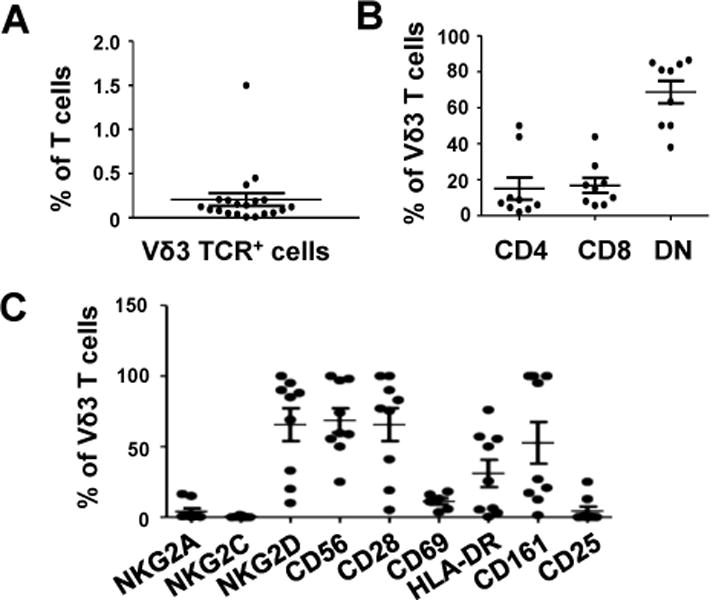
A, Scatterplot showing percentages of freshly-isolated circulating CD3+ cells from 20 healthy donors that express the Vδ3 TCR chain as detected by flow cytometry. B, Percentages of circulating Vδ3 T cells from 9 healthy donors that express CD4, CD8 and neither (DN). C, Percentages of circulating Vδ3 T cells from 9 healthy donors that express NKG2A, NKG2C, NKG2D CD56, CD28, CD69, HLA-DR, CD161 and CD25. Horizontal lines show means.
Expansion of Vδ3 T cells in vitro
Because of their low frequencies in peripheral blood, Vδ3 T cells need to be expanded ex vivo to obtain sufficient numbers for functional studies or for clinical use. We tried a number of T cell expansion protocols and found that a single stimulation of sorted Vδ3 T cells with PHA in the presence of irradiated feeder cells, followed by culturing with IL-2 was optimal. This method yielded up to 25 million Vδ3 T cells from as few as 1,000 cells in 14 days and with purities of >95% (Fig. 2A&B). Phenotypic analysis of Vδ3 T cell lines from 5 donors showed that the CD4/CD8/DN distributions of expanded Vδ3 T cells were similar to those of fresh Vδ3 T cells. Expanded Vδ3 T cells also retained expression of NKG2D, were negative for NKG2C and had lower frequencies of CD56 and CD161 expression than fresh Vδ3 T cells (Fig. 2A&C). Thus, highly pure Vδ3 T cells can be readily expanded by mitogen stimulation in vitro.
Fig. 2. Ex vivo expansion of human Vδ3 T cells.
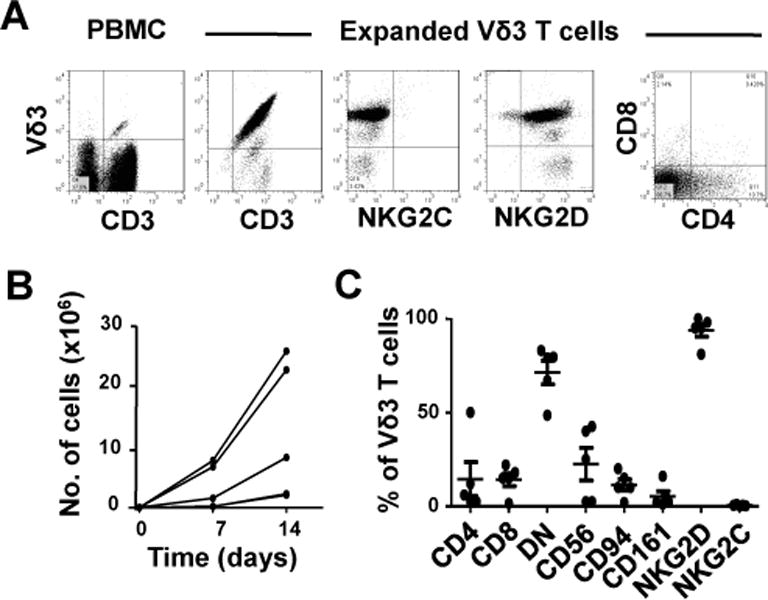
A, Flow cytometric dot plots showing the expression of CD3 and the Vδ3 TCR by fresh PBMC (left panel) and by sorted and expanded Vδ3 T cells (second panel) and the expression of NKG2C (third panel), NKG2D (fourth panel) and CD4 and CD8 (right panel) by expanded Vδ3 T cells. B, Kinetics of mitogen-stimulated Vδ3 T cell expansion starting with 1,000 sorted Vδ3 T cells. C, Percentages of Vδ3 T cells expanded from 5 healthy donors that express CD4, CD8 or neither (DN), CD56, CD94, CD161, NKG2D and NKG2C. Horizontal lines show means.
Vδ3 T cells recognize CD1d
A significant fraction of human T cells, including γδ T cells, recognise autoantigens presented by CD1a, CD1b, CD1c or CD1d (4, 6–8, 22–24). We investigated if expanded Vδ3 T cells could recognise and kill target cells expressing CD1 isotypes by co-culturing them with mock-transfected HeLa cells or HeLa cells expressing transfected CD1a, CD1b, CD1c or CD1d and measuring the expression of the degranulation marker CD107a. In the absence of added glycolipid, Vδ3 T cells degranulated in response to HeLa cells expressing CD1d, but not CD1a, CD1b, or CD1c (Fig. 3A). The requirement for CD1d was confirmed in blocking experiments in which anti-CD1d mAb abrogated degranulation in response to CD1d alone but not CD1d + PMA (Fig. 3B). In contrast, treatment with an anti-Vδ3 mAb did not prevent Vδ3 T cell activation. Future studies are required to determine if this mAb can block or stimulate Vδ3 T cell activation.
Fig. 3. Vδ3 T cells recognize CD1d.
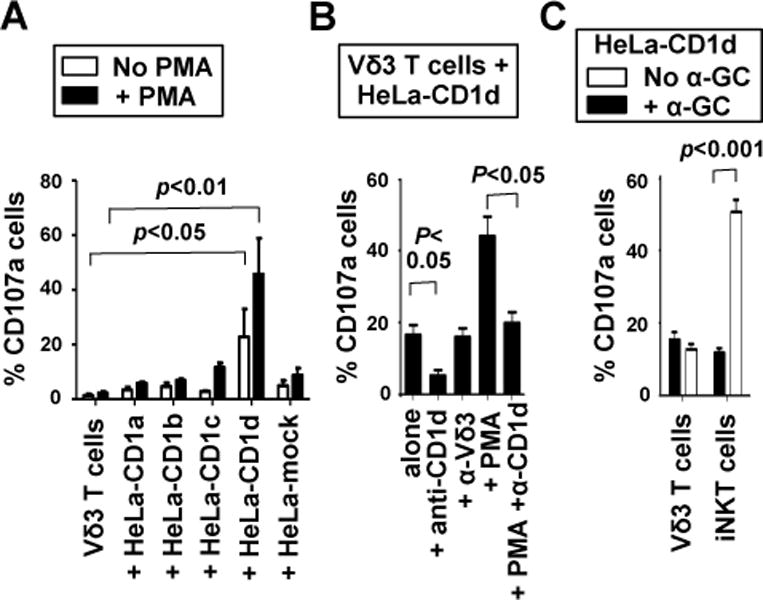
A, Mean percentages of Vδ3 T cells expanded from 5 donors that externalise CD107a after culturing for 4 hours in medium alone, with HeLa cells expressing CD1a, CD1b, CD1c and CD1d, and with mock-transfected HeLa cells in the absence and presence of PMA. B, mean percentages of expanded Vδ3 T cells from 3 donors that degranulate in response to HeLa-CD1d in the absence and presence of PMA and mAbs specific for CD1d (α-CD1d) or Vδ3 (α-Vδ3). C, mean percentages of Vδ3 T cells or iNKT cells from 5 donors that degranulate in response to HeLa-CD1d cells in the absence and presence of α-GC. Error bars show SEM.
Many CD1d-restricted T cells do not express the Vα24Jα18 TCR found on iNKT cells nor recognize the CD1d-binding glycolipid α-GC (4, 8, 24) and are termed type 2 NKT cells. We analysed the co-expression of the Vδ3 and Vα24Jα18 TCR chains by PBMC, expanded Vδ3 T cells or expanded iNKT cells and found that these TCR chains were never co-expressed by the same cells (data not shown), indicating that Vδ3 T cells are not iNKT cells. Vδ3 T cell responses to CD1d were not augmented by adding α-GC as was seen when iNKT cells were used (Fig. 3C), indicating that Vδ3 and iNKT cells have distinct antigen specificities. Thus, some (if not all) Vδ3 T cells fit the definition of type 2 NKT cells. CD1d-restricted activation by other glycolipids, including sulphatide and cardiolipin, has been reported for human Vδ1 T cells (7, 8) and murine γδ T cells (25).
Cytokine production by Vδ3 T cells
A notable feature of innate T cells is their capacity to rapidly secrete large amounts of helper T cell polarising cytokines which can skew adaptive immune responses. iNKT cells can secrete Th1, Th2, Th17 and Treg cytokines, sometimes simultaneously (1, 2, 21), whereas Vγ9Vδ2 T cells most readily produce Th1 cytokines but can be induced under certain conditions to produce Th2 and Th17 cytokines (5, 11) and MAIT cells can produce Th1 and Th17 cytokines (3). We examined intracellular production of IFN-γ, TNF-α, IL-4, IL-10, IL-13 and IL-17 by expanded Vδ3 T cells stimulated with HeLa cells expressing CD1d or PMA and ionomycin. Fig. 4A shows that some Vδ3 T cells produced IFN-γ, TNF-α, IL-4 or IL-17 but not IL-10, indicating that like iNKT cells, they can promote Th1, Th2 and Th17 responses. CD1d-dependent release of IFN-γ and IL-17 by Vδ3 T cells into cell supernatants was also shown by ELISA (Fig. 4B) and found to be blocked by treatment with anti-CD1d, but not isotype control Ab. Thus, like other innate T cells, Vδ3 T cells can regulate adaptive immune responses via production of multiple Th-polarizing cytokines.
Fig. 4. Vδ3 T cells produce multiplecytokines upon stimulation with PMA and ionomycin or CD1d+ cells.
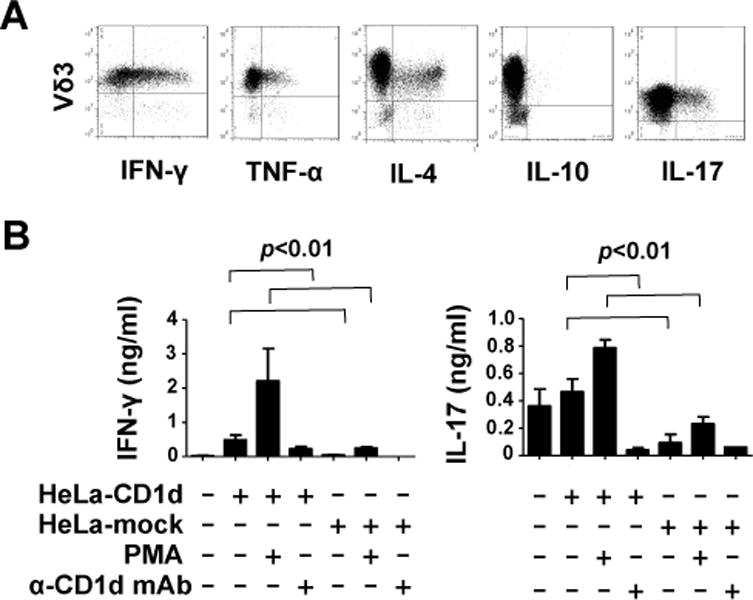
A, Flow cytometric dot plots showing expression of IFN-γ, TNF-α, IL-4, IL-10 and IL-17 by expanded Vδ3 T cell lines after stimulation for 4 hours with PMA and ionomycin. Results are representative of data using expanded Vδ3 T cells from 6 donors. B, Mean levels of IFN-γ and IL-17 released by expanded Vδ3 T cells from 4 donors after stimulation for 24 hours with mock transfected HeLa cells or HeLa-CD1d cells in the absence or presence of PMA and blocking anti-CD1d mAb. No blocking was seen when isotype-matched control Ab was used (not shown). Error bars show SEM.
Dendritic cell maturation by Vδ3 T cells
iNKT cells and Vγ9Vδ2 T cells can drive the differentiation of immature DC into APCs (1, 9, 11) and this property has led to their testing as adjuvants in DC-based immunotherapies (13, 14). We tested if Vδ3 T cells can similarly induce DC maturation in vitro. Immature monocyte-derived DC were co-cultured for 3 days alone or with equal numbers of expanded Vδ3 T cells from 4 donors or LPS and the expression of the APC markers CD40, CD54, CD80, CD83, CCR7 and HLA-DR by DC were analysed by flow cytometry. We found that Vδ3 T cells upregulated CD40, CD83, CD86 and HLA-DR expression by DC to levels comparable to LPS-stimulated DC. CD80, CCR7 and CD54 were not upregulated (Fig. 5A shows data for CD40 and CD86). Inclusion of blocking mAbs showed that Vδ3 T cell mediated DC maturation required CD1d but not CD40-CD40L interactions. Vδ3 T cells also induced IL-10 and IL-12 production by DC (Fig. 5B) and Vδ3 T cell-matured DC induced increased proliferation of naïve alloreactive T cells compared to immature DC (Fig. 5C). These findings argue that some (if not all) Vδ3 T cells promote maturation of DC into APC capable of activating naïve T cells.
Fig. 5. Vδ3 T cells induce dendritic cell (DC) maturation.
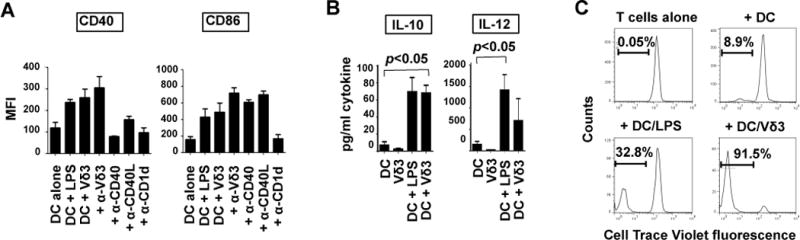
A, Mean fluorescence intensities (MFI4) of expression of CD40 and CD86 by monocyte-derived DC after culturing them for 3 days in medium alone, with LPS or with equal numbers of Vδ3 T cells in the absence and presence of blocking mAbs against CD1d, Vδ3, CD40 and CD40L. Results are means of 5 different DC-Vδ3 T cell combinations. Error bars show SEM. B, Levels of IL-10 and IL-12 released by DC or Vδ3 T cells cultured alone for 2 days, DC cultured with LPS and DC cultured with Vδ3 T cells. Results are means of 3 experiments using different DC and Vδ3 T cells. C, Proliferation of naïve allogeneic T cells in response to medium alone, immature DC, or DC matured for 24 hours with LPS or Vδ3 T cells. T cells were labelled with Cell Trace Violet before adding to the DC. Results show representative flow cytometry histograms (from 4 experiments) showing Cell Trace dye dilution after 6 days.
Concluding remarks
We have identified Vδ3 TCR+ T cells as a novel population of human CD1d-restricted T cells whose glycolipid specificities are distinct from those of iNKT cells. Like iNKT cells, activated Vδ3 T cells kill CD1d+ cells, release Th1, Th2 and Th17 cytokines and promote maturation of DC into APCs. Future studies are required to find out if, like iNKT cells, Vδ3 T cells can be targeted for tumour immunotherapy but two studies (16, 26) have shown that they can kill tumor intestinal epithelial cells but not healthy epithelial cells. Since CD4+, CD8+ and DN subsets of iNKT cells have distinct cytokine profiles (21), it is likely that a functional comparison of CD4+, CD8+ and DN Vδ3 T cell subsets will be required in order to identify the optimal antitumor cells.
Acknowledgments
We thank Conleth Feighery, John Jackson, Jacinta Kelly and Cliona O’Farrelly for helpful discussions on the subject of Vδ3 T cells.
Footnotes
This work was supported by grants from the Irish Health Research Board (BAM, VO’R and PJD), the Irish Research Council (MRD) and the National Childrens Research Centre (AEH). MAE was supported by grants DK066917, CA143748 and CA170194.
Abbreviations used in this paper: α-GC, α-galactosylceramide; α-CD1d, anti-CD1d; DC, dendritic cell; DN, double negative for CD4 and CD8; iNKT, invariant natural killer T; MAIT, mucosal-associated invariant T; MFI, mean fluorescence intensity
References
- 1.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 2.Berzins SP, Smyth MJ, Baxter AG. Presumed guilty: natural killer T cell defects and human disease. Nat Rev Immunol. 2011;11:131–142. doi: 10.1038/nri2904. [DOI] [PubMed] [Google Scholar]
- 3.Le Bourhis L, Guerri L, Dusseaux M, Martin E, Soudais C, Lantz O. Mucosal-associated invariant T cells: unconventional development and function. Trends Immunol. 2011;32:212–218. doi: 10.1016/j.it.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Uldrich AP, Patel O, Cameron G, Pellicci DG, Day EB, Sullivan LC, Kyparissoudis K, Kjer-Nielsen L, Vivian JP, Cao B, Brooks AG, Williams SJ, Illarionov P, Besra GS, Turner SJ, Porcelli SA, McCluskey J, Smyth MJ, Rossjohn J, Godfrey DI. A semi-invariant Vα10+ T cell antigen receptor defines a population of natural killer T cells with distinct glycolipid antigen-recognition properties. Nat Immunol. 2011;12:616–623. doi: 10.1038/ni.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morita CT, Jin C, Sarikonda G, Wang H. Nonpeptide antigens, presentation mechanisms, and immunological memory of human Vγ2Vδ2 T cells: discriminating friend from foe through the recognition of prenyl pyrophosphate antigens. Immunol Rev. 2007;215:59–76. doi: 10.1111/j.1600-065X.2006.00479.x. [DOI] [PubMed] [Google Scholar]
- 6.Spada FM, Grant EP, Peters PJ, Sugita M, Melian A, Leslie DS, Lee HK, van Donselaar E, Hanson DA, Krensky AM, Majdic O, Porcelli SA, Morita CT, Brenner MB. Self-recognition of CD1 by γ/δ T cells: implications for innate immunity. J Exp Med. 2000;191:937–948. doi: 10.1084/jem.191.6.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Russano AM, Bassotti G, Agea E, Bistoni O, Mazzocchi A, Morelli A, Porcelli SA, Spinozzi F. CD1-restricted recognition of exogenous and self-lipid antigens by duodenal γδ+ T lymphocytes. J Immunol. 2007;178:3620–3626. doi: 10.4049/jimmunol.178.6.3620. [DOI] [PubMed] [Google Scholar]
- 8.Bai L, Picard D, Anderson B, Chaudhary V, Luoma A, Jabri B, Adams EJ, Savage PB, Bendelac A. The majority of CD1d-sulfatide-specific T cells in human blood use a semiinvariant Vδ1 TCR. Eur J Immunol. 2012;42:2505–2510. doi: 10.1002/eji.201242531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujii S, Shimizu K, Hemmi H, Steinman RM. Innate Vα14+ natural killer T cells mature dendritic cells, leading to strong adaptive immunity. Immunol Rev. 2007;220:183198. doi: 10.1111/j.1600-065X.2007.00561.x. [DOI] [PubMed] [Google Scholar]
- 10.Galli G, Nuti S, Tavarini S, Galli-Stampino L, De Lalla C, Casorati G, Dellabona P, Abrignani S. CD1d-restricted help to B cells by human invariant natural killer T lymphocytes. J Exp Med. 2003;197:1051–1057. doi: 10.1084/jem.20021616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunne MR, Madrigal-Estebas L, Tobin LM, Doherty DG. (E)-4-hydroxy-3-methyl-but-2 enyl pyrophosphate-stimulated Vγ9Vδ2 T cells possess T helper type 1-promoting adjuvant activity for human monocyte-derived dendritic cells. Cancer Immunol Immunother. 2010;59:1109–1120. doi: 10.1007/s00262-010-0839-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bansal RR, Mackay CR, Moser B, Eberl M. IL-21 enhances the potential of human γδ T cells to provide B-cell help. Eur J Immunol. 2012;42:110–119. doi: 10.1002/eji.201142017. [DOI] [PubMed] [Google Scholar]
- 13.Motohashi S, Okamoto Y, Yoshino I, Nakayama T. Anti-tumor immune responses induced by iNKT cell-based immunotherapy for lung cancer and head and neck cancer. Clin Immunol. 2011;140:167–176. doi: 10.1016/j.clim.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 14.Fournié JJ, Sicard H, Poupot M, Bezombes C, Blanc A, Romagné F, Ysebaert L, Laurent G. What lessons can be learned from γδ T cell-based cancer immunotherapy trials? Cell Mol Immunol. 2013;10:35–41. doi: 10.1038/cmi.2012.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Déchanet J, Merville P, Lim A, Retière C, Pitard V, Lafarge X, Michelson S, Méric C, Hallet MM, Kourilsky P, Potaux L, Bonneville M, Moreau JF. Implication of γδ T cells in the human immune response to cytomegalovirus. J Clin Invest. 1999;103:1437–1449. doi: 10.1172/JCI5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halary F, Pitard V, Dlubek D, Krzysiek R, de la Salle H, Merville P, Dromer C, Emilie D, Moreau JF, Déchanet-Merville J. Shared reactivity of Vδ2− γδ T cells against cytomegalovirus-infected cells and tumor intestinal epithelial cells. J Exp Med. 2005;201:1567–1578. doi: 10.1084/jem.20041851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knight A, Madrigal AJ, Grace S, Sivakumaran J, Kottaridis P, Mackinnon S, Travers PJ, Lowdell MW. The role of Vδ2-negative γδ T cells during cytomegalovirus reactivation in recipients of allogeneic stem cell transplantation. Blood. 2010;116:2164–2172. doi: 10.1182/blood-2010-01-255166. [DOI] [PubMed] [Google Scholar]
- 18.Kabelitz D, Hinz T, Dobmeyer T, Mentzel U, Marx S, Böhme A, Arden B, Rossol R, Hoelzer D. Clonal expansion of Vγ3/Vδ3-expressing γδ T cells in an HIV-1/2-negative patient with CD4 T-cell deficiency. Br J Haematol. 1997;96:266–271. doi: 10.1046/j.1365-2141.1997.d01-2027.x. [DOI] [PubMed] [Google Scholar]
- 19.Bartkowiak J, Kulczyck-Wojdala D, Blonski JZ, Robak T. Molecular diversity of γδ T cells in peripheral blood from patients with B-cell chronic lymphocytic leukaemia. Neoplasma. 2002;49:86–90. [PubMed] [Google Scholar]
- 20.Kenna T, Golden-Mason L, Norris S, Hegarty JE, O’Farrelly C, Doherty DG. Distinct subpopulations of γδ T cells are present in normal and tumor-bearing human liver. Clin Immunol. 2004;113:56–63. doi: 10.1016/j.clim.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 21.O’Reilly V, Zeng SG, Bricard G, Atzberger A, Hogan AE, Jackson J, Feighery C, Porcelli SA, Doherty DG. Distinct and overlapping effector functions of expanded human CD4+, CD8+ and CD4−CD8− invariant natural killer T cells. PLoS One. 2011;6:e28648. doi: 10.1371/journal.pone.0028648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Jong A, Peña-Cruz V, Cheng TY, Clark RA, Van Rhijn I, Moody DB. CD1a-autoreactive T cells are a normal component of the human αβ T cell repertoire. Nat Immunol. 2010;11:1102–1109. doi: 10.1038/ni.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Lalla C, Lepore M, Piccolo FM, Rinaldi A, Scelfo A, Garavaglia C, Mori L, De Libero G, Dellabona P, Casorati G G. High-frequency and adaptive-like dynamics of human CD1 self-reactive T cells. Eur J Immunol. 2011;41:602–610. doi: 10.1002/eji.201041211. [DOI] [PubMed] [Google Scholar]
- 24.Exley MA, He Q, Cheng O, Wang RJ, Cheney CP, Balk SP, Koziel MJ. Cutting edge: Compartmentalization of Th1-like noninvariant CD1d-reactive T cells in hepatitis C virus-infected liver. J Immunol. 2002;168:1519–1523. doi: 10.4049/jimmunol.168.4.1519. [DOI] [PubMed] [Google Scholar]
- 25.Dieudé M, Striegl H, Tyznik AJ, Wang J, Behar SM, Piccirillo CA, Levine JS, Zajonc DM, Rauch J. Cardiolipin binds to CD1d and stimulates CD1d-restricted γδ T cells in the normal murine repertoire. J Immunol. 2011;186:4771–4781. doi: 10.4049/jimmunol.1000921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harly C, Peyrat MA, Netzer S, Déchanet-Merville J, Bonneville M, Scotet E. Up-regulation of cytolytic functions of human Vδ2-γ T lymphocytes through engagement of ILT2 expressed by tumor target cells. Blood. 2011;117:2864–2873. doi: 10.1182/blood-2010-09-309781. [DOI] [PubMed] [Google Scholar]


