Abstract
Objective
Patients with schizophrenia have widespread cognitive impairments, with selective deficits in relational memory. We previously reported a differential relational memory deficit in schizophrenia using the Associative Inference Paradigm (AIP), a task suggested by the Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia (CNTRICS) initiative to examine relational memory. However, the AIP had limited feasibility for testing in schizophrenia due to high attrition of schizophrenia patients during training. Here we developed and tested a revised version of the AIP to improve feasibility.
Method
30 healthy control and 37 schizophrenia subjects received 3 study-test sessions on 3 sets of paired associates: H-F1 (house paired with face), H-F2 (same house paired with new face), and F3-F4 (two novel faces). After training, subjects were tested on the trained, non-inferential Face-Face pairs (F3-F4) and novel, inferential Face-Face pairs (F1-F2), constructed from the faces of the trained House-Face pairs.
Results
Schizophrenia patients were significantly more impaired on the inferential F1-F2 pairs than the non-inferential F3-F4 pairs, providing evidence for a differential relational memory deficit. Only 8 percent of schizophrenia patients were excluded from testing due to poor training performance.
Conclusions
The revised AIP confirmed the previous finding of a relational memory deficit in a larger and more representative sample of schizophrenia patients.
Keywords: memory, associative inference, cognitive deficit, schizophrenia
INTRODUCTION
Widespread cognitive deficits are a prominent feature of schizophrenia (Fioravanti, Carlone, Vitale, Cinti, & Clare, 2005; Heinrichs & Zakzanis, 1998; Kalkstein, Hurford, & Gur, 2010; Kuperberg & Heckers, 2000; Palmer, Dawes, & Heaton, 2009; Saykin et al., 1991). They remain largely unaffected by current pharmacological treatments (Marder & Fenton, 2004; Palmer, et al., 2009) and are a target for the development of new interventions. Meta-analyses have shown that schizophrenia patients exhibit the greatest impairments in the domains of learning, memory, and processing speed (Aleman, Hijman, de Haan, & Kahn, 1999; Dickinson, Ramsey, & Gold, 2007; Fioravanti, et al., 2005; Heinrichs & Zakzanis, 1998; Saykin, et al., 1991). In addition, memory ability has the best predictive power for functional outcome in schizophrenia, making memory an ideal target for drug development and improved quality of life in schizophrenia (Green, 1996; Puig et al., 2008; Velligan, Bow-Thomas, Mahurin, Miller, & Halgunseth, 2000).
Previous research has shown that declarative memory, and in particular episodic memory, is impaired in schizophrenia (Aleman, et al., 1999; Cirillo & Seidman, 2003; Ranganath, Minzenberg, & Ragland, 2008; Weiss & Heckers, 2001), with consistent findings of moderate-to-large effect sizes relative to healthy controls (Danion, Huron, Vidailhet, & Berna, 2007; Heinrichs & Zakzanis, 1998; Leavitt & Goldberg, 2009). Memory for a specific episode requires the binding together of an event with its context, a process that is supported by the hippocampus in healthy individuals (Cohen et al., 1999). Tests of associative or relational memory target this “binding” aspect of memory, and previous studies have shown patients with schizophrenia are especially impaired in this domain (Achim & Lepage, 2003). Specifically, schizophrenia patients show greater memory impairments when asked to remember pairs of items relative to single items(Elvevag, Egan, & Goldberg, 2000; Lepage et al., 2006; Luck et al., 2009), and have significant performance deficits on tests of transitive inference, which can only be solved based on trained relationships between items (Armstrong, Kose, Williams, Woolard, & Heckers, 2010; Coleman et al., 2010; Ongur et al., 2006; Titone, Ditman, Holzman, Eichenbaum, & Levy, 2004). These relational memory impairments have been linked to abnormalities of hippocampal structure and function in schizophrenia (for review see(Heckers & Konradi, 2010)).
The isolation of a selective relational memory deficit poses unique experimental design challenges. On one hand, the comparison of pair versus item memory is often confounded by differential task difficulty (Luck, et al., 2009). On the other hand, the assessment of relational memory ability using inference tasks leads to the exclusion of participants with poor baseline performance, which limits the generalizablity of group differences (Armstrong, et al., 2010; Ongur, et al., 2006; Titone, et al., 2004). We recently published a striking example of the limited generalizabilty of relational memory paradigms in a sample of schizophrenia patients tested with the Associative Inference paradigm (AIP) (Armstrong, et al., 2010). The AIP is one of the relational memory tasks suggested by the Cognitive Neuroscience Treatment Research to Improve Cognition in schizophrenia (CNTRICS) initiative (Ragland et al., 2009) for use in translational cognitive neuroscience studies to facilitate drug development (Carter & Barch, 2007). The AIP had not been previously used with schizophrenia patients, and we found that nearly 35 percent (24/68) of patients had to be excluded for poor performance during the training phase. While we did find a relational memory deficit when comparing the 37 schizophrenia patients who were able to successfully learn the training pairs with 36 healthy controls, the large number of participants excluded highlights the limited feasibility of the AIP in its original form for quantifying a selective relational memory deficit in schizophrenia.
To address this limitation of the AIP, we have revised the task to increase its feasibility for studying cognition in schizophrenia. Major revisions included reducing the size of the training blocks to decrease memory load for training pairs, providing additional feedback during training, and giving participants explicit instructions for solving the inference pairs during test. The goal of these task modifications was to maximize the number of patients with schizophrenia included in the final test of relational memory, thereby increasing the generalizability of our findings to the larger schizophrenia population. We hypothesized that, compared to our original AIP study (Armstrong, et al., 2010), a greater proportion of schizophrenia patients would be able to learn the paired associates during training, but that they would not be able to relationally bind the previously studied pairs during test, due to a selective relational memory deficit.
METHODS
Subjects
We recruited 37 patients with a diagnosis of schizophrenia or schizoaffective disorder and 30 healthy control subjects. The study protocol was approved by the Vanderbilt University Institutional Review Board. Patients were recruited both from Vanderbilt Psychiatric Hospital and from outpatient clinics. Healthy control subjects were recruited by advertisements in the community. Written informed consent was acquired from all subjects after a detailed explanation of the study procedures, and all subjects were paid for their participation. All subjects were assessed by a trained rater using the Structured Clinical Interview for DSM-IV (SCID) (First, Gibbon, Spitzer, & Williams, 1995) and symptom rating scales (Positive and Negative Syndrome Scale (Kay, Fiszbein, & Opler, 1987), Young Mania Scale (Young, Biggs, Ziegler, & Meyer, 1978), and Hamilton Rating Scale for Depression (Hamilton, 1960)). Only subjects without a history of significant head injury, major medical or neurological illness, or current alcohol or other substance abuse within the past three months were included in the study. Healthy control subjects had no Axis I psychiatric disorder and no history of any major neurological or medical illness, substance abuse, or psychotropic medication use. The psychiatric diagnoses were finalized in a diagnostic consensus conference, utilizing information from the structured assessment of the patient, diagnostic assessments of treating physicians, family informants, and past medical records.
Only three patients with schizophrenia were not included in the analysis of the revised associative inference test because they did not reach the training criterion for the premise pairs, set a priori at 70 percent accuracy. The remaining 34 patients (18 with schizophrenia, 16 with schizoaffective disorder) and 30 control subjects were age-and-gender matched and did not differ in parental education (Table 1). A majority of subjects in the current study also participated in the previous AIP study (schizophrenia: 29/37, healthy controls: 18/30) but a significant amount of time passed before completing the revised paradigm (average days that passed between completing the two studies: schizophrenia: 563, range of 346-667 days, healthy controls: 615, range of 341-717 days).
Table 1.
Demographic, clinical, and cognitive characteristics of subjects.
| Healthy Control (n = 30) |
Schizophrenia (n = 34) |
|
|---|---|---|
|
| ||
| Characteristic | Mean ± SD | Mean ± SD |
| Demographics | ||
| Age | 43.70 ± 8.84 | 44.59 ± 9.70 |
| NART IQc | 111.70 ± 7.17 | 107.48 ± 8.07 |
| Educationd | 15.93 ± 2.16 | 13.85 ± 2.51 |
| Parental Education | 12.72 ± 2.89 | 13.34 ± 2.67 |
| Gendera | 17 F / 13 M | 19 F / 15 M |
| Racea | 18 W / 12 B | 20 W / 14 B |
| Clinical Characteristics | ||
| Age of Onset | 22.47 ± 8.43 | |
| Duration of Illness | 22.12 ± 10.28 | |
| HAM-D | 5.26 ± 3.98 | |
| YMRS | 5.44 ± 8.02 | |
| PANSS-positive | 17.38 ± 6.23 | |
| PANSS-negative | 14.24 ± 6.66 | |
| PANSS-general | 26.91 ± 6.03 | |
| PANSS-total | 58.53 ± 13.87 | |
| AIMS | 2.06 ± 2.45 | |
| Chlorpromazine Equivalent | 615.08 ± 307.32 | |
| SCIP (z-scores) b | ||
| Verbal Learning-Immediated | 0.21 ± 1.00 | −1.61 ± 1.58 |
| Working Memoryc | −0.34 ± 1.15 | −1.54 ± 1.57 |
| Verbal Fluency | 0.06 ± 1.09 | −0.07 ± 0.90 |
| Verbal Learning-Delayedc | −0.36 ± 1.01 | −1.09 ± 1.20 |
| Processing Speedd | −0.91 ± 1.26 | −2.29 ± 1.26 |
| Overall Meand | −0.35 ± 0.77 | −1.32 ± 0.88 |
Note: NART, National Adult Reading Test; HAM-D, Hamilton Rating Scale for Depression; YMRS, Young Mania Rating Scale; PANSS, Positive and Negative Syndrome Scale; AIMS, Abnormal Involuntary Movement Scale; SCIP, Screen for Cognitive Impairment in Psychiatry
Chi-square test performed rather than t-test, and sample sizes provided rather than means and standard deviations.
SCIP scores available for 27 out of 30 healthy controls.
Significantly different between groups at p < 0.05
Significantly different between groups at p < 0.001
Demographic, clinical, and cognitive characteristics of the sample are presented in Table 1. The schizophrenia patients were chronically ill (mean duration of illness: 22.12 ± 10.28 years), and moderately symptomatic (mean PANSS total scores: 58.53 ± 13.87). Most schizophrenia patients were medicated at the time of testing with a mean chlorpromazine (CPZ) equivalent of 615.08 mg ± 307.32. Two patients were not taking antipsychotic medication. Patients had significantly fewer years of education (p < 0.001) and lower estimated verbal IQ (p < .05), as measured by the National Adult Reading Test (NART) (Nelson, 1982), than control subjects. Schizophrenia subjects also scored significantly lower on the majority of subscales of a brief cognitive measure, the Screen for Cognitive Impairment in Psychiatry (SCIP) (Purdon, 2005) than our healthy control sample (Table 1).
Apparatus and stimuli
The stimuli were presented on a PC using E-Prime software (version 2.0) (Psychology Software Tools, Inc., 2007), and subjects viewed images on a 20-inch LCD monitor. Stimuli included 30 color photographs of houses sized to 190×142 pixels and 120 color photographs of faces (60 male, 60 female), centered by the bridge of the nose, on white backgrounds with black frame, and sized to 142×190 pixels. All face and house images were gathered from online picture databases.
Experimental task
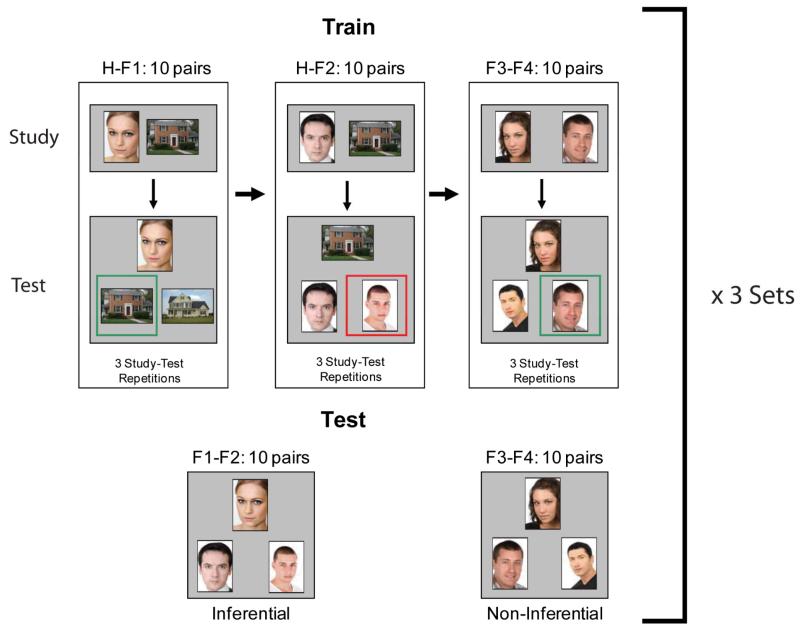
The experimental task is similar to our previously published associative inference paradigm (AIP) (Armstrong, et al., 2010), but significant changes were made to the training-test structure to minimize attrition of schizophrenia patients (Figure 1). Subjects were trained on three conditions. In the first condition, subjects learned a house paired with a face (H-F1), in the next condition subjects learned the same set of houses now paired with a new face of opposite gender (H-F2), and in the final condition, subjects learned two novel faces paired together, one male and one female (F3-F4). Following training, subjects completed a test that included the trained, non-inferential face-face pairs (F3-F4) and a set of novel, inferential face-face pairs that could be linked together because they were paired with the same house during training (F1-F2).
Figure 1.
Experimental Paradigm. Subjects were trained on three types of paired associates: H-F1 (House paired with Face), H-F2 (same House paired with new Face), and F3-F4 (two novel Faces paired together). Within a single study set, subjects completed three study-test sessions on 10 pairs in each condition in a fixed order (H-F1, H-F2, F3-F4). Following training, subjects were tested on the trained, non-inferential F3-F4 pairs as well as a novel set of inferential face-face pairs F1-F2, made up from the faces of the house-face pairs. Subjects then completed 2 more study sets with new face and house stimuli, resulting in a total of 30 unique pairs for each condition. Feedback as indicated by the green (correct) and red (incorrect) boxes was provided during training but not test.
In the original AIP, participants were not told they would be asked to match faces together across identical houses from the H-F1 and H-F2 blocks, but explicit instructions were given in the revised AIP. Although explicit instruction could lead to more elaborative encoding processes, the fundamental construct of relational memory remains unchanged, as flexible manipulation of the H-F1 and H-F2 representations is still necessary to solve the inferential test pairs. In the revised version, after the explanation of task instructions, each subject completed a demonstration of the task, which included practice trials of each training block and the subsequent test block. This was added to increase understanding of and familiarity with the task before beginning the experiment. We also made a major revision to the training structure. Whereas in the original AIP participants learned the 30 pairs from each condition in a single, 30-trial block, in the revised version training-test blocks were broken into three sets of 10 pairs for each condition. This change was implemented to decrease memory load, increase training accuracy of the premise pairs (H-F1, H-F2), and allow for breaks during the experiment while still testing 30 pairs in each condition. A final change from the original task design was to have equivalent training for all three sets of paired associates (three study-test sessions each), rather than only a single training block for the F3-F4 face pairs as in our previous study.
The experiment was composed of 3 training sets, each of which included 10 unique H-F1, H-F2, and F3-F4 pairs. The beginning of each trial was initiated by the participant via a button press. Each training set began with a study block in which 10 H-F1 pairs were presented for 4 seconds. The study block was followed by an immediate, self-paced, two alternative forced-choice test, in which subjects were instructed to pick one of two houses that matched the target face, and feedback was given. This study-test sequence was completed 3 times for the H-F1 pairs (30 study trials, 30 test trials). Then participants completed 3 study-test blocks of H-F2 pairs (30 study trials, 30 test trials) and 3 study-test blocks of F3-F4 pairs (30 study trials, 30 test trials). After completing a full set of 180 training trials (90 study, 90 test with feedback), participants moved immediately to the final inferential memory test. Subjects were tested on the trained face-face pairs (F3-F4, non-inferential) and a novel set of inferential face-face pairs (F1-F2) (Figure 1). The novel face-face set was constructed from the faces of the house-face pairs, and the correct pair could only be inferred through overlapping representations that included the same house from training. The test was self-paced, forced-choice, and pairs were presented in random order with no feedback. For all training and test conditions the correct and incorrect images were equally familiar from training.
This entire sequence was repeated 2 more times with the other stimulus sets, for a grand total of 540 training trials (270 study, 270 test). Each set took about 15 minutes to administer, for a total of 45 minutes for the entire experiment. The order in which subjects received the three sets was randomized.
Statistical analysis
We used repeated measures analysis of variance (ANOVA) to test for (1) effects of group (between-subject) as well as repetition (first, second, or third training block) and pair type (H-F1, H-F2, F3-F4) on accuracy during training, (2) effects of group and face-face pair type (inferential versus non-inferential) on accuracy during test– the crucial comparison, (3) effects of group and face-face pair type (inferential versus non-inferential) on reaction time. We also used bivariate correlations to assess relationships of demographic, clinical, and cognitive variables with memory measures. Effect sizes for analysis of variance are reported as partial eta squared (η2) values.
RESULTS
Accuracy during training
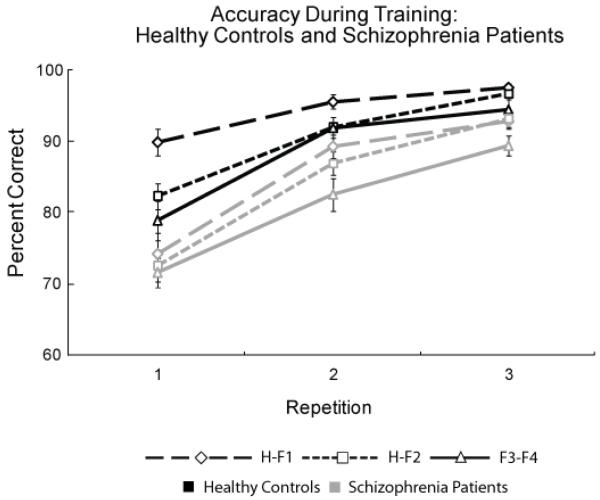
Figure 2 displays the accuracy scores during training. All subjects became more accurate with more practice (main effect of repetition: F (2, 64) = 111.40, p < 0.001, η2 = 0.78) and showed a significant difference in performance across conditions (H-F1 > H-F2 > F3-F4 in overall accuracy; main effect of pair type: F (2, 64) = 21.49, p < 0.001, η2 = 0.41). Overall, healthy control subjects were more accurate than patients (main effect of group: F (1, 64) = 15.75, p < 0.001, η2 = 0.20). We found a repetition by group interaction (F (2, 64) = 4.65, p < 0.05, η2 = 0.10), reflecting a significant difference between the two groups in the slope of the learning curves (healthy controls show the greatest increase in accuracy from repetition 1 to 2, schizophrenia patients show similar changes across all repetitions). There was also a pair by repetition interaction (F (4, 64) = 3.47, p < 0.05, η2 = 0.03), illustrating higher initial performance for H-F1 pairs relative to the other conditions. Finally, we found a significant three-way interaction (F (4, 64) = 2.52, p = 0.05, η2 = 0.05), indicating that the groups differ in the rate of learning the different pair types (in the initial training block, healthy controls show performance differences across pair types whereas patients with schizophrenia show similar accuracy for all pair types).
Figure 2.
Training accuracy data (mean and SE values) for healthy controls (black lines) and schizophrenia patients (gray lines) for house-face (H-F1, diamond with long-dashed line; H-F2, square with short-dashed line) and face-face (F3-F4, triangle with solid line) pairs. Healthy controls show a steep learning curve between the first and second repetitions, indicating rapid early learning whereas schizophrenia subjects show a more gradual learning progression over the three repetitions.
Accuracy during test
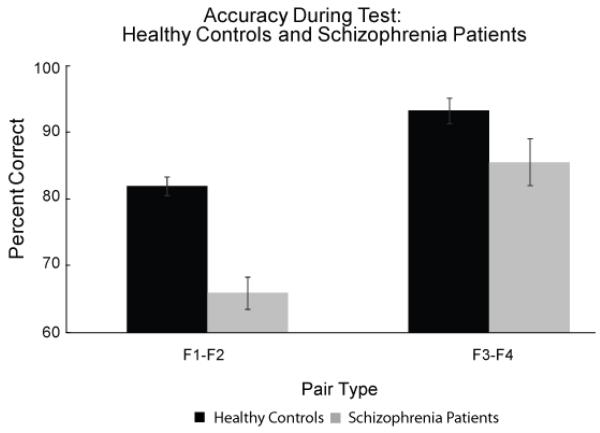
All subjects were tested on their ability to correctly identify the novel, inferential pairs (F1-F2) relative to the previously learned non-inferential face-face pairs (F3-F4) (Figure 3). Healthy control subjects were more accurate than patients in identifying both types of face-face pairs (main effect of group: F (1, 64) = 15.08, p < 0.001, η2 = 0.20) and performance was higher for F3-F4 pairs than F1-F2 pairs (main effect of pair type: F (1, 64) = 75.55, p < 0.001, η2 = 0.55) (Figure 3). Crucially, the between-group differences were significantly greater in the F1-F2 associative inference memory condition (significant pair type by group interaction (F (1, 64) = 5.50, p < 0.05, η2 = 0.08).
Figure 3.
Test accuracy data (mean and SE values) for trained, non-inferential F3-F4 pairs and novel, inferential F1-F2 pairs for healthy control and schizophrenia patients. Relative to healthy controls, schizophrenia patients are more impaired on the inferential F1-F2 pairs than the trained F3-F4 pairs (significant group by pair type interaction), illustrating a particular relational memory deficit in schizophrenia.
The two memory conditions were not matched for task difficulty (i.e., both groups performed better and close to ceiling on the non-inferential F3-F4 pairs). This raises the possibility that the group by pair type interaction described above is driven by differential difficulty, rather than a specific impairment in inferential memory. We conducted 2 additional analyses to address this issue and to isolate differences in inferential memory above and beyond non-inferential memory performance. First, we included F3-F4 accuracy as a covariate and found inferential memory to be impaired in schizophrenia patients relative to controls (F (1, 64) = 4.04, p <0.05, η2 = 0.06). Second, we excluded 5 subjects from each group to create sub-groups matched for non-inferential performance (control 92.0 ± 7.6 %; schizophrenia 89.0 ± 7.8%), gender, age, IQ, and parental education. This ANOVA confirmed our finding of a specific associative inference deficit in schizophrenia (main effect of pair type: F (1, 54) = 65.66, p <0.001, η2 = 0.56; main effect of group: F (1, 54) = 5.28, p < 0.05, η2 = 0.92; pair type by group interaction: F (1, 54) = 4.55, p < 0.05, η2 = 0.08).
Predictors of Memory Performance
Both inferential (F1-F2) and non-inferential (F3-F4) memory performance are related to IQ and cognitive ability (Table 2). IQ is positively correlated with both F1-F2 and F3-F4 performance in schizophrenia patients (F1-F2: r2 = 0.19, p = 0.01; F3-F4: r2 = 0.15, p < 0.05) but only F3-F4 performance in healthy controls (F1-F2: r2 = 0.12, p > 0.05; F3-F4: r2 = 0.32, p = 0.001). Cognitive ability, as assessed by the SCIP, is also related to inferential and non-inferential performance for both healthy controls (F1-F2: r2 = 0.28, p < 0.01; F3-F4: r2 = 0.20, p < 0.05) and patients with schizophrenia (F1-F2: r2 = 0.17, p <0.05; F3-F4: r2 = 0.31, p = 0.001). For SCIP sub-scale correlations, see Table 2.
Table 2.
IQ and cognitive correlations with inferential and non-inferential performance in healthy controls and patients with schizophrenia.
| Healthy Control | Schizophrenia | |||
|---|---|---|---|---|
|
| ||||
| Measure | Inferential | Non-Inferential | Inferential | Non-Inferential |
| IQ | 0.12 | 0.32** | 0.19* | 0.15* |
| Overall SCIP | 0.28 | 0.20 | 0.17* | 0.31** |
| VL-Immediatea | 0.02 | 0.06 | 0.04 | 0.16* |
| Working Memory | 0.32** | 0.22* | 0.18* | 0.15* |
| Verbal Fluency | 0.11 | 0.00 | 0.12* | 0.10 |
| VL-Delayeda | 0.12 | 0.38* | 0.02 | 0.18* |
| Processing Speed | 0.18* | 0.20* | 0.08 | 0.11* |
All cognitive measures assessed by the Screen for Cognitive Impairment in Psychiatry
VL signifies Verbal Learning
p < 0.05
p < 0.01
As task performance is correlated with general cognitive ability and premorbid IQ, it is not surprising that including SCIP and NART scores as covariates affect the ANOVA outcome. Specifically, when including mean SCIP score as a covariate we still find a main effect of pair type (F (1, 64) = 24.65, p < 0.001, η2 = 0.30), but no longer find significant effects of group (F (1, 64) = 1.96, p = 0.167. η2 = 0.03) or pair type by group interaction (F (1, 64) = 1.74, p = 0.192, η2 = 0.03). With the addition of the NART as a covariate, we still find main effects of group (F (1, 64) = 8.54, p = 0.005, η2 = 0.12) and pair type (F (1, 64) = 5.16, p = 0.027, η2 = 0.08), and the pair type by group interaction is marginally significant (F (1, 64) = 3.73, p = 0.058, η2 = 0.06).
Additionally, performance did not differ between schizophrenia subjects who completed the original AIP task (n = 27) and those who did not (n = 7) (no main effect of group (F (1, 32) = 0.002, p = 0.961, η2 = 0.00) or pair type by group interaction (F (1, 32) = 0.59, p = 0.448, η2 = 0.02). However, for controls, subjects who completed the original task (n = 18) performed better on the inferential pairs than those that did not (n = 12) (no main effect of group: F (1, 28) = 3.48, p >0.05, η2 = 0.11, but significant pair type by group interaction: F (1, 28) = 5.46, p < 0.05, η2 = 0.16). Finally, no clinical measure (HAM-D, YMRS, PANSS) was correlated with relational memory performance.
Response latency during test
We analyzed response latencies for correct trials only. Reaction times were longer for inferential pairs (F1-F2) relative to previously trained pairs (F3-F4) (main effect of pair type: F (1, 64) = 100.91, p < 0.001, η2 = 0.62). The pattern of response latencies did not differ between groups (no main effect of group, p = 0.45, η2 = 0.01; no group by pair type interaction, p = 0.49, η2 = 0.01).
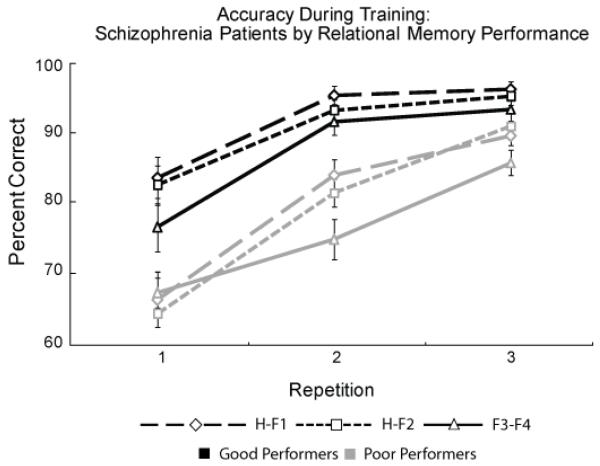
Schizophrenia Performance Groups
As in our previous study (Armstrong, et al., 2010) we split the schizophrenia participants into two groups based on their accuracy in the inferential condition. Good performers (n = 14) were those with equal to or greater than chance performance (designated as 66.67 percent), whereas poor performers (n = 19) had less than chance inference performance (less than 66.67 percent). Figure 4 shows training accuracy for these performance groups. Good performers had higher training accuracy than poor performers (main effect of group: F (1, 34) = 29.56, p < 0.001, η2 = 0.48) and performance significantly varied across conditions (H-F1 > H-F2 > F3-F4 for overall accuracy; main effect of pair type: F (2, 34) = 9.03, p < 0.01, η2 = 0.37). Accuracy increased with repeated exposure (main effect of repetition: F (2, 34) = 82.45, p < 0.001, η2 = 0.80). We also found a significant group by repetition interaction (F (2, 34) = 11.84, p < 0.001, η2 = 0.43) indicating the good performance group showed a steep learning curve from repetition 1 to 2, but a small change from 2 to 3, while the poor performance group has a more gradual learning slope with similar gains in accuracy across all training repetitions. The two performance groups did not differ on any demographic, clinical, or cognitive characteristic listed in Table 1.
Figure 4.
Training accuracy data (mean and SE values) for good (black lines) and poor schizophrenia (gray lines) subjects defined by inferential memory performance above or below chance (Good performance: greater or equal to 66.67%, Bad performance: less than 66.67%). The good performers show higher accuracy overall and rapid early learning compared to poor learners.
DISCUSSION
The purpose of this study was to revise an existing task, the Associative Inference Paradigm (AIP), to increase its feasibility and usefulness for studying relational memory in schizophrenia. The original AIP (Preston, Shrager, Dudukovic, & Gabrieli, 2004), suggested by the CNTRICS initiative, was developed in healthy control subjects. Our previous study of the AIP in schizophrenia revealed that a large proportion of patients failed to reach the learning criterion required to test relational memory (Armstrong, et al., 2010). Here we implemented several modifications to the AIP, including shorter training-test blocks to reduce memory load, feedback throughout the training period, and explicit instructions on how to solve the inference pairs. These task revisions greatly improved the feasibility of AIP in schizophrenia, such that only 8 percent of patients with schizophrenia (3/37) failed to learn the premise pairs during training, as opposed to 35 percent (24/68) in our previous study.
Importantly, we still found impaired relational memory in schizophrenia using the revised AIP, evidenced by the significant group by pair type interaction showing that schizophrenia patients were more impaired on inferential pairs (F1-F2) than non-inferential pairs (F3-F4) compared to healthy controls. Although there were differences in training performance between healthy controls and patients with schizophrenia, all subjects were trained to at least 70 percent accuracy on each of the three training pairs by the final repetition. The two pair types were not matched for difficulty (Chapman & Chapman, 1973; Danion, Rizzo, & Bruant, 1999; Lepage, et al., 2006), which is a common issue in relational memory studies (Danion, et al., 1999; Lepage, et al., 2006), however, additional analyses covarying for and matching on non-inferential performance confirmed that our pattern of results was not simply due to differential task difficulty. Finally, while AIP performance was related to both IQ and cognitive ability, this was expected for an inferential memory task, as memory performance is likely mediated by other cognitive processes such as attention and information processing. Taken together, our results support the hypothesis of a selective inferential memory impairment, relative to memory for explicitly trained stimuli, in schizophrenia (Elvevag, et al., 2000; Hanlon et al., 2005; Lepage, et al., 2006; Ongur, et al., 2006; Titone, et al., 2004; Williams et al., 2010) and make the AIP an attractive paradigm for future functional imaging studies and pharmacological trials.
Although we found a relational memory deficit for the schizophrenia group overall, it is important to note that a sizable number of patients (n = 14) showed intact relational memory, similar to previous findings from our group and others (Armstrong, et al., 2010; Coleman, et al., 2010; Hanlon, et al., 2005). Interestingly, the 14 patients with good inferential performance did not differ from the 19 patients with poor inferential performance on any demographic or clinical variable. While we did find a significant correlation between inferential memory performance and cognitive functioning (total SCIP scores) across all patients with schizophrenia, patients with good and poor inferential ability did not differ on any SCIP measure. Future studies using structural and functional imaging techniques should explore correlates of the relational memory deficits between patient groups, including abnormalities of the hippocampus and frontal cortex (for reviews see (Achim & Lepage, 2005; Heckers & Konradi, 2010)).
A limitation of our study is that all but two of our schizophrenia patients were medicated at the time of testing. While we cannot rule out that pharmacologic treatment contributes to poor memory performance, we did not find a correlation between chlorpromazine equivalent and inferential memory performance in the schizophrenia patients. Future studies should use this revised version of the AIP in unmedicated and first episode psychosis patients.
In summary, we were able to successfully revise the AIP for the study of relational memory in patients with schizophrenia, which greatly improved the feasibility of the task. This provides a good example for the iterative process of implementing experimental designs from the cognitive neuroscience literature in schizophrenia research (Luck & Gold, 2008). We confirmed our hypothesis of a selective deficit of relational memory in schizophrenia using the revised AIP. Future studies using structural and functional neuroimaging methods may provide insight into the neural correlates of these selective memory deficits.
Acknowledgments
The authors would like to thank Dr. Neil Woodward for helpful comments and discussion about this study.
Funding
This work was supported by the National Institutes of Health to Dr. Stephan Heckers (Grant No. RO1-MH070560)
References
- Achim AM, Lepage M. Is associative recognition more impaired than item recognition memory in schizophrenia? A meta-analysis. Brain and Cognition. 2003;53(2):121–124. doi: 10.1016/s0278-2626(03)00092-7. [DOI] [PubMed] [Google Scholar]
- Achim AM, Lepage M. Episodic memory-related activation in schizophrenia: Meta-analysis. British Journal of Psychiatry. 2005;187:500–509. doi: 10.1192/bjp.187.6.500. [DOI] [PubMed] [Google Scholar]
- Aleman A, Hijman R, de Haan EH, Kahn RS. Memory impairment in schizophrenia: A meta-analysis. American Journal of Psychiatry. 1999;156(9):1358–1366. doi: 10.1176/ajp.156.9.1358. [DOI] [PubMed] [Google Scholar]
- Armstrong K, Kose S, Williams L, Woolard A, Heckers S. Impaired associative inference in schizophrenia. Schizophrenia Bulletin. 2010 doi: 10.1093/schbul/sbq145. doi:10.1093/schbul/sbq145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Barch DM. Cognitive neuroscience-based approaches to measuring and improving treatment effects on cognition in schizophrenia: The CNTRICS initiative. Schizophrenia Bulletin. 2007;33(5):1131–1137. doi: 10.1093/schbul/sbm081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP. Problems in the measurement of cognitive deficit. Psychological Bulletin. 1973;79(6):380–385. doi: 10.1037/h0034541. [DOI] [PubMed] [Google Scholar]
- Cirillo MA, Seidman LJ. Verbal declarative memory dysfunction in schizophrenia: From clinical assessment to genetics and brain mechanisms. Neuropsychology Review. 2003;13(2):43–77. doi: 10.1023/a:1023870821631. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Ryan J, Hunt C, Romine L, Wszalek T, Nash C. Hippocampal system and declarative (relational) memory: Summarizing the data from functional neuroimaging studies. Hippocampus. 1999;9(1):83–98. doi: 10.1002/(SICI)1098-1063(1999)9:1<83::AID-HIPO9>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Coleman MJ, Titone D, Krastoshevsky O, Krause V, Huang Z, Mendell NR. Reinforcement ambiguity and novelty do not account for transitive inference deficits in schizophrenia. Schizophrenia Bulletin. 2010;36(6):1187–1200. doi: 10.1093/schbul/sbp039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danion JM, Huron C, Vidailhet P, Berna F. Functional mechanisms of episodic memory impairment in schizophrenia. Canadian Journal of Psychiatry. 2007;52(11):693–701. doi: 10.1177/070674370705201103. [DOI] [PubMed] [Google Scholar]
- Danion JM, Rizzo L, Bruant A. Functional mechanisms underlying impaired recognition memory and conscious awareness in patients with schizophrenia. Archives of General Psychiatry. 1999;56(7):639–644. doi: 10.1001/archpsyc.56.7.639. [DOI] [PubMed] [Google Scholar]
- Dickinson D, Ramsey ME, Gold JM. Overlooking the obvious: a meta-analytic comparison of digit symbol coding tasks and other cognitive measures in schizophrenia. Arch Gen Psychiatry. 2007;64(5):532–542. doi: 10.1001/archpsyc.64.5.532. [DOI] [PubMed] [Google Scholar]
- Elvevag B, Egan MF, Goldberg TE. Paired-associate learning and memory interference in schizophrenia. Neuropsychologia. 2000;38(12):1565–1575. doi: 10.1016/s0028-3932(00)00074-9. [DOI] [PubMed] [Google Scholar]
- Fioravanti M, Carlone O, Vitale B, Cinti ME, Clare L. A meta-analysis of cognitive deficits in adults with a diagnosis of schizophrenia. Neuropsychology Review. 2005;15(2):73–95. doi: 10.1007/s11065-005-6254-9. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW. User’s Guide for the SCID-I: Structured Clinical Interview for DSM-IV Axis I Disorders. Biometrics Research 2 Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? American Journal of Psychiatry. 1996;153(3):321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon FM, Weisend MP, Yeo RA, Huang M, Lee RR, Thoma RJ. A specific test of hippocampal deficit in schizophrenia. Behavioral Neuroscience. 2005;119(4):863–875. doi: 10.1037/0735-7044.119.4.863. [DOI] [PubMed] [Google Scholar]
- Heckers S, Konradi C. Hippocampal pathology in schizophrenia. Current Topics in Behavorial Neuroscience. 2010;4:529–553. doi: 10.1007/7854_2010_43. [DOI] [PubMed] [Google Scholar]
- Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: A quantitative review of the evidence. Neuropsychology. 1998;12(3):426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- Kalkstein S, Hurford I, Gur RC. Neurocognition in schizophrenia. Current Topics in Behavorial Neuroscience. 2010;4:373–390. doi: 10.1007/7854_2010_42. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bulletin. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kuperberg G, Heckers S. Schizophrenia and cognitive function. Current Opinion in Neurobiology. 2000;10(2):205–210. doi: 10.1016/s0959-4388(00)00068-4. [DOI] [PubMed] [Google Scholar]
- Leavitt VM, Goldberg TE. Episodic memory in schizophrenia. Neuropsychology Review. 2009;19(3):312–323. doi: 10.1007/s11065-009-9107-0. [DOI] [PubMed] [Google Scholar]
- Lepage M, Montoya A, Pelletier M, Achim AM, Menear M, Lal S. Associative memory encoding and recognition in schizophrenia: An event-related fMRI study. Biological Psychiatry. 2006;60(11):1215–1223. doi: 10.1016/j.biopsych.2006.03.043. [DOI] [PubMed] [Google Scholar]
- Luck D, Montoya A, Menear M, Achim AM, Lal S, Lepage M. Selective pair recognition memory impairment with no response bias in schizophrenia. Psychiatry research. 2009;169(1):39–42. doi: 10.1016/j.psychres.2008.06.037. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Gold JM. The translation of cognitive paradigms for patient research. Schizophrenia Bulletin. 2008;34(4):629–644. doi: 10.1093/schbul/sbn036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder SR, Fenton W. Measurement and Treatment Research to Improve Cognition in Schizophrenia: NIMH MATRICS initiative to support the development of agents for improving cognition in schizophrenia. Schizophrenia Research. 2004;72(1):5–9. doi: 10.1016/j.schres.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Nelson HE. National Adult Reading Test (NART): Test Manual. NFER-Nelson; Windsor: 1982. [Google Scholar]
- Ongur D, Cullen TJ, Wolf DH, Rohan M, Barreira P, Zalesak M. The neural basis of relational memory deficits in schizophrenia. Archives of General Psychiatry. 2006;63(4):356–365. doi: 10.1001/archpsyc.63.4.356. [DOI] [PubMed] [Google Scholar]
- Palmer BW, Dawes SE, Heaton RK. What do we know about neuropsychological aspects of schizophrenia? Neuropsychology Review. 2009;19(3):365–384. doi: 10.1007/s11065-009-9109-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston AR, Shrager Y, Dudukovic NM, Gabrieli JD. Hippocampal contribution to the novel use of relational information in declarative memory. Hippocampus. 2004;14(2):148–152. doi: 10.1002/hipo.20009. [DOI] [PubMed] [Google Scholar]
- Puig O, Penades R, Gasto C, Catalan R, Torres A, Salamero M. Verbal memory, negative symptomatology and prediction of psychosocial functioning in schizophrenia. Psychiatry Research. 2008;158(1):11–17. doi: 10.1016/j.psychres.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Purdon S. The Screen for Cognitive Impairment in Psychiatry (SCIP): Instructions and three alternate forms. PNL Inc; Edmonton, Canada: 2005. [Google Scholar]
- Ragland JD, Cools R, Frank M, Pizzagalli DA, Preston A, Ranganath C. CNTRICS final task selection: Long-term memory. Schizophrenia Bulletin. 2009;35(1):197–212. doi: 10.1093/schbul/sbn134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C, Minzenberg MJ, Ragland JD. The cognitive neuroscience of memory function and dysfunction in schizophrenia. Biological Psychiatry. 2008;64(1):18–25. doi: 10.1016/j.biopsych.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saykin AJ, Gur RC, Gur RE, Mozley PD, Mozley LH, Resnick SM. Neuropsychological function in schizophrenia. Selective impairment in memory and learning. Archives of General Psychiatry. 1991;48(7):618–624. doi: 10.1001/archpsyc.1991.01810310036007. [DOI] [PubMed] [Google Scholar]
- Titone D, Ditman T, Holzman PS, Eichenbaum H, Levy DL. Transitive inference in schizophrenia: Impairments in relational memory organization. Schizophrenia Research. 2004;68(2-3):235–247. doi: 10.1016/S0920-9964(03)00152-X. [DOI] [PubMed] [Google Scholar]
- Velligan DI, Bow-Thomas CC, Mahurin RK, Miller AL, Halgunseth LC. Do specific neurocognitive deficits predict specific domains of community function in schizophrenia? Journal of Nervous and Mental Disease. 2000;188(8):518–524. doi: 10.1097/00005053-200008000-00007. [DOI] [PubMed] [Google Scholar]
- Weiss AP, Heckers S. Neuroimaging of declarative memory in schizophrenia. Scandinavian Journal of Psychology. 2001;42(3):239–250. doi: 10.1111/1467-9450.00234. [DOI] [PubMed] [Google Scholar]
- Williams LE, Must A, Avery S, Woolard A, Woodward ND, Cohen NJ. Eye movement behavior reveals relational memory impairment in schizophrenia. Biological Psychiatry. 2010;68:617–624. doi: 10.1016/j.biopsych.2010.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. British Journal of Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]