The cooled dual-slot antenna creates a more spherical ablation zone than does a cooled monopole antenna in both ex vivo and in vivo liver models.
Abstract
Purpose:
To compare the performance of a microwave antenna design with two annular slots to that of a monopole antenna design in creating a more spherical ablation zone.
Materials and Methods:
Animal care and use committee approval was obtained before in vivo experiments were performed. Microwave ablation zones were created by using dual-slot and monopole control antennas for 2, 5, and 10 minutes at 50 and 100 W in ex vivo bovine livers. Dual-slot and monopole antennas were then used to create ablation zones at 100 W for 5 minutes in in vivo porcine livers, which also underwent intraprocedural imaging. Ablation diameter, length, and aspect ratio (diameter ÷ length) were measured at gross pathologic examination and compared at each combination of power and time by using the paired Student t test. A P value less than .05 was considered to indicate a significant difference. Aspect ratios closer to 1 reflected a more spherical ablation zone.
Results:
The dual-slot antenna created ablation zones with a higher aspect ratio at 50 W for 2 minutes (0.75 vs 0.53, P = .003) and 5 minutes (0.82 vs 0.63, P = .053) than did the monopole antenna in ex vivo liver tissue, although the difference was only significant at 2 minutes. At 100 W, the dual-slot antenna had a significantly higher aspect ratio at 2 minutes (0.52 vs 0.42, P = .002). In vivo studies showed significantly higher aspect ratios at 100 W for 5 minutes (0.63 vs 0.53, respectively, P = .029). Intraprocedural imaging confirmed this characterization, showing higher rates of ablation zone growth and heating primarily at the early stages of the ablation procedure when the dual-slot antenna was used.
Conclusion:
The dual-slot microwave antenna created a more spherical ablation zone than did the monopole antenna both in vivo and ex vivo liver tissue. Greater control over power delivery can potentially extend the advantages of the dual-slot antenna design to higher power and longer treatment times.
© RSNA, 2013
Introduction
Percutaneous tumor ablation is a minimally invasive procedure that destroys cancerous tissue in situ with cytotoxic thermal energy. Thermal ablation procedures are associated with quicker recovery and fewer complications compared with surgical resection, and these are now critical procedures in the management of early-stage hepatocellular carcinoma, hepatic metastases, renal cell carcinoma, peripheral lung nodules, osteoid osteomas, and osteosarcomas (1,2). Although radiofrequency ablation is the most widely used ablation modality, its use is limited by difficulties in heating charred or dessicated tissue and its poor performance near blood vessels. Such limitations to heating can lead to potentially inadequate ablation zones and a higher rate of local tumor progression than those with resection (2,3). Microwave ablation, on the other hand, has been shown to heat larger volumes of tissue to greater temperatures, potentially increasing procedural efficacy (4–6).
Interstitial antennas deliver power from the microwave generator to the target tissue. The pattern of heating around the antenna varies with antenna design, but high-power delivery can also promote electric field propagation along the proximal shaft during an ablation treatment (7–11). This effect can cause procedural complications such as body-wall burns and can restrict where the applicator can be placed (12–14). Such complications may be reduced by cooling the skin surface and limiting the power or duration of the treatment; however, these techniques add procedural complexity and reduce the size of the ablation zone (15). Antenna cooling can effectively reduce shaft heating but has minimal effect against ablation zone elongation (16–19).
A dual-slot antenna comprising two coaxial, annular slots at the distal tip was recently shown to inhibit proximal electric field propagation and, therefore, to shorten the length of ex vivo ablations (20). However, the antenna in that study was created from relatively malleable coaxial cables without active shaft cooling, making it unsuitable for clinical use. A dual-slot antenna was recently developed that incorporates a rigid, sharpened tip and internal water cooling to facilitate percutaneous use while maintaining its original performance. The goal of this study was to compare the performance of a microwave antenna design with two annular slots to that of a monopole antenna design in creating a more spherical ablation zone.
Materials and Methods
The microwave antenna devices used in this study incorporate aspects of multiple patents pending. No industrial support was provided for this study. Author C.L.B. is a founder, shareholder, and consultant for NeuWave Medical. Study data and publication information were controlled by J.C.
By using a parametric analysis similar to that used in previous work, we designed a dual-slot antenna with a built-in shaft, cooling channels, and a coaxial feed separate from the trocar tip to simplify fabrication. The optimal cooled design provided a heating pattern and power delivery efficiency similar to those of the uncooled design previously described (20). A cooled monopole antenna served as a control antenna for comparison of ablation performance (Fig 1). Both antennas were fabricated by using a thin coaxial antenna (UT-020C; Micro-Coax, Pottstown, Pa), an alumina ceramic tip to enhance rigidity (McDanel Advanced Ceramic Technology, Beaver Falls, Pa), and a 17-gauge steel catheter to create the cooling channel (MicroGroup, Medway, Mass). Antennas were fabricated by two authors (K.A.H. and J.C., each with 2 years of experience).
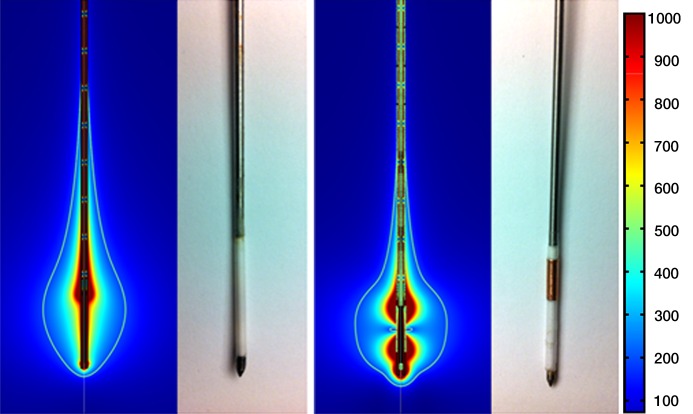
Figure 1:
Numeric modeling image and photograph of monopole antenna (left) and dual-slot antenna (right). In modeling images, color bar represents electric field intensity from 100 to 1000 V/m. Monopole antenna shows electric field emanating from tip of steel catheter; dual-slot antenna shows electric field from each of its slots. White line represents volumetric heating rate of 3 × 104 W/m3, giving a visual approximation of what ablation zone may look like in liver tissue. Note decreased active heating on shaft of dual-slot antenna compared with that of monopole.
Microwave power was delivered by means of a continuous-wave 2.45-GHz magnetron source (MG300; CouberMuegge, Norwalk, Conn) and transferred to the antenna via a 6-foot RG-400 coaxial cable. Cable losses were measured by using a wideband power sensor (Birds Electronic, Solon, Ohio) and were used to recalibrate the generator output to ensure delivery of the prescribed power into the tissue.
Ex Vivo Bovine Liver Ablation
Microwave antenna performance was initially validated by using ex vivo bovine liver tissue. The liver was sectioned into blocks measuring 6 × 6 × 9 cm and was warmed to room temperature before each experiment. The microwave generator was set to deliver 50 W and 100 W for 2, 5, and 10 minutes for each antenna. Six samples were performed (K.A.H. and M.B., both with 2 years of experience) per power and time combination for a total of 36 dual-slot and 36 monopole ablations. The ablation zone was sliced along the antenna axis for gross pathologic analysis. The slices were scanned optically and images were stored for analysis by using ImageJ 1.43u software (US National Institutes of Health, Bethesda, Md). The maximum dimension of the ablation zone transverse to the antenna was defined as the ablation diameter, and the maximum dimension along the antenna insertion path was considered the ablation length. The aspect ratio was defined as the diameter divided by the length. An aspect ratio approaching 1 indicates that the ablation length is approaching that of its diameter, indicating a more spherical ablation zone.
In Vivo Porcine Liver Ablation
All studies were conducted with approval from our institutional animal care and use committee and were compliant with National Research Council guidelines (21). A total of four ablations (two with the dual-slot and two with monopole antennas) were created (J.C. and a veterinary technician, with 2 and 12 years of experience, respectively) by using 100 W for 5 minutes in each of six female domestic swine with a weight range of 80–90 kg (Arlington Farms, Arlington, Wis) for a total of 24 ablations (12 monopole and 12 dual-slot). For each animal, half (n = 2) of the ablations for each experimental arm were performed in the medial lobes and the other half were performed in the lateral lobes to account for differences in perfusion rates. Ablations that protruded beyond the parenchyma of the tissue or in which antenna failure occurred were excluded from the results. After exclusion of incomplete ablation zones, there were eight dual-slot ablations and eight monopole ablation zones in the in vivo study.
Animals were sedated with intramuscular tiletamine hydrochloride and zolazepam hydrochloride (7 mg/kg, Telazol, Fort Dodge, Iowa) and xylazine hydrochloride (2.2 mg/kg, Xyla-Ject; Phoenix Pharmaceutical, St Joseph, Mo). Anesthesia was maintained with inhaled 1%–2% isofluorane (Halocarbon Laboratories, River Edge, NJ). An ear vein was cannulated with a 20-gauge angiographic catheter for administration of intravenous fluids.
After ablation, animals were sacrificed by means of an intravenous injection of pentobarbital sodium and phenytoin sodium (0.2 mL/kg, Beuthanasia-D; Schering-Plough, Kenilworth, NJ). The liver was removed and sectioned along the axis of each antenna. Image analysis was performed in the same way as with ex vivo tissue.
Unenhanced computed tomographic (CT) imaging was used to assess ablation growth in two of the animals. Before the ablation, a roadmap CT scan was obtained of the entire liver. During the ablation, CT data were acquired every 30 seconds. (120 kV, 200 mA, 512 × 512, 1:1 helical pitch, 5 mm section thickness). A threshold was set at 35 HU, which was approximately 16 HU below the background liver attenuation with postprocessing software (Volume Viewer version 3.1; Advantage Windows version 4.5, GE Medical Systems, Wis) to detect and monitor ablation zone area growth at each time point. This value was chosen on the basis of previous CT studies, which showed the average 16-HU difference between an ablated lesion from radiofrequency ablation and the background tissue (22). The final threshold image was compared with gross pathologic results to confirm the validity of using our threshold technique to monitor ablation growth. The central hypoattenuating region, which was heated and gaseous surrounding the emission point, was also monitored, and diameter, length, and overall area were recorded at each time point.
Statistical Analysis
To ensure that there were no probe–lobe interaction effects, we compared the ablation zone characteristics produced by each antenna while accounting for placement in the lobe. Because the effects seemed additive, we averaged the ablation zone dimensions over lobes by probe type to produce a single monopole and dual-slot pair per animal. Differences in mean diameter, length, and aspect ratio between the dual-slot and monopole antenna designs were identified among the power and treatment length combinations by using a paired Student t test. The paired Student t test was considered appropriate after validating approximately equal variances and symmetric distribution among comparison groups. P values less than .05 were considered to indicate a significant difference. Statistical analysis was performed by using Graphpad Prism version 5.04 (La Jolla, Calif) with assistance from the departmental statistician.
Results
Ex Vivo Results
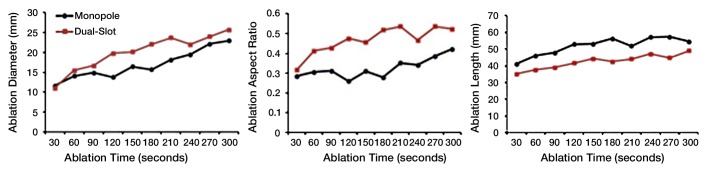
Power and time dose responses of the monopole and dual-slot antennas are shown in Figure 2. At 50 W, the dual-slot antenna created ablation zone diameters similar to those of the monopole at 2 minutes (1.95 cm ± 0.18 vs 1.79 cm ± 0.27, respectively; P = .278), 5 minutes (2.12 cm ± 0.14 vs 2.32 cm ± 0.18; P = .078), and 10 minutes (3.01 cm ± 0.29 vs 3.02 cm ± 0.16; P = .987). With respect to ablation length, the dual-slot antenna created significantly shorter ablation zones compared with those of the monopole antenna at 2 minutes (2.64 cm ± 0.30 vs 3.38 cm ± 0.42; P = .007) and 5 minutes (2.61 cm ± 0.34 vs 3.83 cm ± 0.92; P = .020), but not at 10 minutes (5.14 cm ± 0.64 vs 4.71 cm ± 0.51, respectively; P = .225). Accordingly, the combination of equivalent diameters and shorter ablation lengths led to a greater aspect ratio for dual-slot ablation zones at 2 minutes (0.75 ± 0.11 vs 0.53 ± 0.04, P = .003) and 5 minutes (0.82 ± 0.13 vs 0.63 ± 0.14, P = .053), but not at 10 minutes (0.59 ± 0.07 vs 0.64 ± 0.05, P = .168).
Figure 2a:
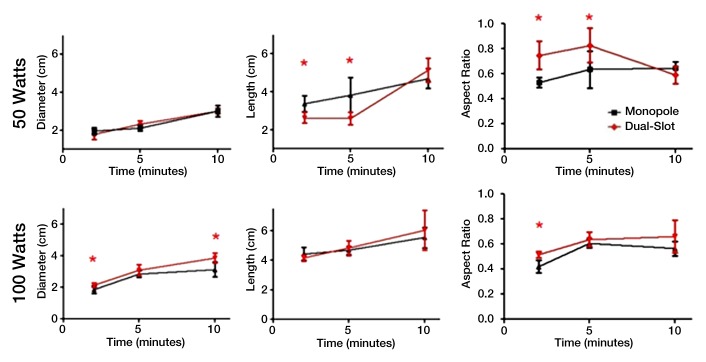
(a) Graphs show ablation diameter, length, and aspect ratio of ex vivo ablations comparing dual-slot with monopole antennas. Solid dots represent sample mean, and horizontal line segments represent standard deviation. * = significant difference (P < .05). (b) Sample ablation zone created by dual-slot (left) and monopole (right) antennas at 100 W for 5 minutes. Dual-slot antenna created significantly shorter and more spherical ablation zone than did monopole antenna at 50 W for 2 and 5 minutes. Dual-slot antenna created a significantly wider and more spherical ablation zone compared with monopole antenna at 100 W for 2 minutes.
Figure 2b:
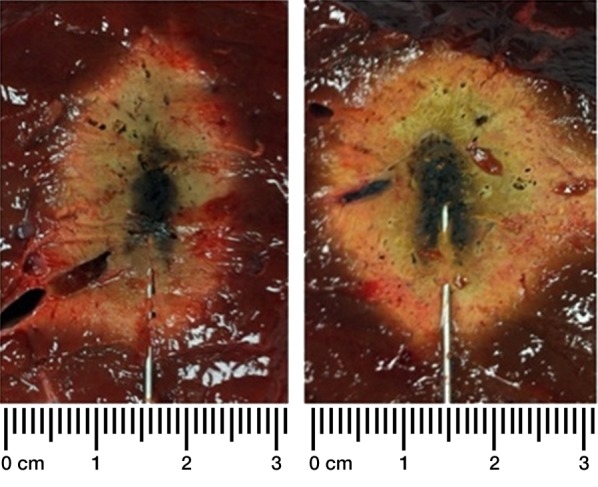
(a) Graphs show ablation diameter, length, and aspect ratio of ex vivo ablations comparing dual-slot with monopole antennas. Solid dots represent sample mean, and horizontal line segments represent standard deviation. * = significant difference (P < .05). (b) Sample ablation zone created by dual-slot (left) and monopole (right) antennas at 100 W for 5 minutes. Dual-slot antenna created significantly shorter and more spherical ablation zone than did monopole antenna at 50 W for 2 and 5 minutes. Dual-slot antenna created a significantly wider and more spherical ablation zone compared with monopole antenna at 100 W for 2 minutes.
At 100 W, the dual-slot antenna created ablation zones with diameters greater than those created by the monopole antenna at all of the time points (2.14 cm ± 0.15 vs 1.86 cm ± 0.24 at 2 minutes, P = .032; 3.07 cm ± 0.35 vs 2.80 cm ± 0.19 at 5 minutes, P = .077; 3.86 cm ± 0.32 vs 3.12 cm ± 0.48 at 10 minutes, P = .017), but the difference was only significant at 2 and 10 minutes. On the other hand, no differences were noted in ablation lengths between the dual-slot and monopole antennas at any of the time points (4.16 cm ± 0.20 vs 4.44 cm ± 0.44 at 2 minutes, P = .186; 4.83 cm ± 0.50 vs 4.62 cm ± 0.31 at 5 minutes, P = .354; 6.04 cm ± 1.35 vs 5.55 cm ± 0.69 at 10 minutes, P = .458). Collectively, the greater ablation diameter of the dual-slot antenna with the equivalent ablation lengths led to a significantly higher aspect ratio than that of the monopole antenna at only 2 minutes (0.52 ± 0.03 vs 0.42 ± 0.05, P = .002), with the aspect ratios being statistically similar at 5 minutes (0.64 ± 0.05 vs 0.61 ± 0.03, P = .191) and 10 minutes (0.66 ± 0.13 vs 0.56 ± 0.06, P = .136).
In Vivo Results
In vivo studies confirmed the trends shown in the ex vivo studies (Fig 3a). The dual-slot antenna created a significantly greater diameter (2.85 cm ± 0.45 vs 2.35 cm ± 0.42, respectively; P = .034) and a slightly shorter length (4.47 cm ± 0.71 vs 4.59 cm ± 0.81, respectively; P = .597), leading to a significantly greater aspect ratio compared with the monopole antenna (0.63 ± 0.08 vs 0.53 ± 0.08, respectively; P = .029).
Figure 3a:
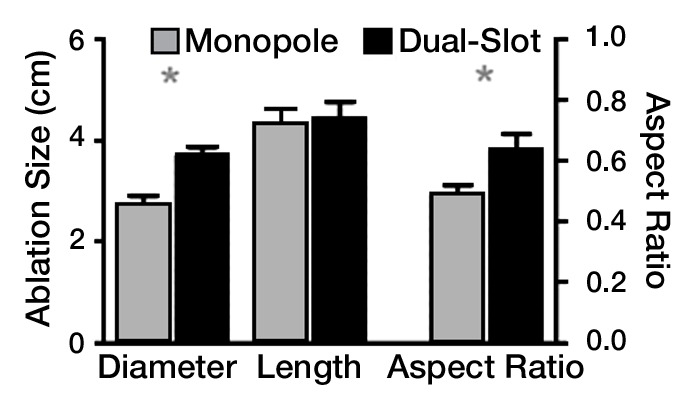
(a) Bar graph shows in vivo diameter, length,, and aspect ratio of dual-slot and monopole antennas at 100 W for 5 minutes. Dual-slot antenna created ablation zones that were significantly wider and more spherical than those created by using monopole antennas. This is consistent with trends found in power and treatment length in ex vivo tissue. * = significant difference (P < .05). (b) Sample intraprocedural images show microwave antenna placement in porcine liver. (c) Intraprocedural monitoring of ablation zone diameter, length, and aspect ratio during in vivo ablation with dual-slot and monopole antennas.
Figure 3b:
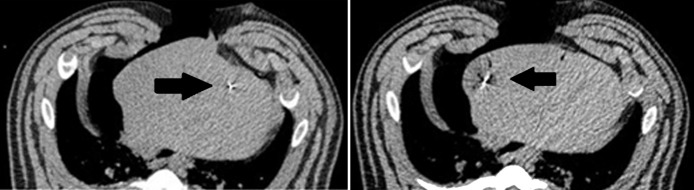
(a) Bar graph shows in vivo diameter, length,, and aspect ratio of dual-slot and monopole antennas at 100 W for 5 minutes. Dual-slot antenna created ablation zones that were significantly wider and more spherical than those created by using monopole antennas. This is consistent with trends found in power and treatment length in ex vivo tissue. * = significant difference (P < .05). (b) Sample intraprocedural images show microwave antenna placement in porcine liver. (c) Intraprocedural monitoring of ablation zone diameter, length, and aspect ratio during in vivo ablation with dual-slot and monopole antennas.
Figure 3c:
(a) Bar graph shows in vivo diameter, length,, and aspect ratio of dual-slot and monopole antennas at 100 W for 5 minutes. Dual-slot antenna created ablation zones that were significantly wider and more spherical than those created by using monopole antennas. This is consistent with trends found in power and treatment length in ex vivo tissue. * = significant difference (P < .05). (b) Sample intraprocedural images show microwave antenna placement in porcine liver. (c) Intraprocedural monitoring of ablation zone diameter, length, and aspect ratio during in vivo ablation with dual-slot and monopole antennas.
Intraprocedural CT with thresholding confirmed these trends, with the final ablation zone of the dual-slot antenna showing a greater diameter and shorter ablation zone than those of the monopole antenna. Intraprocedural CT also showed differences in the ablation zone growth pattern. The diameter of the dual-slot ablation zone was similar to that of the monopole at the start of the procedure but grew much faster than that of the monopole until 4 minutes into the procedure. After this point, the growth rate of the diameter slowed down, while the monopole ablation diameter continued to grow in a linear fashion. The ablation length of the dual-slot antenna was shorter than that of the monopole antenna at nearly every time point, although the growth rate was approximately the same. The combination of the dual-slot antenna creating a wider ablation zone and maintaining a shorter ablation length led to the dual-slot antenna having a greater aspect ratio at every time point during the 5-minute ablation (Fig 3c).
Intraprocedural CT also demonstrated differences in heating pattern, which was demarcated by a coarse outline of a hypoattenuating region (less than –300 HU), most likely representing water vaporization at the interface between tissue and the antenna heating element (Fig 4a). The hypoattenuating region was noted initially near the base of the monopole radiating segment, approximately 20 mm proximal to the antenna tip. The dual-slot antenna created a similar region approximately 5 mm proximal to the tip. That region grew slightly along the proximal antenna shaft but to a lesser degree than that of the monopole. The growth rate of the hypoattenuating area varied between the antenna designs (Fig 4b). The dual-slot antenna created a larger hypoattenuating region at all of the time points than did the monopole, suggesting a higher rate of local heating and subsequent vaporization. In terms of growth rate, both antennas created most of the hypoattenuating region in the first 2 minutes; the dual-slot antenna appeared to plateau within 3 minutes, but the monopole antenna’s hypoattenuating region continued to grow steadily until 5 minutes.
Figure 4a:
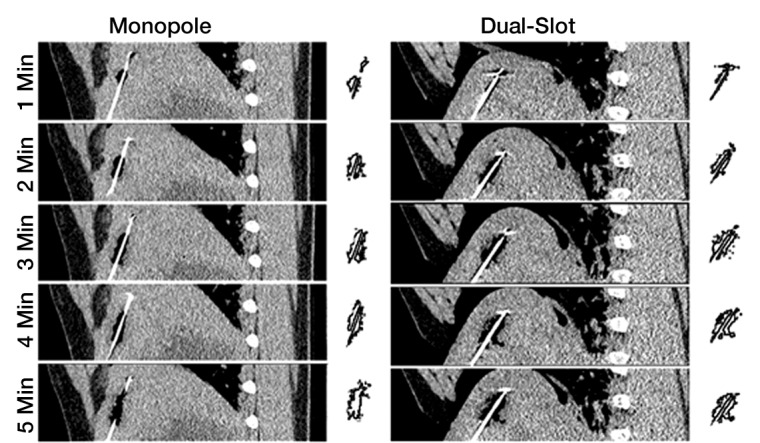
(a) In vivo intraprocedural CT images of monopole (left) and dual slot (right) antennas show differences in hypoattenuating area corresponding to vapor formation around heating element. Hypoattenuating region outlines appear on the right of each CT image. (b) Graph shows comparison of the hypoattenuating cross-sectional areas of dual-slot and monopole antennas at 1-minute intervals. (c) Gross histologic examination shows hypoattenuating region demarcating area of central desiccation in ablation zone. Dual-slot antenna is capable of maintaining hypoattenuating region of growth longer and of creating overall larger hypoattenuating area compared with that of monopole antenna.
Figure 4b:
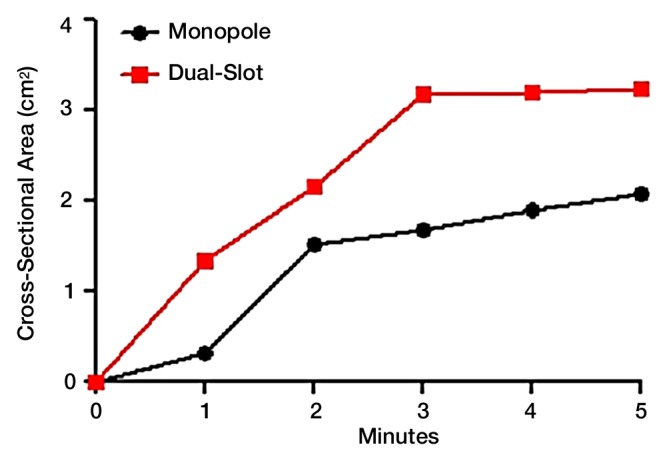
(a) In vivo intraprocedural CT images of monopole (left) and dual slot (right) antennas show differences in hypoattenuating area corresponding to vapor formation around heating element. Hypoattenuating region outlines appear on the right of each CT image. (b) Graph shows comparison of the hypoattenuating cross-sectional areas of dual-slot and monopole antennas at 1-minute intervals. (c) Gross histologic examination shows hypoattenuating region demarcating area of central desiccation in ablation zone. Dual-slot antenna is capable of maintaining hypoattenuating region of growth longer and of creating overall larger hypoattenuating area compared with that of monopole antenna.
Figure 4c:
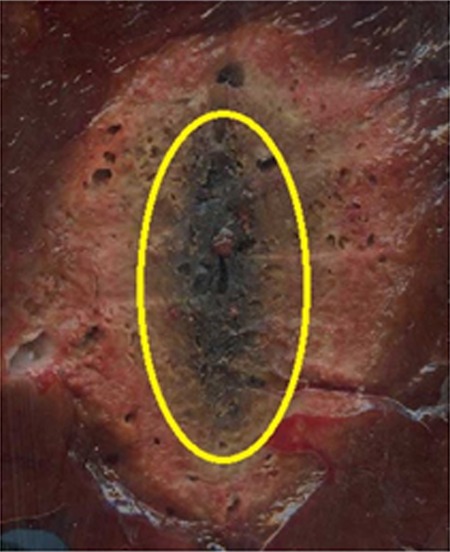
(a) In vivo intraprocedural CT images of monopole (left) and dual slot (right) antennas show differences in hypoattenuating area corresponding to vapor formation around heating element. Hypoattenuating region outlines appear on the right of each CT image. (b) Graph shows comparison of the hypoattenuating cross-sectional areas of dual-slot and monopole antennas at 1-minute intervals. (c) Gross histologic examination shows hypoattenuating region demarcating area of central desiccation in ablation zone. Dual-slot antenna is capable of maintaining hypoattenuating region of growth longer and of creating overall larger hypoattenuating area compared with that of monopole antenna.
Discussion
In our study, we characterized the ablation zone produced by a water-cooled dual-slot antenna in a preclinical liver tissue model. Compared with a cooled monopole antenna, dual-slot ablations had greater diameter-to-length aspect ratios, particularly for treatment times less than 5 minutes. Greater aspect ratios were attributed to shorter ablation lengths at lower power and increased ablation diameters at higher power. Similar trends were observed in vivo, although ablation zones were markedly smaller and more spherical than those made in ex vivo tissue due to the presence of perfusion.
Our results suggest that antenna design may be more effective at actively controlling the ablation shape for the first few minutes of the ablation. After then, effects such as thermal conduction and tissue property changes may eliminate earlier differences. Schramm et al (23) predicted only a small influence of thermal conduction on microwave ablations, but that study did not account for water vaporization or associated heat transfer. Ji et al (24) noted an increase in the ablation zone length over time when accounting for dielectric property changes caused by vaporization and desiccation around the antenna shaft. Our study results appear to have confirmed those findings, as was shown in the lack of difference between dual-slot and monopole ablation aspect ratios at 10 minutes. This evidence suggests that power delivery techniques may need to be tailored to a particular antenna design to optimize treatment results at earlier time points.
Intraprocedural imaging revealed differences in ablation zone growth and vapor formation between both antennas, which may contribute to the final gross pathologic results. The dual-slot ablation length started out shorter than that of the monopole and maintained that difference throughout the procedure, with both ablation zones growing at approximately the same rate. Although our gross pathologic studies revealed only part of the entire ablation growth curve, these imaging results confirmed the ablation trends found in both the in vivo and ex vivo studies.
Zones of extreme hypoattenuation that corresponded to water vaporization in the tissue were also monitored near the ablation applicator (24). The vapor zones corresponded approximately to the centrally blackened, desiccated area in the middle of the ablation zone, which appeared to reach much higher temperatures compared with the transition zone, which was darker but maintained its structure on gross pathologic examination. Similar to the overall ablation trends found in the ex vivo, in vivo, and imaging studies, there was evidence of a dynamic heating profile specific to each antenna design. This region of desiccation has potential implications in postoperative pathology or monitoring in demarcating areas where cells potentially experience thermal fixation (25).
The ablation zones found with the dual-slot antenna were comparable to those found with other cooled antenna designs. Ablation zones with the monopole and dual-slot antennas were both larger than those of the cooled dipole antennas as reported by Sun et al (16) and Zhou et al (26) in both diameter and length at 50 W, but comparable dimensions were reported at the higher powers (80–100 W for 10 minutes). These studies confirm that antenna designs create distinct ablation zones at lower powers but become similar to each other at higher powers. Hines-Peralta et al (19) characterized a cooled large-gauge antenna that created much wider and longer ablation zones compared with the ones reported here, but they used an antenna that was much greater in diameter. Cavagnaro et al (18) reported a validation study with a cooled, choked dipole design, which added a third outer conductor over an entire dipole antenna. Their ex vivo results showed an increasingly spherical ablation zone as ablation length continued up until 15 minutes at lower powers (20–60 W). At shorter time periods, the choked-dipole antenna design created ablation zones that were more elongated, with comparable diameters compared with those created by the dual-slot antenna. Comparisons between ex vivo and in vivo studies were consistent with these previous studies, where the in vivo ablation zones were noticeably smaller than those created in ex vivo tissue. One exception was with the Hines-Peralta study (27), where the in vivo ablation zones were larger than the ex vivo ablation zones for all 150-W ablations less than 10 minutes long. These variations could potentially have been attributed to the condition of the liver or more rapid tissue contraction, which were not evaluated.
There were certain limitations to our study. First, the cooled monopole and dual-slot antennas were fabricated by hand, limiting tolerances to approximately 1 mm and producing some variation between antennas. However, such variations were not likely to influence the treatment more than variations between tissue properties, perfusion rates, and placement locations. Second, the ex vivo and in vivo tissue often had vasculature that disrupted the shape of the ablation zone. Microwave energy is capable of overcoming large vascular heat sinks and expanding the ablation zone through extreme vessel heating, adding variability to our ablation geometry measurements (28). Third, our in vivo studies were limited in that they were not performed in a tumor-specific model, which would have a different vasculature appearance, as well as different thermal and electric properties. However, tumor models for large animals are not widely available or feasible for such characterization studies. Last, our in vivo data were limited to a single power setting. Preliminary testing at 50 W created ablation zones that were highly susceptible to the effect of perfusion and not useful for antenna characterization. The use of higher powers could add further detail and increase the performance of the two antennas.
In conclusion, the cooled dual-slot antenna created more spherical ablation zones than did a cooled monopole antenna in both ex vivo and in vivo liver models. The ablation zones of both antennas became more elongated at higher powers and longer treatment times. Intraprocedural CT studies that use a threshold function to observe the ablation zone growth were not consistent with the gross pathologic results. Further refinement with this method of observing ablation growth at unenhanced CT is required to obtain more consistent monitoring. Intraprocedural studies revealed a distinct hypoattenuating region of vapor that was unique to each antenna design, demonstrating different rates of volumetric ablation growth between the monopole and dual-slot antennas. This vaporization pattern potentially can be used to characterize microwave antenna performance. Differences between antenna designs may gradually diminish with increasing power or treatment duration. Adjusting the power delivery algorithm may more fully exploit the theoretical advantages of specific antenna designs.
Advances in Knowledge.
• The dual-slot microwave antenna can create a more spherical ablation zone than the monopole antenna can in ex vivo and in vivo liver tissue.
• Substantial water vapor is formed and can be visualized with intraprocedural CT imaging to characterize ablation zone growth during microwave heating.
• Differences in heating patterns produced by different antenna designs may be minimized by using high powers or long treatment times.
Implication for Patient Care.
• A more spherical, less elongated ablation zone can potentially lead to increased ablation precision and reduced complications.
Disclosures of Conflicts of Interest: J.C. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: patents pending with support of the Wisconsin Alumni Research Foundation. Other relationships: none to disclose. K.A.H. No relevant conflicts of interest to disclose. M.B. No relevant conflicts of interest to disclose. C.L.B. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: payment for consultancy from NeuWave Medical, Triagenics, and Edwin Hlavka, stock options from NeuWave Medical. Other relationships: none to disclose.
Acknowledgments
The authors thank Lisa Sampson, BS, for her assistance in experimental setup and Alejandro Muñoz del Rio, PhD, for his assistance with statistics.
Received September 21, 2012; revision requested November 19; revision received January 5, 2013; accepted January 21, 2013; final version accepted January 23.
From the 2012 RSNA Annual Meeting.
Funding: This research was supported by the National Institutes of Health (grants 1 R01 CA142737 and UL1TR000427).
References
- 1.Wong R, Frenette C. Updates in the management of hepatocellular carcinoma. Gastroenterol Hepatol (N Y) 2011;7(1):16–24 [PMC free article] [PubMed] [Google Scholar]
- 2.Abu-Hilal M, Primrose JN, Casaril A, McPhail MJ, Pearce NW, Nicoli N. Surgical resection versus radiofrequency ablation in the treatment of small unifocal hepatocellular carcinoma. J Gastrointest Surg 2008;12(9):1521–1526 [DOI] [PubMed] [Google Scholar]
- 3.Ayav A, Germain A, Marchal F, et al. Radiofrequency ablation of unresectable liver tumors: factors associated with incomplete ablation or local recurrence. Am J Surg 2010;200(4):435–439 [DOI] [PubMed] [Google Scholar]
- 4.Andreano A, Huang Y, Meloni MF, Lee FT, Jr, Brace C. Microwaves create larger ablations than radiofrequency when controlled for power in ex vivo tissue. Med Phys 2010;37(6):2967–2973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brace CL. Microwave tissue ablation: biophysics, technology, and applications. Crit Rev Biomed Eng 2010;38(1):65–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brace CL. Radiofrequency and microwave ablation of the liver, lung, kidney, and bone: what are the differences? Curr Probl Diagn Radiol 2009;38(3):135–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Labonte S, Blais A, Legault SR, Ali HO, Roy L. Monopole antennas for microwave catheter ablation. IEEE Trans Microw Theory Tech 1996;44(10):1832–1840 [Google Scholar]
- 8.Hurter W, Reinbold F, Lorenz WJ. A dipole antenna for interstitial microwave hyperthermia. IIEEE Trans Microw Theory Tech 1991;39(6):1048–1054 [Google Scholar]
- 9.Brace CL, Laeseke PF, van der Weide DW, Lee FT. Microwave ablation with a triaxial antenna: results in ex vivo bovine liver. IEEE Trans Microw Theory Tech 2005;53(1):215–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bertram JM, Yang D, Converse MC, Webster JG, Mahvi DM. A review of coaxial-based interstitial antennas for hepatic microwave ablation. Crit Rev Biomed Eng 2006;34(3):187–213 [DOI] [PubMed] [Google Scholar]
- 11.Deardorff DL, Diederich CJ, Nau WH. Control of interstitial thermal coagulation: comparative evaluation of microwave and ultrasound applicators. Med Phys 2001;28(1):104–117 [DOI] [PubMed] [Google Scholar]
- 12.Wolf FJ, Grand DJ, Machan JT, Dipetrillo TA, Mayo-Smith WW, Dupuy DE. Microwave ablation of lung malignancies: effectiveness, CT findings, and safety in 50 patients. Radiology 2008;247(3):871–879 [DOI] [PubMed] [Google Scholar]
- 13.Oshima F, Yamakado K, Nakatsuka A, Takaki H, Makita M, Takeda K. Simultaneous microwave ablation using multiple antennas in explanted bovine livers: relationship between ablative zone and antenna. Radiat Med 2008;26(7):408–414 [DOI] [PubMed] [Google Scholar]
- 14.Dong BW, Liang P, Yu XL, et al. Sonographically guided microwave coagulation treatment of liver cancer: an experimental and clinical study. AJR Am J Roentgenol 1998;171(2):449–454 [DOI] [PubMed] [Google Scholar]
- 15.Liang P, Wang Y. Microwave ablation of hepatocellular carcinoma. Oncology 2007;72(Suppl 1):124–131 [DOI] [PubMed] [Google Scholar]
- 16.Sun Y, Wang Y, Ni X, et al. Comparison of ablation zone between 915- and 2,450-MHz cooled-shaft microwave antenna: results in in vivo porcine livers. AJR Am J Roentgenol 2009;192(2):511–514 [DOI] [PubMed] [Google Scholar]
- 17.Knavel EM, Hinshaw JL, Lubner MG, et al. High-powered gas-cooled microwave ablation: shaft cooling creates an effective stick function without altering the ablation zone. AJR Am J Roentgenol 2012;198(3):W260–W265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cavagnaro M, Amabile C, Bernardi P, Pisa S, Tosoratti N. A minimally invasive antenna for microwave ablation therapies: design, performances, and experimental assessment. IEEE Trans Biomed Eng 2011;58(4):949–959 [DOI] [PubMed] [Google Scholar]
- 19.Hines-Peralta AU, Pirani N, Clegg P, et al. Microwave ablation: results with a 2.45-GHz applicator in ex vivo bovine and in vivo porcine liver. Radiology 2006;239(1):94–102 [DOI] [PubMed] [Google Scholar]
- 20.Brace CL. Dual-slot antennas for microwave tissue heating: parametric design analysis and experimental validation. Med Phys 2011;38(7):4232–4240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Committee for the Update of the Guide for the Care and Use of Laboratory Animals; National Research Council Guide for the care and use of laboratory animals. 8th ed. Washington, DC: National Academies Press, 2010 [Google Scholar]
- 22.Cha CH, Lee FT, Jr, Gurney JM, et al. CT versus sonography for monitoring radiofrequency ablation in a porcine liver. AJR Am J Roentgenol 2000;175(3):705–711 [DOI] [PubMed] [Google Scholar]
- 23.Schramm W, Yang D, Haemmerich D. Contribution of direct heating, thermal conduction and perfusion during radiofrequency and microwave ablation. Conf Proc IEEE Eng Med Biol Soc 2006;1:5013–5016 [DOI] [PubMed] [Google Scholar]
- 24.Ji Z, Brace CL. Expanded modeling of temperature-dependent dielectric properties for microwave thermal ablation. Phys Med Biol 2011;56(16):5249–5264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coad JE, Kosari K, Humar A, Sielaff TD. Radiofrequency ablation causes ‘thermal fixation’ of hepatocellular carcinoma: a post-liver transplant histopathologic study. Clin Transplant 2003;17(4):377–384 [DOI] [PubMed] [Google Scholar]
- 26.Zhou Q, Jin X, Jiao D-C, et al. Microwave ablation: results in ex vivo and in vivo porcine livers with 2450-MHz cooled-shaft antenna. Chin Med J (Engl) 2011;124(20):3386–3393 [PubMed] [Google Scholar]
- 27.Brace CL, Diaz TA, Hinshaw JL, Lee FT., Jr Tissue contraction caused by radiofrequency and microwave ablation: a laboratory study in liver and lung. J Vasc Interv Radiol 2010;21(8):1280–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu NC, Raman SS, Kim YJ, Lassman C, Chang X, Lu DSK. Microwave liver ablation: influence of hepatic vein size on heat-sink effect in a porcine model. J Vasc Interv Radiol 2008;19(7):1087–1092 [DOI] [PubMed] [Google Scholar]