Abstract
Mature adipocytes are generated through the proliferation and differentiation of precursor cells. Our prior studies identified adipocyte progenitors in white adipose tissue (WAT) as Lin−:CD29+:CD34+:Sca-1+:CD24+ (CD24+) cells that are capable of generating functional WAT1. Here, we employ several Cre recombinase mouse models to identify the adipocyte cellular lineage in vivo. While it has been proposed that white adipocytes are derived from endothelial2 and hematopoietic3, 4 lineages, we find that neither of these lineages label white adipocytes. However, platelet-derived growth factor receptor α (PdgfRα)-Cre trace labels all white adipocytes. Analysis of WAT from PdgfRα-Cre reporter mice identifies CD24+ and Lin−:CD29+:CD34+:Sca-1+:CD24− (CD24−) cells as adipocyte precursors. We show that CD24+ cells generate the CD24− population in vivo and the CD24− cells express late markers of adipogenesis. From these data we propose a model where the CD24+ adipocyte progenitors become further committed to the adipocyte lineage as CD24 expression is lost, generating CD24− preadipocytes. This characterization of the adipocyte cellular lineage will facilitate study of the mechanisms that regulate WAT formation in vivo and WAT mass expansion in obesity.
The number of mature adipocytes in white adipose tissue (WAT) of adults is tightly regulated, despite their continual turnover5. As mature adipocytes are post-mitotic6, 7, change in adipocyte number occurs via disruption of the balance between rates of adipogenesis and adipocyte death. Therefore, characterization of the adipocyte cellular lineage is required for mechanistic understanding of WAT homeostasis and growth.
Various methods have been used to study adipocyte precursors ex vivo and in vivo. One common method is to culture the whole stromal-vascular fraction (SVF) from adipose tissues and select cell populations by their adherence to plastic8, 9. The cells derived from this method are referred to as preadipocytes or adipocyte-derived stem cells. However, these cells have not been shown to have de novo adipogenic capacity in vivo and their relationship to adipocyte lineage cells in vivo is not known.
Alternatively, several groups used fluorescence-activated cell sorting (FACS) in a prospective approach to identify adipogenic cell populations from various tissues1, 10-12. Two cell populations derived from WAT, defined by the marker profiles Lin−:CD34+:CD29+:Sca-1+:CD24+ (CD24+) and Lin−:CD34+:CD29+:Sca-1+:CD24− (CD24− ), are adipogenic in vitro but only the CD24+ population is capable of generating a functional WAT depot upon transplantation into a residual WAT depot of lipodystrophic mice1, indicating that the CD24+ population contains adipocyte progenitors. Cells with similar marker profiles have been shown to be adipogenic within the skin10 and skeletal muscle11.
Genetic approaches have also been used to investigate the adipocyte cellular lineage. A previous study showed, through crossing Cadherin-5 (Cdh5)-Cre mice into reporter lines that express cytoplasmic β-galactosidase and GFP, that Cdh5-Cre labels mature adipocytes2, 13, suggesting an endothelial origin for white adipocytes as Cdh5 labels endothelial lineages14. However, for studies of WAT the cellular specificity of reporters that stain the cytoplasm is difficult to delineate given the paucity of cytoplasm in mature adipocytes and the high vascularity of WAT. To overcome this limitation, we employed a mouse strain harboring a fluorescent –membrane dTomato/membrane eGFP (mT/mG) Cre reporter construct that marks Cre excision by a heritable switch from membrane targeted tdTomato expression to membrane targeted eGFP expression15. We crossed this reporter to several mouse lines expressing Cre recombinase from various promoters to more specifically determine the identity of the adipocyte precursors. Whole mount confocal microscopy of WAT from Adiponectin-Cre:mT/mG mice demonstrates GFP expression in mature adipocytes of all WAT depots assayed, with no GFP fluorescence in the absence of Cre expression, indicating that the mT/mG reporter model is appropriate for lineage tracing of mature adipocytes (Fig. 1a and Supplemental Fig. 1a). Flow cytometry analysis of the SVF from mT/mG models (Supplemental Fig. 2a) demonstrates that this model is also suitable for the study of potential precursor populations. However, flow cytometry analysis of Adiponectin-Cre:mT/mG WAT shows there are no GFP+ cells in the SVF (Fig. 1c, Supplemental Fig. 1b), indicating that the Adiponectin promoter is not active in immature adipocyte lineage cells and thus, Adiponectin-Cre mice are not useful for identification of adipocyte precursors in adult WAT.
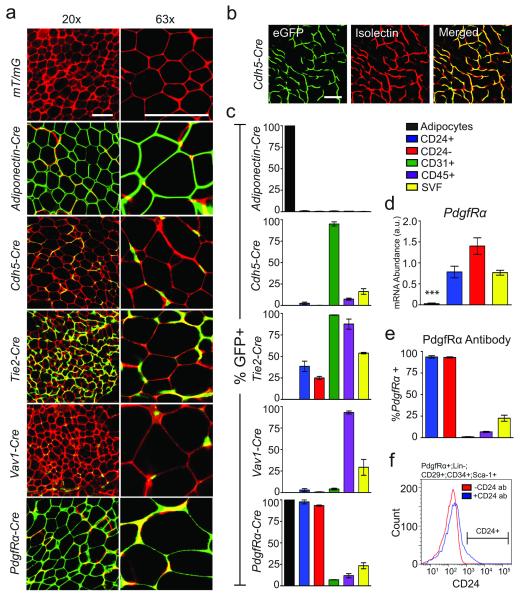
Figure 1.
Adipocytes are derived from PdgfRα+ precursor cells in subcutaneous WAT. (a) Confocal images of whole-mounted SWAT from indicated 4-week old Cre:mT/mG male mice (red: membrane-targeted dTomato; green: membrane-targeted eGFP, indicating Cre excision of dTomato). (b) Confocal images of membrane targeted eGFP and Isolectin GS-IB4 Alexa Fluor 647 staining endothelial cells of Cdh5-Cre:mT/mG SWAT. (c) Quantification of flow cytometry analysis of SVF populations from indicated 4-week old Cre:mT/mG mice (n=3). (d) Quantification of qPCR analysis of PdgfRα in mature adipocytes and FACS sorted SVF, Lin−:CD29+:CD34+:Sca-1+:CD24+ (CD24+) and Lin−:CD29+:CD34+:Sca-1+:CD24− (CD24−) cell populations (n=5 RNA extractions from independently isolated cell samples, *** p<0.001). (e) Quantification of flow cytometry analysis of anti-PdgfRα-PE antibody staining in indicated cell populations from 6-week old male C57BL/6 SWAT (n=3 SWAT SVF preparations). (f) A histogram of the distribution of CD24 staining in PdgfRα+:Lin−:CD29+:CD34+:Sca-1+ cells from (e). All error bars represent S.E.M. All scale bars represent 100 μm.
We next generated Cdh5-Cre:mT/mG mice, using the same mouse line used in the previous study2, to determine if adipocyte precursors within the SVF are derived from Cdh5 expressing cells. While CD31+ endothelial cells were almost completely labeled by Cdh5-Cre, mature adipocytes from WAT depots and brown adipose tissue did not express GFP in this model (Figs. 1a-c, Supplemental Fig. 1). Analysis of another endothelial lineage marker, endothelial-specific receptor tyrosine kinase (Tie2)-Cre, produced similar results (Fig. 1a,c, Supplemental Fig. 1), indicating that adipocytes are not derived from endothelial cells under normal conditions. Due to the high vascularity of WAT and the tight association of capillaries with mature adipocytes, some adipocytes are completely surrounded by GFP+ vasculature, giving the appearance that the adipocyte may be GFP+. However, labeling endothelial cells with isolectin GSIB4 clearly shows that the GFP signal is derived from endothelial cells in Cdh5-Cre and Tie2-Cre and the mature adipocytes are dTomato+ (Fig. 1b and Supplemental Fig. 1c).
It has also been proposed that adipocytes are derived from hematopoietic lineages16-22, and recent studies of transplant and injury models have shown that at least some adipocytes are derived from circulating cells of hematopoietic origin3, 4. However, Tie2-Cre labels nearly all CD45+ cells in WAT, yet does not label mature adipocytes. Similarly, Vav 1 oncogene (Vav1)-Cre, another marker of hematopoietic lineages14, 23, fails to label adipocytes despite near complete label of CD45+ cells in WAT (Fig.1a,c, Supplemental Fig. 1a,b). These data indicate that adipocytes are not normally derived from hematopoietic lineages.
To determine if endothelial or hematopoietic lineages contribute mature adipocytes in the context of obesity we placed Cdh5-Cre and Vav1-Cre mT/mG reporter mice on a high fat diet (HFD). Following 10 weeks of HFD, no GFP positive adipocytes were observed in the Cdh5-Cre WAT, while adipocyte-like GFP positive structures are present in Vav1-Cre WAT (Supplemental Fig. 3). However, all of these GFP positive structures are multi-nucleated and stain positive for macrophage markers F4/80, CD11b, and CD45 (Supplemental Fig. 3a-d) indicating that the GFP fluorescence is not derived from adipocyte membranes but from macrophages forming crown like structures24. These data indicate that hematopoietic and endothelial lineage cells do not contribute to mature adipocyte formation in HFD-induced obesity.
Previous studies demonstrated that PdgfRα is expressed in adipogenic cells from skeletal muscle11, 12. In addition, PdgfRα is expressed in WAT-resident cells that produce brown-like adipocytes in response to beta-adrenergic stimulation and white adipocytes upon HFD feeding25. Therefore, we determined if PdgfRα labels mature adipocytes during normal formation of WAT. PdgfRα-Cre labels all mature adipocytes in all major WAT depots, as indicated by GFP+ adipocyte membranes (Fig. 1a, Supplemental Fig. 1a). As PdgfRα is not expressed in mature adipocytes25 (Fig. 1d), the GFP expression observed in adipocytes of PdgfRα-Cre:mT/mG mice is due to lineage tracing.
We next examined the labeling of SVF cells in PdgfRα-Cre mice to identify potential adipocyte precursors. The SVF of PdgfRα-Cre:mT/mG mice contains a low percentage of GFP+ cells in CD31+ and CD45+ populations. In contrast, both the CD24+ and CD24− cells are nearly completely traced by PdgfRα-Cre (Fig. 1c, Supplemental Fig. 2b) and PdgfRα is expressed in these cell populations (Fig. 1d-f, Supplemental Fig. 2b). This data suggests that both the CD24+ and the CD24− populations are part of the in vivo adipocyte cellular lineage.
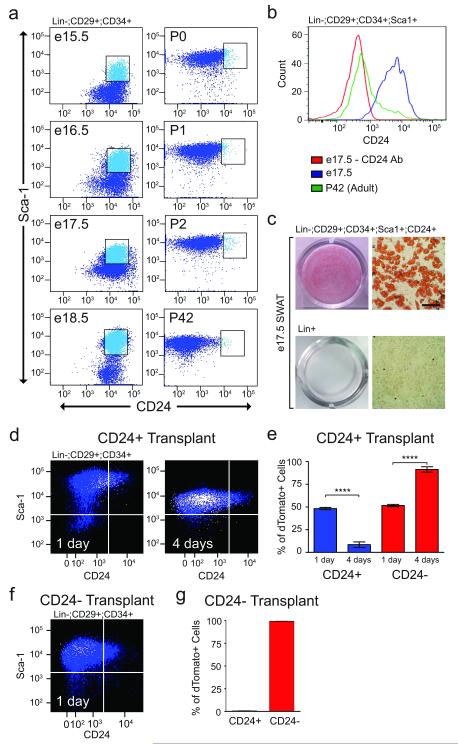
Therefore, we next examined the relationship between the CD24+ and CD24− cells in vivo. During development of subcutaneous WAT (SWAT), lipid filling of differentiating adipocytes is not observed until birth26. Flow cytometry analysis of embryonic SWAT (e15.5-e18.5) shows there is a large population of cells with the cell surface marker profile of CD24+ adipogenic precursors (Fig 2a,b). These embryonic CD24+ cells are 98.62 ± 1.08% PdgfRα+ and are adipogenic in cell culture (Fig 2c), suggesting that they are adipocyte progenitors. Prior to birth there is no discernable CD24− population (Fig 2a). Upon birth (P0) there is a shift in the population profiles, with the appearance of a large population CD24− cells and a simultaneous reduction in the amount of CD24+ cells to levels similar to those observed in adult WAT (Fig. 2a). These population dynamics during development of WAT suggests that the CD24+ cells may generate the CD24− cell population.
Figure 2.
CD24+ adipocyte progenitors give rise to CD24− cells in vivo. (a) Flow cytometry plots of SVF from a time course SWAT development in C57BL/6 mice at the indicated embryonic (e) and post-natal (P) days. Dot plots show Lin−:CD29+:CD34+ cells. (b) CD24 staining in Lin−:CD29+:CD34+:Sca1+ cells from e17.5 and P42 SWAT. (c) Oil Red O staining of differentiated FACS sorted cell populations from e17.5 SWAT. Scale bar represents 100 μm. (d) Representative images of flow cytometry plots of SWAT Lin−:CD29+:CD34+ cells from 6-week old Rag2-/-;IL2Rγ-/-;A-Zip mice at indicated days post transplantation of 100,000 FACS-isolated dTomato+, Lin−:CD29+:CD34+:Sca-1+:CD24+ (CD24+) cells from 6-week old mT/mG mice. Plots show overlay of dTomato+ transplanted cells (white) on Rag2-/-;IL2Rγ-/-;A-Zip recipient SVF cells (blue). (e) Quantification of flow cytometry analysis of dTomato+ CD24+ transplantations into Rag2-/-;IL2Rγ-/-;A-Zip mice (n=4 transplantations, **** p<0.0001, error bar represent S.E.M.). (f). Representative flow cytometry plot of SWAT Lin−:CD29+:CD34+ cells from 6-week old Rag2-/-;IL2Rγ-/-;A-Zip mice at 1 day post transplantation of 100,000 FACS isolated dTomato+, CD24− cells from 6-week old mT/mG mice. The dot plot shows overlay of dTomato+ transplanted cells (white) on Rag2-/-;IL2Rγ-/-;A-Zip recipient SVF cells (blue). (g) Quantification of flow cytometry analysis of dTomato+ CD24− transplantations into Rag2-/- ;IL2Rγ-/-;A-Zip mice (n=3 transplants, error bars represent S.E.M.).
To test this hypothesis we employed an A-Zip transplantation model similar to the assay previously used to demonstrate the capacity of CD24+ cells to reconstitute functional WAT1. In these assays, dTomato-labeled CD24+ cells from mT/mG mice were transplanted into the undeveloped WAT of A-Zip mice and injected tissues were analyzed before the appearance of mature adipocytes, 1-4 days post-injection. When dTomato+:CD24− cells are transplanted, dTomato+ cells recovered one day post-injection are nearly all CD24− with no dTomato+ cells in the CD24+ gate, demonstrating the assay accurately identifies the CD24− population (Fig. 2f, g). In contrast, when dTomato+:CD24+ cells are transplanted, 51.7% of dTomato+ cells are CD24− after one day, while four days post-injection 91.4% of dTomato+ cells are CD24− (Fig. 2d, e). The increasing appearance of CD24− donor cells over time after transplantation of CD24+ adipocyte progenitors indicates that the CD24+ adipocyte progenitors generate the CD24− adipogenic cell population.
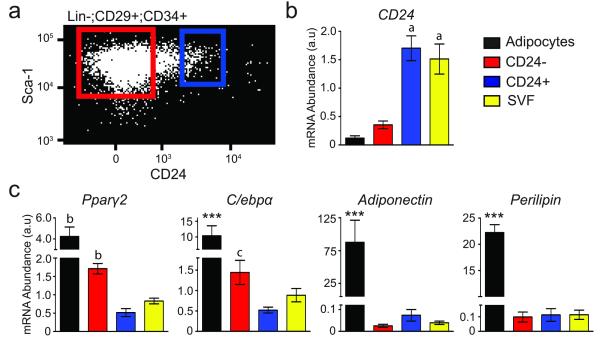
The finding that the CD24− cell population is derived from the CD24+ adipocyte progenitors, coupled with the reduced ability of the CD24− population to generate functional adipose tissue in vivo1, suggests that the CD24− cell population contains cells that are further differentiated towards mature adipocytes. Thus, we performed gene expression analysis on sorted cells to investigate the relative state of differentiation in these adipocyte precursor populations. Real-time PCR shows that CD24 mRNA is enriched in the CD24+ population, validating the sorting of these populations (Fig. 3a,b). CD24 is also highly expressed in unfractionated SVF as CD24 is expressed in several other cell types in SVF, such as B lymphocytes27, and only 0.61% ± 0.12 of SVF cells that express CD24 display the complete cell surface marker profile of adipocyte progenitors. The late adipogenic markers Pparγ2 and C/ebpα are enriched in the CD24− population while markers of mature adipocytes, Adiponectin and Perilipin, are only expressed in isolated mature adipocytes (Fig 3c).
Figure 3.
CD24− cells express late adipogenic genes. (a) A representative dot plot is shown with boxes representing the sort parameters for the CD24+ (blue) and CD24− (red) populations. (b,c) qPCR of CD24 expression, late adipogenic and mature adipocyte specific genes in purified mature adipocytes and FACS isolated cell populations (n=5 RNA extractions from independently isolated cell samples; *** p<0.001 vs. all other populations; a p<0.001 vs. CD24− and adipocytes (Adi); b p<0.001 vs. CD24+ and SVF; c p<0.001 vs. Adi and p<0.05 vs. CD24+; error bars represent S.E.M.).
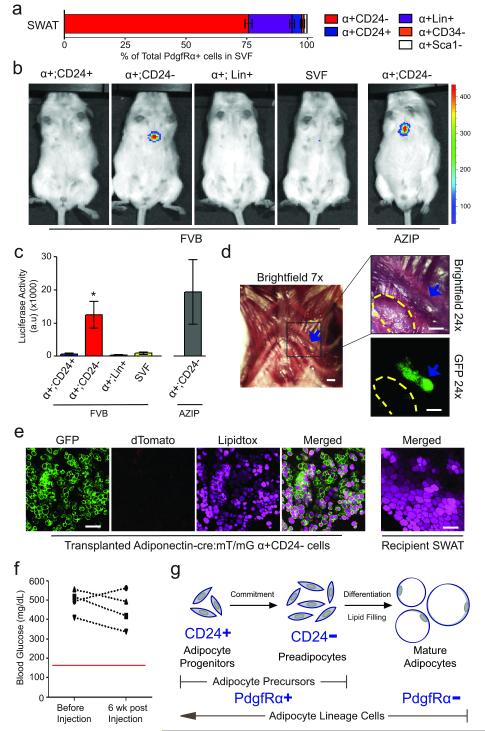
The enrichment of late adipogenic markers in CD24− cells suggests that this population may contain cells that are committed to an adipogenic fate. Thus, we tested the adipogenic capacity of the CD24+ and CD24− cells in a previously developed in vivo adipocyte commitment assay28, 29. PdgfRα+ populations (Fig. 4a, Supplemental Fig. 2b) were sorted from leptin-luciferase mice30 and injected subcutaneously over the sternum of wild type mice, a region where adipose tissue does not normally form. While the PdgfRα+:CD24+ adipocyte progenitors and PdgfRα+:Lin+ cells did not produce luciferase signal, the transplanted PdgfRα+CD24− cells produced significant luciferase signal after 6 weeks, indicative of the formation of mature adipocytes (Fig. 4b, c).
Figure 4.
CD24− cells are further committed to an adipogenic fate. (a) Breakdown of PdgfRα+ (α+) SVF into subpopulations. Flow cytometry plots and percentages are shown in Supplemental Figure 2b. α+:CD24−, α+:CD24+, and α+:Sca1− are Lin−:CD29+:CD34+. α+:CD34− are Lin−:CD29+. (b) Luminescence in 12-week old FVBN/J or A-Zip mice at 6 weeks post subcutaneous sternum transplantation of indicated FACS isolated populations from 6-week old leptin-luciferase BAC transgenic mice. 50,000 cells were transplanted per experiment. (c) Quantification of luminescence from (b) (n=5 for α+:CD24+ and α+:Lin+ transplants into FVB; n=10 for α+:CD24− and SVF transplants into FVB; n=4 for α+:CD24− transplants into AZIP. *p<.05, error bars represent S.E.M.). (d) Brightfield and fluorescent images of tissue formed 6 weeks post subcutaneous sternum transplantation of 2×105 Adiponectin-Cre:mT/mG α+:CD24− cells. Blue arrow indicates tissue formed from transplanted cells. Yellow dashes outline a small piece of recipient SWAT placed on top of sternum as a negative imaging control. Scale bars represent 1 mm. (e) Representative confocal images of Lipidtox stained tissues from (d). Scale bars represent 100 μm. (f) Blood glucose measurement of A-Zip mice before and 6 weeks post subcutaneous sternum transplantation of 50,000 α+CD24− as shown in (b) and (c). (g) A model of in vivo adipogenesis.
Despite the inability of the CD24+ adipocyte progenitors to form mature adipocytes outside of the WAT microenvironment (Fig. 4b, c), previous work has shown that this cell population is capable of reconstituting a functional WAT depot that rescues hyperglycemia when transplanted into the residual perigonadal WAT (GWAT) of A-Zip mice1. The same study also showed that the CD24− cell population has limited adipogenic capacity in this WAT microenvironment. To more directly assess the capacity of CD24− cells to form adipocytes, we performed sternum injections of PdgfRα+:CD24− cells from Adiponectin-Cre:mT/mG mice. These injections resulted in the formation of lipid filled, Adiponectin expressing mature adipocytes (Fig. 4d,e). However, the adipose tissue formed by differentiation of these PdgfRα+:CD24− cells is several fold smaller than the tissue observed in GWAT reconstitution by CD24+ adipocyte progenitors1 in A-Zip mice (Fig. 4d, e). In addition, the adipose tissue resulting from the injection of PdgfRα+:CD24− cells above the sternum of A-Zip mice does not rescue their hyperglycemia (Fig. 4f). The ability of the PdgfRα+:CD24− population to form small amounts of adipose tissue both within and outside of the WAT microenvironment indicates that this population harbors cells that are further to committed to adipogenesis yet have limited adipogenic capacity as compared to CD24+ adipocyte progenitors.
Taken together our data characterize a previously unappreciated cellular lineage of adipocytes within WAT. Our recent prospective studies identified the CD24+ population as a rare cell type in WAT that is capable of forming a functional WAT depot in vivo1. We show here that these CD24+ adipocyte progenitors are indeed a component of the adipocyte lineage in vivo, becoming further committed to the adipocyte lineage as they lose CD24 expression, forming a CD24− preadipocyte population in the course of differentiating into mature adipocytes in vivo (Fig. 4g).
Our data also indicate that adipocytes are derived from PdgfRα expressing cells during the normal establishment of WAT. Previous data has demonstrated that adipocytes formed in response to β-Adrenergic stimulation and HFD are also derived from PdgfRα+ cells that share other markers with the preadipocytes described here, including Sca-1 and CD3425, although this previous study did not investigate the role of the CD24+ adipocyte progenitors. Using Pdgfrα as a single marker of adipocyte precursors, this previous study found that Pdgfrα+ cells are associated with the vasculature25. However, we have shown that the Pdgfrα+ population also contains non- adipogenic cell populations (Fig. 4b, c). Therefore, Pdgfrα expression alone cannot definitively identify adipocyte progenitors within WAT. Similarly, CD24 cannot be used as a sole marker of adipocyte progenitors as only 0.61% of CD24+ cells within WAT are adipocyte progenitors (Supplemental Fig. 4a, b). Additionally, only 17.42% of Pdgfrα+;CD24+ cells within WAT are adipocyte progenitors (Supplemental Fig. 4a, b). These Pdgfrα+;CD24+ cells are dispersed throughout WAT and are not preferentially localized to vasculature (Supplemental Fig. 4c), indicating that precise localization of adipocyte progenitors requires more exclusive markers.
While many factors are known to affect adipocyte formation in vitro, the precise role of these factors in adipocyte dynamics in vivo remains uncharacterized. For example, the expression of Pdgfrα on adipocyte precursors suggests that PDGF signaling plays a role in WAT mass regulation in vivo. However, previous studies of the role of PDGF in adipogenesis in vitro are conflicting, with PDGF signaling either inducing31 or inhibiting32 adipogenesis. The identification of the adipocyte cellular lineage in vivo and the characterization of PdgfRα-Cre mice as a model for genetic manipulation of adipocyte precursors will now permit detailed studies of the cellular and molecular mechanisms that regulate adipogenesis and WAT mass in vivo under various conditions, including the establishment of the adipose tissue ‘set point’33 during development and the expansion of WAT mass in obesity. Determining the mechanisms involved in these different states of WAT mass regulation may lead to the development of novel therapeutic strategies for controlling WAT mass.
Supplementary Material
Acknowledgements
We thank Elise Jeffery, Chris Church, Diane Krause and Valerie Horsley for critical reading of the manuscript and valuable discussions. This work was supported by American Diabetes Association Award 7-12-JF-46, DERC pilot project grant DK045735 and NIDDK grant DK090489 to M.S.R. and NIDDK grant DK041096 to Jeffrey Friedman.
Footnotes
Contributions R.B. and M.S.R. designed and performed experiments, analyzed and interpreted data and wrote the manuscript.
Competing financial interests The authors declare no competing financial interests.
References
- 1.Rodeheffer MS, Birsoy K, Friedman JM. Identification of white adipocyte progenitor cells in vivo. Cell. 2008;135:240–249. doi: 10.1016/j.cell.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 2.Tran KV, et al. The vascular endothelium of the adipose tissue gives rise to both white and brown fat cells. Cell Metab. 2012;15:222–229. doi: 10.1016/j.cmet.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crossno JT, Majka SM, Grazia T, Gill RG, Klemm DJ. Rosiglitazone promotes development of a novel adipocyte population from bone marrow-derived circulating progenitor cells. J Clin Invest. 2006;116:3220–3228. doi: 10.1172/JCI28510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sera Y, et al. Hematopoietic stem cell origin of adipocytes. Exp Hematol. 2009;37:1108–1120. 1120.e1101–1104. doi: 10.1016/j.exphem.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spalding KL, et al. Dynamics of fat cell turnover in humans. Nature. 2008;453:783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- 6.Simon G. Histogenesis, in Adipose Tissue. In: Renold A, Cahill G, editors. Handbook of Physiology. Vol. 5. American Physiology Society; Washington, D.C.: 1965. pp. 101–107. [Google Scholar]
- 7.Pairault J, Green H. A study of the adipose conversion of suspended 3T3 cells by using glycerophosphate dehydrogenase as differentiation marker. Proc Natl Acad Sci U S A. 1979;76:5138–5142. doi: 10.1073/pnas.76.10.5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin CS, et al. Defining adipose tissue-derived stem cells in tissue and in culture. Histol Histopathol. 2010;25:807–815. doi: 10.14670/HH-25.807. [DOI] [PubMed] [Google Scholar]
- 9.Zuk PA, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 10.Festa E, et al. Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell. 2011;146:761–771. doi: 10.1016/j.cell.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joe AW, et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12:153–163. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uezumi A, Fukada S, Yamamoto N, Takeda S, Tsuchida K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol. 2010;12:143–152. doi: 10.1038/ncb2014. [DOI] [PubMed] [Google Scholar]
- 13.Tang W, et al. White fat progenitor cells reside in the adipose vasculature. Science. 2008;322:583–586. doi: 10.1126/science.1156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alva JA, et al. VE-Cadherin-Cre-recombinase transgenic mouse: a tool for lineage analysis and gene deletion in endothelial cells. Dev Dyn. 2006;235:759–767. doi: 10.1002/dvdy.20643. [DOI] [PubMed] [Google Scholar]
- 15.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- 16.Charrière G, et al. Preadipocyte conversion to macrophage. Evidence of plasticity. J Biol Chem. 2003;278:9850–9855. doi: 10.1074/jbc.M210811200. [DOI] [PubMed] [Google Scholar]
- 17.McCollough AW. Evidence of macrophagal origin of adipose cells in the white rat as shown by studies on starved animals. Journal of Morphology. 1944;75:193–201. [Google Scholar]
- 18.Wassermann F. Die Fettorgane des Menchen. Entwicklung, Bau und systematische Stellung des sogenannten Fettgewebes. Zeit. f. Zellforsch. u. Mikr. Anat. 1926;3:325–329. [Google Scholar]
- 19.Latta S.S.a.D.I.R. The reaction of the omental tissue to trypan blue injected intraperitoneally, with special reference to intrarelationship between cell types. American Journal of Anatomy. 1935;56:481–503. [Google Scholar]
- 20.Godina G. Ricerche sullo sviluppo e sulla natura del tessuto adiposo dei bovini. Nota I. Arch. Ital. Anat. 1939;42:455–473. [Google Scholar]
- 21.Cousin B, et al. A role for preadipocytes as macrophage-like cells. FASEB J. 1999;13:305–312. doi: 10.1096/fasebj.13.2.305. [DOI] [PubMed] [Google Scholar]
- 22.White P.a.M.A. Inflammation-Incuced Insulin Resistance in Obesity. Human Kinetics, Champaign; Illinois, USA: 2008. [Google Scholar]
- 23.de Boer J, et al. Transgenic mice with hematopoietic and lymphoid specific expression of Cre. Eur J Immunol. 2003;33:314–325. doi: 10.1002/immu.200310005. [DOI] [PubMed] [Google Scholar]
- 24.Cinti S, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 25.Lee YH, Petkova AP, Mottillo EP, Granneman JG. In vivo identification of bipotential adipocyte progenitors recruited by β3-adrenoceptor activation and high-fat feeding. Cell Metab. 2012;15:480–491. doi: 10.1016/j.cmet.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Birsoy K, et al. Analysis of gene networks in white adipose tissue development reveals a role for ETS2 in adipogenesis. Development. 2011;138:4709–4719. doi: 10.1242/dev.067710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pillai PS, Reynolds SD, Chace JH, Scott DW. Differential expression of a surface antigen recognized by a monoclonal antibody, J11d, on unprimed and primed B cells. J Immunol. 1986;137:791–797. [PubMed] [Google Scholar]
- 28.Mandrup S, Loftus TM, MacDougald OA, Kuhajda FP, Lane MD. Obese gene expression at in vivo levels by fat pads derived from s.c. implanted 3T3-F442A preadipocytes. Proc Natl Acad Sci U S A. 1997;94:4300–4305. doi: 10.1073/pnas.94.9.4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang QQ, Otto TC, Lane MD. Commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc Natl Acad Sci U S A. 2004;101:9607–9611. doi: 10.1073/pnas.0403100101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Birsoy K, et al. Cellular program controlling the recovery of adipose tissue mass: An in vivo imaging approach. Proc Natl Acad Sci U S A. 2008;105:12985–12990. doi: 10.1073/pnas.0805621105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bachmeier M, Löffler G. Influence of growth factors on growth and differentiation of 3T3-L1 preadipocytes in serum-free conditions. Eur J Cell Biol. 1995;68:323–329. [PubMed] [Google Scholar]
- 32.Fitter S, Vandyke K, Gronthos S, Zannettino AC. Suppression of PDGF-induced PI3 kinase activity by imatinib promotes adipogenesis and adiponectin secretion. J Mol Endocrinol. 2012;48:229–240. doi: 10.1530/JME-12-0003. [DOI] [PubMed] [Google Scholar]
- 33.Harris RB. Role of set-point theory in regulation of body weight. FASEB J. 1990;4:3310–3318. doi: 10.1096/fasebj.4.15.2253845. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.