Abstract
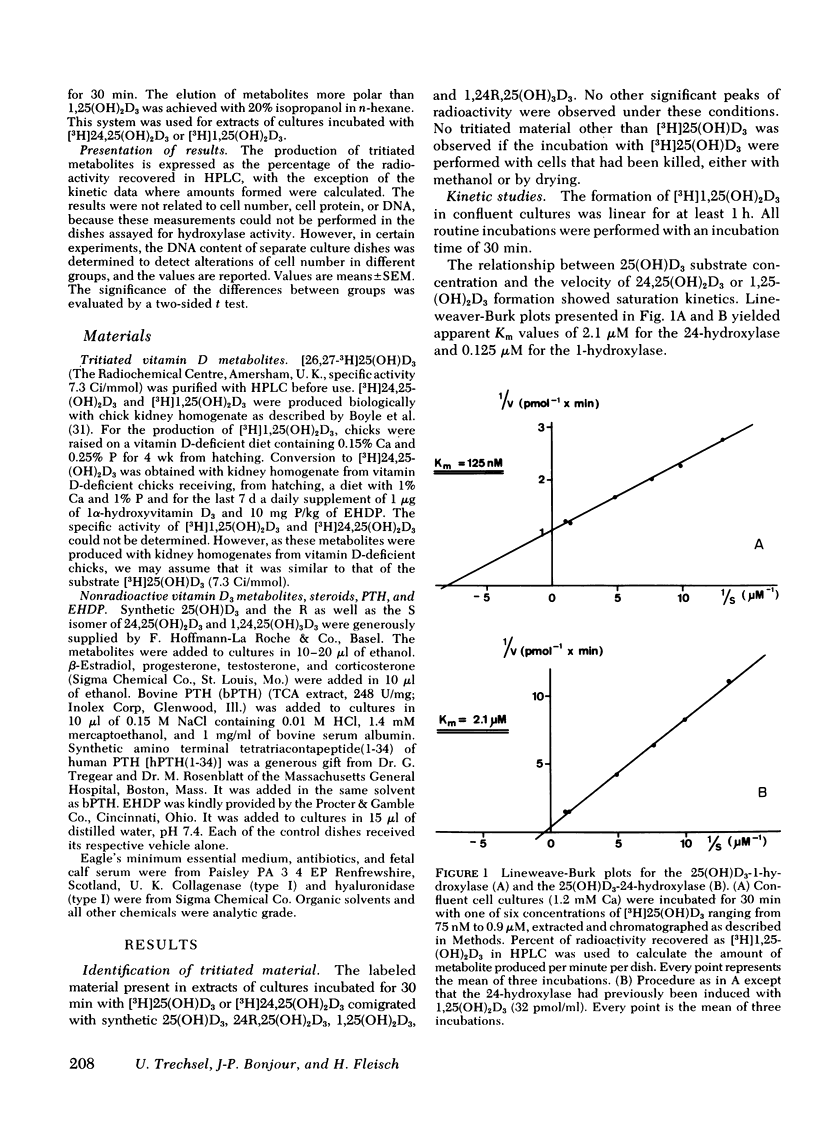
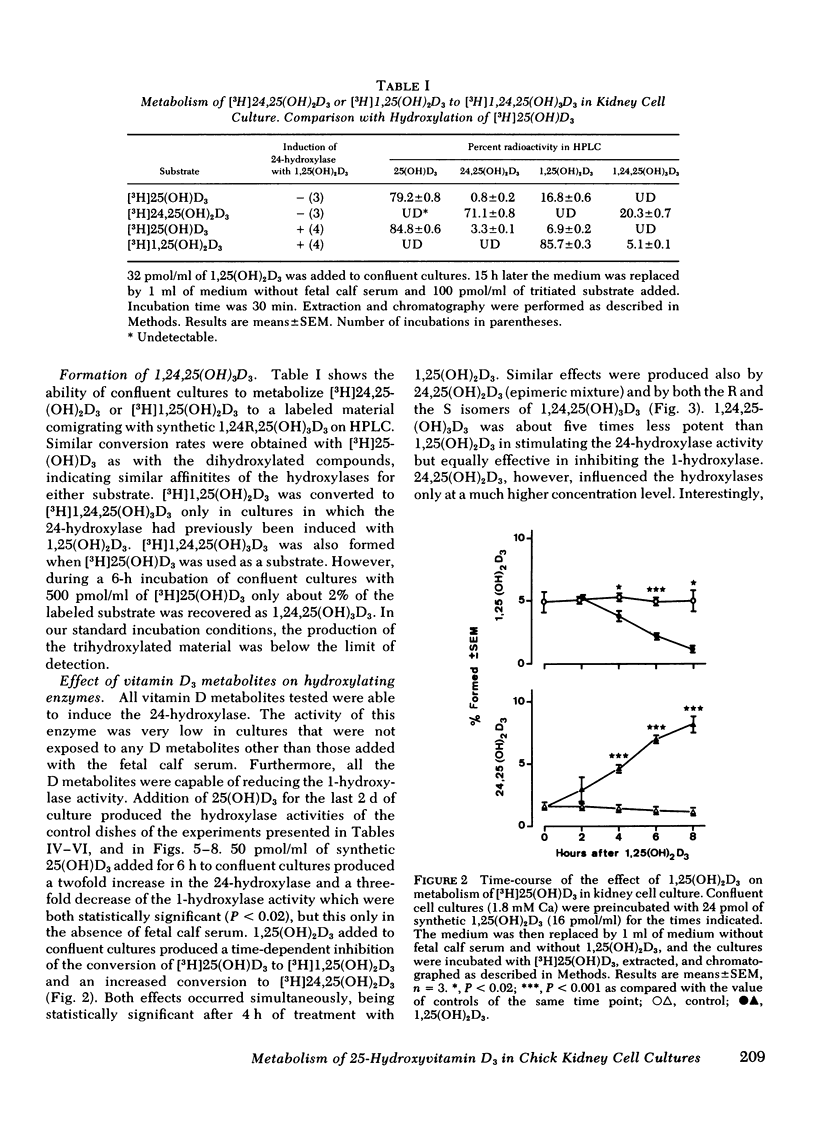
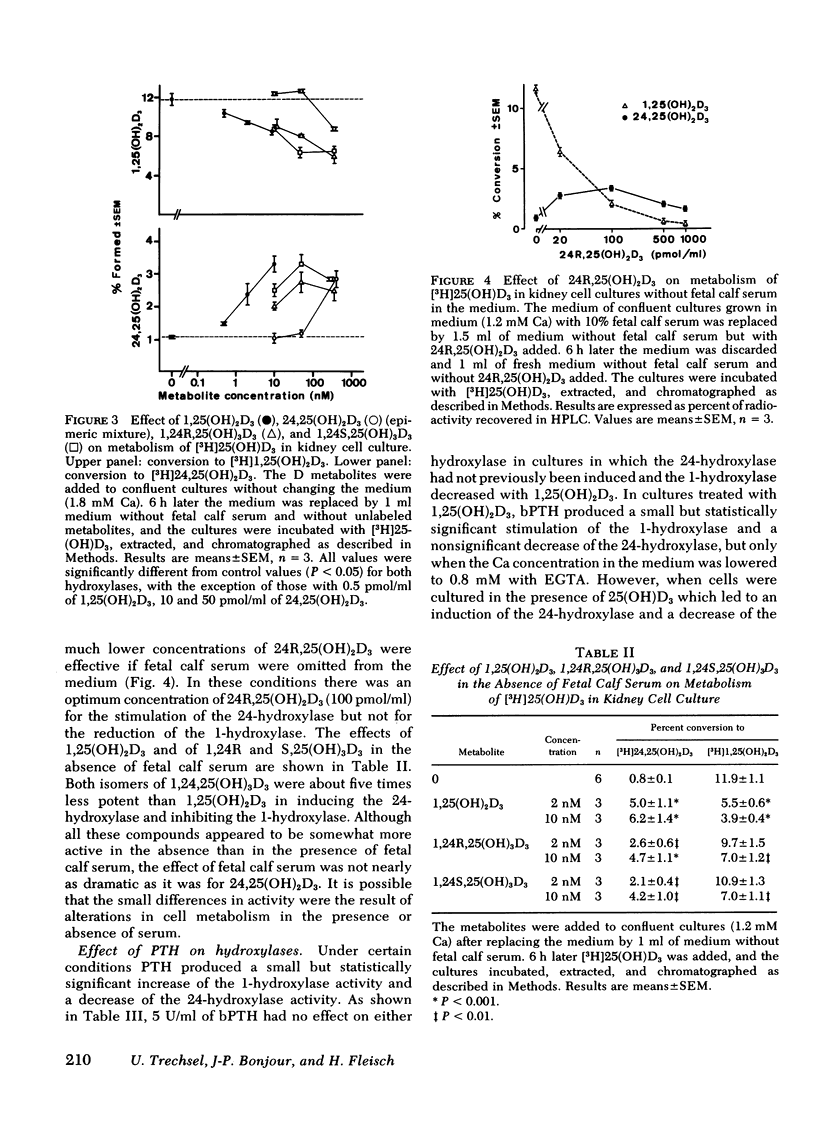
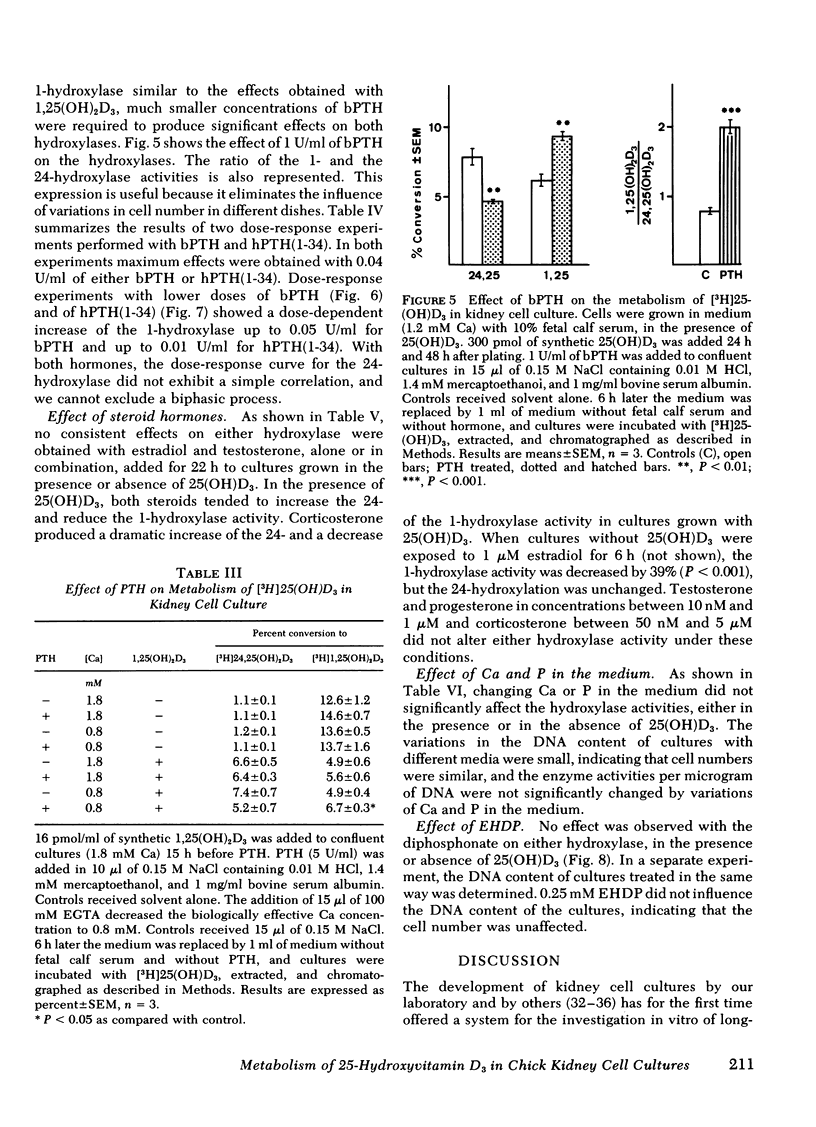
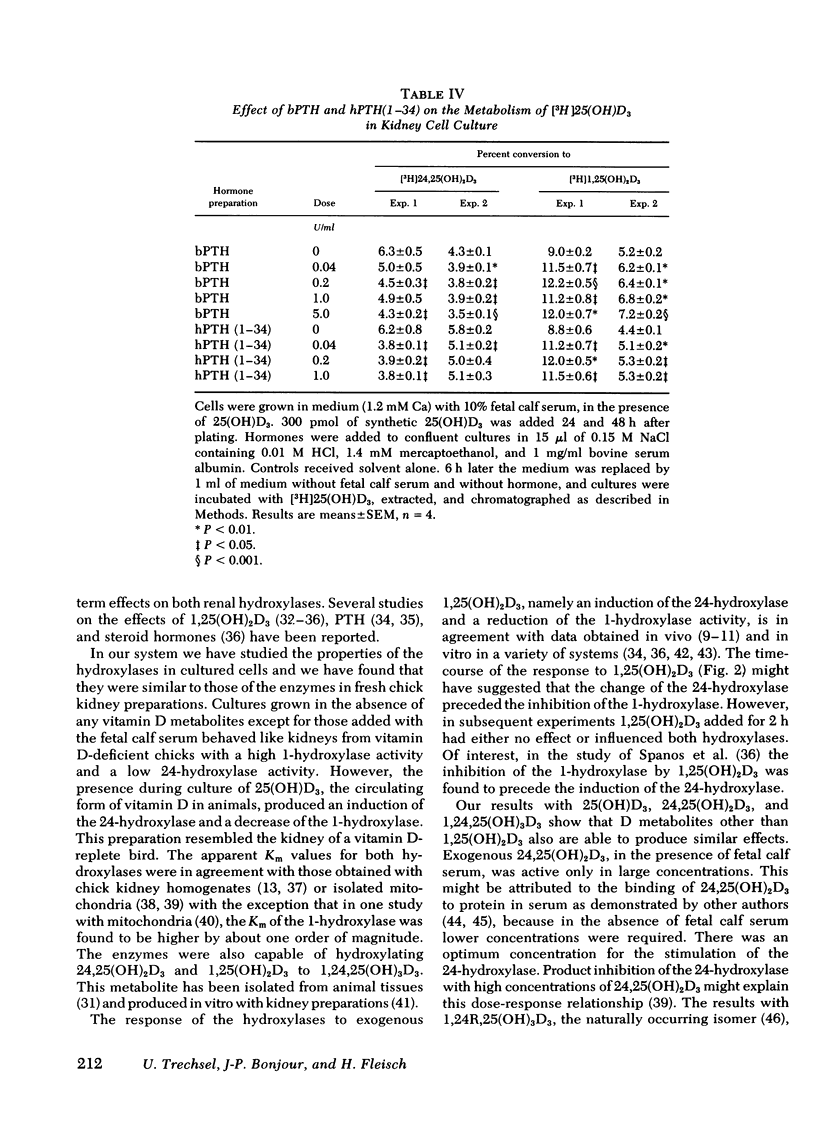
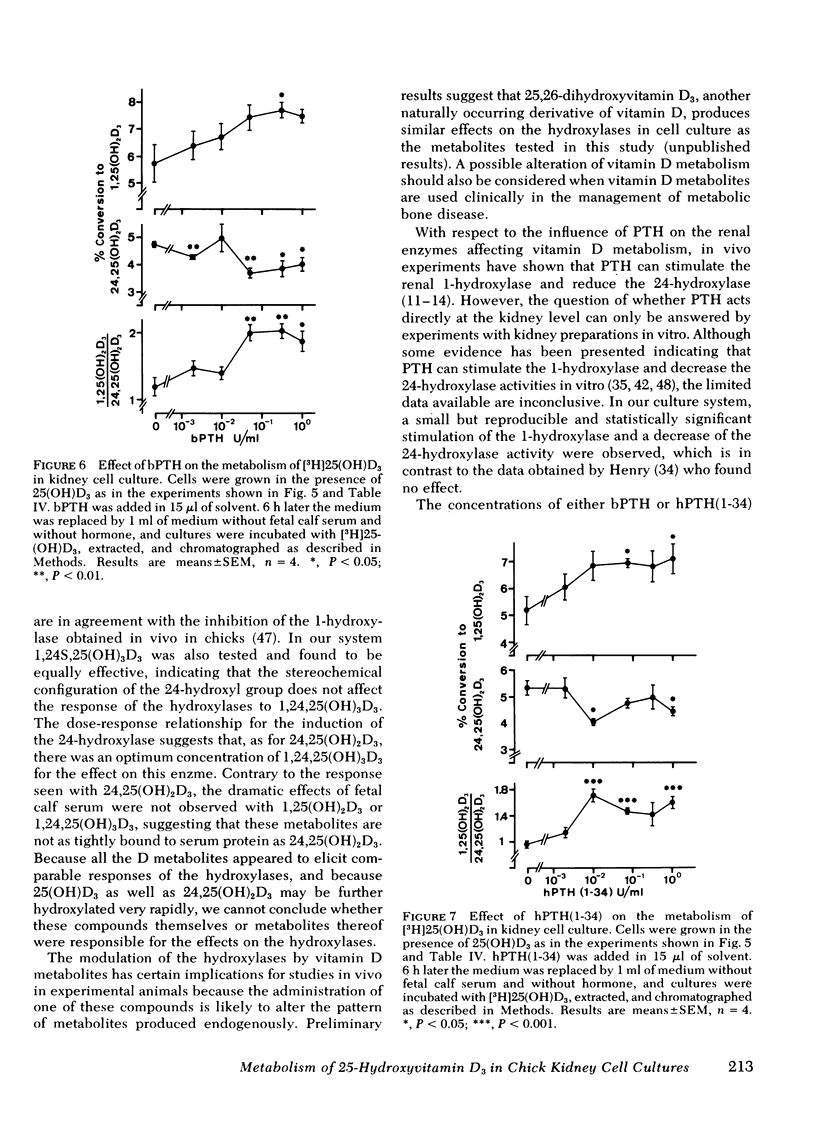
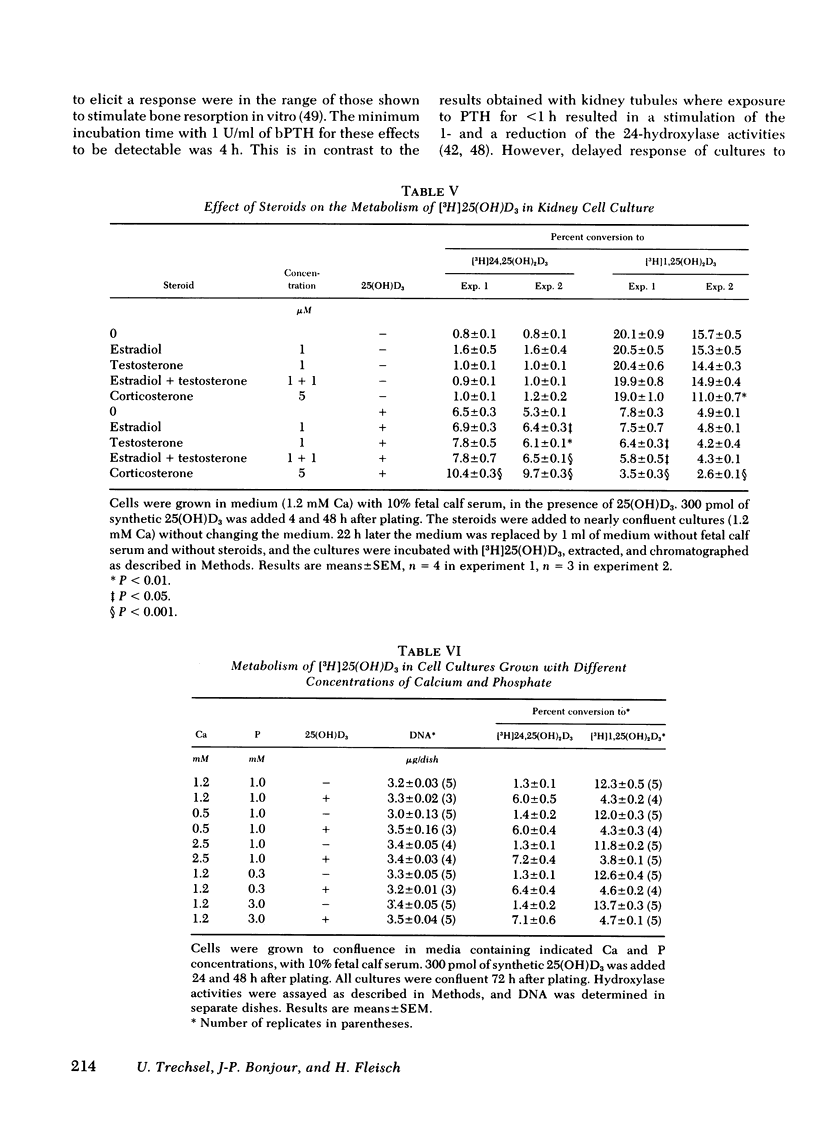
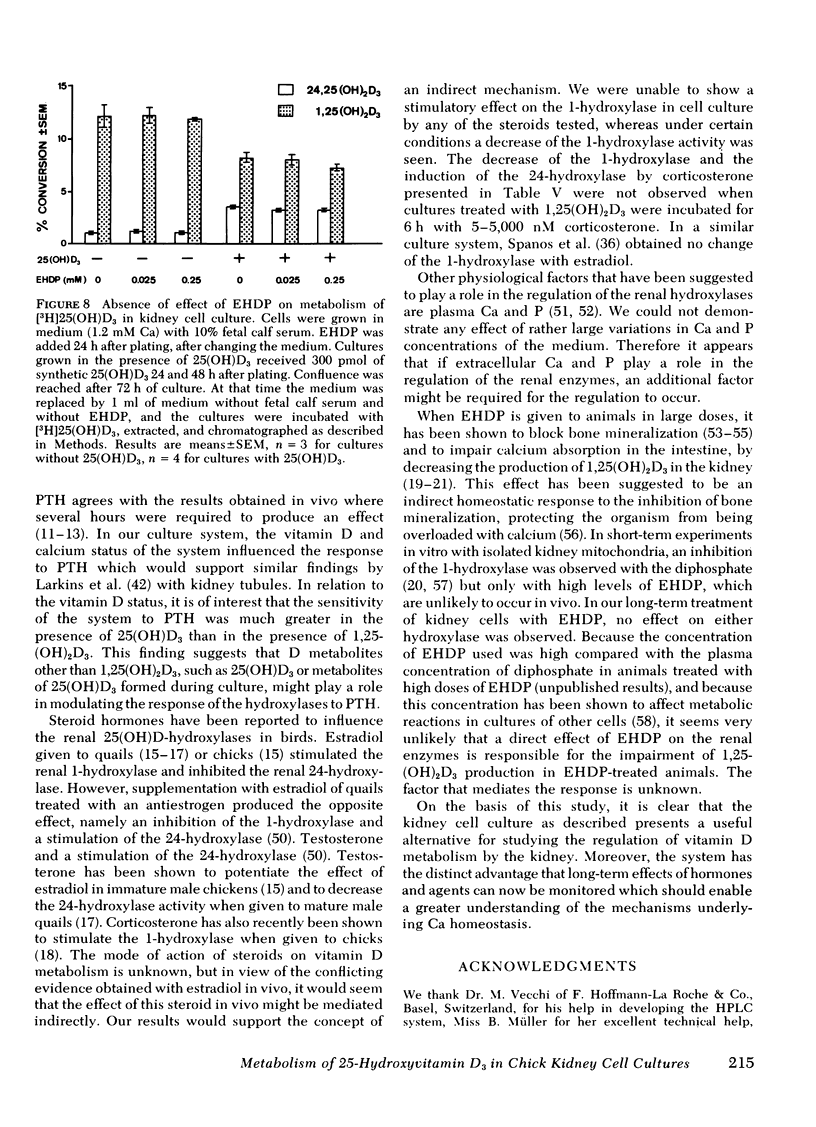
A primary chick kidney cell culture is described, capable of forming 1,25-dihydroxyvitamin D3 [1,25(OH)2D3], 24,25-dihydroxyvitamin D3 [24,25(OH)2D3], and 1,24,25-trihydroxyvitamin D3 [1,24,25(OH)3D3] over several days. The apparent Km values were 0.125 microM for the 1-hydroxylase and 2.1 microM for the 24-hydroxylase. Exogenous 1,25(OH)2D3 decreased 1-hydroxylase and increased 24-hydroxylase within 4 h. 24,25(OH)2D3 produced similar effects, but only in the absence of fetal calf serum. R and S isomers of 1,24,25(OH)3D3 were about fives times less active than 1,25(OH)2D3. Bovine parathyroid hormone stimulated the 1- and reduced the 24-hydroxylase in 6 h, but this only occurred in cultures either previously treated with 1,25(OH)2D3 and EGTA to lower Ca to 0.8 mM or in cultures grown in the presence of 25-hydroxyvitamin D3 (25(OH)D3). Under the latter condition, the sensitivity to bovine parathyroid hormone was enhanced, 0.04 U/ml producing a maximum response. Synthetic aminoterminal tetratriacontapeptide (1-34) human parathyroid hormone was equally effective. In the absence of D metabolites, estradiol for 6 h produced a dose-dependent inhibition of the 1-hydroxylase, but no change in the 24-hydroxylase. Progesterone, testosterone, and corticosterone had no significant effect. In cultures grown in the presence of 25(OH)D3 no reproducible effects were obtained with either 1 microM estradiol or 1 microM testosterone, alone or in combination, but 5 microM corticosterone decreased the 1- and increased the 24-hydroxylase. Changes in Ca and P concentrations of the medium as well as addition of ethane-l-hydroxy-1, 1-diphosphate for 48 h did not affect any of the hydroxylase activities. The modulation of the hydroxylase activities by vitamin D3 metabolites and parathyroid hormone suggests that these factors regulate the renal hydroxylase by direct actions, whereas it would appear that ethane-1-hydroxy-1,1-diphosphate, Ca, P, and steroid may exert their influence indirectly.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baksi S. N., Kenny A. D. Effects of administration of antiestrogen (tamoxifen) in vivo on the metabolism of 25-hydroxyvitamin D3 in vitro in the Japanese quail. Biochem Pharmacol. 1977 Dec 15;26(24):2439–2443. doi: 10.1016/0006-2952(77)90454-3. [DOI] [PubMed] [Google Scholar]
- Baksi S. N., Kenny A. D. Vitamim D3 metabolism in immature Japanese quail: effects of ovarian hormones. Endocrinology. 1977 Oct;101(4):1216–1220. doi: 10.1210/endo-101-4-1216. [DOI] [PubMed] [Google Scholar]
- Baxter L. A., Deluca H. F., Bonjour J. P., Fleisch H. A. Inhibition of vitamin D metabolism by ethane-1-hydroxyl-1, 1-diphosphonate. Arch Biochem Biophys. 1974 Oct;164(2):655–662. doi: 10.1016/0003-9861(74)90077-0. [DOI] [PubMed] [Google Scholar]
- Bikle D. D., Rasmussen H. A comparison of the metabolism of 25-hydroxyvitamin D3 by chick renal tubules, homogenates and mitochondria. Biochim Biophys Acta. 1974 Oct 8;362(3):439–447. doi: 10.1016/0304-4165(74)90139-1. [DOI] [PubMed] [Google Scholar]
- Boyle I. T., Gray R. W., DeLuca H. F. Regulation by calcium of in vivo synthesis of 1,25-dihydroxycholecalciferol and 21,25-dihydroxycholecalciferol. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2131–2134. doi: 10.1073/pnas.68.9.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle I. T., Miravet L., Gray R. W., Holick M. F., Deluca H. F. The response of intestinal calcium transport to 25-hydroxy and 1,25-dihydroxy vitamin D in nephrectomized rats. Endocrinology. 1972 Mar;90(3):605–608. doi: 10.1210/endo-90-3-605. [DOI] [PubMed] [Google Scholar]
- Boyle I. T., Omdahl J. L., Gray R. W., DeLuca H. F. The biological activity and metabolism of 24,25-dihydroxyvitamin D 3 . J Biol Chem. 1973 Jun 25;248(12):4174–4180. [PubMed] [Google Scholar]
- Castillo L., Tanaka Y., DeLuca H. F., Sunde M. L. The stimulation of 25-hydroxyvitamin D3-1 alpha-hydroxylase by estrogen. Arch Biochem Biophys. 1977 Feb;179(1):211–217. doi: 10.1016/0003-9861(77)90105-9. [DOI] [PubMed] [Google Scholar]
- Colston K. W., Evans I. M., Galante L., MacIntyre I., Moss D. W. Regulation of vitamin D metabolism: factors influencing the rate of formation of 1,25-dihydroxycholecalciferol by kidney homogenates. Biochem J. 1973 Jul;134(3):817–820. [PMC free article] [PubMed] [Google Scholar]
- DeLuca H. F., Schnoes H. K. Metabolism and mechanism of action of vitamin D. Annu Rev Biochem. 1976;45:631–666. doi: 10.1146/annurev.bi.45.070176.003215. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Edelstein S., Lawson D. E., Kodicek E. The transporting proteins of cholecalciferol and 25-hydroxycholecalciferol in serum of chicks and other species. Partial purification and characterization of the chick proteins. Biochem J. 1973 Nov;135(3):417–426. doi: 10.1042/bj1350417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fast D. K., Felix R., Dowse C., Neuman W. F., Fleisch H. The effects of diphosphonates on the growth and glycolysis of connective-tissue cells in culture. Biochem J. 1978 Apr 15;172(1):97–107. doi: 10.1042/bj1720097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser D. R. Advances in the knowledge of the metabolism of vitamin D. Proc Nutr Soc. 1975 Sep;34(2):139–143. doi: 10.1079/pns19750024. [DOI] [PubMed] [Google Scholar]
- Fraser D. R., Kodicek E. Regulation of 25-hydroxycholecalciferol-1-hydroxylase activity in kidney by parathyroid hormone. Nat New Biol. 1973 Feb 7;241(110):163–166. doi: 10.1038/newbio241163a0. [DOI] [PubMed] [Google Scholar]
- Garabedian M., Holick M. F., Deluca H. F., Boyle I. T. Control of 25-hydroxycholecalciferol metabolism by parathyroid glands. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1673–1676. doi: 10.1073/pnas.69.7.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser A. B., Morgan D. B., Fleisch H. A., Richelle L. J. The influence of two diphosphonates on calcium metabolism in the rat. Clin Sci. 1972 Jul;43(1):31–45. doi: 10.1042/cs0430031. [DOI] [PubMed] [Google Scholar]
- Gray R. W., Omdahl J. L., Ghazarian J. G., DeLuca H. F. 25-Hydroxycholecalciferol-1-hydroxylase. Subcellular location and properties. J Biol Chem. 1972 Dec 10;247(23):7528–7532. [PubMed] [Google Scholar]
- Haddad J. G., Jr, Min C., Walgate J., Hahn T. Competition by 24,25-dihydroxycholecalciferol in the competitive protein binding radioassay of 25-hydroxycalciferol. J Clin Endocrinol Metab. 1976 Sep;43(3):712–715. doi: 10.1210/jcem-43-3-712. [DOI] [PubMed] [Google Scholar]
- Henry H. L. Metabolism of 25-hydroxy-vitamin D3 by primary cultures of chick kidney cells. Biochem Biophys Res Commun. 1977 Jan 24;74(2):768–774. doi: 10.1016/0006-291x(77)90368-0. [DOI] [PubMed] [Google Scholar]
- Henry H. L., Midgett R. J., Norman A. W. Regulation of 25-hydroxyvitamin D3-1-hydroxylase in vivo. J Biol Chem. 1974 Dec 10;249(23):7584–7592. [PubMed] [Google Scholar]
- Hill L. F., Lumb G. A., Mawer E. B., Stanbury S. W. Indirect inhibition of the biosynthesis of 1,25-dihydroxycholecalciferol in rats treated with a diphosphonate. Clin Sci. 1973 Apr;44(4):335–347. doi: 10.1042/cs0440335. [DOI] [PubMed] [Google Scholar]
- Holick M. F., Kleiner-Bossaller A., Schnoes H. K., Kasten P. M., Boyle I. T., DeLuca H. F. 1,24,25-Trihydroxyvitamin D3. A metabolite of vitamin D3 effective on intestine. J Biol Chem. 1973 Oct 10;248(19):6691–6696. [PubMed] [Google Scholar]
- Jones G., DeLuca H. F. High-pressure liquid chromatography: separation of the metabolites of vitamins D2 and D3 on small-particle silica columns. J Lipid Res. 1975 Nov;16(6):448–453. [PubMed] [Google Scholar]
- Juan D., DeLUCA H. F. The regulation of 24,25-dihydroxyvitamin D3 production in cultures of monkey kidney cells. Endocrinology. 1977 Oct;101(4):1184–1193. doi: 10.1210/endo-101-4-1184. [DOI] [PubMed] [Google Scholar]
- King W. R., Francis M. D., Michael W. R. Effect of disodium ethane-1-hydroxy-1, 1-diphosphonate on bone formation. Clin Orthop Relat Res. 1971;78:251–270. doi: 10.1097/00003086-197107000-00021. [DOI] [PubMed] [Google Scholar]
- Knutson J. C., DeLuca H. F. 25-Hydroxyvitamin D3-24-hydroxylase. Subcellular location and properties. Biochemistry. 1974 Mar 26;13(7):1543–1548. doi: 10.1021/bi00704a034. [DOI] [PubMed] [Google Scholar]
- Larkins R. G., MacAuley S. J., MacIntyre I. Feedback control of vitamin D metabolism by a nuclear action of 1,25-dihydroxycholecalciferol on the kidney. Nature. 1974 Nov 29;252(5482):412–414. doi: 10.1038/252412a0. [DOI] [PubMed] [Google Scholar]
- Larkins R. G., MacAuley S. J., Rapoport A., Martin T. J., Tulloch B. R., Byfield P. G., Matthews E. W., MacIntyre I. Effects of nucleotides, hormones, ions, and 1,25-dihydroxycholecalciferon on 1,25-dihydroxycholecalciferol production in isolated chick renal tubules. Clin Sci Mol Med. 1974 May;46(5):569–582. doi: 10.1042/cs0460569. [DOI] [PubMed] [Google Scholar]
- Leyva A., Jr, Kelley W. N. Measurement of DNA in cultured human cells. Anal Biochem. 1974 Nov;62(1):173–179. doi: 10.1016/0003-2697(74)90378-9. [DOI] [PubMed] [Google Scholar]
- Mawer E. B., Backhouse J., Hill L. F., Lumb G. A., De Silva P., Taylor C. M., Stanbury S. W. Vitamin D metabolism and parathyroid function in man. Clin Sci Mol Med. 1975 May;48(5):349–365. doi: 10.1042/cs0480349. [DOI] [PubMed] [Google Scholar]
- Norman A. W., Henry H. 1,25-Dihydroxycholecalciferol--a hormonally active form of vitamin D. Recent Prog Horm Res. 1974;30(0):431–480. [PubMed] [Google Scholar]
- Omdahl J. L., DeLuca H. F. Regulation of vitamin D metabolism and function. Physiol Rev. 1973 Apr;53(2):327–372. doi: 10.1152/physrev.1973.53.2.327. [DOI] [PubMed] [Google Scholar]
- Procsal D. A., Henry H. L., Friedlander E. J., Norman A. W. Studies on the mode of action of calciferol. Biological activity of 1 alpha, 24R,25-trihydroxyvitamin D3 in the chick. Arch Biochem Biophys. 1977 Feb;179(1):229–234. doi: 10.1016/0003-9861(77)90107-2. [DOI] [PubMed] [Google Scholar]
- RAISZ L. G. BONE RESORPTION IN TISSUE CULTURE. FACTORS INFLUENCING THE RESPONSE TO PARATHYROID HORMONE. J Clin Invest. 1965 Jan;44:103–116. doi: 10.1172/JCI105117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raisz L. G., Trummel C. L., Holick M. F., DeLuca H. F. 1,25-dihydroxycholecalciferol: a potent stimulator of bone resorption in tissue culture. Science. 1972 Feb 18;175(4023):768–769. doi: 10.1126/science.175.4023.768. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Wong M., Bikle D., Goodman D. B. Hormonal control of the renal conversion of 25-hydroxycholecalciferol to 1,25-dihydroxycholecalciferol. J Clin Invest. 1972 Sep;51(9):2502–2504. doi: 10.1172/JCI107065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds J. J., Holick M. F., De Luca H. F. The role of vitamin D metabolites in bone resorption. Calcif Tissue Res. 1973;12(4):295–301. doi: 10.1007/BF02013742. [DOI] [PubMed] [Google Scholar]
- Schenk R., Merz W. A., Mühlbauer R., Russell R. G., Fleisch H. Effect of ethane-1-hydroxy-1,1-diphosphonate (EHDP) and dichloromethylene diphosphonate (Cl 2 MDP) on the calcification and resorption of cartilage and bone in the tibial epiphysis and metaphysis of rats. Calcif Tissue Res. 1973 Mar 12;11(3):196–214. doi: 10.1007/BF02547219. [DOI] [PubMed] [Google Scholar]
- Shain S. A. In vitro metabolism of 25-hydroxycholecalciferol by chick intestinal and renal cell preparations. Identification of a metabolic product as 1,25-dihydroxycholecalciferol and delineation of its metabolic fate in intestinal cells. J Biol Chem. 1972 Jul 10;247(13):4393–4403. [PubMed] [Google Scholar]
- Spanos E., Barrett D. I., Chong K. T., MacIntyre I. Effect of oestrogen and 1,25-dihydroxycholecalciferol on 25-hydroxycholecalciferol metabolism in primary chick kidney-cell cultures. Biochem J. 1978 Jul 15;174(1):231–236. doi: 10.1042/bj1740231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanos E., Colston K. W., Macintyre I. Effect of glucocorticoids on vitamin D metabolism. FEBS Lett. 1977 Mar 15;75(1):73–76. doi: 10.1016/0014-5793(77)80056-2. [DOI] [PubMed] [Google Scholar]
- Tahaka Y., Lorenc R. S., DeLuca H. F. The role of 1,25-dihydroxyvitamin D3 and parathyroid hormone in the regulation of chick renal 25-hydroxyvitamin D3-24-hydroxylase. Arch Biochem Biophys. 1975 Dec;171(2):521–526. doi: 10.1016/0003-9861(75)90061-2. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Castillo L., DeLuca H. F. Control of renal vitamin D hydroxylases in birds by sex hormones. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2701–2705. doi: 10.1073/pnas.73.8.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y., Castillo L., DeLuca H. F. The 24-hydroxylation of 1,25-dihydroxyvitamin D3. J Biol Chem. 1977 Feb 25;252(4):1421–1424. [PubMed] [Google Scholar]
- Tanaka Y., DeLuca H. F. Stimulation of 24,25-dihydroxyvitamin D3 production by 1,25-dihydroxyvitamin D3. Science. 1974 Mar;183(130):1198–1200. doi: 10.1126/science.183.4130.1198. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Deluca H. F. The control of 25-hydroxyvitamin D metabolism by inorganic phosphorus. Arch Biochem Biophys. 1973 Feb;154(2):566–574. doi: 10.1016/0003-9861(73)90010-6. [DOI] [PubMed] [Google Scholar]
- Taylor C. M., Hughes S. E., de Silva P. Competitive protein binding assay for 24,25-dihydroxycholecalciferol. Biochem Biophys Res Commun. 1976 Jun 21;70(4):1243–1249. doi: 10.1016/0006-291x(76)91035-4. [DOI] [PubMed] [Google Scholar]
- Taylor C. M., Mawer E. B., Reeve A. The effects of a diphosphonate and dietary calcium on the metabolism of vitamin D3 (cholecalciferol) in the chick. Clin Sci Mol Med. 1975 Nov;49(5):391–400. doi: 10.1042/cs0490391. [DOI] [PubMed] [Google Scholar]
- Trechsel U., Schenk R., Bonjour J. P., Russell R. G., Fleisch H. Relation between bone mineralization, Ca absorption, and plasma Ca in phosphonate-treated rats. Am J Physiol. 1977 Mar;232(3):E298–E305. doi: 10.1152/ajpendo.1977.232.3.E298. [DOI] [PubMed] [Google Scholar]
- Wong R. G., Norman A. W., Reddy C. R., Coburn J. W. Biologic effects of 1,25-dihydroxycholecalciferol (a highly active vitamin D metabolite) in acutely uremic rats. J Clin Invest. 1972 May;51(5):1287–1291. doi: 10.1172/JCI106923. [DOI] [PMC free article] [PubMed] [Google Scholar]



