Abstract
The concentration and composition of wheat gluten proteins and the presence, concentration and location of cysteine residues therein are important for wheat flour quality. However, it is difficult to identify gluten proteins, as they are an extremely polymorphic mixture of prolamins. We here present methods for cysteine labeling of wheat prolamins with 4-vinylpyridine (4-VP) and iodoacetamide (IDAM) which, as compared to label-free analysis, substantially improve identification of cysteine-containing peptides in enzymic prolamin digests by electrospray ionization - tandem mass spectrometry. Both chymotrypsin and thermolysin yielded cysteine-containing peptides from different gluten proteins, but more proteins could be identified after chymotryptic digestion. In addition, to the best of our knowledge, we were the first to label prolamins with isotope coded affinity tags (ICAT), which are commonly used for quantitative proteomics. However, more peptides were detected after labeling gluten proteins with 4-VP and IDAM than with ICAT.
Wheat is one of the major food raw materials with a typical worldwide production of 7 · 108 tonnes per year1. The wide use of wheat flour for food applications is mainly the result of the properties of its particular storage proteins. This complex mixture of similar but distinct proteins is referred to as prolamins. In the wheat kernel, prolamins are present as gliadins and glutenins. Upon mixing wheat flour with water, these proteins develop into gluten, a viscoelastic protein network. Gluten has a great impact on the quality of a whole range of cereal-based foods2,3.
Prolamins are extremely polymorphic due to gene mutation into many allelic forms. Native gliadins in wheat flour are monomers and subdivided into α-, γ-, and ω-gliadins. They contain no (ω) or only (α and γ) intramolecular disulfide bonds. The glutenins occur as polymers of glutenin subunits (GS), which contain free thiol groups, intramolecular, and intermolecular disulfide bonds. The intermolecular disulfide bonds are responsible for the polymeric nature of glutenins. A distinction is made between high molecular weight (HMW) and low molecular weight (LMW) GS4. The latter are subdivided into typical (m-, s- or i-type) LMW-GS and gliadin-like LMW-GS, which are in fact α-, γ-, or ω-gliadins with an additional or missing cysteine residue as a result of a point mutation5. Common or bread wheat (Triticum aestivum L.) is a hexaploid species. A single bread wheat variety contains 6 genes for HMW-GS, 20 or more for LMW-GS, 29 or more for γ-gliadins, up to 150 for α-gliadins, and at least 5 for ω-gliadins. Not all of these genes are expressed4,6,7,8,9,10,11,12. Even for wheat flour from a single wheat cultivar, the protein composition and corresponding amino acid sequences are rarely entirely known.
There are still important gaps in the knowledge on post-translational modifications leading to the disulfide structure during the synthesis of prolamins in the growing plant4,13. Whether HMW- and LMW-GS separately form linear backbones and whether only few cross-links between HMW- and LMW-GS exist, remains to be confirmed14. Also, disulfide bond formation and thiol/disulfide interchange reactions between GS during dough development and bread baking are not well understood15. Even though all individual gluten proteins and their subunits probably contribute in some way to gluten functionality in food, one group is of particular interest, namely the HMW-GS16. Strategies to improve gluten and, hence, wheat quality include manipulation of the number and composition of HMW-GS by inserting additional genes encoding mutant and wild-type proteins17,18. In addition, typical m-, s- or i-type LMW-GS contribute to glutenin polymers as they can form two intermolecular SS bonds with LMW-GS or HMW-GS, while gliadin-like LMW-GS are assumed to have a negative impact on visco-elastic properties as they act as chain terminators during polymerization5,19. Information on the location of cysteine residues in GS is required to understand the contribution of specific prolamins to gluten properties5,16. Additionally, quantification of prolamins and their cysteine-containing peptides is useful for evaluating gluten functionality. Apart from that, there is still a demand for good, reliable and sensitive methods to unambiguously identify gluten in foods, because such proteins trigger an inflammatory reaction in celiac patients20. With respect to prolamin quantification in (so-called) gluten-free products, Tanner et al.21 outlined the problems associated with solely relying on the enzyme-linked immuno sorbent assay (ELISA) and highlighted the need for development of new sensitive and selective quantitative assays such as liquid chromatography-mass spectrometry (LC-MS).
Electrospray ionization (ESI) MS and matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) MS have contributed to assessing prolamin heterogeneity and gluten structure determination22. As such, Lutz et al.23 introduced ESI-MS/MS with alternating electron transfer dissociation (ETD) and collision-induced dissociation (CID) to detect disulfide-linked prolamin peptide ions. However, this technique requires prior knowledge of the location of cysteine residues. Elucidation of the gluten network structure and evaluation of gluten functionality still require means to facilitate identification, but also quantification of prolamins and localization of their cysteine-residues. Camafeita et al.24,25 presented a system allowing quantification of small quantities of wheat gliadins using MALDI-TOF-MS. However, they acknowledged the need for a procedure to accurately quantify total gluten.
Despite the proteomic research already performed on cereal storage proteins, analysis of wheat prolamins still faces several challenges. Because of their high molecular weights and high levels of glutamine, proline and hydrophobic amino acids, gluten proteins are not extractable in the salt buffers typically used for protein characterization3. Furthermore, trypsin, the enzyme of choice in most proteomic studies because of its reliability and specificity, is not suited in the case of cereal storage proteins20. Sequences of gluten proteins have few sites for tryptic hydrolysis, as they are low in arginine and lysine2,26. In addition, the repetitive character of gluten protein sequences results in a substantial sequence redundancy. Finally, the low cysteine levels in gluten proteins [ca. 2.2 mol%27] further complicate identification of cysteine-labeled gluten peptides, despite their importance for flour quality.
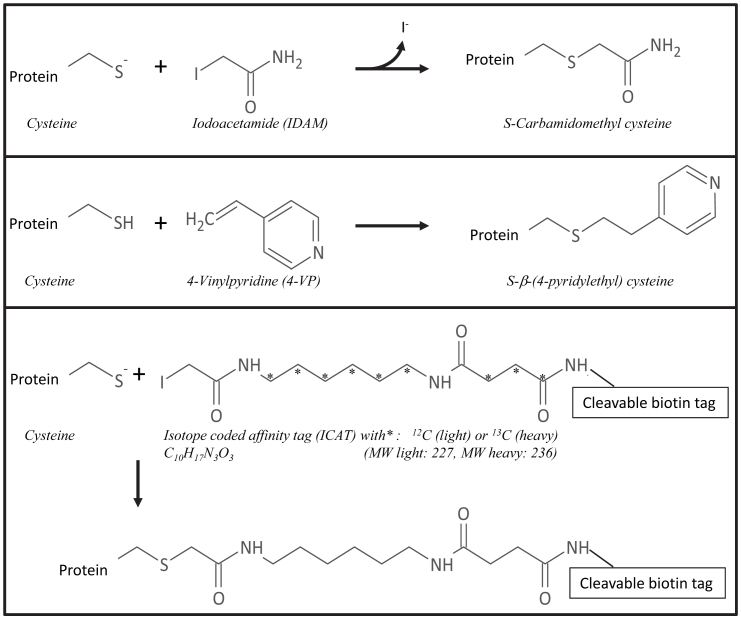
Cysteine alkylation is a successful strategy to improve the identification28 and even quantification29,30 of cysteine residues via cysteine-containing peptides31. A variety of cysteine derivatizing agents is available, most often based on the reaction with iodoacetyl groups (with iodine as a good leaving group) or with double bonds (for Michael-type addition reactions)29. The popular derivatizing agents iodoacetamide (IDAM) and 4-vinylpyridine (4-VP) transform cysteine residues in S-carbamidomethyl cysteine and S-β-(4-pyridylethyl) cysteine, respectively28 (Figure 1). Cysteine residues can also be labeled with isotope-coded affinity tags (ICAT) and subsequently quantified (Figure 1). ICAT labeling was originally designed for quantitative analysis of complex protein mixtures32, but is now also used to determine the redox state of cysteine residues in proteins33,34,35.
Figure 1. Schematic overview of cysteine labeling reactions.
Even though literature reports on various cysteine labeling techniques for standard proteins28, the lack of extractability of gluten proteins in the buffers typically used for derivatization reactions (see above) complicates cysteine labeling. For instance, when Islam et al.36 studied the expression of wheat seed proteins in relation to chromosome deletion under the standard conditions of ICAT labeling, they identified only non-gluten proteins, almost all of them with molecular weights below 30,000. In a comparable effort to quantify highly hydrophobic membrane proteins, Ramus et al.37 noted the need for specialized buffers for ICAT labeling. In the specific case of gluten proteins, both the sample extraction procedure and labeling conditions, be it with IDAM, 4-VP or ICAT, remain to be optimized.
Overall, localization of cysteine residues and detection of cysteine-containing gluten peptides with MS/MS are laborious and difficult. Given the importance of gluten protein identification and the localization of cysteine residues for understanding end product quality of wheat-based food products, this paper explores the potential of cysteine labeling (4-VP, IDAM, ICAT) for identifying prolamins and quantifying cysteine-containing gluten peptides.
Results
Optimal conditions for cysteine labeling of wheat prolamins
Gluten proteins have limited extractability in most buffers and solvents due to their extremely high level of glutamine, which forms strong hydrogen bonds, the high level of hydrophobic amino acids, and low levels of amino acids with ionizable side chains. In order to obtain complete cysteine labeling, it was of the utmost importance to maximize gluten protein extraction. Therefore, extraction conditions were optimized. An efficient extraction system for wheat storage proteins is 50% (v/v) 1-propanol containing a reducing agent with extraction at 60°C38. Alkylation of cysteine with IDAM requires high pH, since cysteine reacts as thiolate anion39. Complete extractability of wheat flour proteins was achieved by shaking for 30 min at 60°C in a Tris/HCl buffer [0.5 mol/L buffer (pH 8.5), diluted to 50% (v/v) with 1-propanol] containing tris-(2-carboxyethyl)phosphine (TCEP). TCEP is a reducing agent suitable for almost complete extraction of prolamins40. The buffer was apt for labeling with either IDAM or 4-VP. Because IDAM and 4-VP are unstable and light-sensitive, solutions were prepared immediately before use and alkylation was performed in the dark. At very alkaline pH, IDAM also reacts with amino groups of lysine and histidine41. Here, over-alkylation was substantially reduced if not prevented by strictly controlling alkylation time (30 min), temperature (37°C) and pH (8.5). Amino acid analysis of alkylated wheat flour proteins confirmed that, under these conditions, all cysteine residues had reacted with IDAM with no side reactions such as N-alkylation (results not shown).
Impact of cysteine labeling on detection of cysteine-containing peptides
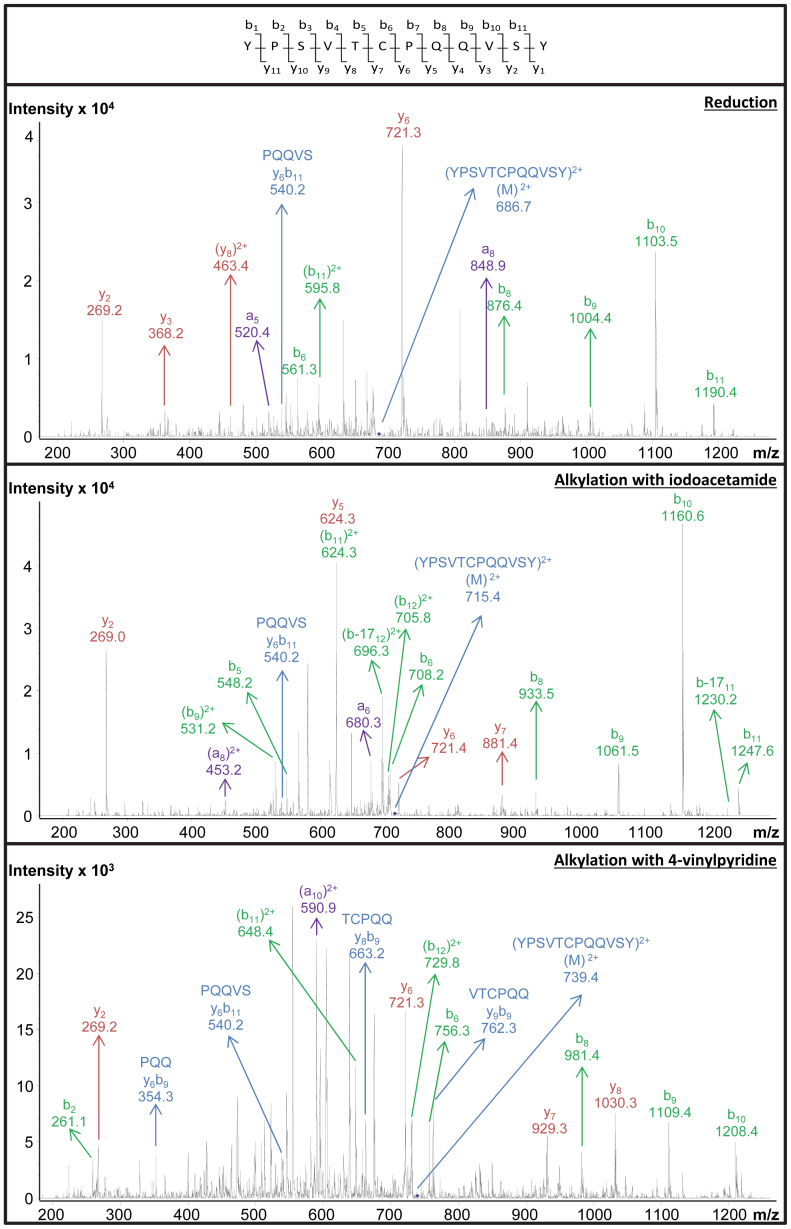
Wheat prolamins were extracted under the conditions described above. Cysteine residues were then (i) reduced, (ii) reduced and labeled with IDAM or (iii) reduced and labeled with 4-VP. The protein mixtures were hydrolyzed with chymotrypsin (37°C, 24 h, pH 7.8, enzyme/substrate: 1/50) and separated on a C18 reversed phase (RP) column with 0.1% (v/v) formic acid as the mobile phase. Based on comparison of the MS/MS data with theoretical peptide sequences of the wheat flour storage proteome, (i) three, (ii) twenty-four, or (iii) sixteen cysteine-containing peptides were detected, respectively (Table 1). Figure 2 shows an example of MS/MS spectra which allowed identifying the peptide YPSVTCPQQVSY. This peptide was identified with and without prior alkylation. The MS/MS spectra of the alkylated peptides resulted in a Mascot score exceeding 40, which indicated identity or extensive similarity (p < 0.05). The MS/MS spectrum of the reduced peptide had a Mascot score higher than 15 and was manually confirmed based on independent fragment peaks matching the theoretical peptide fragments. In the spectra of the two alkylated peptides and the non-alkylated one, especially b-fragments with a high m/z value led to successful identification. The detection of YPSVTCPQQVSY also allowed investigating the impact of alkylation on retention time on a C18 RP column. The retention time increased in the following order: 4-VP < IDAM < free thiol, which is in agreement with Jiang et al.42. Under acidic conditions, 4-VP and IDAM labeled peptides are more hydrophilic than their non-alkylated equivalents, and in the presence of formic acid 4-VP labeled peptides elute earlier than their IDAM labeled equivalents42. It should be emphasized that YPSVTCPQQVSY was one of the few cysteine-containing peptides detected after reduction and no labeling. Considerably more cysteine-containing peptides were found after labeling with either IDAM or 4-VP, which can be ascribed to the prevention of thiol group oxidation when converting this functional group into stable thioethers.
Table 1. Gluten peptides containing cysteine, S-carbamidomethyl cysteine, or S-β-(4-pyridylethyl) cysteine, detected by LC-MS/MS after chymotryptic digestion of a reduced/unalkylated or reduced/alkylated protein extract of wheat flour (cv. Akteur).
| Reduction | Alkylation with iodoacetamide | Alkylation with 4-vinylpyridine | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amino acid sequence | Mr | M/z | Score | RT (min) | Mr | M/z | Score | RT (min) | Mr | M/z | Score | RT (min) |
| ALETLPAMCNVY | 1380.6 | 691.3 | 24 | 55.1 | 1428.7 | 715.4 | 19 | 50.1 | ||||
| CCQQL | 707.3 | 708.2 | 44 | 18.4 | ||||||||
| CCQQLL | 820.4 | 821.3 | 68 | 33.9 | ||||||||
| CCQQLW | 893.4 | 894.3 | 63 | 41.3 | ||||||||
| CNVY | 602.3 | 603.3 | 17 | 16.9 | ||||||||
| CSTTIAPF | 895.4 | 896.4 | 19 | 45.1 | ||||||||
| CSVNVPL | 835.4 | 836.3 | 30 | 36.0 | ||||||||
| CTIAPF | 707.3 | 708.3 | 34 | 44.6 | 755.4 | 756.3 | 35 | 37.9 | ||||
| CTIAPVGF | 863.4 | 864.4 | 59 | 48.6 | 911.5 | 456.8 | 78 | 42.5 | ||||
| CTIAPVGIF | 976.5 | 977.5 | 47 | 58.9 | 1024.5 | 513.3 | 34 | 51.6 | ||||
| ELEACQQVVDQQL | 1606.8 | 804.3 | 15 | 23.1 | ||||||||
| GQCSF | 597.2 | 598.2 | 25 | 20.9 | ||||||||
| GQCVSQPQQQL | 1271.6 | 636.8 | 40 | 28.4 | 1319.6 | 660.9 | 20 | 25.6 | ||||
| GQCVSQPQQQSQQQL | 1742.8 | 872.4 | 53 | 27.4 | ||||||||
| IPCMDVVL | 993.5 | 497.9 | 20 | 51.3 | ||||||||
| IPEQSRCQAIH | 1280.6 | 641.4 | 17 | 22.2 | ||||||||
| LGECCSRL | 993.4 | 497.7 | 68 | 25.9 | ||||||||
| LLQQCNPVSL | 1218.6 | 610.4 | 22 | 41.7 | ||||||||
| LQQCNPVSL | 1057.5 | 529.7 | 44 | 39.1 | 1105.6 | 553.9 | 42 | 35.2 | ||||
| LQQQCIPVAM | 1234.6 | 618.3 | 16 | 40.6 | ||||||||
| NPCKNFL | 891.4 | 446.8 | 24 | 17.6 | ||||||||
| QCEREL | 833.4 | 417.7 | 39 | 15.9 | ||||||||
| QCERELQEL | 1203.6 | 602.7 | 136 | 32.4 | ||||||||
| QQCNPVSL | 944.4 | 945.4 | 46 | 34.7 | ||||||||
| QQLNPCKL | 942.5 | 943.4 | 15 | 38.4 | ||||||||
| QQQLIPCMDVVL | 1442.7 | 722.4 | 30 | 66.7 | 1490.8 | 746.4 | 16 | 60.4 | ||||
| QQQLNPCKNIL | 1354.7 | 678.4 | 28 | 29.2 | ||||||||
| SQQQPCSQQQQQPL | 1683.8 | 842.9 | 534 | 25.4 | 1731.8 | 578.4 | 71 | 23.4 | ||||
| SQVCF | 639.3 | 640.2 | 23 | 32.1 | ||||||||
| VPPDCSTINVPY | 1360.6 | 681.3 | 22 | 46.5 | ||||||||
| VPPECSIM | 979.5 | 490.8 | 25 | 34.5 | ||||||||
| WQSSCH | 803.3 | 804.3 | 17 | 43.2 | ||||||||
| YPSVTCPQQVSY | 1370.6 | 686.3 | 20 | 45.3 | 1427.6 | 714.9 | 47 | 41.9 | 1475.7 | 738.8 | 42 | 38.8 |
Figure 2. CID MS/MS spectra of the cysteine-containing peptide YPSVTCPQQVSY.
The peptide was found in a chymotryptic digest of a wheat (cv. Akteur) flour extract. Top: unlabeled (reduced). Middle: IDAM-labeled. Bottom: 4-VP-labeled.
Average MS/MS sequence coverages of wheat flour proteins were not significantly different after reduction (71.2%), or reduction followed by labeling with either IDAM (69.2%) or 4-VP (71.3%). Apparently, labeling of cysteine residues of wheat flour proteins specifically facilitates the detection of the labeled peptides, but has little or no impact on the overall detection of gluten peptides. With all wheat prolamins having repetitive sequences, their identification mainly depends on peptides with unique sequences in the N or C-terminal domains. Since no N-terminal wheat prolamin sequences were detected except for those from some HMW-GS, it was investigated whether wheat prolamin identification could rely on detecting specific cysteine-containing peptides. For every detected cysteine-containing peptide, its occurrence in various protein types was evaluated (Table 2). The following protein types were considered: various HMW-GS [referred to by their Payne score43], m-, s- and i-type LMW-GS (typical LMW-GS), α- and γ-like LMW-GS (gliadin-like LMW-GS), and α- and γ-gliadins. For instance, the peptide YPSVTCPQQVSY is unique for HMW-GS 1Dx5, while QCEREL is found in all HMW-GS except in some HMW-GS from the B genome (1Bx7 and 1Bx17)44. In contrast, the peptide CCQQL occurs in both in wheat prolamins and wheat albumins. One identified peptide (SQVCF) contained the additional cysteine residue specific for an α-like LMW-GS. The other detected LMW-GS peptides were only present in typical m-, s- or i- type LMW-GS (e.g. LQQQCIPVAM), or both in typical and gliadin-like LMW-GS (e.g. CSVNVPL). The detected α- and γ-gliadin peptides all contained cysteine residues involved in intramolecular cross-links in native wheat gluten. Some of them are specific for either α- or γ-gliadin (e.g. CTIAPVGF), but most of them also occur in gliadin-like LMW-GS (e.g. QQCNPVSL). Overall, peptides specific for each prolamin type, except for D-LMW-GS, were detected. D-LMW-GS or ω-bound gliadins are ω-gliadins with only one cysteine residue and they account for less than 1% of the total protein content in wheat flour45. This makes the detection of cysteine containing D-LMW-GS peptides difficult. The chymotryptic peptides from GS are a good basis for an MS study of changes in the SS structure during glutenin synthesis in the developing wheat plant. In the context of gluten functionality, it is helpful that various prolamin types were detected, especially because gliadins, LMW-GS and HMW-GS have a different impact on the gluten network5,19. Also in the context of celiac disease it is essential that methodologies are available for detecting all types of wheat prolamins. Focusing solely on labeled cysteine-containing peptides offers the possibility to rapidly assess gluten composition in general and that of individual proteins responsible for gluten intolerance in particular.
Table 2. Occurrence of cysteine-containing peptides from the wheat storage proteome detected by LC-MS/MS after chymotryptic digestion of wheat flour (cv. Akteur) using MS/MS.
| Amino acid sequence | Present in | Detected after |
|---|---|---|
| ALETLPAMCNVY | α-like LMW-GS, α-gliadin | Labeling with IDAM or 4-VP |
| CCQQL1,* | By9, Dy10, m-, s-, and i-type LMW-GS, α- and γ-like LMW-GS, α- and γ-gliadins | Labeling with IDAM |
| CCQQLL | s-type LMW-GS, α-gliadin | Labeling with IDAM |
| CCQQLW | α-like LMW-GS, α-gliadin | Labeling with IDAM |
| CNVY* | α- and γ-like LMW-GS, α- and γ-gliadin | Labeling with 4-VP |
| CSTTIAPF | α-like LMW-GS, α-gliadin | Labeling with IDAM |
| CSVNVPL | m-, s-, and i-type LMW-GS, α- and γ-like LMW-GS | Labeling with 4-VP |
| CTIAPF | α-like LMW-GS, α-gliadin | Labeling with IDAM or 4-VP |
| CTIAPVGF | α-gliadin | Labeling with IDAM or 4-VP |
| CTIAPVGIF | α-like LMW-GS, α-gliadin | Labeling with IDAM or 4-VP |
| ELEACQQVVDQQL2 | Bx7 | Labeling with 4-VP |
| GQCSF | m-, s- and i-type LMW-GS | Labeling with IDAM |
| GQCVSQPQQQL | m-, s- and i-type LMW-GS | Labeling with IDAM or 4-VP |
| GQCVSQPQQQSQQQL | m- and s-type LMW-GS, γ-like LMW-GS | Labeling with IDAM |
| IPCMDVVL | α-like LMW-GS, α-gliadin | Labeling with 4-VP |
| IPEQSRCQAIH | α-like LMW-GS, α-gliadin | Reduction |
| LGECCSRL | Puroindoline a | Labeling with IDAM |
| LLQQCNPVSL | γ−like LMW-GS, γ-gliadin | Labeling with 4-VP |
| LQQCNPVSL | γ−like LMW-GS, γ-gliadin | Labeling with IDAM or 4-VP |
| LQQQCIPVAM | i-type LMW-GS | Labeling with 4-VP |
| NPCKNFL | γ−like LMW-GS, γ-gliadin | Labeling with IDAM |
| QCEREL3 | Ax1, Dx5, By9, Dy10 | Labeling with IDAM |
| QCERELQEL4 | Dx5 | Labeling with IDAM |
| QQCNPVSL | γ−like LMW-GS, γ-gliadin | Labeling with IDAM |
| QQLNPCKL | m-type LMW-GS | Reduction |
| QQQLIPCMDVVL | α-like LMW-GS, α-gliadin | Labeling with IDAM or 4-VP |
| QQQLNPCKNIL | γ−like LMW-GS, γ-gliadin | Labeling with IDAM |
| SQQQPCSQQQQQPL | s-type LMW-GS | Labeling with IDAM or 4-VP |
| SQVCF | α-like LMW-GS | Labeling with IDAM |
| VPPDCSTINVPY | γ−like LMW-GS, γ-gliadin | Labeling with IDAM |
| VPPECSIM | γ−like LMW-GS, γ-gliadin | Labeling with 4-VP |
| WQSSCH | m-, s- and i-type LMW-GS | Labeling with IDAM |
| YPSVTCPQQVSY | Dx5 | Reduction or labeling with IDAM or 4-VP |
1Also present in other y-type HMW-GS.
2Also present in other Bx-type HMW-GS.
3Also present in other HMW-GS.
4Also present in other Dx-type HMW-GS.
*Also present in non-wheat prolamins that are toxic to celiac patients.
Chymotryptic versus thermolytic digestion
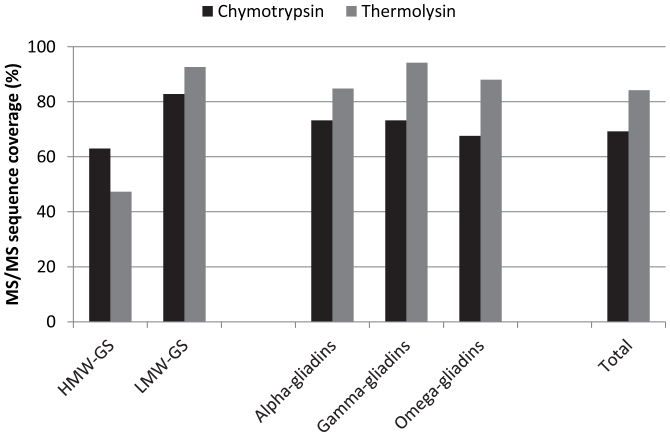
Besides chymotrypsin also thermolysin is used in bottom-up proteomics of gluten20. Trypsin, the enzyme commonly used to hydrolyze proteins prior to MS analysis, was not used in this study because theoretical in-silico digests of gluten proteins showed that the amount of detectable cysteine-containing peptides (m/z range 200–2000) after tryptic digestion was only 15 to 30% of that after chymotryptic or thermolytic digestion due to the low abundance of lysine and arginine residues. After reduction and cysteine alkylation with IDAM, gluten proteins were digested with chymotrypsin or thermolysin, and the detection of chymotryptic and thermolytic gluten peptides was compared. More cysteine-containing gluten peptides were found after chymotryptic (24) than after thermolytic digestion (15) (Table 3). This difference was noted for all prolamin types, except for γ-gliadins and γ-like LMW-GS. Different cysteine-residues were found in both cases. Hence, to detect as many cysteine-containing peptides as possible by LC-MS, it proved advisable to perform both chymotryptic as well as thermolytic digestion. This is in line with Vensel et al.26 who demonstrated that the use of multiple enzymes increased the number of wheat proteins identified. The highest MS/MS full protein sequence coverage was obtained after thermolytic digestion, except in the case of HMW-GS (Figure 3). To conclude, for a single analysis of the entire wheat storage proteome, chymotryptic digestion is expected to provide most information.
Table 3. S-carbamidomethyl cysteine-containing gluten peptides, detected by LC-MS/MS after thermolytic digestion of a reduced/alkylated protein extract of wheat flour (cv. Akteur).
| Amino acid sequence | Mr | M/z | Score | RT (min) | Present in |
|---|---|---|---|---|---|
| LCCQQ | 707.3 | 708.3 | 36 | 11.8 | α-like LMW-GS, α-gliadin |
| LGQC | 476.2 | 477.2 | 20 | 10.8 | m-, s- and i-type LMW-GS |
| LGQCS | 563.2 | 564.2 | 16 | 11.0 | m-, s- and i-type LMW-GS, γ-like LMW-GS |
| LKACQQ | 746.4 | 747.3 | 16 | 22.4 | Dx-type HMW-GS |
| LNPCKN* | 744.4 | 373.3 | 18 | 11.9 | γ-like LMW-GS, γ-gliadin |
| LQQCKP | 772.4 | 773.4 | 16 | 10.8 | γ-like LMW-GS, γ-gliadin |
| LQQCNP | 758.3 | 759.3 | 28 | 15.5 | γ-like LMW-GS, γ-gliadin |
| LQQQCIP | 885.4 | 886.5 | 32 | 30.4 | i-type LMW-GS |
| LQQQCSP | 859.4 | 860.4 | 40 | 16.4 | m-, s- and i-type LMW-GS |
| VRPDCST | 833.4 | 834.4 | 18 | 11.4 | α-like LMW-GS, α-gliadin |
| VSAKCRS | 806.4 | 404.3 | 17 | 5.7 | γ-like LMW-GS, γ-gliadin |
| VSPDCST | 764.3 | 765.3 | 23 | 14.0 | γ-like LMW-GS, γ-gliadin |
| VYIPPYCT | 1011.5 | 1012.5 | 23 | 42.0 | α-like LMW-GS, α-gliadin |
| VYVPPDCST | 1036.5 | 1037.5 | 32 | 28.2 | γ-like LMW-GS, γ-gliadin |
| VYVPPECS | 949.4 | 950.4 | 44 | 29.2 | γ-like LMW-GS, γ-gliadin |
*Also present in non-wheat prolamins that are toxic to celiac patients.
Figure 3. Average MS/MS sequence coverages (%) of wheat gluten proteins.
Wheat (cv. Akteur) gluten proteins were reduced, labeled with IDAM, and subsequently digested with either chymotrypsin or thermolysin.
ICAT labeling of cysteine residues from gluten proteins
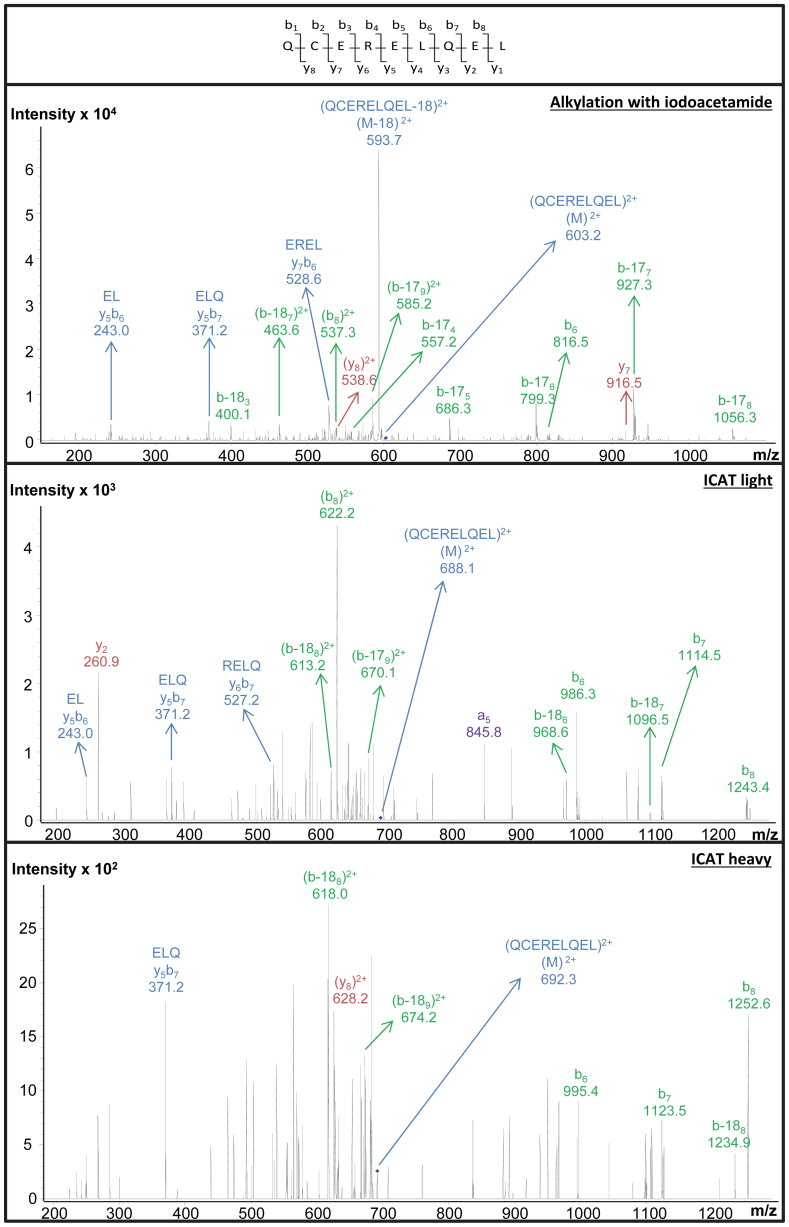
Another task in proteomics is protein quantification46,47. This is especially challenging in the case of gluten proteins, given their limited solubility. In this study, ICAT was used to label wheat prolamins (Figure 1). The manufacturer's protocol (Cleavable ICAT Reagent Kit for Protein Labeling) recommends dissolving the proteins in Tris/HCl buffer (0.05 mol/L, pH 8.5) containing 0.1% SDS, and subsequently labeling them in the same buffer with cleavable ICAT reagent in acetonitrile. This procedure allowed identifying cysteine-containing peptides of water-soluble proteins, such as bovine serum albumin, but it failed to do so in the case of wheat flour proteins (results not shown). Even when isolated prolamin fractions were subjected to the manufacturer's protocol, no cysteine-containing peptides were detected, probably because prolamins were not soluble under these conditions. Since derivatization reactions with IDAM and ICAT are both based on reaction of a thiol residue with an IDAM group, ICAT labeling for wheat flour proteins was optimized based on the procedure for IDAM labeling as described above. Wheat flour proteins were first reduced by shaking for 30 min at 60°C in 0.500 mol/L Tris/HCl buffer (pH 8.5) diluted to 50% (v/v) with 1-propanol and containing 0.001 mol/L reducing agent (TCEP). The buffer contained no sodium dodecyl sulfate, which would compromise the labeling reaction48. The protein solution was subsequently labeled with either light or heavy cleavable ICAT reagent (Figure 1) in this buffer for 2 h at 37°C. This procedure is further referred to as the new protocol. When the new protocol was applied to bovine serum albumin, chymotryptic digestion produced a number of detectable cysteine-containing peptides (Table 4). The Tris/HCl buffer was thus suitable for ICAT labeling. Even though labeling under these conditions yielded many S-carbamidomethylated prolamin peptides after chymotryptic digestion (Table 1), no ICAT-labeled gluten peptides were detected by LC-MS. However, no unlabeled cysteine-containing gluten peptides were found either. Hence, it is reasonable to assume that cysteine residues were successfully labeled with ICAT in the Tris/HCl buffer, but that the technical problems had their origin in the subsequent enzymic hydrolysis or affinity chromatography. We here reasonably assumed that ICAT labeling did not affect the rate and specificity of chymotrypsin, since the enzyme acts at peptide bonds of amino acid residues other than cysteine49. While isolation of labeled peptides by biotin-avidin affinity chromatography significantly reduces the complexity of the peptide mixture, it sometimes suffers from non-specific binding of cysteine-free peptides and/or irreversible adsorption of some cysteine-peptides29. More cysteine-containing peptides were derived from bovine serum albumin after IDAM than after ICAT labeling. Considering the lower cysteine level in prolamins [ca. 2.2 mol%27] than in bovine serum albumin [6.0 mol%50], it cannot be excluded that inefficient affinity chromatography resulted in the lack of detection of cysteine-containing peptides in the case of gluten. Therefore, samples were analyzed again, this time focusing on the peptides that had been detected after S-carbamidomethylation. Only compounds with m/z values corresponding to the doubly or triply charged ion of potential ICAT labeled peptides were fragmented. One peptide (QCERELQEL) was now detected. Figure 4 shows the corresponding MS/MS spectra. Quantification of cysteine-containing peptides by ICAT labeling of wheat storage proteins remains difficult. Alternative labeling agents include 2D-,13C- and 18O-coded reagents based on 2- or 4-VP51, IDAM52, or iodoacetic acid53. Some of these reagents are commercially available, e.g. 4-VP-d3, 2-VP-d7, IDAM-d4, iodoacetic acid-13C2. Others can be readily prepared52,53.
Table 4. ICAT-labeled cysteine-containing peptides from bovine serum albumin, detected after a single LC-MS/MS run. Cysteine residues were labeled according to the new protocol and all peptides were manually confirmed as described in the materials and methods section.
| Amino acid sequence | Mr | z | M/z | Score | RT (min) |
|---|---|---|---|---|---|
| AEDKDVCKNY | Light: 1410.6 | 2+ | 706.3 | 97 | 20.8 |
| Heavy: 1419.6 | 710.8 | 37 | 20.8 | ||
| 3+ | 471.3 | 69 | 20.9 | ||
| 474.3 | 15 | 20.8 | |||
| CDEF | Light: 739.3 | 1+ | 740.2 | 20 | 29.2 |
| Heavy: 748.3 | 749.3 | 38 | 29.2 | ||
| ECADDRADL | Light: 1233.5 | 2+ | 617.7 | 16 | 26.2 |
| Heavy: 1242.5 | 622.3 | 28 | 26.3 | ||
| 3+ | 412.3 | 15 | 26.2 | ||
| 415.3 | 15 | 26.1 |
Figure 4. CID MS/MS spectra of the cysteine-containing peptide QCERELQEL.
The peptide was found in a chymotryptic digest of a wheat (cv. Akteur) flour extract. Top: IDAM-labeled. Middle: light ICAT-labeled. Bottom: heavy ICAT-labeled.
Discussion
Results in this paper show that labeling of cysteine-containing wheat prolamin with 4-VP or IDAM facilitates the detection of the peptides using LC-MS/MS with CID. The proposed methods to identify cysteine-peptides in an unknown flour mixture form a basis for further elucidating wheat prolamin structures as a result of redox-based post-translational modifications during plant development, but also during food processing which often includes heating.
Cysteine labeling for quantitative proteomics of wheat storage proteins can further be helpful to evaluate gluten functionality and to develop alternative methods to detect gluten contamination in so-called gluten-free products. For example, the current calculation of gluten content by considering it to be twice the gliadin content is invalid when assessing levels of contamination by gluten in so-called gluten-free products54.
Labeling wheat prolamins with ICAT reagent was successful but complicated. 2H-, 13C- and 18O-coded reagents are probably better alternatives for quantification purposes. Further work exploring the opportunities of cysteine labeling to accurately determine gluten levels would be extremely valuable. The combination of isotopic labeling and MS is a promising tool for detection and quantification of specific cysteine-containing prolamin peptides as well as entire prolamins.
Methods
Materials
Wheat kernels of the cv. Akteur (harvest 2009) were milled into patent flour with a Bühler MLU 202 Mill (Uzwil, Switzerland) and the flour sifted through a 0.2 mm screen. The Cleavable ICAT Reagent Kit for Protein Labeling was purchased from AB Sciex (Darmstadt, Germany). All solvents, chemicals and reagents were at least of analytical grade. Water was deionized by a Millipore-O Milli-Q purification system.
Cysteine labeling
Cysteine residues of the Akteur wheat flour proteins were reduced and subsequently alkylated with IDAM, 4-VP, or ICAT. Reduction was performed by shaking 8 mg wheat flour (equivalent to 1 mg protein containing about 0.2 μmol cysteine) for 30 min at 60°C under nitrogen atmosphere in 320 μL Tris/HCl buffer (0.50 mol/L, pH 8.5), diluted to 50% (v/v) with 1-propanol and 8 μL reducing reagent (0.050 mol/L TCEP). The mixtures were centrifuged (10 min, 10 · 103 g) and the residues discarded. Alkylation with IDAM was performed by adding 12 μL 0.50 mol/L IDAM solution to the supernatant (1 mg protein) and shaking for 30 min at 37°C in the dark. For 4-VP alkylation, 0.5 μL pure 4-VP was added and mixtures (1 mg protein) were shaken for 30 min at 60°C. ICAT labeling was performed by 120 min reaction at 37°C of ICAT reagents (1 unit which labels about 50 μmol cysteine under the present conditions) with protein (1 mg) dissolved in the denaturing buffer containing reducing reagent. Because a modified denaturing buffer was used, labeling conditions were different from those described in the manufacturer's protocol (Cleavable ICAT Reagent Kit for Protein Labeling). All labeling steps were performed under nitrogen atmosphere.
Amino acid analysis
Gluten amino acids were liberated by acid hydrolysis and separated by high-performance anion-exchange chromatography with integrated pulsed amperometric detection (HPAEC-IPAD) as described by Rombouts et al.27. Freeze dried samples (5.0 mg protein, db) were heated (24 h, 110°C) in 1.0 ml 6.0 mol/L HCl containing 0.1% phenol and 3.0 mmol/L norleucine (as internal standard). Reaction mixtures were subsequently diluted (200-fold) in deionised water and filtered (Millex-GP, 0.22 μm, polyethersulfone; Millipore). Amino acids (injection volume 25 μl, flow rate 0.25 ml/min, 30°C) were separated on an AminoPac PA10 column (250 × 2 mm; Dionex Benelux, Amsterdam, The Netherlands) using a Dionex BioLC system (Dionex, Sunnyvale, CA, USA) equipped with Chromeleon Version 6.70 software (Dionex Benelux). Gradient conditions and detection waveform were as previously described27. Amino acid levels were expressed on dry matter protein (μmol/g protein) and calculated from the relative peak areas of the amino acid to norleucine. Relative standard deviations did not exceed 10%. Relative standard deviations did not exceed 10%.
Protein digestion
Prior to digestion, protein solutions were evaporated to dryness. For chymotryptic digestion, 1.000 mL chymotrypsin solution [0.020 mg α-chymotrypsin/mL and 0.040 mol/L urea in 0.100 mol/L Tris/HCl (pH 7.8)] was added and the mixture was shaken for 24 h at 37°C. For thermolytic digestion, 1.000 mL thermolysin solution [0.100 mg thermolysin/mL and 0.020 mol/L CaCl2.· 6H2O in 0.200 mol/L Tris/HCl (pH 6.5)] was added and the mixture was shaken for 16 h at 37°C. Digestions were stopped by lowering the pH to 1.4 by adding 3.0 μL trifluoroacetic acid (TFA).
Purification of peptides
After reduction (followed by labeling with IDAM or 4-VP) and subsequent digestion, peptides were purified by solid phase extraction on Strata-X-C devices (Phenomenex, Aschaffenburg, Germany). Solid phase extraction cartridges were conditioned with methanol (1.0 mL) and equilibrated with 1.0 mL 0.1% TFA. The digests (1.0 mL) were loaded and the cartridges washed with 50% (v/v) methanol (5 × 1.0 mL). Peptides were eluted with 1.0 mL 2.0 mol/L ammonia, which was then evaporated. Samples with reduced, S-carbamidomethylated or S-pyridylethylated cysteine residues were dissolved in 1.0 mL 0.1% (v/v) formic acid. Samples with ICAT labeled cysteine residues were further purified by affinity chromatography. Avidin cartridges were activated with 2.0 mL elute buffer (30% (v/v) acetonitrile containing 0.4% (v/v) TFA) and 2.0 mL load buffer (pH 7.2, 0.020 mol/L sodium phosphate, 0.300 mol/L sodium chloride). The peptides, dissolved in 1.0 mL load buffer, were loaded and the cartridges washed with 0.5 mL load buffer, 1.0 mL 0.010 mol/L sodium phosphate buffer (pH 7.2) containing 0.150 mol/L sodium chloride), 1.0 mL 0.050 mol/L ammonium bicarbonate (pH 8.3) containing 20% (v/v) methanol and 1.0 mL milliQ water. Biotinylated peptides were eluted with 0.80 mL elution buffer, which was then evaporated to dryness. ICAT labeled peptides were incubated for 120 min at 37°C with 90 μL of TFA to cleave the biotin moiety. Samples were evaporated to dryness and dissolved in 50 μL 0.1% formic acid.
LC-MS/MS
LC-MS/MS experiments were performed on an HCT-Ultra PTM ion trap MS (Bruker Daltonics, Bremen, Germany) with CID, coupled with an UltiMate 3000 HPLC (Dionex, Idstein, Germany) system equipped with an Aeris PEPTIDE 3.6 μ XB-C18 column (2.10 × 150 mm; Phenomenex). The MS contained a spherical ion trap with an ESI interface running in the positive mode (capillary voltage: −4000 V; capillary exit voltage: 158.5 V; skimmer voltage: 40 V). Nitrogen was used as drying (8.0 L/min, 325°C) and nebulizing gas (0.2 MPa). The mobile phases for LC separation were (A) 0.1% (v/v) formic acid and (B) 0.1% (v/v) formic acid in acetonitrile. The following gradient was used: (i) linear from 0 to 32% B over 80 min; (ii) linear from 32 to 90% B over 5 min; (iii) isocratic at 90% B for 9 min; (iv) linear from 90 to 0% B in 1 min; (v) isocratic at 0% B for 10 min. The flow rate was 0.2 mL/min, injection volume was 5 μL, and column temperature was 30°C. The scan range was m/z 200 to 2000 (smart target value: 200,000; maximum acquisition time: 100 ms), and CID-MS2 scan steps were performed on precursor ions using the AutoMS/MS mode (fragmentation amplitude: 1.0 V; collision gas: helium).
Analysis of MS results
MS/MS data in a Mascot Generic File (*.mgf) using the Bruker Daltonics Data Analysis 3.4 software were analyzed using the MS/MS ions search module of Mascot software (Matrix Science, London, UK) based on the SwissProt database 2012 (taxonomic category: Viridiplantae; peptide mass tolerance: ± 0.1%; fragment mass tolerance: ± 0.5 Da; peptide charges: 1+, 2+ and 3+; monoisotopic ions). MS/MS data (*.mgf) were also compared with a database containing gluten sequences from literature26 and the NCBI database (National Library of Medicine, Bethesda, MD, USA) using the SequenceEditor module of the Bruker Biotools software 3.2 (m/z range: 200–3,000; peptide and MS/MS tolerance: ± 0.5; peptide charges: 1+, 2+ and 3+; monoisotopic ions). Potential chymotrypsin cleavage sites were entered as follows: at the C-terminal end of Phe, His, Leu, Met, Trp and Tyr, but not before Pro. Possible thermolysin cleavage sites were entered as follows: at the N-terminal end of Val, Ile, Ala, Leu, Met and Phe, if not preceding Pro. Two missed cleavages by the enzymes were allowed. S-pyridylethyl and S-carbamidomethyl were set as fixed modifications on cysteine in the corresponding samples, and oxidation at methionine was used as variable modifications in all samples. Charge state deconvolution and deisotoping were not performed. The presence of detected peptides in wheat prolamins was evaluated by performing a blast search on the Uniprot database.
Criteria for peptide identification
Individual ion scores based on peptide mass fingerprints were calculated as −10 × log(P), where P is the probability that the observed match is a random event. Individual ion scores > 40 were considered to indicate identity or extensive similarity (p < 0.05). All peptide identifications with scores between 15 and 40 were manually validated, essentially following the rules of Chen et al.55. For doubly or triply charged ions consisting of x amino acids, at least x-2 isotopically resolved independent fragment peaks were required to match the theoretical peptide fragments. Only a-, b- or y-ions, or associated peaks arising due to water or amine loss were considered as daughter ions of a peptide, and either the b- or y-ion series were required to confirm at least three consecutive amino acids in the peptide sequence. Only one isotopically resolved peak with an intensity higher than one third of the maximum intensity, an m/z value larger than that of the doubly/triply charged parent ion and not matching a theoretical peptide fragment, were allowed. For single charged ions, either the b-ion or y-ion series were required to confirm at least three consecutive amino acids in the peptide sequence. Large mass errors among the fragment ions and low/no intensity spectral peaks at the position of a readily cleavable proline residue caused a peptide to be rejected.
Author Contributions
Conceived and designed the experiments: I.R., B.L., M.B., P.K. Performed the experiments: B.L., M.B. Analyzed the data: I.R. Contributed reagents/materials/analysis tools: P.K., J.D. Wrote the paper: I.R.
Acknowledgments
Ine Rombouts and Bert Lagrain wish to acknowledge the Research Foundation – Flanders (FWO, Brussels, Belgium) for their positions as postdoctoral fellows and financial support during a research stay at the German Research Centre for Food Chemistry (Freising, Germany). The authors would like to thank Ines Otte and Sami Kaviani-Nejad (German Research Center for Food Chemistry, Freising, Germany) for excellent technical assistance and help with the MS measurements.
References
- Faostat. Food and Agricultural commodities production, <faostat.fao.org> (2011). Date of access 8th of July 2013. [Google Scholar]
- Dupont F. M., Vensel W. H., Tanaka C. K., Hurkman W. J. & Altenbach S. B. Deciphering the complexities of the wheat flour proteome using quantitative two-dimensional electrophoresis, three proteases and tandem mass spectrometry. Proteome Sci. 9, 10 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delcour J. A. et al. Wheat gluten functionality as a quality determinant in cereal-based food products. Annu. Rev. Food Sci. Technol. 3, 469–492 (2012). [DOI] [PubMed] [Google Scholar]
- Wieser H. Chemistry of gluten proteins. Food Microbiol. 24, 115–119 (2007). [DOI] [PubMed] [Google Scholar]
- D'Ovidio R. & Masci S. The low-molecular-weight glutenin subunits of wheat gluten. J. Cereal Sci. 39, 321–339 (2004). [Google Scholar]
- Anderson O. D. & Greene F. C. The characterization and comparative analysis of high-molecular-weight glutenin genes from genomes A and B of a hexaploid bread wheat. Theor. Appl. Genet. 77, 689–700 (1989). [DOI] [PubMed] [Google Scholar]
- Anderson O. D., Gu Y. Q., Kong X., Lazo G. R. & Wu J. The wheat omega-gliadin genes: structure and EST analysis. Funct. Integr. Genomics 9, 397–410 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPont F. M., Vensel W., Encarnacao T., Chan R. & Kasarda D. D. Similarities of omega gliadins from Triticum urartu to those encoded on chromosome 1A of hexaploid wheat and evidence for their post-translational processing. Theor. Appl. Genet. 108, 1299–1308 (2004). [DOI] [PubMed] [Google Scholar]
- Gianibelli M. C., Larroque O. R., MacRitchie F. & Wrigley C. W. Biochemical, genetic, and molecular characterization of wheat glutenin and its component subunits. Cereal Chem. 78, 635–646 (2001). [Google Scholar]
- Gu Y. Q. et al. Genomic organization of the complex alpha-gliadin gene loci in wheat. Theor. Appl. Genet. 109, 648–657 (2004). [DOI] [PubMed] [Google Scholar]
- Qi P. F. et al. The gamma-gliadin multigene family in common wheat (Triticum aestivum) and its closely related species. BMC Genomics. 10, 168 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salentijn E. M. et al. Tetraploid and hexaploid wheat varieties reveal large differences in expression of alpha-gliadins from homoeologous Gli-2 loci. BMC Genomics. 10, 48 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnie C., Sultan A. & Grasser K. D. From protein catalogues towards targeted proteomics approaches in cereal grains. Phytochemistry. 72, 1145–1153 (2011). [DOI] [PubMed] [Google Scholar]
- Wieser H., Bushuk W. & MacRitchie F. In: Gliadin and Glutenin. The Unique Balance of Wheat Quality (eds Wrigley, C. W.,Békés, F. & Bushuk, W.) Ch. 7, 213–240 (American Association of Cereal Chemists, 2006). [Google Scholar]
- Naeem H. A., Paulon D., Irmak S. & MacRitchie F. Developmental and environmental effects on the assembly of glutenin polymers and the impact on grain quality of wheat. J. Cereal Sci. 56, 51–57 (2012). [Google Scholar]
- Shewry P. R., Tatham A. S., Barro F., Barcelo P. & Lazzeri P. Biotechnology of breadmaking: unraveling and manipulating the multi-protein gluten complex. Biotechnology (N Y). 13, 1185–1190 (1995). [DOI] [PubMed] [Google Scholar]
- Miflin B., Napier J. & Shewry P. Improving plant product quality. Nat. Biotechnol. 17, 13–14 (1999).9920253 [Google Scholar]
- Payne P. I. Genetics of wheat storage proteins and the effect of allelic variation on bread-making quality. Annu. Rev. Plant Phys. 38, 141–153 (1987). [Google Scholar]
- Kasarda D. D. In: Wheat is unique (ed Pomeranz, Y.) 277–302 (American Association of Cereal Chemistry, 1989). [Google Scholar]
- Šalplachta J., Marchetti M., Chmelík J. & Allmaier G. A new approach in proteomics of wheat gluten: combining chymotrypsin cleavage and matrix-assisted laser desorption/ionization quadrupole ion trap reflectron tandem mass spectrometry. Rapid Commun. Mass Spectrom. 19, 2725–2728 (2005). [DOI] [PubMed] [Google Scholar]
- Tanner G. J., Colgrave M. L., Blundell M. J., Goswami H. P. & Howitt C. A. Measuring hordein (gluten) in beer – A comparison of ELISA and mass spectrometry. PLoS ONE 8, e56452 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamone G., De Caro S., Di Luccia A., Addeo F. & Ferranti P. Proteomic-based analytical approach for the characterization of glutenin subunits in durum wheat. J. Mass Spectrom. 44, 1709–1723 (2009). [DOI] [PubMed] [Google Scholar]
- Lutz E., Wieser H. & Koehler P. Identification of disulfide bonds in wheat gluten proteins by means of mass spectrometry/electron transfer dissociation. J. Agric. Food Chem. 60, 3708–3716 (2012). [DOI] [PubMed] [Google Scholar]
- Camafeita E., Alfonso P., Acevedo B. & Mendez E. Sample preparation optimization for the analysis of gliadins in food by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. J. Mass Spectrom. 32, 444–449 (1997). [Google Scholar]
- Camafeita E. et al. Selective identification by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry of different types of gluten in foods made with cereal mixtures. J. Chromatogr. A 823, 299–306 (1998). [DOI] [PubMed] [Google Scholar]
- Vensel W. H., Dupont F. M., Sloane S. & Altenbach S. B. Effect of cleavage enzyme, search algorithm and decoy database on mass spectrometric identification of wheat gluten proteins. Phytochemistry. 72, 1154–1161 (2011). [DOI] [PubMed] [Google Scholar]
- Rombouts I. et al. Wheat gluten amino acid composition analysis by high-performance anion-exchange chromatography with integrated pulsed amperometric detection. J. Chromatogr. A 1216, 5557–5562 (2009). [DOI] [PubMed] [Google Scholar]
- Sechi S. & Chait B. T. Modification of cysteine residues by alkylation. A tool in peptide mapping and protein identification. Anal. Chem. 70, 5150–5158 (1998). [DOI] [PubMed] [Google Scholar]
- Leitner A. & Lindner W. Current chemical tagging strategies for proteome analysis by mass spectrometry. J. Chromatogr. B 813, 1–26 (2004). [DOI] [PubMed] [Google Scholar]
- Tao W. A. & Aebersold R. Advances in quantitative proteomics via stable isotope tagging and mass spectrometry. Curr. Opin. Biotechnol. 14, 110–118 (2003). [DOI] [PubMed] [Google Scholar]
- Paulech J., Solis N. & Cordwell S. J. Characterization of reaction conditions providing rapid and specific cysteine alkylation for peptide-based mass spectrometry. Biochim. Biophys. Acta 1834, 372–379 (2013). [DOI] [PubMed] [Google Scholar]
- Gygi S. P. et al. Quantitative analysis of complex protein mixtures using isotope-coded affinity tags. Nat. Biotechnol. 17, 994–999 (1999). [DOI] [PubMed] [Google Scholar]
- Sethuraman M. et al. Isotope-coded affinity tag (ICAT) approach to redox proteomics: identification and quantitation of oxidant-sensitive cysteine thiols in complex protein mixtures. J. Proteome Res. 3, 1228–1233 (2004). [DOI] [PubMed] [Google Scholar]
- Leichert L. I. et al. Quantifying changes in the thiol redox proteome upon oxidative stress in vivo. Proc. Natl. Acad. Sci. U. S. A. 105, 8197–8202 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Santamarina S. et al. The oxidized thiol proteome in fission yeast--optimization of an ICAT-based method to identify H2O2-oxidized proteins. J Proteomics. 74, 2476–2486 (2011). [DOI] [PubMed] [Google Scholar]
- Islam N., Tsujimoto H. & Hirano H. Wheat proteomics: relationship between fine chromosome deletion and protein expression. Proteomics 3, 307–316 (2003). [DOI] [PubMed] [Google Scholar]
- Ramus C., Gonzalez de Peredo A., Dahout C., Gallagher M. & Garin J. An optimized strategy for ICAT quantification of membrane proteins. Mol. Cell. Proteomics 5, 68–78 (2006). [DOI] [PubMed] [Google Scholar]
- Marchylo B. A., Kruger J. E. & Hatcher D. W. Quantitative reversed-phase high-performance liquid chromatographic analysis of wheat storage proteins as a potential quality prediction tool. J. Cereal Sci. 9, 113–130 (1989). [Google Scholar]
- Lundell N. & Schreitmuller T. Sample preparation for peptide mapping - A pharmaceutical quality-control perspective. Anal. Biochem. 266, 31–47 (1999). [DOI] [PubMed] [Google Scholar]
- Gessendorfer B., Wieser H. & Koehler P. Optimisation of a solvent for the complete extraction of prolamins from heated foods. J. Cereal Sci. 52, 331–332 (2010). [Google Scholar]
- Boja E. S. & Fales H. M. Overalkylation of a protein digest with iodoacetamide. Anal. Chem. 73, 3576–3582 (2001). [DOI] [PubMed] [Google Scholar]
- Jiang X., Shamshurin D., Spicer V. & Krokhin O. V. The effect of various S-alkylating agents on the chromatographic behavior of cysteine-containing peptides in reversed-phase chromatography. J. Chromatogr. B 915–916, 57–63 (2013). [DOI] [PubMed] [Google Scholar]
- Payne P. I., Nightingale M. A., Krattiger A. F. & Holt L. M. The relationship between HMW glutenin subunit composition and the bread-making quality of british-grown wheat-varieties. J. Sci. Food Agric. 40, 51–65 (1987). [Google Scholar]
- Shewry P. R. et al. In: Wheat: Chemistry and Technology ((eds Khan, K & Shewry, P. R.) 223–298 (AACC International, 2009). [Google Scholar]
- Wieser H. Comparative investigations of gluten proteins from different wheat species I. Qualitative and quantitative composition of gluten protein types. Eur. Food Res. Technol. 211, 262–268 (2000). [Google Scholar]
- Bantscheff M., Schirle M., Sweetman G., Rick J. & Kuster B. Quantitative mass spectrometry in proteomics: a critical review. Anal. Bioanal. Chem. 389, 1017–1031 (2007). [DOI] [PubMed] [Google Scholar]
- Kline K. G. & Sussman M. R. Protein quantitation using isotope-assisted mass spectrometry. Annu. Rev. Biophys. 39, 291–308 (2010). [DOI] [PubMed] [Google Scholar]
- Smolka M. B., Zhou H., Purkayastha S. & Aebersold R. Optimization of the isotope-coded affinity tag-labeling procedure for quantitative proteome analysis. Anal. Biochem. 297, 25–31 (2001). [DOI] [PubMed] [Google Scholar]
- Turecek F. Mass spectrometry in coupling with affinity capture-release and isotope-coded affinity tags for quantitative protein analysis. J. Mass Spectrom. 37, 1–14 (2002). [DOI] [PubMed] [Google Scholar]
- Hirayama K., Akashi S., Furuya M. & Fukuhara K. Rapid confirmation and revision of the primary structure of bovine serum albumin by ESIMS and Frit-FAB LC/MS. Biochem. Biophys. Res. Commun. 173, 639–646 (1990). [DOI] [PubMed] [Google Scholar]
- Sebastiano R., Citterio A., Lapadula M. & Righetti P. G. A new deuterated alkylating agent for quantitative proteomics. Rapid Commun. Mass Spectrom. 17, 2380–2386 (2003). [DOI] [PubMed] [Google Scholar]
- Nelson K. J., Day A. E., Zeng B. B., King S. B. & Poole L. B. Isotope-coded, iodoacetamide-based reagent to determine individual cysteine pK(a) values by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Anal. Biochem. 375, 187–195 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. & Kaltashov I. A. A new strategy of using O18-labeled iodoacetic acid for mass spectrometry-based protein quantitation. J. Am. Soc. Mass Spectr. 23, 1293–1297 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieser H. & Koehler P. Is the calculation of the gluten content by multiplying the prolamin content by a factor of 2 valid? Eur. Food Res. Technol. 229, 9–13 (2009). [Google Scholar]
- Chen Y., Kwon S. W., Kim S. C. & Zhao Y. Integrated approach for manual evaluation of peptides identified by searching protein sequence databases with tandem mass spectra. J. Proteome Res. 4, 998–1005 (2005). [DOI] [PubMed] [Google Scholar]