Abstract
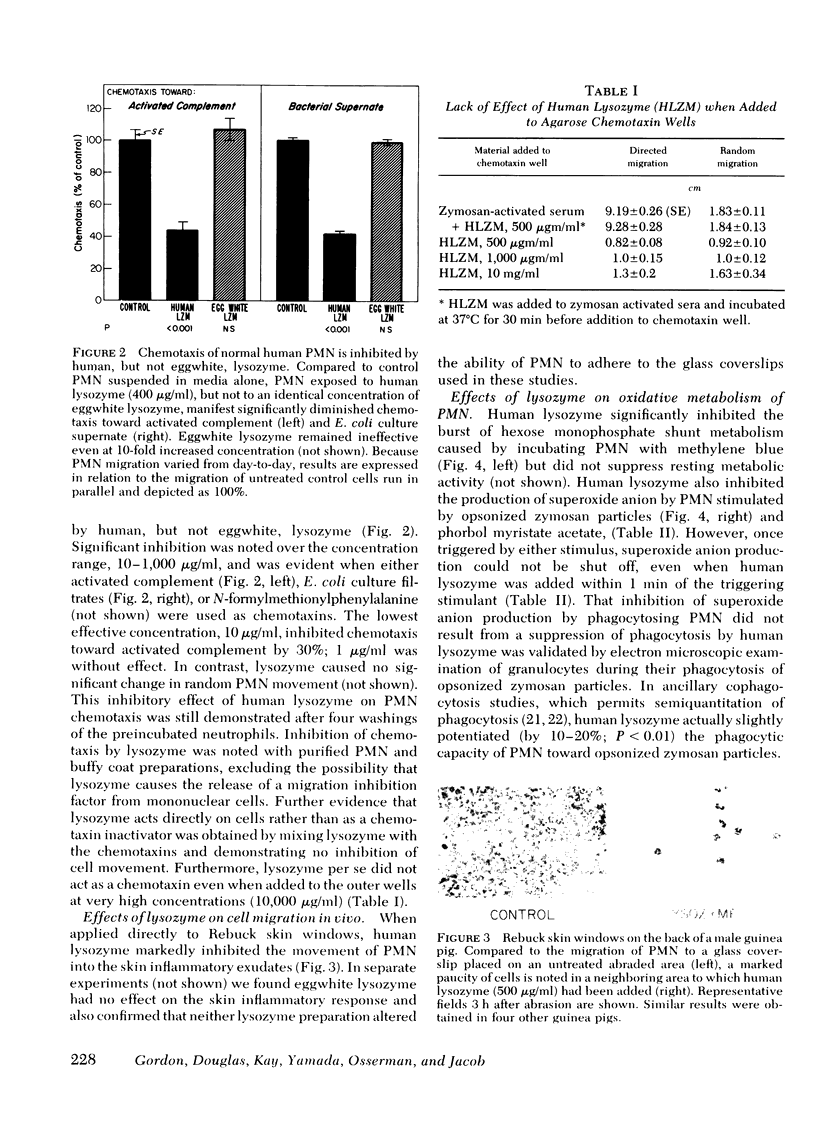
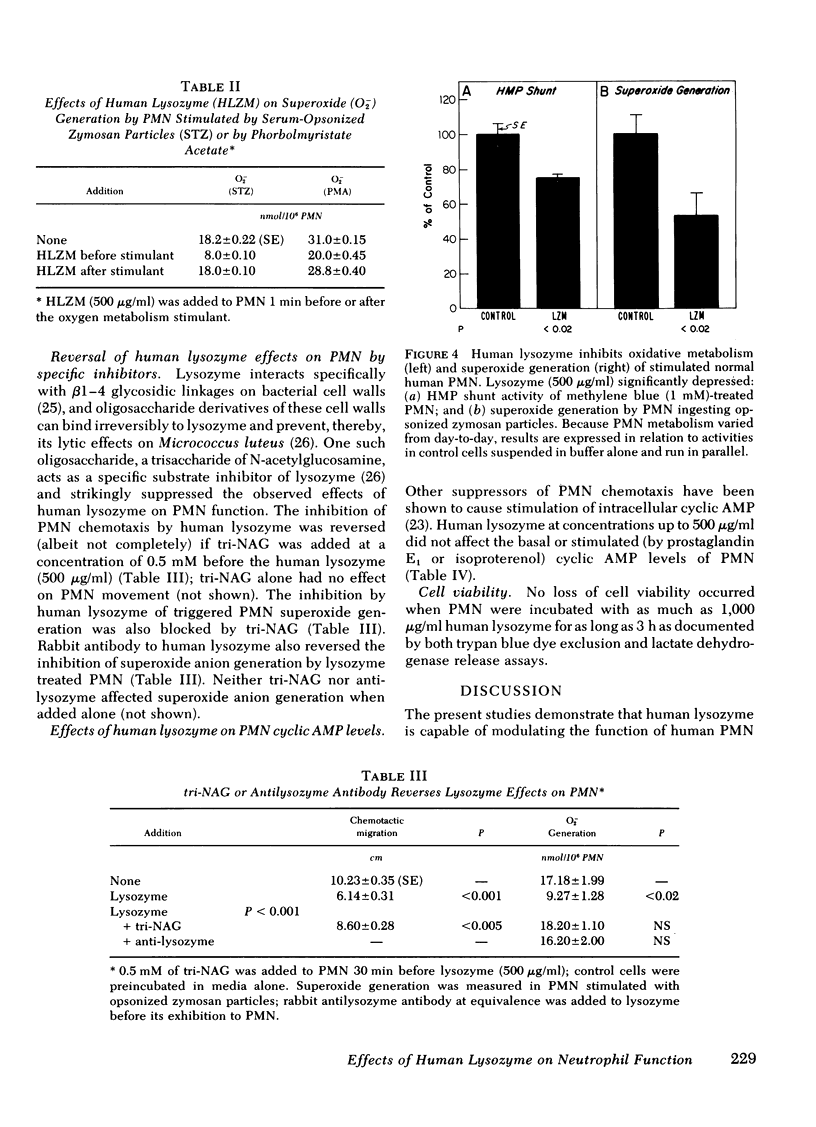
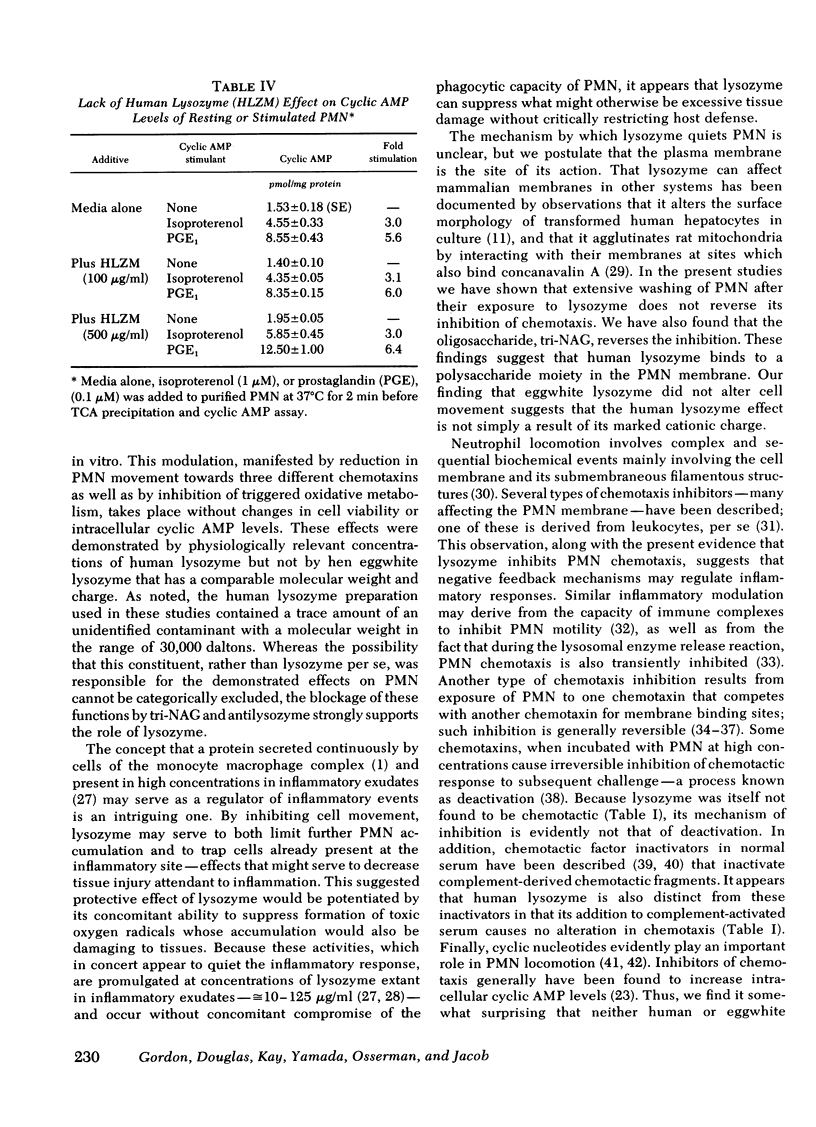
Host responses to infectious organisms should be modulated so that tissue-damaging products of inflammatory cells do not produce excessive destruction of normal tissue. Lysozyme, which is continuously secreted by monocytes, which, in turn, migrate relatively late to inflammatory areas, was found to significantly dampen several responses of neutrophils to inflammatory stimulants. Thus, human lysozyme obtained and purified from the urine of patients with monocytic leukemia (but not its structurally similar and comparably cationic analogue, eggwhite lysozyme) depresses chemotaxis of normal neutrophils to activated complement, bacterial supernate, and N-formylmethionyl-phenylalanine. In addition, human (but not eggwhite) lysozyme depresses oxidative metabolism (hexose monophosphate shunt activity) and superoxide generation of neutrophils. The specificity of the suppressive effects was indicated by inhibition studies with rabbit antihuman lysozyme antibody, and with the trisaccharide of N-acetylglucosamine, a specific inhibitor of lysozyme. The results suggest that lysozyme, a product of inflammatory cells themselves, may function in a negative feedback system to modulate the inflammatory response.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berenberg J. L., Ward P. A. Chemotactic factor inactivator in normal human serum. J Clin Invest. 1973 May;52(5):1200–1206. doi: 10.1172/JCI107287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozna J. P., Senior R. M., Kreutzer D. L., Ward P. A. Chemotactic factor inactivators of human granulocytes. J Clin Invest. 1977 Dec;60(6):1280–1288. doi: 10.1172/JCI108887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannarozzi N. A., Malawista S. E. Phagocytosis by human blood leukocytes measured by the uptake of 131I-labeled human serum albumin: inhibitory and stimulatory effects of cytochalasin B. Yale J Biol Med. 1973 Jun;46(3):177–189. [PMC free article] [PubMed] [Google Scholar]
- Chang Y. H. Studies on phagocytosis. I. Uptake of radio-iodinated (131-I) human serum albumin as a measure of the degree of phagocytosis in vitro. Exp Cell Res. 1969 Jan;54(1):42–48. doi: 10.1016/0014-4827(69)90290-0. [DOI] [PubMed] [Google Scholar]
- Chipman D. M., Sharon N. Mechanism of lysozyme action. Science. 1969 Aug 1;165(3892):454–465. doi: 10.1126/science.165.3892.454. [DOI] [PubMed] [Google Scholar]
- Estensen R. D., Hill H. R., Quie P. G., Gogan N., Goldberg N. D. Cyclic GMP and cell movement. Nature. 1973 Oct 26;245(5426):458–460. doi: 10.1038/245458a0. [DOI] [PubMed] [Google Scholar]
- Glick A. D., Ranhand J. M., Cole R. M. Degradation of group A streptococcal cell walls by egg-white lysozyme and human lysosomal enzymes. Infect Immun. 1972 Sep;6(3):403–413. doi: 10.1128/iai.6.3.403-413.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetzl E. J., Austen K. F. A neutrophil-immobilizing factor derived from human leukocytes. I. Generation and partial characterization. J Exp Med. 1972 Dec 1;136(6):1564–1580. doi: 10.1084/jem.136.6.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. M., Roos D., Kaplan H. B., Weissmann G. Complement and immunoglobulins stimulate superoxide production by human leukocytes independently of phagocytosis. J Clin Invest. 1975 Nov;56(5):1155–1163. doi: 10.1172/JCI108191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S., Todd J., Cohn Z. A. In vitro synthesis and secretion of lysozyme by mononuclear phagocytes. J Exp Med. 1974 May 1;139(5):1228–1248. doi: 10.1084/jem.139.5.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch G. E., Nichols W. K., Hill H. R. Cyclic nucleotide changes in human neutrophils induced by chemoattractants and chemotactic modulators. J Immunol. 1977 Aug;119(2):450–456. [PubMed] [Google Scholar]
- Henson P. M. Membrane receptors on neutrophils. Immunol Commun. 1976;5(9):757–774. doi: 10.3109/08820137609047618. [DOI] [PubMed] [Google Scholar]
- Hill H. R., Estensen R. D., Quie P. G., Hogan N. A., Goldberg N. D. Modulation of human neutrophil chemotactic responses by cyclic 3',5'-guanosine monophosphate and cyclic 3',5'-adenosine monophosphate. Metabolism. 1975 Mar;24(3):447–456. doi: 10.1016/0026-0495(75)90124-9. [DOI] [PubMed] [Google Scholar]
- JEANLOZ R. W., SHARON N., FLOWERS H. M. THE CHEMICAL STRUCTURE OF A DISACCHARIDE ISOLATED FROM MICROCOCCUS LYSODEIKTICUS CELL WALL. Biochem Biophys Res Commun. 1963 Sep 10;13:20–25. doi: 10.1016/0006-291x(63)90155-4. [DOI] [PubMed] [Google Scholar]
- Jensen R. S., Tew J. G., Donaldson D. M. Extracellular beta-lysin and muramidase in body fluids and inflammatory exudates. Proc Soc Exp Biol Med. 1967 Feb;124(2):545–549. doi: 10.3181/00379727-124-31785. [DOI] [PubMed] [Google Scholar]
- Kay N. E., Bumol T. F., Douglas S. D. Effect of phagocytosis and Fc receptor occupancy on complement-dependent neutrophil chemotaxis. J Lab Clin Med. 1978 May;91(5):850–856. [PubMed] [Google Scholar]
- Keusch G. T., Douglas S. D., Mildvan D., Hirschman S. Z. 14 C-glucose oxidation in whole blood: a clinical assay for phagocyte dysfunction. Infect Immun. 1972 Mar;5(3):414–415. doi: 10.1128/iai.5.3.414-415.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maderazo E. G., Ward P. A., Woronick C. L., Kubik J., DeGraff A. C., Jr Leukotactic dystunction in sarcoidosis. Ann Intern Med. 1976 Apr;84(4):414–419. doi: 10.7326/0003-4819-84-4-414. [DOI] [PubMed] [Google Scholar]
- Nelson R. D., Quie P. G., Simmons R. L. Chemotaxis under agarose: a new and simple method for measuring chemotaxis and spontaneous migration of human polymorphonuclear leukocytes and monocytes. J Immunol. 1975 Dec;115(6):1650–1656. [PubMed] [Google Scholar]
- Osserman E. F., Klockars M., Halper J., Fischel R. E. Effects of lysozyme on normal and transformed mammalian cells. Nature. 1973 Jun 8;243(5406):331–335. doi: 10.1038/243331a0. [DOI] [PubMed] [Google Scholar]
- Osserman E. F., Lawlor D. P. Serum and urinary lysozyme (muramidase) in monocytic and monomyelocytic leukemia. J Exp Med. 1966 Nov 1;124(5):921–952. doi: 10.1084/jem.124.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REBUCK J. W., CROWLEY J. H. A method of studying leukocytic functions in vivo. Ann N Y Acad Sci. 1955 Mar 24;59(5):757–805. doi: 10.1111/j.1749-6632.1955.tb45983.x. [DOI] [PubMed] [Google Scholar]
- Schiffmann E., Corcoran B. A., Wahl S. M. N-formylmethionyl peptides as chemoattractants for leucocytes. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1059–1062. doi: 10.1073/pnas.72.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secemski I. I., Lehrer S. S., Lienhard G. E. A transition state analog for lysozyme. J Biol Chem. 1972 Aug 10;247(15):4740–4748. [PubMed] [Google Scholar]
- Senn H. J., Chu B., O'Malley J., Holland J. F. Experiemental and clinical studies on muramidase (lysozyme). I. Muramidase activity of normal human blood cells and inflammatory exudates. Acta Haematol. 1970;44(2):65–77. doi: 10.1159/000208664. [DOI] [PubMed] [Google Scholar]
- Showell H. J., Freer R. J., Zigmond S. H., Schiffmann E., Aswanikumar S., Corcoran B., Becker E. L. The structure-activity relations of synthetic peptides as chemotactic factors and inducers of lysosomal secretion for neutrophils. J Exp Med. 1976 May 1;143(5):1154–1169. doi: 10.1084/jem.143.5.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P. A., Becker E. L. The deactivation of rabbit neutrophils by chemotactic factor and the nature of the activatable esterase. J Exp Med. 1968 Apr 1;127(4):693–709. doi: 10.1084/jem.127.4.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P. A. Chemotoxis of mononuclear cells. J Exp Med. 1968 Nov 1;128(5):1201–1221. doi: 10.1084/jem.128.5.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P. A., Lepow I. H., Newman L. J. Bacterial factors chemotactic for polymorphonuclear leukocytes. Am J Pathol. 1968 Apr;52(4):725–736. [PMC free article] [PubMed] [Google Scholar]
- Williams L. T., Snyderman R., Pike M. C., Lefkowitz R. J. Specific receptor sites for chemotactic peptides on human polymorphonuclear leukocytes. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1204–1208. doi: 10.1073/pnas.74.3.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond S. H. Chemotaxis by polymorphonuclear leukocytes. J Cell Biol. 1978 May;77(2):269–287. doi: 10.1083/jcb.77.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]





