Abstract
To explore the role of the human intestine as a source of apolipoproteins, we have studied intestinal lipoproteins and apoprotein secretion in two subjects with chyluria (mesenteric lymphatic—urinary fistulae). After oral corn oil, apolipoprotein A-I (apoA-I) and apolipoprotein A-II (apoA-II) output in urine increased in parallel to urinary triglyceride. One subject, on two occasions, after 40 g of corn oil, excreted 8.4 and 8.6 g of triglyceride together with 196 and 199 mg apoA-I and on one occasion, 56 mg apoA-II. The other subject, after 40 g corn oil, excreted 0.3 g triglyceride and 17.5 mg apoA-I, and, after 100 g of corn oil, excreted 44.8 mg apoA-I and 5.8 mg apoA-II. 14.5±2.1% of apoA-I and 17.7±4.3% of apoA-II in chylous urine was in the d < 1.006 fraction (chylomicrons and very low density lipoprotein). Calculations based on the amount of apoA-I and apoA-II excreted on triglyceride-rich lipoproteins revealed that for these lipid loads, intestinal secretion could account for 50 and 33% of the calculated daily synthetic rate of apoA-I and apoA-II, respectively. Similarly, subject 2 excreted 48-70% and 14% of the calculated daily synthetic rate of apoA-I and apoA-II, respectively.
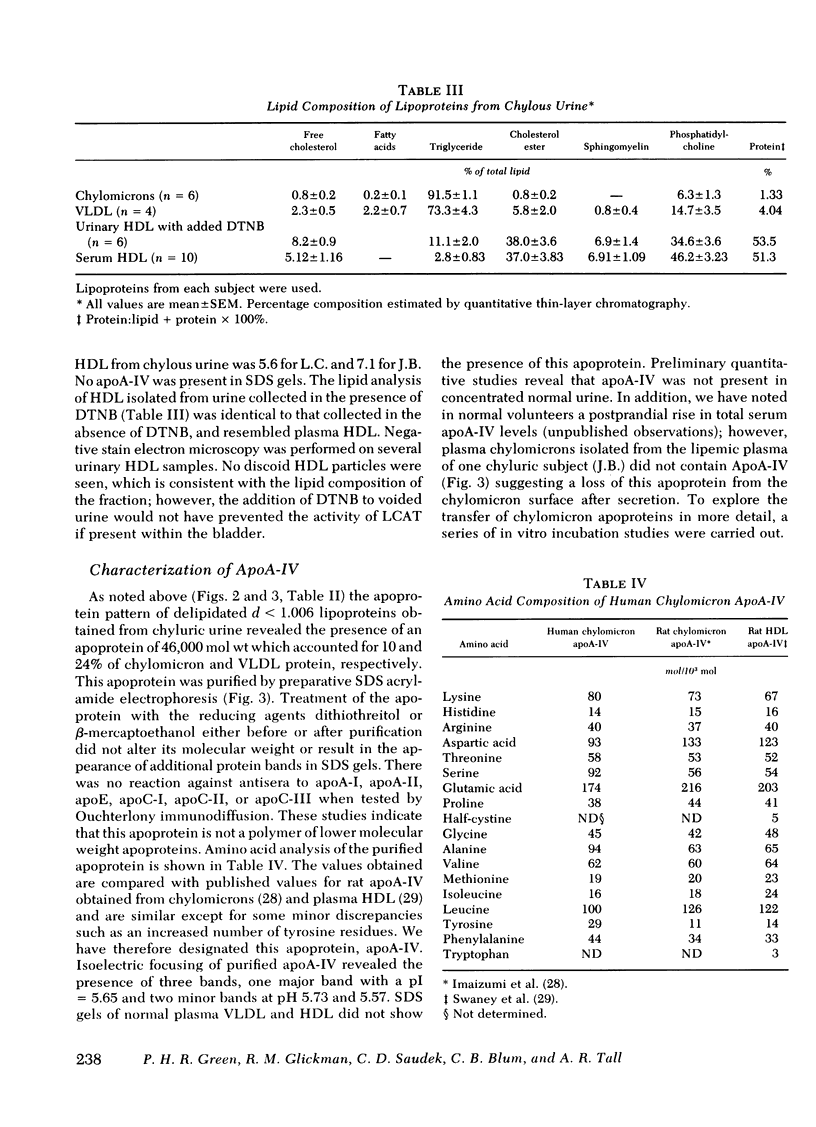
Chylous urine contained chylomicrons, very low density lipoproteins and high density lipoproteins, all of which contained apoA-I. Chylomicrons and very low density lipoproteins contained a previously unreported human apoprotein of 46,000 mol wt. We have called this apoprotein apoA-IV because of the similarity of its molecular weight and amino acid composition to rat apoA-IV. In sodium dodecyl sulfate gels, chylomicron apoproteins consisted of apoB 3.4±0.7%, apoA-IV 10.0±3.3%, apoE 4.4±0.3%, apoA-I 15.0±1.8%, and apoC and apoA-II 43.3±11.3%. Very low density lipoprotein contained more apoB and apoA-IV and less apoC than chylomicrons. Ouchterlony immunodiffusion of chylomicron apoproteins revealed the presence of apoC-I, apoC-II, and apoC-III. In contrast, plasma chylomicrons isolated during a nonchyluric phase revealed a markedly altered chylomicron apoprotein pattern when compared with urinary chylomicrons. The major apoproteins in plasma chylomicrons were apoB, apoE, and the C peptides: no apoA-I or apoA-IV were present in sodium dodecyl sulfate gels indicating that major changes in chylomicron apoproteins occur during chylomicron metabolism. When incubated in vitro with plasma, urinary chylomicrons lost apoA-I and apoA-IV and gained apoE and apoC. Loss of apoA-I and apoA-IV was dependent upon the concentration of high density lipoproteins in the incubation mixture.
These studies demonstrate that the human intestine secretes significant amounts of apoA-I and apoA-II during lipid absorption. Subsequent transfer of apoproteins from triglyceride-rich lipoproteins to other plasma lipoproteins may represent a mechanism whereby the intestine contributes to plasma apoprotein levels.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albers J. J., Wahl P. W., Cabana V. G., Hazzard W. R., Hoover J. J. Quantitation of apolipoprotein A-I of human plasma high density lipoprotein. Metabolism. 1976 Jun;25(6):633–644. doi: 10.1016/0026-0495(76)90060-3. [DOI] [PubMed] [Google Scholar]
- BLOMSTRAND R., THORN N. A., AHRENS E. H., Jr The absorption of fats, studied in a patient with chyluria. I. Clinical investigation. Am J Med. 1958 Jun;24(6):958–966. doi: 10.1016/0002-9343(58)90348-6. [DOI] [PubMed] [Google Scholar]
- Blum C. B., Levy R. I., Eisenberg S., Hall M., 3rd, Goebel R. H., Berman M. High density lipoprotein metabolism in man. J Clin Invest. 1977 Oct;60(4):795–807. doi: 10.1172/JCI108833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung M. C., Albers J. J. The measurement of apolipoprotein A-I and A-II levels in men and women by immunoassay. J Clin Invest. 1977 Jul;60(1):43–50. doi: 10.1172/JCI108767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing D. T. Photodensitometry in the thin-layer chromatographic analysis of neutral lipids. J Chromatogr. 1968 Nov 5;38(1):91–99. doi: 10.1016/0021-9673(68)85011-3. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Felker T. E., Fainaru M., Hamilton R. L., Havel R. J. Secretion of the arginine-rich and A-I apolipoproteins by the isolated perfused rat liver. J Lipid Res. 1977 Jul;18(4):465–473. [PubMed] [Google Scholar]
- Glickman R. M., Green P. H., Lees R. S., Lux S. E., Kilgore A. Immunofluorescence studies of apolipoprotein B in intestinal mucosa. Absence in abetalipoproteinemia. Gastroenterology. 1979 Feb;76(2):288–292. [PubMed] [Google Scholar]
- Glickman R. M., Green P. H., Lees R. S., Tall A. Apoprotein A-I synthesis in normal intestinal mucosa and in Tangier disease. N Engl J Med. 1978 Dec 28;299(26):1424–1427. doi: 10.1056/NEJM197812282992602. [DOI] [PubMed] [Google Scholar]
- Glickman R. M., Green P. H. The intestine as a source of apolipoprotein A1. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2569–2573. doi: 10.1073/pnas.74.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman R. M., Kirsch K. The apoproteins of various size classes of human chylous fluid lipoproteins. Biochim Biophys Acta. 1974 Nov 5;371(1):255–266. doi: 10.1016/0005-2795(74)90175-5. [DOI] [PubMed] [Google Scholar]
- Green P. H., Tall A. R., Glickman R. M. Rat intestine secretes discoid high density lipoprotein. J Clin Invest. 1978 Feb;61(2):528–534. doi: 10.1172/JCI108963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton R. L., Williams M. C., Fielding C. J., Havel R. J. Discoidal bilayer structure of nascent high density lipoproteins from perfused rat liver. J Clin Invest. 1976 Sep;58(3):667–680. doi: 10.1172/JCI108513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi K., Fainaru M., Havel R. J. Composition of proteins of mesenteric lymph chylomicrons in the rat and alterations produced upon exposure of chylomicrons to blood serum and serum proteins. J Lipid Res. 1978 Aug;19(6):712–722. [PubMed] [Google Scholar]
- Katz S. S., Shipley G. G., Small D. M. Physical chemistry of the lipids of human atherosclerotic lesions. Demonstration of a lesion intermediate between fatty streaks and advanced plaques. J Clin Invest. 1976 Jul;58(1):200–211. doi: 10.1172/JCI108450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostner G., Holasek A. Characterization and quantitation of the apolipoproteins from human chyle chylomicrons. Biochemistry. 1972 Mar 28;11(7):1217–1223. doi: 10.1021/bi00757a016. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laurell C. B. Quantitative estimation of proteins by electrophoresis in agarose gel containing antibodies. Anal Biochem. 1966 Apr;15(1):45–52. doi: 10.1016/0003-2697(66)90246-6. [DOI] [PubMed] [Google Scholar]
- Marsh J. B. Apoproteins of the lipoproteins in a nonrecirculating perfusate of rat liver. J Lipid Res. 1976 Jan;17(1):85–89. [PubMed] [Google Scholar]
- Ockner R. K., Hughes F. B., Isselbacher K. J. Very low density lipoproteins in intestinal lymph: origin, composition, and role in lipid transport in the fasting state. J Clin Invest. 1969 Nov;48(11):2079–2088. doi: 10.1172/JCI106174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ockner R. K., Hughes F. B., Isselbacher K. J. Very low density lipoproteins in intestinal lymph: role in triglyceride and cholesterol transport during fat absorption. J Clin Invest. 1969 Dec;48(12):2367–2373. doi: 10.1172/JCI106203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETERSON M. L. On the reesterification of fatty acids during absorption of fat: studies in patients with chyluria. Gastroenterology. 1963 Jun;44:774–786. [PubMed] [Google Scholar]
- Rachmilewitz D., Albers J. J., Saunders D. R., Fainaru M. Apoprotein synthesis by human duodenojejunal mucosa. Gastroenterology. 1978 Oct;75(4):677–682. [PubMed] [Google Scholar]
- Schaefer E. J., Jenkins L. L., Brewer H. B., Jr Human chylomicron apolipoprotein metabolism. Biochem Biophys Res Commun. 1978 Jan 30;80(2):405–412. doi: 10.1016/0006-291x(78)90691-5. [DOI] [PubMed] [Google Scholar]
- Schonfeld G., Bell E., Alpers D. H. Intestinal apoproteins during fat absorption. J Clin Invest. 1978 Jun;61(6):1539–1550. doi: 10.1172/JCI109074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd J., Packard C. J., Patsch J. R., Gotto A. M., Jr, Taunton O. D. Effects of dietary polyunsaturated and saturated fat on the properties of high density lipoproteins and the metabolism of apolipoprotein A-I. J Clin Invest. 1978 Jun;61(6):1582–1592. doi: 10.1172/JCI109078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore V. G., Shore B., Lewis S. B. Isolation and characterization of two threonine-poor apolipoproteins of human plasma high density lipoproteins. Biochemistry. 1978 May 30;17(11):2174–2179. doi: 10.1021/bi00604a023. [DOI] [PubMed] [Google Scholar]
- Swaney J. B., Reese H., Eder H. A. Polypeptide composition of rat high density lipoprotein: characterization by SDS-gel electrophoresis. Biochem Biophys Res Commun. 1974 Jul 24;59(2):513–519. doi: 10.1016/s0006-291x(74)80010-0. [DOI] [PubMed] [Google Scholar]
- Weisgraber K. H., Bersot T. P., Mahley R. W. Isolation and characterization of an apoprotein from the d less than 1.006 lipoproteins of human and canine lymph homologous with the rat A-IV apoprotein. Biochem Biophys Res Commun. 1978 Nov 14;85(1):287–292. doi: 10.1016/s0006-291x(78)80041-2. [DOI] [PubMed] [Google Scholar]
- Weisgraber K. H., Mahley R. W. Apoprotein (E--A-II) complex of human plasma lipoproteins. I. Characterization of this mixed disulfide and its identification in a high density lipoprotein subfraction. J Biol Chem. 1978 Sep 10;253(17):6281–6288. [PubMed] [Google Scholar]
- Windmueller H. G., Herbert P. N., Levy R. I. Biosynthesis of lymph and plasma lipoprotein apoproteins by isolated perfused rat liver and intestine. J Lipid Res. 1973 Mar;14(2):215–223. [PubMed] [Google Scholar]
- Windmueller H. G., Spaeth A. E. Fat transport and lymph and plasma lipoprotein biosynthesis by isolated intestine. J Lipid Res. 1972 Jan;13(1):92–105. [PubMed] [Google Scholar]
- Wu A. L., Windmueller H. G. Identification of circulating apolipoproteins synthesized by rat small intestine in vivo. J Biol Chem. 1978 Apr 25;253(8):2525–2528. [PubMed] [Google Scholar]





