Abstract
Objective:
The objective of this study was to determine which aspects of executive functions are most affected in behavioral variant frontotemporal dementia (bvFTD) and best differentiate this syndrome from Alzheimer disease (AD).
Methods:
We compared executive functions in 22 patients diagnosed with bvFTD, 26 with AD, and 31 neurologically healthy controls using a conceptually driven and comprehensive battery of executive function tests, the NIH EXAMINER battery (http://examiner.ucsf.edu).
Results:
The bvFTD and the AD patients were similarly impaired compared with controls on tests of working memory, category fluency, and attention, but the patients with bvFTD showed significantly more severe impairments than the patients with AD on tests of letter fluency, antisaccade accuracy, social decision-making, and social behavior. Discriminant function analysis with jackknifed cross-validation classified the bvFTD and AD patient groups with 73% accuracy.
Conclusions:
Executive function assessment can support bvFTD diagnosis when measures are carefully selected to emphasize frontally specific functions.
Alzheimer disease (AD) and behavioral variant frontotemporal dementia (bvFTD) are neurodegenerative diseases that affect executive functions.1,2 Executive functions support goal-oriented behavior and include working memory, inhibition, mental flexibility, fluency, self-monitoring, and organizing appropriate social behavior.3–5 It is unknown whether the executive impairments are the same in both disorders, or whether differences could assist with differential diagnosis.
According to a recent meta-analysis of 94 studies, the frequently used executive function tests do not reliably differentiate FTD and AD.6 Several of the studies included progressive aphasia patients with bvFTD, making the results difficult to interpret. Some studies have identified select measures of rule violation, poor planning, perseveration, letter fluency relative to category fluency, and organizing appropriate social behavior that are more impaired in bvFTD than AD.7–11
Patients with AD and bvFTD may exhibit different patterns of executive deficits arising from the distinct anatomical targets of each disease. In bvFTD, the ventromedial prefrontal cortex is targeted, it progresses into the lateral prefrontal cortex,12 and it attenuates connectivity with the frontoinsular cortex.13 In AD, the parietal lobes are severely targeted and connectivity with medial parieto-occipital regions is reduced; the frontal lobes are relatively spared, initially.14,15 Accordingly, we hypothesized that executive functions relying on frontoparietal networks would similarly impaired in bvFTD and AD, because they encompass the primary targets of each disease. We hypothesized that patients with bvFTD would be more impaired in executive functions with primarily frontal substrates that rely less critically on the parietal lobes.
METHODS
Subjects.
The NIH EXAMINER battery and the Mini-Mental State Examination (MMSE)16 were administered to 31 normal controls (NCs), 26 patients with AD, and 22 patients with bvFTD at the Mayo Medical Center or the University of California, San Francisco (UCSF) Memory and Aging Center. Patients who met probable research diagnostic criteria17,18 as determined by neurologic, cognitive, and functional evaluations via their participation in other research projects at Mayo or UCSF, and who scored >18 on the MMSE, were referred to the study. The diagnostic evaluations were conducted at a separate research visit and did not include any of the measures from the NIH EXAMINER.
Neurologically healthy controls underwent neurologic and cognitive screening to verify health status. Participants were excluded if they had a history of major psychiatric illness, current substance abuse disorder, ongoing cancer treatment, metabolic abnormalities, known HIV, major systemic medical illness, history of traumatic brain injury with >30 minutes’ loss of consciousness, seizure disorder, cortical stroke, or diagnosis of developmental learning disability. The MMSE cutoff for exclusion was ≤18 for patients and 26 for NCs. From an original sample of 52 NCs that included many individuals with a high level of education, we included only 31 in order to match them to the patients on education.
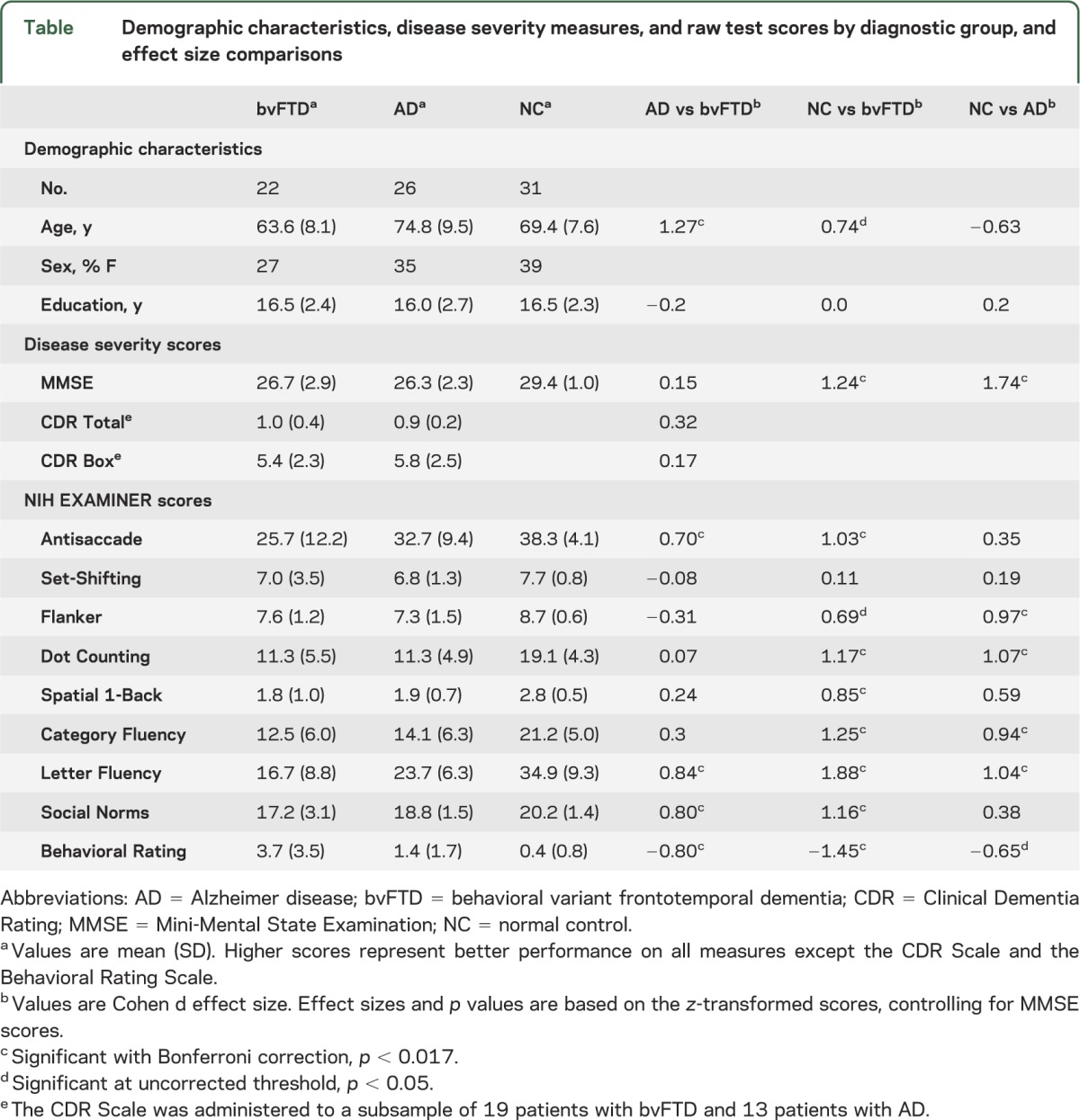
Demographic variables were compared using analysis of variance with Tukey post hoc tests and α = 0.05 (table). The patients with bvFTD were younger than those with AD and the controls, and the patients with AD were older than the controls. There were no group differences in sex or education. The patients with bvFTD and AD scored lower than the NCs on the MMSE, but did not differ from each other. The patients also scored similarly to each other on the Clinical Dementia Rating Scale, which was administered to a subsample of 13 patients with AD and 19 patients with bvFTD as a measure of disease severity.
Table.
Demographic characteristics, disease severity measures, and raw test scores by diagnostic group, and effect size comparisons
Executive function assessment.
Participants were administered the NIH EXAMINER battery in a quiet room and using a standard 15.4-in. Dell Latitude D830 laptop. Three measures of controlled attention (the Antisaccade Test, the Set-Shifting Test, and the Flanker Test), 2 measures of working memory (the Spatial 1-Back and the Dot Counting Test), 2 measures of verbal fluency (Letter Fluency and Category Fluency), and 2 measures of social behavior (the Social Norms Questionnaire and the Behavioral Rating Scale) were administered and are described briefly with missing data issues in appendix e-1 on the Neurology® Web site at www.neurology.org. Detailed descriptions of the tests and the forms for the Social Norms Questionnaire and the Behavioral Rating Scale are available at http://examiner.ucsf.edu.
Standard protocol approvals, registrations, and patient consents.
Written informed consent was obtained from each participant before testing and after a complete explanation of the specifics of the study. The study was approved by the UCSF and the Mayo Medical Center committees on human research.
Data analysis.
Statistical analyses were performed using PASW 17.0 for Windows (SPSS Inc., Chicago, IL). The NIH EXAMINER variables were analyzed for violations of normality and homogeneity of variance. Because of negative skew, the Antisaccade Test and the Behavioral Rating Scale were log transformed. The NIH EXAMINER variables were transformed into z scores relative to NC performance. Mean z scores for the groups on each task, therefore, reflect the degree of impairment compared with controls. Analyses of covariance were performed to determine group differences in the z-transformed EXAMINER scores. MMSE scores were used as a covariate to control for dementia severity. Pairwise comparisons with Bonferroni correction by analysis were used to evaluate differences among adjusted means; p values <0.017 for these comparisons were considered significant.
A discriminant function analysis was performed to determine how well patients with AD could be distinguished from patients with bvFTD based on their EXAMINER test scores. The variables that differentiated AD from bvFTD were included as predictors. Before analysis, univariate outliers were tested for using standard scores, multivariate outliers were tested for using Mahalanobis distance (α = 0.001), and homogeneity of the variance-covariance matrices was tested for using Box's M (α = 0.05). A jackknifed classification procedure was performed to predict each patient's diagnostic group using the functions derived from all cases other than that case, and with prior probabilities computed from sample sizes.
RESULTS
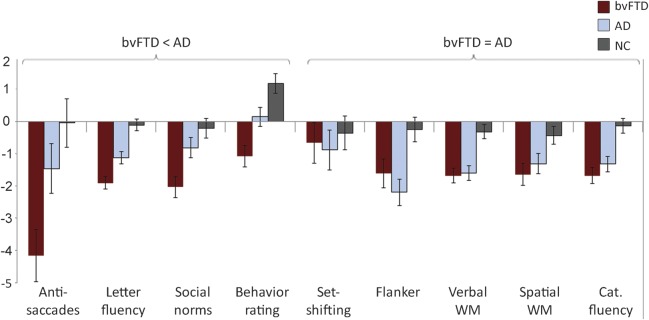
The raw mean scores and SDs of test performance by group are presented in the table. The estimated marginal means and standard errors after MMSE adjustment and z-score transformation are depicted in the figure, with low scores representing poor performance on all measures.
Figure. Estimated marginal means and standard errors of z-transformed scores after adjusting for MMSE scores.
Lower scores represent worse performance on all measures. AD = Alzheimer disease; bvFTD = behavioral variant frontotemporal dementia; cat. = category; MMSE = Mini-Mental State Examination; NC = normal control; WM = working memory.
The bvFTD and AD patients did not differ in their performance on the Flanker Test, the Dot Counting Test, the Spatial 1-Back Test, the Category Fluency Test, or the Set-Shifting Test. Compared with NCs, both bvFTD and AD groups showed impairment on the Dot Counting Test and the Category Fluency Test, the patients with AD were impaired on the Flanker Test, and the patients with bvFTD were impaired on the Spatial 1-Back Test. On the Set-Shifting Test, the patient and NC groups did not differ.
The patients with bvFTD were more impaired than the patients with AD and the NCs on the Antisaccade Test, the Letter Fluency Test, the Social Norms Questionnaire, and the Behavioral Rating Scale. On these tests, the AD group differed from NCs only on the Letter Fluency Test.
On the Behavioral Rating Scale, the bvFTD patients were scored as having more severe behaviors than the AD patients and the NCs. The patients with AD tended to exhibit more severe behaviors than the NCs, but this difference did not exceed Bonferroni correction. All 9 behaviors were rated as present in 25% or more of the patients with bvFTD (appendix e-1). In contrast, only 2 behaviors, perseveration and distractibility, were rated as present in 25% or more of patients with AD.
To determine whether the patients with AD would exhibit fewer impairments relative to an older NC group that was more closely matched on age, we reran the analyses of covariance with a subset of 21 closely age-matched controls. The same measures were significant and no additional measures reached significance.
Discriminant function analysis was performed to predict bvFTD vs AD diagnosis including the measures with significant patient group differences, that is, the Antisaccade Test, the Letter Fluency Test, the Social Norms Questionnaire, and the Behavioral Rating Scale. Two bvFTD cases and 2 AD cases were excluded from the analysis because of missing data. One univariate outlier was detected on the Antisaccade Test and was winsorized to one point more extreme than the next most extreme score. No multivariate outliers were detected and Box's M was not significant. The discriminant function was significant (χ2 = 11.29, df = 4, p = 0.02). The standardized canonical discriminant function coefficients were as follows: Letter Fluency 0.58, Behavioral Rating Scale 0.32, Social Norms Questionnaire 0.28, and Antisaccade 0.18. With the use of a jackknifed cross-validation procedure, 73% of the cases were correctly classified, including 79% of the patients with AD and 65% of the patients with bvFTD. A logistic regression analysis yielded similar results (75% of cases correctly classified).
Four of the 10 items on the Behavioral Rating Scale correspond to diagnostic criteria for bvFTD: socially inappropriate, lack of social/emotional engagement, motor stereotypies, and perseverative,17 so it is not surprising that patients with bvFTD were rated as having more severe behaviors on this scale than those with AD or NCs. We repeated the discriminant function analysis with this measure removed to determine whether the other measures provided useful classification information. The discriminant function was significant (χ2 = 9.11, df = 3, p = 0.03), and correctly classified 65% of the cases, including 71% of the patients with AD and 59% of the patients with bvFTD.
DISCUSSION
The purpose of this study was to investigate the pattern of executive function deficits in bvFTD vs AD with a comprehensive and conceptually based battery of executive function tests. The patients with bvFTD exhibited a pervasive pattern of executive dysfunction that included impairments in spatial and verbal working memory, letter and category fluency, antisaccade accuracy, social decision-making, and social behavior. The patients with AD exhibited a more circumscribed pattern of executive function impairments involving verbal working memory, letter fluency, category fluency, and controlled attention (Flanker). A comparison of the 2 patient groups revealed that antisaccade accuracy, letter fluency, social decision-making, and social behavior were significantly more impaired in bvFTD than in AD. Using a jackknifed cross-classification procedure, 73% of patients were classified correctly using these variables.
The widespread executive deficits observed in the bvFTD patient sample support the item in the new International Consensus Diagnostic Criteria for bvFTD that emphasizes executive function impairments.17 Integrity of the lateral prefrontal cortex is important for the cognitive aspects of executive functions, including verbal fluency,19 antisaccade accuracy,20,21 working memory,22,23 and controlled attention.24,25 Although integrity of both the lateral and ventromedial prefrontal cortex is important for social decision-making and behavior, the ventromedial aspects are most critical.26–28 Patients with bvFTD usually exhibit impairments in social behavior before cognitive aspects of executive functions, which probably reflects the spread of the disease from the ventromedial to dorsolateral prefrontal cortex.12 The presence of executive dysfunction without behavioral disturbances is not even sufficient for a possible bvFTD diagnosis.17 When we removed the Behavioral Rating Scale from the cross-validation, the classification based on antisaccade accuracy, letter fluency, and social decision-making was still significant. Its accuracy decreased from 73% to 65%, however, which highlights the importance of behavioral observations in addition to cognitive test performance when distinguishing these patients.
In contrast to the pervasive executive function deficits observed in the bvFTD patient sample, the patients with AD showed more circumscribed impairments on tests of verbal fluency, verbal working memory, and controlled attention. These tests are not frontally specific but rather require cognitive functions that depend heavily on parietal and temporal cortex,19,23,29 regions significantly affected in AD.15 For example, the AD and the bvFTD patients were similarly impaired on category fluency, but the AD patients were less impaired than the bvFTD patients on letter fluency. This relative pattern of letter-based and category-based verbal fluency replicates prior studies and is consistent with the idea that category fluency requires greater access to semantic stores and places more demands on temporal lobe function, whereas letter fluency places relatively more demands on frontal function.10,29–31 Similarly, verbal working memory tests such as the Dot Counting Test rely not only on frontally mediated updating processes, but also on a phonologic short-term store that is mediated by the inferior parietal and superior temporal cortices.23,32 Parietal-frontal networks underlie attentional control and are important for good performance on the Flanker paradigm.25 Although we expected both groups to be impaired on the Set-Shifting Test, neither was impaired after the MMSE adjustment. This lack of group differences may be attributable to the diffuse neuroanatomical substrates of set-shifting and to the performance variability in the patients.24 The patients with AD exhibited a trend for higher ratings on the Behavioral Rating Scale, specifically perseveration and distractibility, but no elevations on the other 7 items. In fact, none of the AD patients in our sample were rated to lack social engagement or to exhibit inappropriate social behavior, consistent with the preservation of social and emotional sensitivity typical of this disease.11
The MMSE was the best available measure of global cognitive functioning and it was included as a covariate in the group difference analyses. This task samples orientation, memory, visuospatial skills, and language skills and has been shown to decline with neurodegenerative disease progression. Because good MMSE performance relies more heavily on memory and language than executive functions, patients with bvFTD may be more functionally impaired relative to patients with AD at a given MMSE level. Using the MMSE as a covariate, however, allowed us to control for global cognition without removing the executive function variance of interest.
Although the diagnostic groups significantly differed in age, age was not included as a covariate in the analyses. Controlling for age would have removed disease-related, rather than age-related, variance. The bvFTD patients were the youngest group, and despite their young age, they exhibited more pervasive executive impairments than the AD patients. As such, their executive impairments cannot be attributed to age differences. The AD patients were the oldest group, which meant we might have overestimated the extent of their executive function impairments due to AD, but comparing them to a closely matched NC sample did not change the results.
In this study, we comprehensively evaluated executive functions in patients diagnosed with AD or bvFTD using the NIH EXAMINER, a new battery of tests developed by the National Institute of Neurological Disorders and Stroke. This conceptually driven test battery is designed to comprehensively and efficiently evaluate executive functions with an emphasis on controlled attention, working memory, fluency, and social behavior. Patients diagnosed with bvFTD showed pervasive executive dysfunction on this battery, and their deficits exceeded those of patients diagnosed with AD on measures of antisaccade accuracy, letter fluency, social decision-making, and social behavior. These 4 measures were able to accurately classify 73% of patients, suggesting that careful selection of executive function tests can assist in differential diagnosis. In particular, frontally specific executive function measures may be most useful in this regard. Although the patients diagnosed with AD did evidence executive function impairments, these impairments were more circumscribed and involved tests that emphasize nonfrontal as well as frontal functions, including verbal fluency, verbal working memory, and controlled attention.
Supplementary Material
ACKNOWLEDGMENT
The development of the NIH EXAMINER and data collection was supported by the National Institute of Neurological Disorders and Stroke (HHSN271200623661C), and additional support was provided by NIA K23AG037566, P01AG019724, P50AG023501, R01AG022983, 1R01AG032289, and R01AG031278, NIMH R25 MH 07154, and the Hellman Family Foundation. The authors thank the study participants for their time and efforts.
GLOSSARY
- AD
Alzheimer disease
- bvFTD
behavioral variant frontotemporal dementia
- MMSE
Mini-Mental State Examination
- NC
normal control
- UCSF
University of California, San Francisco
Footnotes
Editorial, page 2174
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
K.L. Possin contributed to study design. She was the primary investigator responsible for statistical analyses and interpretation, and for manuscript preparation. D. Feigenbaum contributed to study design, statistical analyses/interpretation, and manuscript drafting. K.P. Rankin contributed to study design and manuscript revision. G.E. Smith contributed to study design, data analysis, and manuscript revision. A.L. Boxer contributed to study design and manuscript revision. K. Wood contributed to analysis of the data and revising the manuscript, in addition to subject recruitment and data acquisition. S.M. Hanna contributed to analysis of the data, subject recruitment, and data acquisition. B.L. Miller contributed to study design, interpretation of analyses, and manuscript revision. J.H. Kramer oversaw all aspects of this project including study design, data interpretation, and manuscript revision.
STUDY FUNDING
This study was sponsored by the NIH (National Institute of Neurological Disorders and Stroke HHSN271200623661C; NIA K23AG037566, P01AG019724, P50AG023501, and R01AG031278; and NIMH R25 MH 07154).
DISCLOSURE
K.L. Possin receives support from NIA K23AG037566, the Hellman Family Foundation, and the Michael J. Fox Foundation. D. Feigenbaum reports no disclosures. K.P. Rankin receives support from NIA R01A-AG029577, R01A-AG00688, and 2P01-AG 019724-11, as well as from the Hillblom Foundation. G.E. Smith receives research support from NIH grants RR 024150, HD073062, and AG16574. He uses and bills for neuropsychological assessment in his clinical practice (25%). He receives honoraria for serving on the University of Wisconsin Alzheimer’s Disease Center external advisory board. A.L. Boxer has been a consultant for Plexikkon, Phloronol, Registrat-Mapi, EnVivo, Neurophage, and iPierian, receives research support from Allon Therapeutics, Bristol-Myers Squibb, EnVivo, Janssen, Forest, Pfizer, and Genentech, and is funded by NIH grants R01AG038791 and R01AG031278, the John Douglas French Foundation, Alzheimer's Drug Discovery Foundation, the Association for Frontotemporal Degeneration, the Silicon Valley Foundation, the Agouron Institute, and the Tau Research Consortium. K. Wood and S.M. Hanna report no disclosures. B.L. Miller receives grant support from the NIH/NIA and has nothing to disclose related to this article. Dr. Miller serves as a consultant for TauRx, Allon Therapeutics, Lilly USA LLC, and Siemens Medical Solutions. He has also received a research grant from Novartis. He is on the Board of Directors for the John Douglas French Foundation for Alzheimer's Research and for The Larry L. Hillblom Foundation. J.H. Kramer receives research support from NIH P50AG023501, R01AG022983, and R01AG032289. He receives honoraria for serving on the University of Indiana Alzheimer's Disease Center external advisory board. He receives royalties from the California Verbal Learning Test. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Schroeter ML, Vogt B, Frisch S, et al. Executive deficits are related to the inferior frontal junction in early dementia. Brain 2012;135(pt 1):201–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stopford CL, Thompson JC, Neary D, Richardson AM, Snowden JS. Working memory, attention, and executive function in Alzheimer's disease and frontotemporal dementia. Cortex 2012;48:429–446 [DOI] [PubMed] [Google Scholar]
- 3.Cummings J, Miller BL. Conceptual and clinical aspects of the frontal lobes. In: Miller BL, Cummings JL, editors. The Human Frontal Lobes. New York: The Guilford Press; 2007:12–24 [Google Scholar]
- 4.Glascher J, Adolphs R, Damasio H, et al. Lesion mapping of cognitive control and value-based decision making in the prefrontal cortex. Proc Natl Acad Sci USA 2012;109:14681–14686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gleichgerrcht E, Torralva T, Roca M, Manes F. Utility of an abbreviated version of the executive and social cognition battery in the detection of executive deficits in early behavioral variant frontotemporal dementia patients. J Int Neuropsychol Soc 2010;16:687–694 [DOI] [PubMed] [Google Scholar]
- 6.Hutchinson AD, Mathias JL. Neuropsychological deficits in frontotemporal dementia and Alzheimer's disease: a meta-analytic review. J Neurol Neurosurg Psychiatry 2007;78:917–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carey CL, Woods SP, Damon J, et al. Discriminant validity and neuroanatomical correlates of rule monitoring in frontotemporal dementia and Alzheimer's disease. Neuropsychologia 2008;46:1081–1087 [DOI] [PubMed] [Google Scholar]
- 8.Possin KL, Chester SK, Laluz V, et al. The frontal-anatomic specificity of design fluency repetitions and their diagnostic relevance for behavioral variant frontotemporal dementia. J Int Neuropsychol Soc 2012;18:834–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson JC, Stopford CL, Snowden JS, Neary D. Qualitative neuropsychological performance characteristics in frontotemporal dementia and Alzheimer's disease. J Neurol Neurosurg Psychiatry 2005;76:920–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rascovsky K, Salmon DP, Hansen LA, Thal LJ, Galasko D. Disparate letter and semantic category fluency deficits in autopsy-confirmed frontotemporal dementia and Alzheimer's disease. Neuropsychology 2007;21:20–30 [DOI] [PubMed] [Google Scholar]
- 11.Shany-Ur T, Rankin KP. Personality and social cognition in neurodegenerative disease. Curr Opin Neurol 2012;24:550–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seeley WW, Crawford R, Rascovsky K, et al. Frontal paralimbic network atrophy in very mild behavioral variant frontotemporal dementia. Arch Neurol 2008;65:249–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou J, Greicius MD, Gennatas ED, et al. Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer's disease. Brain 2010;133(pt 5):1352–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du AT, Schuff N, Kramer JH, et al. Different regional patterns of cortical thinning in Alzheimer's disease and frontotemporal dementia. Brain 2007;130(pt 4):1159–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rabinovici GD, Seeley WW, Kim EJ, et al. Distinct MRI atrophy patterns in autopsy-proven Alzheimer's disease and frontotemporal lobar degeneration. Am J Alzheimers Dis Other Demen 2007;22:474–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198 [DOI] [PubMed] [Google Scholar]
- 17.Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011;134(pt 9):2456–2477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging–Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:263–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robinson G, Shallice T, Bozzali M, Cipolotti L. The differing roles of the frontal cortex in fluency tests. Brain 2012;135(pt 7):2202–2214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boxer AL, Garbutt S, Rankin KP, et al. Medial versus lateral frontal lobe contributions to voluntary saccade control as revealed by the study of patients with frontal lobe degeneration. J Neurosci 2006;26:6354–6363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mirsky JB, Heuer HW, Jafari A, et al. Anti-saccade performance predicts executive function and brain structure in normal elders. Cogn Behav Neurol 2011;24:50–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qi XL, Katsuki F, Meyer T, et al. Comparison of neural activity related to working memory in primate dorsolateral prefrontal and posterior parietal cortex. Front Syst Neurosci 2010;4:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D'Esposito M. From cognitive to neural models of working memory. Philos Trans R Soc Lond B Biol Sci 2007;362:761–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pa J, Possin KL, Wilson SM, et al. Gray matter correlates of set-shifting among neurodegenerative disease, mild cognitive impairment, and healthy older adults. J Int Neuropsychol Soc 2010;16:640–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luks TL, Oliveira M, Possin KL, et al. Atrophy in two attention networks is associated with performance on a Flanker task in neurodegenerative disease. Neuropsychologia 2010;48:165–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ibanez A, Manes F. Contextual social cognition and the behavioral variant of frontotemporal dementia. Neurology 2012;78:1354–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog Neurobiol 2004;72:341–372 [DOI] [PubMed] [Google Scholar]
- 28.Chang SW, Gariépy JF, Platt ML. Neuronal reference frames for social decisions in primate frontal cortex. Nat Neurosci 2013;16:243–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baldo JV, Schwartz S, Wilkins D, Dronkers NF. Role of frontal versus temporal cortex in verbal fluency as revealed by voxel-based lesion symptom mapping. J Int Neuropsychol Soc 2006;12:896–900 [DOI] [PubMed] [Google Scholar]
- 30.Libon DJ, Xie SX, Wang X, et al. Neuropsychological decline in frontotemporal lobar degeneration: a longitudinal analysis. Neuropsychology 2009;23:337–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gourovitch ML, Kirkby BS, Goldberg TE, et al. A comparison of rCBF patterns during letter and semantic fluency. Neuropsychology 2000;14:353–360 [DOI] [PubMed] [Google Scholar]
- 32.Buchsbaum BR, Olsen RK, Koch P, Berman KF. Human dorsal and ventral auditory streams subserve rehearsal-based and echoic processes during verbal working memory. Neuron 2005;48:687–697 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.