Abstract
Sea ice, a characteristic feature of polar waters, is home to diverse microbial communities. Sea-ice picoeukaryotes (unicellular eukaryotes with cell size <3 μm) have received little attention compared with diatoms that dominate the spring bloom in Arctic first-year sea ice. Here, we investigated the abundance of all picoeukaryotes, and of 11 groups (chlorophytes, cryptophytes, bolidophytes, haptophytes, Pavlovaphyceae, Phaeocystis spp., pedinellales, stramenopiles groups MAST-1, MAST-2 and MAST-6 and Syndiniales Group II) at 13 first-year sea-ice stations localized in Barrow Strait and in the vicinity of Cornwallis Island, Canadian Arctic Archipelago. We applied Catalyzed Reporter Deposition–Fluorescence In Situ Hybridization to identify selected groups at a single cell level. Pavlovaphyceae and stramenopiles from groups MAST-2 and MAST-6 were for the first time reported from sea ice. Total numbers of picoeukaryotes were significantly higher in the vicinity of Cornwallis Island than in Barrow Strait. Similar trend was observed for all the groups except for haptophytes. Chlorophytes and cryptophytes were the dominant plastidic, and MAST-2 most numerous aplastidic of all the groups investigated. Numbers of total picoeukaryotes, chlorophytes and MAST-2 stramenopiles were positively correlated with the thickness of snow cover. All studied algal and MAST groups fed on bacteria. Presence of picoeukaryotes from various trophic groups (mixotrophs, phagotrophic and parasitic heterotrophs) indicates the diverse ecological roles picoeukaryotes have in sea ice. Yet, >50% of total sea-ice picoeukaryote cells remained unidentified, highlighting the need for further study of functional and phylogenetic sea-ice diversity, to elucidate the risks posed by ongoing Arctic changes.
Keywords: first-year sea ice, picoeukaryotes, chlorophytes, cryptophytes, stramenopiles, Syndiniales Group II
Introduction
Sea ice is the key feature that strongly influences the abiotic and biotic components of Arctic marine ecosystems. Presence of the sea-ice cover and overlying snow drastically reduces transmission of photosynthetic active radiance to the water column (Perovich et al., 1993; Mundy et al., 2007), which at times limits phytoplankton production (Fortier et al., 2002; Glud et al., 2007). On the other hand, sea ice provides a unique habitat for diverse and highly productive microbial communities (Gradinger, 2009; Deming, 2010). Ice algae form the base of the polar food web before the melting of sea ice (for example, (Conover et al., 1986; Werner, 1997), as ice-algal fatty acids are essential for the development of key Arctic zooplankton species (Soreide et al., 2010).
The rapid and dramatic changes taking place in the Arctic cryosphere include a decline in multi-year ice extent, with an estimated pan-Arctic monthly averaged ice volume loss of about 70% in September 2012 (Polar Science Center). Moreover, the ice-covered season is shortening due to delayed ice formation and advanced melt (Polyakov et al., 2012). A recent ice–ocean regional model predicts spring ice breakup to advance by as much as 2–5 weeks in the Canadian Arctic Archipelago (Sou and Flato, 2009). This may pose a particular threat to the Arctic ecosystem, as the Archipelago is the region where the highest ice-algal biomasses are reported in the Arctic, associated with strong mixing and replenishment of nutrients at the ice-water interface (Cota et al., 1987; Michel et al., 2006). An exhaustive review of dominant physical and biological processes in the Canadian Arctic Archipelago and recent changes in this area can be found in Michel et al. (2006).
In the Arctic, sea-ice microbial biomass is concentrated in the bottom section of first-year sea ice (Laurion et al., 1995). Pennate diatoms are major component of the spring ice-algal bloom. Other unicellular eukaryotes contribute substantially to protists' biomass in bottom-ice communities in winter, but they typically constitute only a minor fraction of the biomass during the spring bloom (Riedel et al., 2008; Niemi et al., 2011). In result, they have received substantially less attention than sea-ice diatoms, and their composition and role are largely understudied. The phylogenetic diversity of pico- (cell size <3 μm) and nano- (cells size 3–20 μm) eukarytoes in the sea ice has been studied only recently (Eddie et al., 2010; Bachy et al., 2011; Majaneva et al., 2012; Comeau et al., 2013), but reliable identification and enumeration is still lacking. Knowledge on the distribution and abundance of sea-ice protists is essential to understand and predict possible changes in microbial communities that will have profound impacts on Arctic marine food webs (Davidson et al., 2010). Although genomic sequencing provides insights into processes, it is also essential to gain information on the abundance and community structure of picoeukaryotes. Hybridization with fluorescently labeled specific oligonucleotide probes is an invaluable tool for reliable detection and enumeration of morphologically undistinguishable microbial phylotypes in environmental samples (Amann and Fuchs, 2008).
The aim of this study was to determine the community structure of picoeukaryotes in first-year sea ice in the Canadian Arctic Archipelago during the spring period, and to elucidate environmental factors affecting their distribution. We applied Catalyzed Reporter Deposition–Fluorescence In Situ Hybridization (CARD–FISH) technique to detect and enumerate 12 groups of flagellates, including groups that so far have not been reported from the sea ice.
Materials and methods
Samples collection
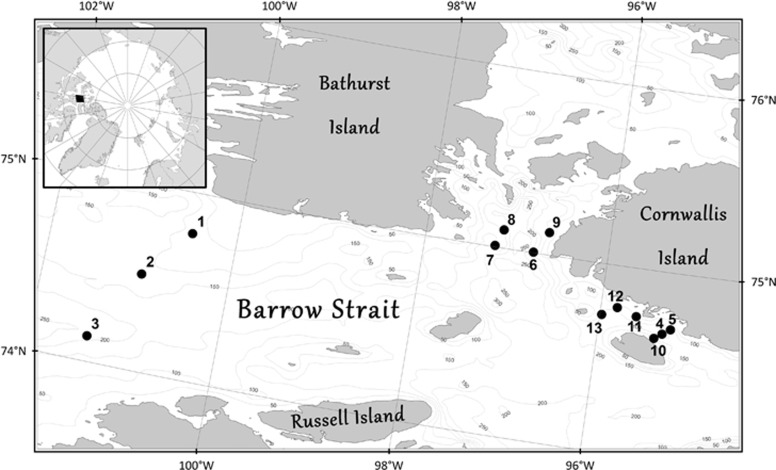
The sampling was carried out from 9–17 May 2010 in Canadian Arctic Archipelago. A total of 13 first-year sea-ice stations (three stations in Barrow Strait and 10 in the vicinity of Cornwallis Island: Resolute Passage and McDougall Sound) were visited by snowmobile or helicopter, and sampled for biochemical measurements in bottom ice (Figure 1). Routine physical measurements included snow and ice thickness measurements at the coring sites. Because of technical obstacles, we could not routinely measured under-ice photosynthetic active radiance radiation.
Figure 1.
Location of the sampling stations in Barrow Strait (stations 1–3), and in the vicinity of Cornwallis Island (stations 4–13), Canadian Arctic Archipelago in May 2010. The inset shows the location of the sampling area in the Canadian High Arctic (black rectangle). Isobaths (in meters) are shown.
At each station five to seven cores were collected using a manual ice corer (Mark II coring system, 9 cm internal diameter, Kovacs Enterprises, Lebanon, NH, USA). The bottom 3 cm of each core were immediately cut off with a stainless steel saw. Three of the cores collected at a station were processed for biological analyses: They were melted together in a dark isothermal container upon the addition of 0.22 μm-filtered surface seawater collected at the time of sampling (dilution factor: 3.7–6 times), to reduce osmotic shock of sea-ice microorganisms (Bates and Cota, 1986). The remaining two to four cores were pooled in sterile Whirlpack bags, slowly (24–36 h) melted without addition of seawater, and processed for nutrient analysis.
Chlorophyll a, nutrients and bacterial numbers
Duplicate subsamples (20–50 ml) were filtered onto Whatman GF/F (GE Healthcare, Little Chalfont, UK) glass–fiber filters (nominal pore size 0.7 μm; referred to as total Chl a) or onto 5 μm Nuclepore polycarbonate membrane filters (referred to as chl a>5 μm; used to approximate biomass of sea-ice algae), extracted during 24 h in 90% acetone at 5 °C in the dark. Chl a concentrations were determined on a 10-AU Turner Designs (Sunnyvale, CA, USA) fluorometer calibrated with chl a extract from Anacystic nidulans (Sigma, Oakville, ON, Canada) (Parsons et al., 1984). Chl a>5 μm fraction excluded all picoeukaryotes (cell size <3 μm), and was, therefore, used for statistical analysis.
Samples for nutrients analysis (NO3+NO2, NO2, PO4 and SiOH4) were filtered through precombusted (450 °C during 24 h) Whatman GF/F filters and immediately frozen in liquid nitrogen. They were analyzed on a Seal's Autoanalyzer 3 (NO3+NO2, NO2, PO4; Mequon, WI, USA) and on a Technicon II Autoanalyzer (SiOH4; Sydney, NSW, Australia).
Samples for bacterial numbers were prefiltered through 40 μm plankton net, preserved with glutaraldehyde (final concentration 0.1%) and frozen in liquid nitrogen. They were thawed in a 30 °C water bath and stained with SYBR-Green I in tris-EDTA buffer (10 mℳTris, 1 mℳEDTA; Sigma) (Belzile et al., 2008, Lebaron et al., 1998). After cooling to ambient temperature, subsamples were analyzed with a FACSort flow cytometer (Becton and Dickinson, San Jose, CA, USA) equipped with an air-cooled argon laser (15 mW). The excitation wavelength was set at 488 nm to provide optimum excitation wavelength for SYBR-Green I 495 nm (Marie et al., 1997).
Picoeukaryotes community composition
Aliquotes of 50–100 ml were prefiltered through 20 μm plankton net, and fixed with alkaline Lugol's solution, formalin (final concentration 2%) and 3% sodium thiosulphate (Sherr et al., 1989). Samples were filtered onto polycarbonate membrane filters (pore size 0.8 μm, diameter 25 mm, Millipore, Billerica, MA, USA), washed with sterile deionized water, air-dried and stored at −20 °C. CARD–FISH followed the procedure by Pernthaler et al. (2004), modified as described in Piwosz and Pernthaler (2010). Permeabilization step by enzymatic digestion was omitted. Filters were embedded in 0.1% agarose, and incubated 20 min in 0.01 M HCl. Filters were cut into 12 sections, which were hybridized at 35 °C for 3 h, and washed at 37 °C for 30 min. The list of 12 applied oligonucleotide probes and hybridization conditions are given in Table 1, and probe sequences and coverage in Supplementary Table S1. The numbers of MAST-1 stramenopiles were estimated with all three probes (NS1A, NS1B and NS1C) mixed together. Amplification with tyramides (Sigma) labeled with carboxyfluorescein (Molecular Probes, Invitrogen, Eugene, OR, USA) was performed at 37 °C for 30 min. The hybridized samples were mounted in glycerol medium containing 4′,6-diamidino-2-phenylindole dihydrochloride (1 μg ml−1) to counterstain the cells. Preparations were stored at −20 °C. They were examined under × 1000 magnification by epifluorescence microscopy (Olympus BX 50) under green/blue excitation. At least 400 hybridized cells were counted for the general eukaryotic probe Euk 516, and 100 cells were counted with other probes. Whenever these numbers could not be reached due to the low abundance of hybridized cells, the complete filter section was counted. Cells size was constantly monitored with an eyepiece New Porton measuring graticule NG12, and only cells <3 μm were counted. Coverage with specific probes is given in relation to the cell numbers obtained with the Euk516 probe.
Table 1. List of probes used in this study.
| Probe name | Target group | Formamide in hybridization buffer (%) | Reference |
|---|---|---|---|
| Euk516 | All eukaryotes | 20 | Amann et al. (1990) |
| Chlo02 | Chlorophyta | 40 | Simon et al. (2000) |
| CryptB | Cryptophyceae | 50 | Metfies and Medlin (2007) |
| Bolido02 | Bolidophyceae | 40 | Guillou et al. (1999b) |
| Prym02 | Haptophyta | 40 | Simon et al. (2000) |
| Pavlova01 | Pavlovaphyceae | 40 | Eller et al. (2007) |
| Phaeo02 | Phaeocystis spp. | 50 | Zingone et al. (1999) |
| Ped675 | Pedinellales | 45 | Piwosz and Pernthaler (2010) |
| NS1A, B and C | MAST-1 stramenopiles | 45 | Massana et al. (2006) |
| NS2 | MAST-2 stramenopiles | 45 | Massana et al. (2006) |
| MAST-6 | MAST-6 stramenopiles | 35 | Piwosz and Pernthaler (2010) |
| Alv01 | Syndiniales Group II | 50 | Chambouvet et al. (2008) |
Optimal hybridization conditions were maintained by varying the concentration of formamide in hybridization buffer at the constant temperature (37 °C). Formamide concentration was increased for all probes, except for Ped675 and MAST-6, compared with the original methodology due to lower hybridization temperature applied in this study (+20% formamide for every 10 °C decrease in hybridization temperature).
The food vacuole content was examined for the most numerous groups and stations: total eukaryotes (all stations), chlorophytes (all stations), cryptophytes (all stations), bolidophytes (stations 8, 9), haptophytes (stations 1–3), Pavlovaphyceae (station 1), stramenopiles MAST-2 (stations 4–7, 10 and 11) and MAST-6 (station 5). 25–50 hybridized cells were examined by epifluorescence microscopy (AxioImager.M2, Carl Zeiss, Oberkochen, Germany). The presence of bacteria in food vacuoles was assessed based on their 4′,6-diamidino-2-phenylindole dihydrochloride signal at ultraviolet excitation. Prey items were counted only if they were in the focal plane of a flagellate cell to ensure that they had not settled onto the surface of the examined cell or did not shine through from underneath (Piwosz and Pernthaler, 2010).
All variables measured in ice cores were corrected for dilution when applicable. Numbers of bacterial and picoeukarote cells are presented in areal (cells m−2) and volume (cells m−3) concentrations to facilitate comparison with available literature.
Statistical procedures
Mann–Whitney U-test was used to compare measured variables between stations from Barrow Strait and in the vicinity of Cornwallis Island. Correlations between the groups of picoeukaryotes and environmental variables were calculated with Spearman rank correlation test.
Similarity analysis of the samples was based on the Bray–Curtis index (Bray and Curtis, 1957), calculated from (i) the untransformed abundances, (ii) percentage contribution to the total picoeukaryote numbers estimated with probe Euk516, of ten studied populations (with exclusion of total picoeukaryotes, and of Pavlovaphyceae and Phaeocystis spp. that were enumerated only at the stations in Barrow Strait). Calculations were performed using the PRIMER6 software (PRIMER-E Ltd, Plymouth, UK).
Results
Environmental variables
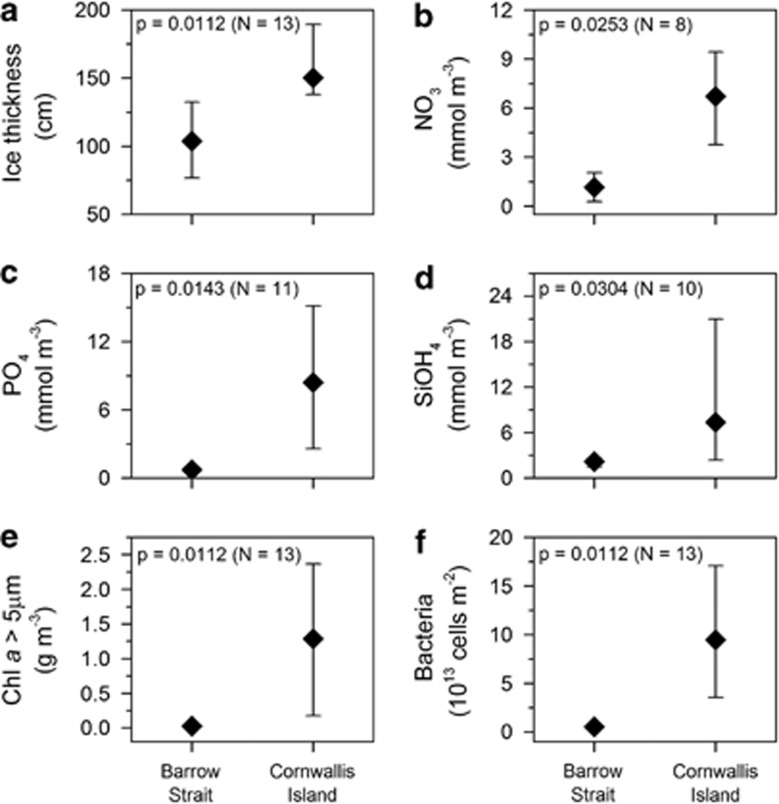
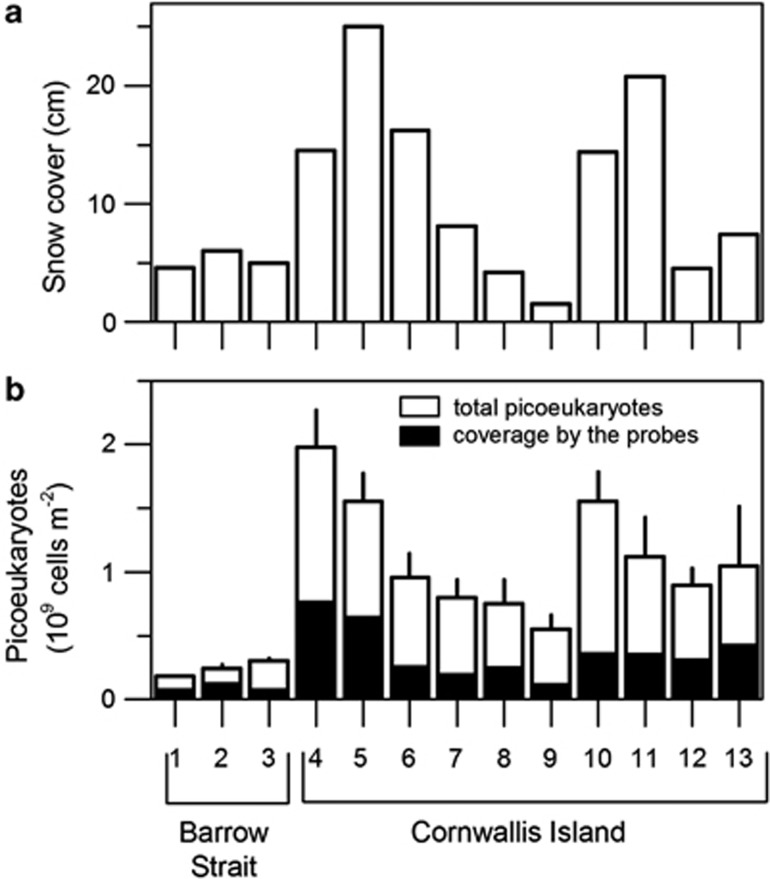
Physico–chemical conditions differed significantly between the stations in Barrow Strait and the vicinity of Cornwallis Island (Mann–Whitney U-test, P<0.05, Figure 2). Drift pack ice present in Barrow Strait was significantly thinner (77–132 cm) than land-fast ice in the vicinity of Cornwallis Island (138–189 cm; Figure 2a, Supplementary Table S2). Concentrations of inorganic nutrients in sea ice were significantly lower in Barrow Strait (NO3: 0.3–2.0 mmol m−3; PO4: 0.5–1.0 mmol m−3; SiOH4: 1.5–2.7 mmol m−3) than in the vicinity of Cornwallis Island (NO3: 3.8–9.4 mmol m−3; PO4: 2.6–15.2 mmol m−3; SiOH4: 2.4–21.0 mmol m−3; Figures 2b–d), except for NO2 (0.07–0.08 mmol m−3 in Barrow Strait and 0.03–0.09 mmol m−3 in the vicinity of Cornwallis Island). Similar trend was observed for concentration of nutrients in water column under the sea ice (Supplementary Table S2). Thickness of snow cover ranged from 1.5 to 25.0 cm (Figure 3a), but the differences between Barrow Strait and the vicinity of Cornwallis Island were not significant.
Figure 2.
Comparison of (a) ice thickness, (b) NO3 concentration, (c) orthophosphate concentration, (d) silicate concentration, (e) chl a (fraction >5 μm) concentration and (f) bacterial numbers measured in bottom sea ice at stations in Barrow Strait and in the vicinity of Cornwallis Island. Significance level (P) from Mann–Whitney U-test, and number of observations (N) are indicated.
Figure 3.
(a) Thickness of snow cover at 13 first-year sea-ice stations, Canadian Arctic Archipelago (b) Numbers of picoeukaryotes detected with probe Euk516. Shaded area depicts the proportions of cells hybridized with specific probes (see Figure 4). Error bars are s.d. of triplicate determinations.
The average bottom ice total chl a concentration was >30-fold higher in the vicinity of Cornwallis Island (10.3–59.5 mg m−2; 364.0–2438.8 mg m−3) than in Barrow Strait (0.6–1.5 mg m−2; 28.9–64.9 mg m−3, Supplementary Table S2). Chl a>5 μm made up >90% of total chl a, except for stations two (79%) and five (<20%), and also was significantly higher in the vicinity of Cornwallis Island (3.7–53.5 mg m−2; 178.0–2367.2 mg m−3) than in Barrow Strait (0.5–0.7 mg m−2; 22.9–35.8 mg m−3 (Figure 2e). Numbers of bacteria were more than two orders of magnitude higher in the vicinity of Cornwallis Island (3.6–17.1 × 1013 cells m−2; 13.6–77.6 × 1014 cells m−3) than in Barrow Strait (5.0–6.5 × 1012 cells m−2; 2.1–3.2 × 1014 cells m−3, Figure 2f, Supplementary Table S2).
Picoeukaryotes in sea ice
The total numbers of sea-ice picoeukaryotes ranged over one order of magnitude, from 0.2 to 2.0±1.3 × 109 cells m−2 (0.9–8.7±1.3 × 1010 cells m−3, Figure 3b). A total of 32–48% (average 39.8%) of cells contained at least one bacterium inside food vacuoles (Table 2). Significantly higher numbers of picoeukaryotes were observed in the vicinity of Cornwallis Island than in Barrow Strait (P<0.05). 20–50% of total picoeukaryote numbers could be detected with the specific probes used (Figure 3b). The observed cells, hybridized with Euk516 and group specific probes, had similar morphology (rounded or slightly elongated cells, 1.5–3 μm in diameter).
Table 2. Food vacuole content of the studied populations of picoeukaryotes.
| Group | Cells with at least one bacterium in food vacuoles (%) | Number of investigated cells | Number of investigated samples |
|---|---|---|---|
| All picoeukaryotes | 39.8 | 650 | 13 |
| Chlorophyta | 7.7 | 520 | 13 |
| Cryptophyceae | 15.4 | 480 | 12 |
| Bolidophyceae | 17.5 | 80 | 2 |
| Haptophyta | 30.8 | 91 | 3 |
| Pavlovaphyceae | 44.0 | 25 | 1 |
| MAST-2 stramenopiles | 29.4 | 211 | 6 |
| MAST-6 stramenopiles | 20.0 | 25 | 1 |
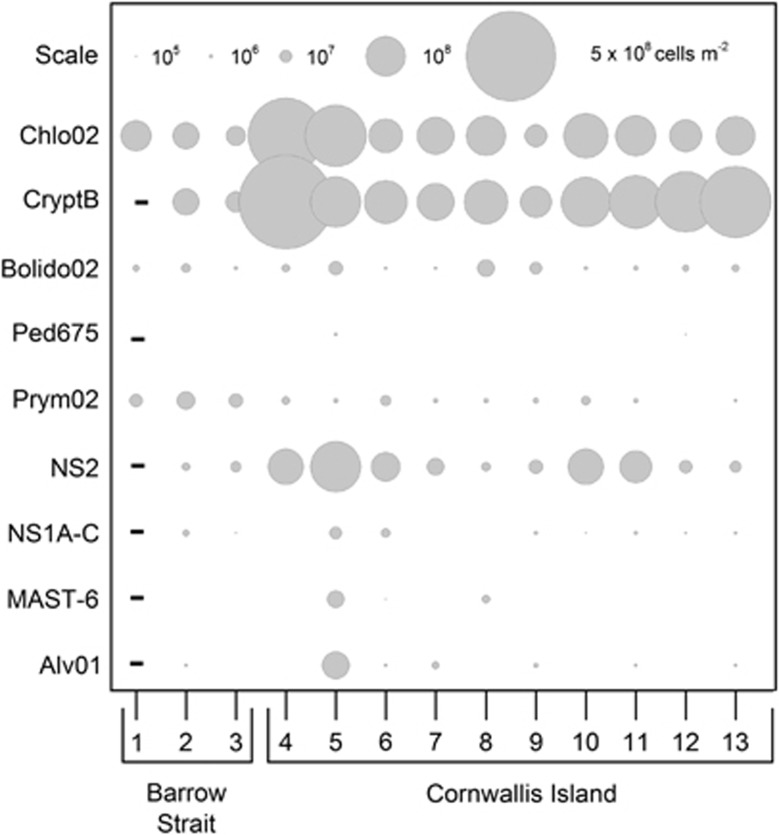
The most numerous picoeukaryote groups at all the stations visited were chlorophytes and cryptophytes (Figure 4). Cell numbers for these two groups ranged from 2.5±0.6 to 35±3 × 107 cells m−2 (1.0±0.2 to 12±1 × 109 cells m−3). The contribution to total picoeukaryote numbers was 5–30% for chlorophytes and 9–30% for cryptophytes. Less than 15% (average 7.7%) of chlorophytes contained bacteria in food vacuoles (Table 2). Percentage of cryptophytes that actively grazed bacteria ranged from 2.5 to 35.0% (average 15.4%, Table 2).
Figure 4.
Distribution of the investigated groups of picoeukaryotes at 13 first-year sea-ice stations, Canadian Arctic Archipelago. Chlo02—Chlorophyta; CryptB—Cryptophyceae; Bolido02—Bolidophyceae; Ped675—Pedinellales, Prym02—Haptophyta; NS2—marine stramenopiles MAST-2; NS1A-C—marine stramenopiles MAST-1A, B & C; MAST-6—marine stramenopiles MAST-6; Alv01—Syndiniales Group II. ‘−' indicates no data, lack of data point indicates samples that were analyzed, but no cells were detected.
Bolidophytes were detected at all the stations visited. Their numbers varied from 0.6 to 18 × 106 cells m−2 (3.9 to 67 × 107 cells m−3, Figure 4), and their contribution to picoeukaryote numbers was <1–2.5%. About 17.5% of the investigated bolidophytes cells contained bacteria in food vacuoles (Table 2).
Pedinellids were present only at stations five and 12 at very low numbers (<8.2 × 105 cells m−2; 3.9 × 107 cells m−3; Figure 4). Their contribution to total picoeukaryote numbers did not exceed 1%.
Numbers of haptophytes ranged from 0.8 to 20±13 × 106 cells m−2 (3.2–91±59 × 107 cells m−3, Figure 4). In contrast to other investigated groups, haptophytes were more numerous in Barrow Strait (stations one–three), where their contribution to total picoeukaryotes numbers was up to 8%. At these stations about 31% of haptophyte cells contained bacteria in food vacuoles (Table 2). The Barrow Strait stations were further investigated for the presence of class Pavlovaphyceae and genus Phaeocystis. Pavlovaphyceae were relatively numerous at station one (6.8 × 106 cells m−2, 3.1 × 107 cells m−3), where they contributed ca. 4% of total picoeukaryote numbers, and 63% of haptophyte numbers. About 44% of cells had at least single bacterium ingested (Table 2). At stations two and three numbers of Pavlovaphyceae were 1.2 × 106 cells m−2 (5.5 × 107 cells m−3) and 9.9 × 105 cells m−2 (4.2 × 107 cells m−3), respectively. Phaeocystis spp. was only detected at station one, in very low numbers (8.8 × 105 cells m−2, 4.3 × 107 cells m−3).
Heterotrophic stramenopiles from the uncultured lineage MAST-2 were found at all the stations visited (Figure 4), with numbers ranging from 4.2±2.3 × 106 to 1.6±1.5 × 108 cells m−2 (0.2±0.1–7.6±7.1 × 109 cells m−3). They were most numerous in the vicinity of Cornwallis Island, where their contribution to total picoeukaryote numbers was 3.9–10.3%. About 30% of the investigated cells actively grazed bacteria (Table 2). Stramenopiles from MAST-1 lineage were at least one order of magnitude less numerous than MAST-2 (0.2–9.3 × 106 cells m−2; or 8.1 × 106–4.4 × 108 cells m−3), and were undetectable at stations four, 7 and 8 (Figure 4). MAST-6 stramenopiles were found only at stations five, six and eight. Their numbers were very low (<1.8 × 107 cells m−2; 9.0 × 108 cells m−3), and their contribution to total picoeukaryote numbers did not exceed 1% (Figure 4). About 20% of population contain bacteria in food vacuoles (Table 2).
Dinospores (that is, invasive single-cell stage) of parasitic Syndiniales Group II were generally absent or present in low numbers, except for station five (4.6±0.8 × 107 cells m−2; 2.2±0.3 × 109 cells m−3), where they contributed ca. 3% to the total numbers of picoeukaryotes (Figure 4). Vermiforms (that is multicellular stage released from a host's cell) were not observed.
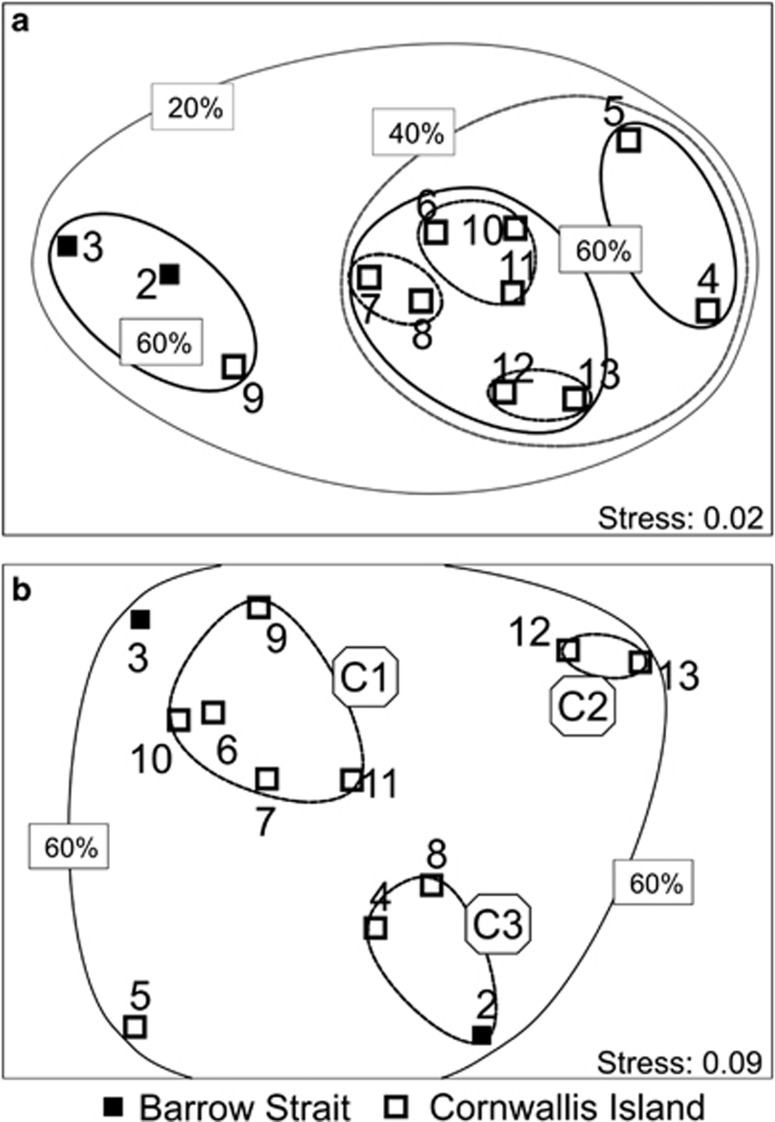
The observed differences between Barrow Strait and the vicinity of Cornwallis Island with respect to the numbers of the picoeukaryotic groups studied were also established by non-metric multidimensional scaling (Figure 5a). However, station nine also grouped with stations two and three from the Barrow Strait at 60% of similarity. The remaining stations grouped at 40% of similarity, but at 60% stations four and five were separated. Non-metric multidimensional scaling analysis performed on percentage contributions of the investigated groups to total numbers of picoeukaryotes resulted in different grouping (Figure 5b). All stations could be grouped at similarity level 60%. At 80% of similarity level, three cluster could be defined: C1 (stations 6, 7 and 9–11), C2 (stations 12, 13) and C3 (stations 2, 4 and 8). Stations 3 and 5 did not fell into any of clusters. Cluster C1 could be characterized by moderate contribution of chlorophytes (5.5–10.5%), cryptophytes (10–16%) and of MAST-2 stramenopiles (2–5%), cluster C1 was formed by stations with very high contribution of cryptophytes (>25%), and cluster C3 was characterized by high contribution of chlorophytes (>12%), cryptophytes (>15%) and for station two and eight also bolidophytes (>2%). Station three was quite similar to stations grouped in cluster C1, but had higher percentage contribution of haptophytes (>4%). Station five, on the other hand, was different from the other stations, as it have the highest percentage contribution of MAST-2 (>10%), MAST-6 (>1%) stramenopiles, and of Syndiniales Group II (3%).
Figure 5.
Configuration plots of non-metric multidimensional scaling based on Bray-Curtis similarity. (a) untransformed numbers (b) percentage contribution to total picoeukaryote numbers. Lines encircle stations at percentage similarity level given in rectangles, unlabeled lines corresponds to 80% of similarity. Labels in hexagons in panel (b) are names for three clusters defined at 80% similarity level (see text).
Correlations with abiotic and biotic variables
The total numbers of picoeukaryotes were positively and significantly correlated with thickness of snow cover (Spearman rank correlation test, Table 3). However, among eleven of the investigated picoeukaryote groups, only chlorophytes and MAST-2 stramenopiles were also correlated with this variable. Sea-ice chl a>5 μm was positively correlated with sea-ice concentrations of NO2−+NO3− and PO43+ (Table 3). Chl a>5 μm was further positively correlated with numbers of bacteria, picoeukaryotes, chlorophytes and MAST-2 stramenopiles (Table 3). Inorganic nutrients and bacterial numbers were not correlated with any of the studied picoeukaryote groups.
Table 3. Correlation matrix for selected environmental variables and picoeucaryote groups.
| Parameter | Picoeukaryotes (Euk516) | Chlorophyta (Chlo02) | Cryptophyta (CryptB) | Bolidophyceae (Bolido02) | Haptophyta (Prym02) | MAST-1 (NS1 A-C) | MAST-2 (NS2) | Chlorophyll-a (>5 μm) |
|---|---|---|---|---|---|---|---|---|
| Ice thickness | 0.10 | 0.21 | 0.28 | 0.45 | −0.49 | −0.32 | 0.02 | 0.31 |
| Snow cover | 0.72** | 0.66* | 0.38 | −0.27 | 0.04 | 0.39 | 0.76** | 0.41 |
| Chlorophyll-a (>5 μm) | 0.64* | 0.57* | 0.39 | −0.33 | −0.15 | −0.27 | 0.59* | — |
| Bacteria | 0.30 | 0.37 | 0.03 | 0.08 | −0.07 | −0.05 | 0.41 | 0.72** |
| NO3+NO2 | 0.37 | 0.50 | 0.46 | 0.57 | −0.39 | −0.27 | 0.28 | 0.78* |
| PO4 | 0.28 | 0.50 | 0.15 | 0.21 | −0.21 | −0.36 | 0.39 | 0.75** |
| SiOH4 | 0.15 | 0.56 | 0.30 | 0.36 | −0.41 | −0.49 | 0.11 | 0.56 |
| Chlorophyta (Chlo02) | 0.88** | — | ||||||
| Cryptophyta (CryptB) | 0.80** | 0.72** | — | |||||
| Bolidophyceae (Bolido02) | −0.05 | 0.21 | 0.17 | — | ||||
| Haptophyta (Prym02) | −0.26 | −0.24 | −0.62* | −0.07 | — | |||
| MAST-1 (NS1 A–C) | 0.01 | −0.10 | 0.08 | 0.15 | 0.15 | — | ||
| MAST-2 (NS2) | 0.85** | 0.70* | 0.44 | −0.16 | −0.08 | 0.19 | — |
Spearman rank correlation coefficients are given. Significance level of bold entries is marked by asterisks: *P<0.05, **P<0.01.
Three most abundant groups: chlorophytes, cryptophytes and MAST-2 stramenopiles were significantly correlated with total picoeukaryote numbers (Table 3). Numbers of chlorophytes were also positively correlated with numbers of cryptophytes and MAST-2 stramenopiles. Finally, numbers of cryptophytes and haptophytes were negatively correlated (Table 3).
Discussion
Arctic sea ice is an ecosystem threatened by ongoing global warming. Positive feedbacks, including decrease in surface albedo due to ice- and snow-melting, accelerate the climate change in this region. In result, the Arctic temperature is rising two to three times faster than the global temperature (Trenberth et al., 2007). First-year sea ice is replacing rapidly vanishing multi-year ice, becoming dominant form of ice in the Arctic (Polyakov et al., 2012). These changes affect the whole Arctic ecosystem, as it is first-year sea ice that supports most active algae communities: an important source of nutrition for both, pelagic and benthic organisms. Larger area of the first-year sea ice cover is predicted to increase energy available for pelagic organisms, but to lower quality of organic matter exported to the bottom (Wassmann and Reigstad, 2011). These scenarios, however, do not account for possible changes in microbial processes within sea ice, which are still inadequately described. Picoeukaryotes are among the least known protistan groups in the sea ice. The first studies on the diversity of sea-ice protists (not only picoeukaryotes) have shown sea-ice communities to consist of various phylotypes (Eddie et al., 2010; Bachy et al., 2011; Majaneva et al., 2012; Comeau et al., 2013). Our study complements these recent results with the first report on the quantitative distribution of particular groups of picoeukaryotes in first-year sea ice, including percentage of cells actively feeding by phagocytosis. However, as every method, CARD–FISH also suffers from limitations. The most important are: group coverage by a probe (percentage of sequences within a group that are targeted by the probe), and hits outside a group (Amann and Fuchs, 2008). All but two probes used in our study has coverage >80% (Supplementary Table S1), and all but one (Bolido02) matches all sequences retrieved from sea ice in the previous studies (Majaneva et al., 2012). This indicates that use of CARD–FISH technique enabled reliable estimation of numbers of most of the investigated groups, providing baseline data on the distribution and relative importance of these groups in the sea ice.
Importance of picoeukaryotes in sea ice
The total numbers of picoeukaryotes in sea ice, estimated with the Euk516 probe (Figure 3b), were at least an order of magnitude higher than numbers of small protists (<5 μm) determined either by flow cytometry (0.4–3.1 × 109 cells m−3, Różańska et al., 2008) or epifluorescent microscopy (0–6.6 × 109 cells m−3, (Riedel et al., 2007; Bachy et al., 2011). Flow cytometry was applied to enumerate only plastidic protists, which explains the observed discrepancy. Microscopic discrimination between 4′,6-diamidino-2-phenylindole dihydrochloride-stained picoeukaryotes and large bacteria is challenging. Application of CARD–FISH and Euk516 probe significantly increased detection of picoeukaryotes in the North Sea (Beardsley et al., 2005), and likely accounted for the higher numbers in our study.
Plastidic eukaryotes other than diatoms are minor component of sea-ice communities during the spring algal bloom (Riedel et al., 2007). Chlorophytes and cryptophytes were the most numerous groups (Figure 4). Sequences of chlorophytes were also retrieved from multi-year sea ice close to the North Pole (Bachy et al., 2011), and first-year sea ice in the Beaufort Sea (Eddie et al., 2010; Comeau et al., 2013) and the Baltic Sea (Majaneva et al., 2012). These phylotypes were related to Chlamydomonas, Mantoniella, Bathycoccus, Pyramimonas and Microsomonas. The cold-adapted picoplanktonic Micromonas strain CCMP2099, which is the most frequent chlorophycean phylotype in Arctic waters (Terrado et al., 2011), grows at low light regimes (Lovejoy et al., 2007). The observed positive correlation between numbers of chlorophytes and snow cover thickness (Table 2), may indicate that also sea-ice chlorophytes are adapted to low light levels, and could be photoinhibited under low snow cover.
Cryptophytes were the second most numerous group (Figure 4). Cryptophyte phylotypes related to Teleaulax and Hemiselmis were found in first-year sea ice in the Canadian Beaufort Sea (Comeau et al., 2013) and the Baltic Sea (Majaneva et al., 2012), but not in multiyear ice close to the North Pole (Bachy et al., 2011). Sequences of cryptophytes have been recovered from size-fractionated (0.2–3 μm) Arctic waters (Terrado et al., 2011). Our results show that picocryptophytes are very numerous also in sea ice.
The presence of bolidophytes in our samples is particularly interesting. This recently discovered picoeukaryotes (Guillou et al., 1999a) form one phylogenetic clade with Parmalean algae Triparma (Ichinomiya et al., 2011), which is also targeted by probe Bolido02. Phylotypes related to bolidophytes were previously reported from pancake sea ice (Majaneva et al., 2012). This young form of sea ice contains many pelagic protists entrapped during freezing (Różańska et al., 2008). We confirmed that bolidophytes may survive winter in sea ice until spring. Considering that probe Bolido02 has mismatches to bolidophycean sequences of Arctic origin, the actual numbers of these organisms in sea ice could be higher than reported here.
HPLC-based studies of sea-ice algae showed photosynthetic prymnesiophytes to be relatively abundant during spring bloom in sea ice in the Canadian Beaufort Sea (Alou-Font et al., 2013). In contrast, our results showed haptophytes to be minor component of picoeukaryotic communities in sea ice, especially in the vicinity of the Cornwallis Island (Figure 4). Haptophycean sequences were also relatively rare in diversity studies, and these affiliated with Chrysochromulina sp., which is not a picoeukaryotic genus (cell size >6 μm, (Throndsen et al., 2007)). There is no report on Phaeocystis spp. and Pavlovaphyceae in sea ice (Eddie et al., 2010; Bachy et al., 2011; Majaneva et al., 2012; Comeau et al., 2013). The latter was relatively abundant at station one in the Barrow Strait, where they contributed in >60% to numbers of picohaptophytes.
Pedinellids were present at two station only, and in low numbers (Figure 4). Most pedinellids are nano-sized organisms (4–15 μm, (Throndsen et al., 2007; Piwosz and Pernthaler, 2010)), and sequences of pedinellids were rare in sea-ice samples (Eddie et al., 2010; Bachy et al., 2011; Majaneva et al., 2012; Comeau et al., 2013). This indicates these flagellates may be uncommon in sea ice.
Heterotrophic nanoeukaryotes generally make up a major part of non-diatom sea-ice communities in spring (Riedel et al., 2007). Majority of sequences of heterotrophic eukaryotes retrieved from sea ice affiliated with ciliates, dinoflagellates, Syndiniales groups I and II and cercozoans (Eddie et al., 2010; Bachy et al., 2011; Majaneva et al., 2012; Comeau et al., 2013). Strains of Cryothecomonas have been frequently isolated from polar waters and sea ice (Thaler and Lovejoy, 2012), and related sequences form substantial part of 18S RNA gene libraries from sea ice (Bachy et al., 2011; Majaneva et al., 2012; Comeau et al., 2013). Cryothecomonas probes were unavailable at the time of this study (Thaler and Lovejoy, 2012), thus we could not enumerate this group. Cryothecomonas-related protists are considered to grazed on other protists, and their cell size is usually above picoeukaryotic range (2.5–29 μm, Thaler and Lovejoy, 2012) Therefore, it could be possible that they had been not very numerous in the picoeukaryotic fraction.
We found two groups of bacterivorous picoeukaryotes: MAST-2 and MAST-1 (Massana et al., 2006). MAST-1 flagellates appear to be cosmopolitan with preference towards polar waters (Lovejoy et al., 2006), and MAST-1C phylotypes were also reported from sea ice in the Baltic Sea (Majaneva et al., 2012). However, MAST-1 flagellates were not very prominent in sea ice in the Canadian Arctic Archipelago (Figure 4). Because of these low numbers, we did not increased the resolution of our study by separately applying probes specific for every clade (NS1A, NS1B and NS1C Table 1 and Supplementary Table S1). In contrast, MAST-2 flagellates were relatively numerous in first-year sea ice (Figure 4). They have been reported to be present in the water column worldwide in very low numbers (<106 cells m−3), including the Arctic and Antarctic regions (Massana et al., 2006), but not in sea ice (Bachy et al., 2011; Majaneva et al., 2012; Comeau et al., 2013). We also found MAST-6 stramenopiles, but in very low numbers. These flagellates have been reported to have two morphotypes, both outside the picoekuoryotic range (Piwosz and Pernthaler, 2010), and were not reported from sea ice (Bachy et al., 2011; Majaneva et al., 2012; Comeau et al., 2013). The MAST-6 probe coverage has decreased to <50% since its publication (Supplementary Table S1), which negatively affected our estimates for this group.
Finally, we also confirmed the presence of parasitic Syndiniales Group II, which often substantially contribute to 18S rRNA gene libraries from sea ice (Bachy et al., 2011; Majaneva et al., 2012; Comeau et al., 2013). These flagellates were generally present in low numbers except for station five, where they were abundant (Figure 4). The coverage of the probe Alv01 (Chambouvet et al., 2008) decreased since its publication (Supplementary Table S1), hence the actual numbers were likely higher than reported here. Presence of vermiforms would have confirmed whether this group is indeed parasitic for sea-ice algae. However, the absence of vermiforms may have resulted from distortion during samples processing. Sea ice could be also a source of parasites to water column upon melting.
Our studies on numbers and distribution of picoeukaryotic groups in sea ice enabled deduction on functional roles of picoeukaryotes in sea ice. We confirmed the presence of bacterivorous phylotypes from groups MAST-1 and MAST-2 (Massana et al., 2006). Bacterivory is well-documented in sea ice (Laurion et al., 1995; Riedel et al., 2007). MAST-1 and MAST-2 numbers were not correlated with numbers of bacteria, even though almost 30% of MAST-2 cells contained bacterial cells in food vacuoles (Table 2). Nevertheless, numbers of MAST-2 were significantly correlated with numbers of chlorophytes (Table 3). This would indicate that other picoeukaryotes may be more important food sources than bacteria, and agrees with the view that bacteria in sea ice are rather bottom-up than top-down controlled (Vezina et al., 1997; Riedel et al., 2007).
Mixotrophic algae also may contribute substantially to overall bacterivory (Zubkov and Tarran, 2008). Mixotrophic phylotypes made up 16% of 18S rRNA libraries (Comeau et al., 2013), but the contribution of mixotrophic algae to total bacterivory in sea ice remains unknown. All investigated groups of algae were found to contain bacteria in food vacuoles (Table 2). Their widespread presence in sea ice and their apparent independence from light conditions (deduced from the lack of correlation with snow cover thickness, Table 3), suggested potential mixotrophic nutrition in sea ice. Such strategy may be advantageous due to low nutrients' concentrations, but surplus of bacteria in sea ice in spring (Vezina et al., 1997; Riedel et al., 2007). On the other hand, percentage contribution of algal cells containing bacteria inside food vacuoles was generally <20%, except for less numerous haptophytes (Table 2). Moreover, none of these groups correlated with bacterial numbers (Table 3). Thus, importance of mixotrophy by picoeukaryotes still remained to be confirmed.
The observed co-occurrence of phylogenetically and functionally diverse (that is, autotrophs, mixotrophic and heterotrophic phagotrophs and parasites) groups reflects the varied roles picoeukaryotes have in sea-ice communities. However, the specific probes used in this study identified only 20–50% of total picoeukaryotes enumerated with the probe Euk516. Therefore, a large part of the picoeukaryote communities still remains to be characterized and likely comprise cells affiliated with Fungi, Amoebozoa and Rhizaria (for example, Cryothecomans (Eddie et al., 2010; Bachy et al., 2011; Majaneva et al., 2012; Comeau et al., 2013)). This emphasizes the need for more research on diversity and roles of picoeukaryotes in sea-ice food webs.
Impact of environmental factors on numbers and distribution of picoeukaryotes in sea ice
Numbers of picoeukaryotes significantly differed between the two investigated regions (Figure 3b). The reason could have been (i) much higher nutrients' concentrations in the vicinity of Cornwallis Island than in Barrow Strait (Figure 2, Supplementary Table S2), (ii) difference in ice type (land-fast ice vs drift pack ice, Supplementary Table S2). These factors likely caused the observed difference in biomass of sea-ice algae (Figure 2). Drift pack ice and land-fast ice have been shown to bear distinct protistan communities (Comeau et al., 2013). Higher nutrients concentrations resulted in higher values of chl a>5 μm (Table 3). As sea-ice algae are fueling an active microbial food web by providing carbon sources (Vezina et al., 1997; Riedel et al., 2007), higher biomasses of sea-ice algae were associated with higher numbers of bacteria, total numbers of picoeukaryotes, chlorophytes and MAST-2 stramenopiles (Table 3). However, concentrations of nutrients were not directly correlated with total numbers of picoeukaryotes or with numbers of any of the investigated groups. The only variable significantly correlated with numbers of picoeukaryotes (and of chlorophytes and MAST-2 stramenopiles, Table 3) was thickness of snow cover. Snow cover limits light available for sea-ice organisms, which likely was the factor that directly influenced picoeukaryote communities. Complex relationships, shifting from positive to negative correlations at lower (<20 cm) and higher (>20 cm) snow cover depths respectively, have been reported in the same region (Mundy et al., 2005).
Non-metric multidimensional scaling analysis did not resemble spatial distribution of stations (Figure 5), neither could be matched to any of the investigated environmental factors. Although one of the reasons could be low taxonomic resolution of our data, it may also points to possible effect of interspecies interaction on distribution of the investigated groups and picoeukaryote community composition. This view was also supported by the observed correlations between different groups of picoeukaryotes (Table 2).
Conclusion
This study showed that picoeukaryotes are important members of sea-ice communities, with diverse functional roles that support complex microbial food webs within the ice. We found auto- and mixo-trophic picoalgae (chlorophytes, cryptophytes, haptophytes and bolidophytes), heterotrophic flagellates (stramenopiles MAST-1 and MAST-2) and parasites (Syndiniales Group II) to be potentially numerous component of microbial communities in Arctic first-year sea ice in spring. The distribution of some, but not all, groups of picoeukaryotes in bottom sea ice was related to snow cover thickness, highlighting the complexity and dynamic nature of the sea-ice physical and biological environment. Our data on the distribution and functional roles of picoeukaryotes in first-year sea ice provide a baseline for future investigations of changes in picoeukaryotic communities. Presently, the absence of comparable data sets on sea-ice Arctic picoeukaryote communities limits our understanding of Arctic ecosystem.
Acknowledgments
This study was supported by IGS (International Governance Strategy, Fisheries and Oceans Canada) and the National Science Engineering Research Council (NSERC) Discovery Grants to CM. Logistical support was provided by Polar Continental Shelf Program (PCSP, Natural Resources Canada). Our sincere thanks go to the PCSP manager and staff for their invaluable help during the field program. We also thank Kelly Hille for help in the field, Line McLaughlin for nutrient analyses, Claude Belzile for flow cytometry analyses and Lena Szymanek for preparing the map with location of the sampling stations (Figure 1). This study was also supported by a grant from the Polish Ministry of Science and Higher Education, grant no 695/N-ARCTICNET/2010/0. This is a contribution of the Freshwater Institute, Fisheries and Oceans Canada, the National Marine Fisheries Research Institute (Poland), the Polish Academy of Sciences and the Arctic in Rapid Transition (ART) Initiative.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on The ISME Journal website (http://www.nature.com/ismej)
Supplementary Material
References
- Alou-Font E, Mundy CJ, Roy S, Gosselin M, Augusti S. Snow cover affects ice algae pigment composition in the coastal Arctic Ocean during spring. Mar Ecol Prog Ser. 2013;474:89–104. [Google Scholar]
- Amann R, Fuchs BM. Single-cell identification in microbial communities by improved fluorescence in situ hybridization techniques. Nat Rev Microbiol. 2008;6:339–348. doi: 10.1038/nrmicro1888. [DOI] [PubMed] [Google Scholar]
- Amann RI, Binder BJ, Olson RJ, Chisholm SW, Devereux R, Stahl DA. Combination of 16S ribosomal-RNA-targeted oligonucleotide probes with flow-cytometry for analyzing mixed microbial populations. Appl Environ Microbiol. 1990;56:1919–1925. doi: 10.1128/aem.56.6.1919-1925.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachy C, Lopez-Garcia P, Vereshchaka A, Moreira D. Diversity and vertical distribution of microbial eukaryotes in the snow, sea ice and seawater near the north pole at the end of the polar night. Front Microbiol. 2011;2:106. doi: 10.3389/fmicb.2011.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates SS, Cota GF. Fluorescence induction and photosynthetic responses of Arctic algae to sample treatment and salinity. J Phycol. 1986;22:421–429. [Google Scholar]
- Beardsley C, Knittel K, Amann R, Pernthaler J. Quantification and distinction of aplastidic and plastidic marine nanoplankton by fluorescence in situ hybridization. Aquat Microb Ecol. 2005;41:163–169. [Google Scholar]
- Belzile C, Brugel S, Nozais C, Gratton Y, Demers S. Variations of the abundance and nucleic acid content of heterotrophic bacteria in Beaufort Shelf waters during winter and spring. J Mar Syst. 2008;74:946–956. [Google Scholar]
- Bray JR, Curtis JT. An ordination of the upland forest communities of southern Wisconsin. Ecol Monogr. 1957;27:326–349. [Google Scholar]
- Chambouvet A, Morin P, Marie D, Guillou L. Control of toxic marine dinoflagellates blooms by serial parasitic killers. Science. 2008;322:1254–1257. doi: 10.1126/science.1164387. [DOI] [PubMed] [Google Scholar]
- Comeau AM, Philippe B, Thaler M, Gosselin M, Poulin M, Lovejoy C.2013Protists in Arctic drift and land-fast ice J Phycol;e-pub ahead of print 27 Nov 2012;doi: 10.1111/jpy.12026 [DOI] [PubMed]
- Conover RJ, Herman AW, Prinsenberg SJ, Harris LR. Distribution and feeding by copepod Pseudocalanus under fast ice during the Arctic spring. Science. 1986;232:1245–1247. doi: 10.1126/science.232.4755.1245. [DOI] [PubMed] [Google Scholar]
- Cota GF, Prinsenberg SJ, Bennett EB, Loder JW, Lewis MR, Anning JL, et al. Nutrient fluxes during extended blooms of Arctic ice algae. J Geophys Res-Oceans. 1987;92:1951–1962. [Google Scholar]
- Davidson AT, Scott FJ, Nash GV, Wright SW, Raymond B. Physical and biological control of protistan community composition, distribution and abundance in the seasonal ice zone of the Southern Ocean between 30 and 80 degrees E. Deep-Sea Res Part II. 2010;57:828–848. [Google Scholar]
- Deming JW.2010Sea ice bacteria and virusesIn: Thomas D, Dieckmann GS (eds)Sea Ice2nd edn.Wiley-Blackwell: Oxford, UK; 247–282. [Google Scholar]
- Eddie B, Juhl A, Krembs C, Baysinger C, Neuer S. Effect of environmental variables on eukaryotic microbial community structure of land-fast Arctic sea ice. Environ Microbiol. 2010;12:797–809. doi: 10.1111/j.1462-2920.2009.02126.x. [DOI] [PubMed] [Google Scholar]
- Eller G, Toebe K, Medlin LK. Hierarchical probes at various taxonomic levels in the Haptophyta and a new division level probe for the Heterokonta. J Plankton Res. 2007;29:629–640. [Google Scholar]
- Fortier M, Fortier L, Michel C, Legendre L. Climatic and biological forcing of the vertical flux of biogenic particles under seasonal Arctic sea ice. Mar Ecol-Prog Ser. 2002;225:1–16. [Google Scholar]
- Glud RN, Rysgaard S, Kühl M, Hansen JW.2007The sea ice in Young Sound: implications for carbon cyclingIn: Rysgaard S, Glud RN (eds)Carbon Cycling in Arctic Marine Ecosystems: Case Study Young Sound. Meddelelser om Grønland The Commission for Scientific Research in Greenland: Copenhagen; 61–86. [Google Scholar]
- Gradinger R. Sea-ice algae: major contributors to primary production and algal biomass in the Chukchi and Beaufort Seas during May/June 2002. Deep-Sea Res Part II. 2009;56:1201–1212. [Google Scholar]
- Guillou L, Chretiennot-Dinet M-J, Medlin LK, Claustre H, Loiseaux-Goer S, Vaulot D. Bolidomonas: a new genus with two species belonging to a new algal class, the Bolidophyceae (Heterkonta) J Phycol. 1999a;35:368–381. [Google Scholar]
- Guillou L, Moon-van der Staay S-Y, Claustre H, Partensky F, Vaulot D. Diversity and abundance of Bolidophyceae (Heterokonta) in two oceanic regions. Appl Environ Microbiol. 1999b;65:4528–4536. doi: 10.1128/aem.65.10.4528-4536.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinomiya M, Yoshikawa S, Kamiya M, Ohki K, Takaichi S, Kuwata A. Isolation and characterization of Parmales (Heterokonta/Heterokontophyta/Stramenopiles) from the Oyashio region, western North Pacific. J Phycol. 2011;47:144–151. doi: 10.1111/j.1529-8817.2010.00926.x. [DOI] [PubMed] [Google Scholar]
- Laurion I, Demers S, Vézina FA. The microbial food web associated with the ice algal assemblage: biomass and bacterivory of nanoflagellate protozoans in Resolute Passage (High Canadian Arctic) Mar Ecol Prog Ser. 1995;120:77–87. [Google Scholar]
- Lebaron P, Parthuisot N, Catala P. Comparison of blue nucleic acid dyes for flow cytometric enumeration of bacteria in aquatic systems. Appl Environ Microbiol. 1998;64:1725–1730. doi: 10.1128/aem.64.5.1725-1730.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovejoy C, Massana R, Pedros-Alio C. Diversity and ditribution of marine microbial eukaryotes in the Arctic Ocean and adjacent seas. Appl Environ Microbiol. 2006;72:3085–3095. doi: 10.1128/AEM.72.5.3085-3095.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovejoy C, Vincent WF, Bonilla S, Roy S, Martineau M-J, Terrado R, et al. Distribution, phylogeny, and growth of cold-adapted picoprasinophytes in arctic seas. J Phycol. 2007;43:78–89. [Google Scholar]
- Majaneva M, Rintala J-M, Piisila M, Fewer DP, Blomster J. Comparison of wintertime eukaryotic community from sea ice and open water in the Baltic Sea, based on sequencing of the 18S rRNA gene. Polar Biol. 2012;35:875–889. [Google Scholar]
- Marie D, Partensky F, Jacquet S, Vaulot D. Enumeration and cell cycle analysis of natural populations of marine picoplankton by flow cytometry using the nucleic acid stain SYBR Green I. Appl Environ Microbiol. 1997;63:186–193. doi: 10.1128/aem.63.1.186-193.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massana R, Terrado R, Forn I, Lovejoy C, Pedros-Alio C. Distribution and abundance of uncultured heterotrophic flagellates in the world oceans. Environ Microbiol. 2006;8:1515–1522. doi: 10.1111/j.1462-2920.2006.01042.x. [DOI] [PubMed] [Google Scholar]
- Metfies K, Medlin L. Refining cryptophyte identification with DNA-microarrays. J Plankton Res. 2007;12:1071–1075. [Google Scholar]
- Michel C, Ingram RG, Harris LR. Variability in oceanographic and ecological processes in the Canadian Arctic Archipelago. Prog Oceanogr. 2006;71:379–401. [Google Scholar]
- Mundy CJ, Barber DG, Michel C. Variability of snow and ice thermal, physical and optical properties pertinent to sea ice algae biomass during spring. J Mar Syst. 2005;58:107–120. [Google Scholar]
- Mundy CJ, Ehn JK, Barber DG, Michel C. Influence of snow cover and algae on the spectral dependence of transmitted irradiance through Arctic landfast first-year sea ice. J Geophys Res-Oceans. 2007;112:C03007. [Google Scholar]
- Niemi A, Michel C, Hille K, Poulin M. Protist assemblages in winter sea ice: setting the stage for the spring ice algal bloom. Polar Biol. 2011;34:1803–1817. [Google Scholar]
- Parsons T, Maita Y, Lalli CM. A Manual of Chemical and Biological Methods for Seawater Analysis. Pergamon Press: Toronto; 1984. [Google Scholar]
- Pernthaler A, Pernthaler J, Amann R. Sensitive multi-color fluorescence in situ hybridization for the identification of environmental microorganisms. Mol Microbial Ecol Manual. 2004;3:711–726. [Google Scholar]
- Perovich DK, Cota GF, Maykut GA, Grenfell TC. Biooptical observations of 1st-year Arctic sea-ice. Geophys Res Lett. 1993;20:1059–1062. [Google Scholar]
- Piwosz K, Pernthaler J. Seasonal population dynamics and trophic role of planktonic nanoflagellates in coastal surface waters of the Southern Baltic Sea. Environ Microbiol. 2010;12:364–377. doi: 10.1111/j.1462-2920.2009.02074.x. [DOI] [PubMed] [Google Scholar]
- Polyakov IV, Walsh JE, Kwok R. Recent changes of Arctic multiyear sea ice coverage and the likely causes. Bull Amer Meteorol Soc. 2012;93:145–151. [Google Scholar]
- Riedel A, Michel C, Gosselin M. Grazing of large-sized bacteria by sea-ice heterotrophic protists on the Mackenzie Shelf during the winter-spring transition. Aquat Microb Ecol. 2007;50:25–38. [Google Scholar]
- Riedel A, Michel C, Gosselin M, LeBlanc B. Winter-spring dynamics in sea-ice carbon cycling in the coastal Arctic Ocean. J Mar Syst. 2008;74:918–932. [Google Scholar]
- Różańska M, Poulin M, Gosselin M. Protist entrapment in newly formed sea ice in the Coastal Arctic Sea. J Mar Syst. 2008;74:901. [Google Scholar]
- Sherr BF, Sherr EB, Pedros-Alio C. Simultaneous measurement of bacterio-plankton production and protozoan bacterivory in estuarine water. Mar Ecol-Prog Ser. 1989;54:209–219. [Google Scholar]
- Simon N, Campbell L, Ornolfsdottir E, Groben R, Guillou L, Lange M, et al. Oligonucleotide probes for the identification of three algal groups by dot blot and fluorescent whole-cell hybridization. J Eukaryot Microbiol. 2000;47:76–84. doi: 10.1111/j.1550-7408.2000.tb00014.x. [DOI] [PubMed] [Google Scholar]
- Soreide JE, Leu E, Berge J, Graeve M, Falk-Petersen S. Timing of blooms, algal food quality and Calanus glacialis reproduction and growth in a changing Arctic. Global Change Biol. 2010;16:3154–3163. [Google Scholar]
- Sou T, Flato G. Sea ice in the Canadian Arctic Archipelago: modeling the past (1950–2004) and the future (2041–60) J Clim. 2009;22:2181–2198. [Google Scholar]
- Terrado R, Medrinal E, Dasilva C, Thaler M, Vincent WF, Lovejoy C. Protist community composition during spring in an Arctic flaw lead polynya. Polar Biol. 2011;34:1901–1914. [Google Scholar]
- Thaler M, Lovejoy C. Distribution and diversity of a Protist predator Cryothecomonas (Cercozoa) in Arctic marine waters. J Eukaryot Microbiol. 2012;59:291–299. doi: 10.1111/j.1550-7408.2012.00631.x. [DOI] [PubMed] [Google Scholar]
- Throndsen J, Hasle GR, Tangen K.2007Heterokontophyta—other classesIn: Throndsen J, Hasle GR, Tangen K (eds)Phytoplankton of Norwegian Coastal Waters Almater Forlag AS: Oslo; 213–217. [Google Scholar]
- Trenberth KE, Jones PD, Ambenje P, Bojariu R, Easterling D, Klein Tank A, et al. Observations: Surface and Atmospheric Climate Change. 2007.
- Vezina AF, Serge D, Laurion I, SimeNgando T, Juniper SK, Devine L. Carbon flows through the microbial food web of first-year ice in Resolute Passage (Canadian High Arctic) J Mar Syst. 1997;11:173–189. [Google Scholar]
- Wassmann P, Reigstad M. Future Arctic ocean seasonal Ice zones and implications for Pelagic-Benthic coupling. Oceanography. 2011;24:220–231. [Google Scholar]
- Werner I. Grazing of Arctic under-ice amphipods on sea-ice algae. Mar Ecol-Prog Ser. 1997;160:93–99. [Google Scholar]
- Zingone A, Chretiennot-Dinet MJ, Lange M, Medlin L. Morphological and genetic characterization of Phaeocystis cordata and P. jahnii (Prymnesiophyceae), two new species from the Mediterranean Sea. J Phycol. 1999;35:1322–1337. [Google Scholar]
- Zubkov MV, Tarran GA. High bacterivory by the smallest phytoplankton in the North Atlantic Ocean. Nature. 2008;455:224–226. doi: 10.1038/nature07236. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.