Abstract
Pulmonary hypertension is defined as an increased systolic pulmonary pressure of >30 mm Hg, and it shows a 40% prevalence in hemodialysis patients due to vascular access (both central venous catheter and arteriovenous fistula). Secondary pulmonary hypertension in chronic kidney disease patients is strictly related to pulmonary circulation impairment together with chronic volume overload and increased levels of cytokines and growth factors, such as FGF, PDGF, and TGF-β, leading to fibrosis. Endothelial dysfunction, together with lower activation of NOS, increased levels of serum endothelin and fibrin storages, involves an extensive growth of endothelial cells leading to complete obliteration of pulmonary vessels. Pulmonary hypertension has no pathognomonic and distinctive symptoms and signs; standard transthoracic echocardiography allows easy assessment of compliance of the right heart chambers. The therapeutic approach is based on traditional drugs such as digitalis-derived drugs, vasodilatory agents (calcium channel blockers), and oral anticoagulants. New pharmacological agents are under investigation, such as prostaglandin analogues, endothelin receptor blockers, and phosphodiesterase-5 inhibitors.
Key Words : Cardiovascular disease, Chronic kidney disease, Pulmonary hypertension, Right heart failure
Background
Pulmonary hypertension is defined as an increased systolic pulmonary pressure of >30 mm Hg. Primary pulmonary hypertension represents an uncommon clinical diagnosis with a prevalence of 2 per million cases all over the world. Table 1 shows the clinical classification of pulmonary hypertension as it was proposed by the World Symposium on Pulmonary Hypertension and confirmed by the World Health Organization (WHO; table 2).
Table 1.
Executive summary of the World Symposium on Primary Pulmonary Hypertension
| 1 | Clinical classification of pulmonary arterial hypertension | |
| 1.1 | Idiopatic pulmonary arterial hypertension | |
| Sporadic | ||
| Familiar | ||
| 1.2 | Pulmonary arterial hypertension associated with: | |
| Connective tissue disease | ||
| Congenital heart disease | ||
| Portal hypertension | ||
| HIV infection | ||
| Anorexigens and other toxic drugs | ||
| Persistent pulmonary hypertension of the newborn | ||
| 2 | Pulmonary venous hypertension | |
| 2.1 | Left-sided atrial or ventricular heart disease | |
| 2.2 | Extrinsic compression of central pulmonary veins | |
| 2.3 | Pulmonary veno-occlusion disease | |
| 3 | Pulmonary hypertension associated with disorders of the respiratory system and/or hypoxemia | |
| 3.1 | Chronic obstructive pulmonary disease | |
| 3.2 | Interstitial lung disease | |
| 3.3 | Sleep-disordered breathing | |
| 3.4 | Chronic exposure to high altitude | |
| 3.5 | Cystic fibrosis | |
| 4 | Pulmonary hypertension due to chronic thrombotic and/or embolic disease | |
| 4.1 | Thromboembolic obstruction of proximal pulmonary arteries | |
| 4.2 | Thromboembolic obstruction of distal pulmonary arteries | |
| Pulmonary embolism | ||
| ‘In situ’ thrombosis | ||
| Thalassemia | ||
| 5 | Pulmonary hypertension due to disorders directly affecting pulmonary vasculature | |
| 5.1 | Inflammatory disease | |
| Schistosomiasis/sarcoidosis | ||
| 5.2 | Pulmonary capillary hemangiomatosis | |
| [37]. | ||
Table 2.
WHO functional classification of pulmonary hypertension
| Class I | Patients without limitation of physical activity. Ordinary physical activity does not cause undue dyspnea or fatigue, chest pain or syncope. |
| Class II | Patients with slight limitation of physical activity. They are comfortable at rest. Ordinary physical activity causes undue dyspnea or fatigue, chest pain or near syncope. |
| Class III | Patients with marked limitation of physical activity. They are comfortable at rest. Less than ordinary activity causes undue dyspnea or fatigue, chest pain or near syncope |
| Class IV | Patients with inability to carry out any physical activity without symptoms. Patients manifest signs of right heart failure. Dyspnea and/or fatigue may even be present at rest. Discomfort is increased by any physical activity. |
Available data report a prevalence of pulmonary hypertension of 40% in hemodialysis patients and of 10% in peritoneal dialysis and pre-dialysis patients [1]. According to our experience, it is quite unusual to observe pathologic plasmin-α2-antiplasmin (PAP) values in uremic patients, while assessment of right ventricular dysfunction in hemodialysis patients is more effective if performed by cardiac ultrasound tricuspid annulus plane systolic excursion (TAPSE) evaluation. Recently, published data reported that about 20% of hemodialysis patients with arteriovenous fistula (AVF) show abnormal TAPSE values (<15 mm), while central venous catheter carriers present normal values [2].
In chronic hemodialysis patients, the presence of an arteriovenous shunt, as in the case of an arteriovenous fistula, involves a preload increase on the right heart chambers with adverse potential long-term effects on their performance [3]. These patients often show right ventricular and atrial volumes close to the maximum allowed (and above) before hemodialysis treatment; volumes return to normal ranges at the end of dialysis treatment due to reduction of extracellular fluid volumes [3].
Therefore, in our patients, we attend to a reduction of the left ventricular telediastolic volume with a concomitant increase in the ejection fraction that indicates beneficial effects of hemodialysis therapy on cardiac performance [3,4]. Otherwise, at present, there are no significant clinical data available on chronic kidney disease (CKD) patients on pre-dialysis. At the same time, according to our personal clinical and echocardiographic experience, it is common to observe abnormalities of echocardiographic parameters of right ventricular function before abnormalities of parameters linked to left ventricular systolic function [5]. Some patients without any pulmonary comorbidity (i.e. chronic bronchitis, pulmonary fibrosis) can show a TAPSE index reduction and increased atrial-ventricular volumes and pressures when their diastolic left ventricular parameters (E/A ratio) are still in normal ranges [6].
Pathophysiology
Secondary pulmonary hypertension in CKD patients' pathophysiology is still complex and not completely clear. We usually observe pulmonary circulation impairment together with chronic volume overload, connective tissue diseases, acquired and congenital cardiopathies, HIV infection, hepatic cirrhosis with portal hypertension, and all chronic comorbidities with increased pressures in the left heart side [5]. As described above, it is crucial to provide early and careful diagnosis based upon a multidisciplinary approach involving any therapeutic method able to delay pathophysiological events leading to pulmonary hypertension.
CKD patients have two peculiar clinical features: anemia and (in most of them) arteriovenous fistula; both factors lead to an increased preload on the right heart chambers [1]. Pulmonary hypertension can lead to increased levels of cytokines and growth factors, such as FGF, PDGF, and TGF-β, with concomitant pulmonary angiotensin-converting enzyme (ACE) activation. As a consequence, we assist to abnormal smooth muscle cell proliferation until fibrosis [7,8]. Endothelial dysfunction, together with a lower activation of nitric oxide synthase (NOS), increased levels of serum endothelin and fibrin storages, could involve an extensive growth of endothelial cells until complete obliteration of pulmonary vessels [9]. Myointimal proliferation, intimal laminated (both concentric and eccentric) fibrosis, thrombosis, and arterial obliteration are typical pathological features of disease progression [9].
Nephrologists have to remember that the right ventricle has a thin muscle equipment because it usually works with low blood pressure and is unable to force high vascular resistances. Together with pulmonary hypertension due to pressure overload, once the right heart chambers lose their distensibility, this leads to tricuspid regurgitation and further right heart volume overload [10].
Diagnosis
Pulmonary hypertension has no pathognomonic and distinctive symptoms and signs. At the onset, we usually observe mild to moderate dyspnea (80% of patients), fatigue, hypotensive crisis, and Raynaud's phenomenon. These clinical findings are usually observed in CKD patients, too, and nephrologists could undervalue secondary diagnosis of pulmonary hypertension [11].
Evaluation of systolic pulmonary pressure is easily performed by standard transthoracic echocardiography. AHA (American Heart Association) and SIEC (Italian Society of Cardiovascular Echography) guidelines list it among the essential parameters to investigate in basic echocardiography [12]. Preliminary B-mode echocardiography, however, allows the detection of some ultrasound aspects that could suggest pulmonary hypertension:
(1) right heart chamber dilation;
(2) ‘D’-shape of the right ventricle by flattening of the interventricular septum;
(3) absence of inferior vena cava inhalatory collapse.
The M-mode view will show small or absent ‘a’ (atrial) wave.
The diagnostic accuracy of these echocardiographic findings is quite poor, both with respect to specificity and sensibility. Therefore, they are all qualitative features and they do not allow the evaluation of the degree of right ventricular impairment and of the response to pharmacological therapy [12]. It is crucial to complete the echocardiographic test with a Doppler evaluation; assessment of pulmonary arterial pressure is performed by tricuspid and pulmonary regurgitation speed assay. Tricuspid regurgitation speed (VTR) is evaluated by four-chamber echocardiographic apical view with ultrasound probe following transvalvular flow to collect regurgitation volume [13]. VTR reveals the systolic pressure gradient between the two right heart sections. If right atrial pressure (RAP) is added, pulmonary artery systolic pressure (PASP) is obtained:
PASP = 4 (VTR)2 + RAP
RAP can be estimated based on basic transthoracic echocardiography with inhalatory collapse in the inferior vena cava diameter. When the inferior vena cava diameter decreases by >50% with inhalation, right atrium pressure is <10 mm Hg; if inferior vena cava inhalatory collapse is <50%, right atrium pressure is >10 mm Hg. Therefore, if the right atrium is dilated (area >15 cm2), RAP is 15 mm Hg at least [14]. It is important to underline that the issues discussed above are not validated for presence of pulmonary valve stenosis or any other mechanical obstruction to right ventricle outflow.
Pulmonary hypertension is characterized by a VTR ≥2 m/s (the normal value is <1 m/s). Telediastolic pulmonary regurgitation speed (VPR) represents a telediastolic pressure gradient between the pulmonary artery and the right ventricle. At the end of diastole, right ventricular pressure should be the same as RAP:
PAEDP = 4 × VPR2 + RAP
where PAEDP is telediastolic pulmonary arterial pressure [13].
Pulmonary arterial pressure is usually increased in patients with high left ventricle inflow pressures; pulsed-wave Doppler study of the mitral valve allows the identification of a restrictive pattern of transmitral flow (increase of E wave, decrease of A wave, and deceleration time of E wave, E/A ratio >2) [15].
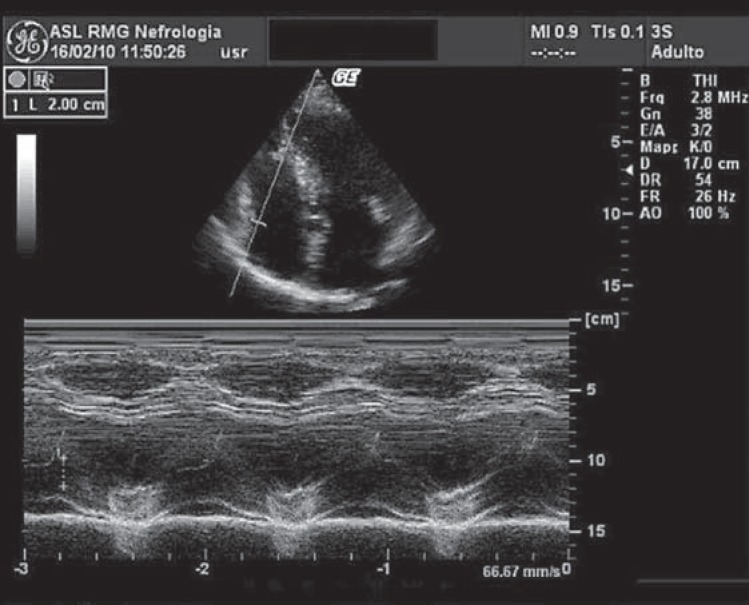
Right ventricular function is often assessed by measuring upper-caudal excursion capability of the tricuspid valve annulus during ventricular systole (TAPSE). TAPSE index can be easily evaluated on M-mode echocardiography without referring to tissue Doppler and has good reproducibility among echocardiographic physicians (fig. 1) [16]. A compliant right ventricle allows wide excursion of the tricuspid annulus, while this is prevented or strongly restricted in progressive right heart impairment and failure. TAPSE is well related with diastolic function of the right ventricle and blood inflow towards the right atrium. TAPSE also represents a good prognostic marker to stratify cardiovascular risk and it is not affected by heart rate and rhythm. Thus, TAPSE can also be performed in patients with tachycardia or atrial fibrillation in which evaluation of diastolic function is prevented by only one atrial wave on transmitral Doppler. TAPSE scoring also represents a cardiovascular prognostic index together with the NYHA (New York Heart Association) classification [16].
Fig. 1.
M-mode echocardiography on long-axis parasternal view: TAPSE index evaluation (normal TAPSE value of 20 mm).
According to our experience, single hemodialysis treatment does not affect PAP values, while it significantly affects TAPSE values as a consequence of volume overload reduction following ultrafiltration, which is not related to the myocardial stunning hypothesis because ejection fraction improves after the hemodialysis session [2]. Our results seem to confirm those of Beigi et al. [17] both concerning long-term effects of arteriovenous fistulae on cardiac output and pulmonary circulation, and epidemiological data on the prevalence of pathological PAP values [3].
Therapy
First of all, patients with a diagnosis of pulmonary hypertension benefit from lifestyle changes such as cessation of smoking and starting mild physical practice. As nephrologists, we have to take care of blood pressure control, optimal and stable hemoglobin levels, and proper management of dry weight.
Concerning pharmacological treatment, scientific evidence and references in the literature have focused on digoxin, diuretics, and calcium channel blockers. Digoxin can exert a favorable hemodynamic effect when given acutely to patients with right ventricular failure due to pulmonary hypertension [18]. Further evidence demonstrates that digoxin can induce a drop-off in noradrenalin circulating levels, one of the biochemical agents involved in the development and progression of pulmonary hypertension. Employment of digitalis-derived drugs has to be strictly monitored in CKD patients, especially in hemodialysis patients [20], with recurrent sampling of digoxin, heart rate, and electrocardiography evaluation [4].
Volume overload represents a crucial step in the pathophysiological pathways to pulmonary hypertension. Loop diuretics, thiazides, and aldosterone antagonists can be helpful in reducing volume overload in patients with effective diuresis, paying attention to serum potassium levels [19]. Extracorporeal ultrafiltration represents a precious tool for nephrologists, giving them further chances to team up with other physicians.
The use of oral anticoagulants is recommended in pulmonary hypertensive patients as underlined by retrospective data from the Mayo Clinic collected in patients in whom therapy with calcium channel blockers failed. Patients who underwent warfarin treatment showed 91% 1-year and 47% 3-year survival rates versus 62 and 31%, respectively, for patients who did not receive warfarin therapy. Recommended doses were similar to those employed in venous thromboembolism prophylaxis (international normalized ratio between 2 and 3) [21].
Low-molecular-weight heparin represents a valid alternative because of its inhibitory effects on smooth muscle cell proliferation [21]. Because of early reports of a reduction in pulmonary arterial pressure following acute administration of vasodilatory agents, it was presumed that they could be the gold standard in the treatment of portopulmonary hypertension (PPH) patients. A common pathway by which vasodilators work can be found in the reduction of intracellular calcium in vascular smooth muscle cells; therefore, most vasodilatory agents demonstrate growth inhibitory properties of smooth muscle cells in culture [22]. Among vasodilatory agents, calcium channel blockers are most common, but some pharmacological properties, such as a negative inotropic effect on right ventricular function and reflex sympathetic stimulation, can worsen underlying pulmonary hypertension and increase resting heart rate [22]. On the other hand, it has been reported that 10-20% of PHH patients treated with high doses of calcium channel blockers exhibit a dramatic reduction in pulmonary arterial pressure and pulmonary vascular resistance. Patients' quality of life was restored with improved functional class and increased rate of survival [22].
Prostaglandin analogues include many pharmacological agents helpful in modulating arterial pulmonary resistance. Among them, epoprostenol [23] has been shown to improve quality of life, PPH-related symptoms, exercise tolerance, hemodynamics, and survival rates because of vasodilatory and antithrombotic effects and related effects in normalizing cardiac output. Continuous administration through a central venous catheter and therapy-related side effects (central venous catheter and exit site infections, flushing, headache, nausea, and diarrhea) have limited the widespread use of epoprostenol.
Treprostinil is a prostacyclin analog quite similar to epoprostenol, stable at room temperature, with a neutral pH and a longer half-life (3-4 h). In a large randomized trial, treprostinil was effective in increasing walking distance, improving exercise tolerance, and reducing dyspnea. It can be administered through continuous subcutaneous infusion without needing central venous catheter insertion [24].
Iloprost, another prostacyclin analog, has been administered by inhalation, showing an acute hemodynamic effect quite similar to nitric oxide. Chronic administration leads to patients' improvement in exercise and pulmonary hemodynamics [25].
Beraprost is a prostacyclin oral analog that has been tested in randomized double-blind controlled multicenter trials. It provides improved exercise capacity and symptom relief, such as dyspnea at rest. Beraprost is associated with frequent side effects, such as headache, flushing, and diarrhea [26], which limit high-dose administration.
Bosentan is an endothelin receptor blocker able to improve exercise tolerance and pulmonary hemodynamics. The recommended dosage is 125 mg twice a day in PPH patients with functional class III-IV [27].
Phosphodiesterase-5 inhibitors (vardenafil and sildenafil) can achieve pulmonary vasodilation by enhancing sustained levels of cyclic guanosine monophosphate (cGMP) and nitric oxide.
Safety and long-term efficacy of these drugs are currently under further investigation [28].
Conclusions
Pulmonary hypertension is a common and pernicious clinical condition in CKD stage 3-5 patients [29,30,31,32,33,34]. Vascular access choice and dry weight management can modify the natural history of the disease, and nephrologists have to be well informed about patients' pulmonary pressures and related pharmacological treatments. Our preliminary data seem to display a well-attested trend to early right ventricular impairment even in early stages of CKD [35,36]. Proper management of right ventricular function impairment could represent a new challenge for cardionephrologists in the 21st century.
Disclosure Statement
The authors declare that they have no conflicts of interest.
References
- 1.Yigla M, Nakhoul F, Sabag A, Tov N, Gorevich B, Abassi Z, Reisner SA. Pulmonary hypertension in patients with end-stage renal disease. Chest. 2003;123:1577–1582. doi: 10.1378/chest.123.5.1577. [DOI] [PubMed] [Google Scholar]
- 2.Di Lullo L, Floccari F, Polito P. Right ventricular diastolic function in dialysis patients could be affected by vascular access. Nephron Clin Pract. 2011;118:c258–c262. doi: 10.1159/000321867. [DOI] [PubMed] [Google Scholar]
- 3.Poulikakos D, Theti D, Pau V, Banerjee D, Jones D. The impact of arteriovenous fistula creation in pulmonary hypertension: measurement of pulmonary pressures by right heart catheterization in a patient with respiratory failure following arteriovenous fistula creation. Hemodial Int. 2012;16:553–555. doi: 10.1111/j.1542-4758.2012.00674.x. [DOI] [PubMed] [Google Scholar]
- 4.Paneni F, Gregori M, Ciavarella GM, Sciarretta S, De Biase L, Marino L, Tocci G, Principe F, Domenici A, Luciani R, Punzo G, Menè P, Volpe M. Right ventricular dysfunction in patients with end-stage renal disease. Am J Nephrol. 2010;32:432–438. doi: 10.1159/000320755. [DOI] [PubMed] [Google Scholar]
- 5.Pabst S, Hammerstingl C, Hundt F, Gerhardt T, Grohé C, Nickenig G, Woitas R, Skowasch D. Pulmonary hypertension in patients with chronic kidney disease on dialysis and without dialysis: results of the Pepper-study. PLoS One. 2012;7:e35310. doi: 10.1371/journal.pone.0035310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Serra W, Chetta A, Santilli D, Mozzani F, Dall'Aglio PP, Olivieri D, Cattabiani MA, Ardissino D, Gherli T. Echocardiography may help detect pulmonary vasculopathy in the early stages of pulmonary artery hypertension associated with systemic sclerosis. Cardiovasc Ultrasound. 2010;8:25. doi: 10.1186/1476-7120-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bogaard HJ, Al Husseini A, Farkas L, Farkas D, Gomez-Arroyo J, Abbate A, Voelkel NF. Severe pulmonary hypertension: the role of metabolic and endocrine disorders. Pulm Circ. 2012;2:148–154. doi: 10.4103/2045-8932.97592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wedgwood S, Devol JM, Grobe A, Benavidez E, Azakie A, Fineman JR, Black SM. Fibroblast growth factor-2 expression is altered in lambs with increased pulmonary blood flow and pulmonary hypertension. Pediatr Res. 2007;61:32–36. doi: 10.1203/01.pdr.0000250013.77008.28. [DOI] [PubMed] [Google Scholar]
- 9.Cracowski JL, Leuchte HH. The potential of biomarkers in pulmonary arterial hypertension. Am J Cardiol. 2012;110(6 suppl):32S–38S. doi: 10.1016/j.amjcard.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 10.Mutlak D, Aronson D, Lessick J, Reisner SA, Dabbah S, Agmon Y. Functional tricuspid regurgitation in patients with pulmonary hypertension: is pulmonary artery pressure the only determinant of regurgitation severity? Chest. 2009;135:115–121. doi: 10.1378/chest.08-0277. [DOI] [PubMed] [Google Scholar]
- 11.Galiè N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, Beghetti M, Corris P, Gaine S, Gibbs JS, Gomez-Sanchez MA, Jondeau G, Klepetko W, Opitz C, Peacock A, Rubin L, Zellweger M, Simonneau G, ESC Committee for Practice Guidelines (CPG) Guidelines for the diagnosis and treatment of pulmonary hypertension: the task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT) Eur Heart J. 2009;30:2493–2537. doi: 10.1093/eurheartj/ehp297. [DOI] [PubMed] [Google Scholar]
- 12.Hammerstingl C, Schueler R, Bors L, Momcilovic D, Pabst S, Nickenig G, Skowasch D. Diagnostic value of echocardiography in the diagnosis of pulmonary hypertension. PLoS One. 2012;7:e38519. doi: 10.1371/journal.pone.0038519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Otto CM. Textbook of Clinical Echocardiography. ed 2. Elsevier Saunders; 2004. Pulmonary Heart Disease: Echocardiographic Approach; pp. 250–252. [Google Scholar]
- 14.Brennan JM, Blair JE, Goonewardena S, Ronan A, Shah D, Vasaiwala S, Kirkpatrick JN, Spencer KT. Reappraisal of the use of inferior vena cava for estimating right atrial pressure. J Am Soc Echocardiogr. 2007;20:857–861. doi: 10.1016/j.echo.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Wong SP, Otto CM. Echocardiographic findings in acute and chronic pulmonary disease. In: Otto CM, editor. The Practice of Clinical Echocardiography. ed 3. WB Saunders; 2002. pp. 739–760. [Google Scholar]
- 16.Di Lullo L, Floccari F, Rivera R, Granata A, D'Amelio A, Logias F, Otranto G, Villani A, Santoboni A, Malaguti M, Timio M, Fiorini F. The nephrologist and the role of ultrasound imaging in the diagnosis of cardiorenal syndrome. G Ital Nefrol. 2012;29:321–327. [PubMed] [Google Scholar]
- 17.Beigi AA, Sadeghi AM, Khosravi AR, Karami M, Masoudpour H. Effects of the arteriovenous fistula on pulmonary artery pressure and cardiac output in patients with chronic renal failure. J Vasc Access. 2009;10:160–166. doi: 10.1177/112972980901000305. [DOI] [PubMed] [Google Scholar]
- 18.Rich S, Seidlitz M, Dodin E, et al. The short term effects of digoxin in patients with right ventricular dysfunction from pulmonary hypertension. Chest. 1998;114:787. doi: 10.1378/chest.114.3.787. [DOI] [PubMed] [Google Scholar]
- 19.Sitbon O, Humbert M, Simonneau G. Primary pulmonary hypertension: current therapy. Prog Cardiovasc Disease. 2002;45:115. doi: 10.1053/pcad.2002.128449. [DOI] [PubMed] [Google Scholar]
- 20.Kiykim AA, Horoz M, Ozcan T, Yildiz I, Sari S, Genctoy G. Pulmonary hypertension in hemodialysis patients without arteriovenous fistula: the effect of dialyzer composition. Ren Fail. 2010;32:1148–1152. doi: 10.3109/0886022X.2010.516854. [DOI] [PubMed] [Google Scholar]
- 21.Barst RJ, Gibbs JS, Ghofrani HA, Hoeper MM, McLaughlin VV, Rubin LJ, Sitbon O, Tapson VF, Galiè N. Updated evidence-based treatment algorithm in pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54:S78–S84. doi: 10.1016/j.jacc.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sitbon O, Humbert M, Jaïs X, Ioos V, Hamid AM, Provencher S, Garcia G, Parent F, Hervé P, Simonneau G. Long-term response to calcium channel blockers in idiopathic pulmonary arterial hypertension. Circulation. 2005;111:3105–3111. doi: 10.1161/CIRCULATIONAHA.104.488486. [DOI] [PubMed] [Google Scholar]
- 23.Sitbon O, Humbert M, Nunes H, et al. Long-term intravenous epoprostenol infusion in primary pulmonary hypertension. J Am Coll Cardiol. 2002;40:780. doi: 10.1016/s0735-1097(02)02012-0. [DOI] [PubMed] [Google Scholar]
- 24.Benza RL, Rayburn BK, Tallaj JA, Pamboukian SV, Bourge RC. Treprostinil-based therapy in the treatment of moderate-to-severe pulmonary arterial hypertension: long-term efficacy and combination with bosentan. Chest. 2008;134:139–145. doi: 10.1378/chest.07-2111. [DOI] [PubMed] [Google Scholar]
- 25.Olschewski H, Simonneau G, Galiè N, Higenbottam T, Naeije R, Rubin LJ, et al. Inhaled iloprost for severe pulmonary hypertension. N Eng J Med. 2002;347:322–329. doi: 10.1056/NEJMoa020204. [DOI] [PubMed] [Google Scholar]
- 26.Galiè N, Humbert M, Vachiéry JL, Vizza CD, Kneussl M, Manes A, Sitbon O, Torbicki A, Delcroix M, Naeije R, Hoeper M, Chaouat A, Morand S, Besse B, Simonneau G. Effects of beraprost sodium, an oral prostacyclin analogue, in patients with pulmonary arterial hypertension: a randomized, double-blind, placebo-controlled trial. J Am Coll Card. 2002;39:1496–1502. doi: 10.1016/s0735-1097(02)01786-2. [DOI] [PubMed] [Google Scholar]
- 27.Galiè N, Rubin LJ, Hoeper M, Jansa P, Al-Hiti H, Meyer GMB, Chiossi E, Kusic-Pajic A, Simonneau G. Treatment of patients with mildly symptomatic pulmonary arterial hypertension with bosentan (EARLY study): a double-blind, randomised controlled trial. Lancet. 2008a;371:2093–2100. doi: 10.1016/S0140-6736(08)60919-8. [DOI] [PubMed] [Google Scholar]
- 28.Ghofrani HA, Wiedemann R, Rose F, Schermuly RT, Olschewski H, Weissmann N, Gunther A, Walmrath D, Seeger W, Grimminger F. Sildenafil for treatment of lung fibrosis and pulmonary hypertension: a randomised controlled trial. Lancet. 2002b;360:895–900. doi: 10.1016/S0140-6736(02)11024-5. [DOI] [PubMed] [Google Scholar]
- 29.Yigla M, Abassi Z, Reisner SA, Nakhoul F. Pulmonary hypertension in hemodialysis patients: an unrecognized threat. Semin Dial. 2006;19:353–357. doi: 10.1111/j.1525-139X.2006.00186.x. [DOI] [PubMed] [Google Scholar]
- 30.Abdelwhab S, Elshinnawy S. Pulmonary hypertension in chronic renal failure patients. Am J Nephrol. 2008;28:990–997. doi: 10.1159/000146076. [DOI] [PubMed] [Google Scholar]
- 31.Yigla M, Fruchter O, Ahronson D, Yanay N, Reisner SA, Lewin M, Nakhoul F. Pulmonary hypertension is an independent predictor of mortality in hemodialysis patients. Kidney Int. 2009;75:969–975. doi: 10.1038/ki.2009.10. [DOI] [PubMed] [Google Scholar]
- 32.Fruchter O, Yigla M. Predictors of long-term survival in elderly patients hospitalized for acute exacerbation of chronic obstructive pulmonary disease. Respirology. 2008;13:851–855. doi: 10.1111/j.1440-1843.2008.01367.x. [DOI] [PubMed] [Google Scholar]
- 33.Abassi Z, Nakhoul F, Khankin E, Reisner SA, Yigla M. Pulmonary hypertension in chronic dialysis patients with arteriovenous fistula: pathogenesis and therapeutic prospective. Curr Opin Nephrol Hypertens. 2006;15:353–360. doi: 10.1097/01.mnh.0000232874.27846.37. [DOI] [PubMed] [Google Scholar]
- 34.Beigi AA, Sadeghi AM, Khosravi AR, Karami M, Masoudpour H. Effects of the arteriovenous fistula on pulmonary artery pressure and cardiac output in patients with chronic renal failure. J Vasc Access. 2009;10:160–166. doi: 10.1177/112972980901000305. [DOI] [PubMed] [Google Scholar]
- 35.Di Lullo L, Floccari F, Granata A, Fiorini F, D'Amelio A, Gorini A, Malaguti M, Polito P. Ecocardiografia e funzione ventricolare destra in pazienti affetti da IRC in stadio III NKF. Congresso Nazionale di Nefrologia, Genova, 21-24 Settembre 2011. Giornale Italiano di Nefrologia. 2011;28:S53–S60. [Google Scholar]
- 36.Floccari F, Granata A, Rivera R, Marrocco F, Santoboni A, Malaguti M, Andrulli S, Di Lullo L.Echocardiography and right ventricular function in NKF stage III-IV chronic kidney disease: ultrasound nephrologists’ role. JUS dx..org/10.1016/j.jus.2012.09.003, in press. [DOI] [PMC free article] [PubMed]
- 37.Classification of Pulmonary Hypertension in Braunwald's Heart Disease . A Textbook of Cardiovascular Medicine. ed 9. Saunders; 2011. pp. 1706–1707. [Google Scholar]