Abstract
Purpose
Arterial media calcification (AMC) is often the only vascular calcification (VC) present in young patients with chronic renal failure and its presence is associated with higher mortality rates. Currently, X-ray imaging (as a standard approach) is able to show AMC in areas without diffuse overlapping arterial intimal calcification (AIC), but X-ray imaging only allows us to identify this lesion when the vessel is widely calcified. The aim of this study was to evaluate the possibility of using ultrasonography as opposed to X-rays to visualize AMC in patients with chronic renal failure. Patients and Methods: In this cross-sectional study, we examined 105 patients (chronic kidney disease stage IV: 19 patients, hemodialysis: 48 patients, renal transplant: 26 patients; mean age: 54 ± 14 years; 65 males and 40 females); B-mode ultrasonography was performed to detect AMC or AIC on the superficial femoral artery (SFA). As a control, plain radiography of the thigh was performed in all patients.
Results
Upon ultrasonography investigation, 12 subjects were excluded due to diffuse VC on the SFA that did not permit a distinction between AMC and AIC. In the remaining 93 patients, AMC was detected on the SFA in 43 patients using ultrasonography and in 20 patients using the standard approach. The sensitivity and specificity of the standard approach for the detection of AMC on the SFA were 47 and 100%, respectively. The positive and negative predictive values of the standard approach were 1 and 0.68, respectively.
Conclusion
Ultrasonography is able to detect AMC better than the X-ray approach, focusing on individuals at higher risk.
Key Words : Vascular biology, Endothelium, Smooth muscle, Basic science, Cardiovascular disease, Dialysis, Chronic kidney disease, General nephrology, Kidney imaging, General nephrology
Introduction
Vascular calcification (VC) increases the risk of cardiovascular mortality in subjects with chronic renal failure [1,2]. There are 2 types of VC: arterial intimal calcification (AIC) and arterial media calcification (AMC). The first type is associated with stenosis and/or obstruction of the vessel, while the second type is associated with arterial stiffness, increased pulse pressure and cardiac overload. At the present time, most devices employed to study VC take advantage of electron beam computed tomography (CT) and multislice spiral CT, which allow the physician to assess the development of VC via a quantitative calcium score over time, but do not allow the distinction between intimal and medial calcification [1,2]. However, the primary disadvantages of such techniques are the high risk of radiation exposure for patients [3] and the high cost. Furthermore, the technology can only be employed in specialized medical centers which are not physically capable of providing services to the majority of subjects affected by chronic renal failure. However, the use of non-invasive alternative techniques such as ultrasonography, echocardiography and radiology is highly recommended.
Several studies have been performed to detect VC [1,2,4] and AIC [5,6,7,8,9]. However, only a few reports have investigated AMC [7,8,9], which is the most common type of arterial calcification in young patients on dialysis [10,11] and in patients with diabetes [9]. Currently, radiology modalities are the only imaging methods that can be used to distinguish between intima and media calcification in non-overlapping areas of vessels. Recently, however, ultrasonography has also been used for this purpose [12,13]. Ultrasonography is widely available in nephrology units permitting to evaluate the development of VC patients affected by chronic kidney disease (CKD).
The aim of our study was to evaluate the presence of AMC using B-mode ultrasonography as opposed to X-rays and to evaluate the factors associated with the presence of AMC in patients with chronic renal failure.
Patients and Methods
Patients
In this cross-sectional study, we investigated 105 patients with chronic renal failure (mean age: 54 ± 14 years; 65 males and 40 females); 19 of the 105 patients had CKD (stage IV), 57 were undergoing treatment with chronic hemodialysis and 29 had undergone a renal transplant.
Ultrasonography and X-Ray Imaging
B-mode ultrasonography (Toshiba Corevision SSA-350A) with a linear transducer (7.5 MHz) was performed to detect intimal or medial calcification on the superficial femoral artery (SFA). The SFA was investigated from the origin to the inferior third of the thigh to identify the presence of AIC, determined by the presence of a hyperechoic lesion >50% larger than the neighboring site of the arterial wall with shadowing [5,6], and to detect AMC in segments without plaque. We considered significant AMC, if the vessel displayed hyperechoic linear deposition in the intima-media thickness [12]. Deposits were considered as either moderate (spots or isolated segments) or severe (more segments).
As a control, plain radiography of the thigh was performed in all patients, and the presence of the ‘railroad track’ sign was considered a positive sign of AMC [7,8,9]. Ultrasonography and X-ray imaging were independently evaluated by A.M. and L.P., who were both blinded to the clinical data.
Statistical Analysis
For categorical variables, we calculated the frequencies and percentages to describe the distribution of the study population's characteristics; in this case, we performed the χ2 test to evaluate differences in the frequency distribution between the AMC-positive and -negative groups. For continuous variables, we calculated the means and standard deviations to describe the distribution of the study population's characteristics; in this case, we used the non-parametric Kruskal-Wallis equality-of-populations rank test to evaluate differences in the frequency distribution between the AMC-positive and -negative groups.
We further calculated the sensitivity, specificity and positive and negative predictive values of the standard X-ray approach in comparison with ultrasonography for the detection of AMC. We also used κ statistics to measure the reliability between ultrasonography and X-rays in the analysis of AMC. The κ value indicates the degree of observer agreement and can range from −1 to +1. A κ value of 0 indicates a total absence of agreement among observers, while a κ value of 1 indicates a perfect agreement between observers. To measure the degree of agreement between X-ray and ultrasonography modalities in the diagnosis of AMC, we measured the level of agreement according to these criteria: κ <0.40 (poor agreement), 0.40 < κ < 0.75 (fair to good agreement), and κ >0.75 (excellent agreement).
Logistic regression models were performed to identify variables associated with the presence of AMC diagnosed with ultrasonography. The presence/absence of AMC was the dependent variable. In these models, we considered the following independent variables: diabetes, smoking habits, hypertension, age, gender, duration of dialysis, posttransplant ultrasonography timing, and the presence of AIC on the SFA.
The statistical analyses were performed using Stata 9.2 software.
Results
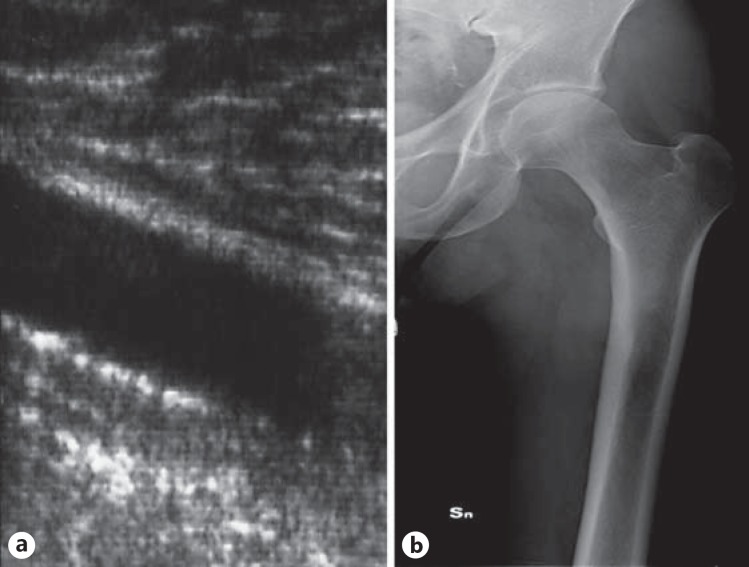
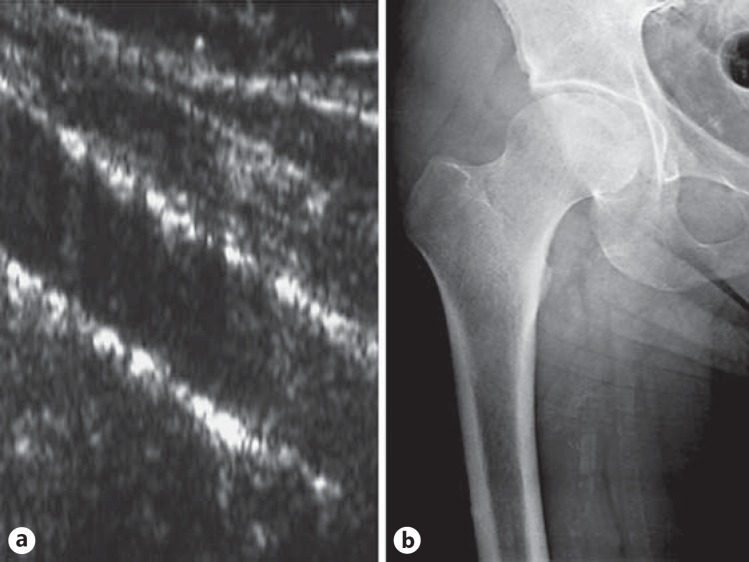
Upon ultrasonography investigation, 12 subjects were excluded due to diffuse AIC on the SFA, which made was it impossible to distinguish between AIC and AMC. The clinical characteristics of the remaining 93 patients according to the presence/absence of AMC detected by ultrasonography are listed in table 1. AMC was detected on the SFA in 43 patients (46%) with ultrasonography and in 20 patients (21%) with X-ray imaging. With ultrasonography, isolated AIC was detected in 14 patients (15%), namely in 6 patients with and 8 patients without AMC, respectively. X-ray imaging was negative for AMC in 18 of the 20 patients in whom ultrasonography revealed moderate deposition (fig. 1). In 18 of the 23 patients with severe AMC as detected by ultrasonography, X-ray imaging was positive (fig. 2). No patients who were found to be negative for AMC based on ultrasonography were positive when examined radiographically.
Table 1.
Demographic and clinical characteristics of the patients (n = 93)
| AMC absent (n = 50) | AMC present (n = 43) | p value | |
|---|---|---|---|
| Male/female gender, n (%) | 20 (60.0)/30 (40.0) | 17 (39.5)/26 (60.5) | 0.96 |
| Mean age ± SD, years | 50.8 ± 12.8 | 56.0 ± 14.9 | 0.08 |
| Diabetes, yes (%) | 4 (8.0) | 11 (25.6) | 0.02 |
| Smoking habit, yes (%) | 23 (46.0) | 14 (32.6) | 0.19 |
| Hypertension, yes (%) | 35 (70.0) | 27 (62.8) | 0.46 |
| Chronic renal failure, yes (%) | 13 (26.0) | 6 (13.9) | 0.15 |
| Hemodialysis, yes (%) | 21 (42.0) | 27 (62.8) | 0.05 |
| Transplant, yes (%) | 16 (32.0) | 10 (23.3) | 0.35 |
| Mean dialysis vintage ± SD, months | 37.1 ± 48.0 | 69.2 ± 76.0 | 0.01 |
| Mean time since renal transplant ± SD, months | 29.3 ± 57.1 | 20.1 ± 41.2 | 0.38 |
Fig. 1.
Moderate AMC detected with ultrasonography on the SFA (a); X-ray imaging is negative in the same patient (b).
Fig. 2.
Severe AMC (on both walls) detected with ultrasonography on the SFA (a); X-ray imaging is positive in the same patient (b).
In table 2, we report the distribution of the sensitivity, specificity, negative and positive predictive values associated with the detection of AMC using either X-ray imaging or ultrasonography. The sensitivity and specificity of X-ray imaging compared to ultrasonography for the detection of AMC on the SFA were 47 and 100%, respectively. The positive and negative predictive values of X-ray imaging for the diagnosis of AMC were 100 and 68%, respectively. The agreement between the results obtained by X-ray imaging and ultrasonography was 75.3%, while the expected agreement by chance was 52.1%. The κ value, which measures the degree of observer agreement, was 0.48; this value suggests a good agreement between X-ray imaging and ultrasonography in the diagnosis of AMC.
Table 2.
Sensitivity, specificity, negative and positive predictive values of X-ray imaging for the detection of AMC in comparison to ultrasonography
| AMC (detected with X-ray imaging) | AMC (detected with ultrasonography) |
|||
|---|---|---|---|---|
| yes | no | total | ||
| Yes | 20 | 0 | 20 | positive predictive value = 100% |
| No | 23 | 50 | 73 | negative predictive value = 68% |
| Total | 43 | 50 | 93 | |
| sensitivity = 47% | specificity = 100% | |||
Table 3 presents the results of logistic regression models used to evaluate factors associated with the presence of AMC (moderate and severe) detected with ultrasonography. Univariate analysis revealed a higher probability of AMC for patients who had been on dialysis for a longer period of time (p = 0.02) and for patients with diabetes (p = 0.03). We also observed a higher probability of AMC with each year of increasing age (p = 0.07). When we adjusted for other variables, only diabetes was associated with the presence of linear calcification (i.e. AMC). We did not find any association between AMC and sex, smoking, hypertension or AIC on the SFA. Considering patients affected by CKD stage IV (19 patients), AMC was absent in 13 vessels, partially present in 4 (2 diabetics) and diffuse in 2 (both diabetics) at the time of ultrasonographic examination. When excluding diabetic patients, only 2 of the 15 vessels were affected by AMC. X-ray imaging was negative in all subjects.
Table 3.
Factors associated with the presence of AMC detected with ultrasonography
| Variable | Crude OR | 95% CI | p value | Adjusted OR* | 95% CI | p value |
|---|---|---|---|---|---|---|
| Diabetes (yes vs. no) | 3.95 | 1.16–13.53 | 0.03 | 6.63 | 1.64–26.78 | 0.01 |
| Smoking (yes vs. no) | 0.57 | 0.24–1.32 | 0.19 | 0.70 | 0.24–2.02 | 0.51 |
| Hypertension (yes vs. no) | 0.72 | 0.30–1.72 | 0.46 | 1.12 | 0.33–3.81 | 0.86 |
| Age (each year more) | 1.03 | 1.00–1.06 | 0.07 | 1.03 | 0.99–1.07 | 0.19 |
| Sex (men vs. women) | 1.02 | 0.44–2.35 | 0.96 | 1.21 | 0.45–3.30 | 0.71 |
| Hemodialysis versus CRF | 2.79 | 0.91–8.56 | 0.07 | 1.78 | 0.43–7.44 | 0.43 |
| Renal transplant versus CRF | 1.35 | 0.39–4.72 | 0.63 | 0.62 | 0.14–8.52 | 0.92 |
| Dialysis vintage (each month more) | 1.01 | 1.00–1.02 | 0.02 | 1.01 | 1.00–1.02 | 0.09 |
| Time since transplant (each month more) | 1.00 | 0.99–1.00 | 0.39 | 1.00 | 0.98–1.02 | 0.99 |
| AIC on the SFA (yes vs. no) | 0.85 | 0.27–2.68 | 0.78 | 0.90 | 0.20–4.10 | 0.89 |
All variables have been adjusted for each other. CRF = Chronic renal failure.
Discussion
AMC on the SFA is associated with increased cardiovascular morbidity and mortality [7,9] and medial calcifications typically affect young patients with chronic renal failure on dialysis [7,8] and diabetics [9]. Moe et al. [10] and, more recently, Coen et al. [11] showed that in relatively young individuals, such as those undergoing a kidney transplant, coronary arterial calcifications as detected by CT are correlated with calcification in the medial layer of the proximal inferior epigastric arteries. In the study by Moe et al. on 39 patients (10 diabetics; mean age: 45 ± 13 years), 11 vessels were positive for AMC and only 1 was positive for AIC [10]. In the study by Coen et al. (44 patients, no diabetics; mean age: 48 ± 14 years), AMC was seen in 33 patients, while no patients exhibited AIC [11]. Schlieper et al. (30 patients, 1 diabetic; mean age: 49 ± 10 years) detected calcifications in the media of iliac arteries by von Kossa staining in 16 tissue samples, while intimal calcification and atherosclerotic plaques were absent [14]. Our sample population was older (mean age: 54 ± 14 years), thereby yielding a higher incidence of AIC (14 patients), which was tied in part to different vessels examined (SFA vs. epigastric artery). AMC was detected in 20 patients using X-ray imaging in comparison to 43 patients using ultrasonography (positive predictive value: 100%). Furthermore, 73 patients were found to be negative for AMC based on X-ray imaging compared to 50 patients using ultrasonography (negative predictive value: 68%).
This is the first study to compare these 2 imaging techniques for the evaluation of AMC. Twelve patients with diffuse AIC were excluded from the analysis, because it was not possible to definitely exclude the co-existence of AMC. In fact, similar to X-ray imaging, ultrasonography cannot identify AMC in the presence of overlapping AIC, but this is not a limitation of the method. Gross et al. [15] demonstrated that in CKD associated with calcified plaques, there was also involvement of the underlying media layer.
Ultrasonography [12,13] can be used to detect medial calcifications; it is the only technique that allows the physician to distinguish the different layers of the arterial wall. This finding, if confirmed by other authors, could prove ultrasonography to be an alternative method for the diagnosis of AMC. X-ray imaging only allows us to identify this lesion when the vessel is widely calcified. Multislice spiral CT and electron beam CT do not distinguish between the 2 types of calcification.
In our study, AMC observed with ultrasonography was associated with the duration of dialysis and with diabetes, as described by other authors who employed radiologic imaging [7,9]. As previously reported [12], SFA is an ideal blood vessel to scan because it is superficial, linear and, due to its length, offers the possibility of detecting AMC in patients with isolated intimal calcifications (14 patients in our sample group). Coll et al. [13] showed hyperechoic linear lesions at the level of the lumen-intimal surface as the most frequent ultrasonographic sign in dialysis patients. Although in our experience this type of calcification is more easily identified in the femoral intimal and media layers, we are confident regarding the potential role of ultrasonography in identifying this calcification.
It is still a matter of discussion as to whether intimal and medial calcifications should be considered as distinct entities with different pathogenesis or as a single nosological entity [16,17]. Unlike intimal calcification, which requires the development of a pre-existing atherosclerotic plaque, a linear non-obstructive calcific deposition between the intima and media layers can develop in the absence of lipid deposits or inflammatory cells in the proximity of the lesion [10,17]. Although AMC and AIC may occur simultaneously in the same patient, AMC can be found in the absence of AIC [7,8]. In our study, AMC was not associated with AIC on the SFA.
VC is often described in patients with renal disease before undergoing dialysis therapy [2,4], but so far no studies have been suitable for distinguishing AMC from AIC. Shroff et al. [18] found that the vessel calcium load was significantly elevated in 34 children with chronic renal failure (10 pre-dialysis and 24 dialysis patients) in comparison to controls, but calcifications in the media and along the internal elastic lamina were detectable by von Kossa staining only in dialysis patients. Our data confirm, as discussed by Shroff et al. [18], that AMC is not frequent in pre-dialysis patients (excluding diabetic patients).
Calcium-sensing receptor and vitamin D receptor have been identified in vascular smooth muscle cells [19,20]. Considering the essential role of vascular smooth muscle cells in the pathogenesis of AMC, it could be important to identify, in an early stage, patients who develop this shape of arterial calcification in order to start drugs such as cinacalcet [21] or paricalcitol [22].
This study has several limitations. First, there is the small sample size and the cross-sectional nature of the study. Second, there is the lack of a histological verification of the location and composition of calcification in the vessels evaluated by ultrasonography and radiology. Third, there is the lack of data on mineral metabolism and treatment. Fourth, ultrasonography is not a fully validated method for the diagnosis of AMC.
Despite all limitations, this study supports some important findings. First, ultrasonography is able to detect not only AIC, but also AMC. Second, X-ray imaging cannot recognize AMC as done by ultrasonography, and therefore AMC in CKD is more common than expected. AMC is the main VC that affects young people with chronic renal failure, and ultrasonography can be used to detect it earlier than X-ray imaging, which will aid in the treatment of high-risk patients.
References
- 1.Raggi P, Boulay A, Chasan-Taber S, et al. Cardiac calcification in adult hemodialysis patients. A link between end-stage renal disease and cardiovascular disease? J Am Coll Cardiol. 2002;39:695–701. doi: 10.1016/s0735-1097(01)01781-8. [DOI] [PubMed] [Google Scholar]
- 2.Sigrist MK, Taal MW, Bungay P, McIntyre CW. Progressive vascular calcification over 2 years is associated with arterial stiffening and increased mortality in patients with stages 4 and 5 chronic renal disease. Clin J Am Soc Nephrol. 2007;2:1241–1248. doi: 10.2215/CJN.02190507. [DOI] [PubMed] [Google Scholar]
- 3.De Mauri A, Brambilla M, Chiarinotti D, Matheoud R, Carriero A, De Leo M. Estimated radiation exposure from medical imaging in hemodialysis patients. J Am Soc Nephrol. 2011;22:571–578. doi: 10.1681/ASN.2010070784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Russo D, Palmiero G, De Blasio AP, Balletta MM, Andreucci VE. Coronary artery calcification in patients with CRF not undergoing dialysis. Am J Kidney Dis. 2004;44:1024–1030. doi: 10.1053/j.ajkd.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 5.Blacher J, Guerin AP, Pannier B, Marchais SJ, London GM. Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension. 2001;38:938–942. doi: 10.1161/hy1001.096358. [DOI] [PubMed] [Google Scholar]
- 6.Cozzolino M, Galassi A, Biondi ML, et al. Serum fetuin-A levels link inflammation and cardiovascular calcification in hemodialysis patients. Am J Nephrol. 2006;26:423–429. doi: 10.1159/000095782. [DOI] [PubMed] [Google Scholar]
- 7.London GM, Guerin AP, Marchais SJ, Metivier F, Pannier B, Adda H. Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant. 2003;18:1731–1740. doi: 10.1093/ndt/gfg414. [DOI] [PubMed] [Google Scholar]
- 8.Damjanovic T, Djuric Z, Schlieper G, et al. Clinical features of hemodialysis patients with intimal versus medial calcifications. J Nephrol. 2009;22:358–366. [PubMed] [Google Scholar]
- 9.Lehto S, Niskanen L, Suhonen M, Ronnemaa T, Laasko M. Medial artery calcification – a neglected harbinger of cardiovascular complications in non-insulin dependent diabetes mellitus. Arterioscler Thromb Vasc Biol. 1996;16:978–983. doi: 10.1161/01.atv.16.8.978. [DOI] [PubMed] [Google Scholar]
- 10.Moe SM, O'Neil KD, Duan D, et al. Medial artery calcification in ESRD patients is associated with deposition of bone matrix proteins. Kidney Int. 2002;61:638–647. doi: 10.1046/j.1523-1755.2002.00170.x. [DOI] [PubMed] [Google Scholar]
- 11.Coen G, De Paolis P, Ballanti P, et al. Peripheral artery calcifications evaluated by histology correlate to those detected by CT: relationship with fetuin-A and FGF-23. J Nephrol. 2011;24:313–321. doi: 10.5301/JN.2010.5818. [DOI] [PubMed] [Google Scholar]
- 12.Marinelli A, Orlandi L, Stivali G. C-reactive protein levels are associated with arterial media calcifications in nondiabetic patients with end-stage renal disease on long-term hemodialysis. Clin Nephrol. 2011;76:425–434. doi: 10.5414/cn107003. [DOI] [PubMed] [Google Scholar]
- 13.Coll B, Betri A, Montserrat Martínez-Alonso M, et al. Large artery calcification on dialysis patients is located in the intima and related to atherosclerosis. Clin J Am Soc Nephrol. 2011;6:303–310. doi: 10.2215/CJN.04290510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schlieper G, Aretz A, Verberckmoes SC, et al. Ultrastructural analysis of vascular calcifications in uremia. J Am Soc Nephrol. 2010;21:689–696. doi: 10.1681/ASN.2009080829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gross ML, Meyer HP, Ziebart H, et al. Calcification of coronary intima and media: immunohistochemistry, backscatter imaging, and X-ray analysis in renal and nonrenal patients. Clin J Am Soc Nephrol. 2007;2:121–134. doi: 10.2215/CJN.01760506. [DOI] [PubMed] [Google Scholar]
- 16.Amman K. Media calcification and intima calcification are distinct entities in chronic kidney disease. Clin J Am Soc Nephrol. 2008;3:1599–1605. doi: 10.2215/CJN.02120508. [DOI] [PubMed] [Google Scholar]
- 17.Drüeke TB. Arterial intima and media calcification: distinct entities with different pathogenesis or all the same? Clin J Am Soc Nephrol. 2008;3:1583–1584. doi: 10.2215/CJN.03250708. [DOI] [PubMed] [Google Scholar]
- 18.Shroff RC, McNair R, Figg N, et al. Dialysis accelerates medial vascular calcification in part by triggering smooth muscle cell apoptosis. Circulation. 2008;118:1748–1757. doi: 10.1161/CIRCULATIONAHA.108.783738. [DOI] [PubMed] [Google Scholar]
- 19.Smajilovic S, Hansen JL, Christoffersen TE, et al. Extracellular calcium sensing in rat aortic vascular smooth muscle cells. Biochem Biophys Res Commun. 2006;348:1215–1223. doi: 10.1016/j.bbrc.2006.07.192. [DOI] [PubMed] [Google Scholar]
- 20.Wu-Wong JR, Nakane M, Ma J, et al. VDR-mediated gene expression patterns in resting human coronary artery smooth muscle cells. J Cell Biochem. 2007;100:1395–1405. doi: 10.1002/jcb.21133. [DOI] [PubMed] [Google Scholar]
- 21.Koleganova N, Piecha G, Ritz E, et al. A calciomimetic (R-568), but not calcitriol, prevents vascular remodeling in uremia. Kidney Int. 2009;75:60–71. doi: 10.1038/ki.2008.490. [DOI] [PubMed] [Google Scholar]
- 22.Mizobuchi M, Finch JL, Martin DR, Slatopolsky E. Differential effects of vitamin D receptor activators on vascular calcification in uremic rats. Kidney Int. 2007;72:709–715. doi: 10.1038/sj.ki.5002406. [DOI] [PubMed] [Google Scholar]