Abstract
Background
Several methods have been developed to assess the hydration status in chronic hemodialysis (HD) patients. The aim of this study was to compare body bioimpedance spectroscopy (BIS) with ultrasound (US) lung comet score (ULCs), B-type natriuretic peptide (BNP) and inferior vena cava diameter (IVCD) by US for the estimation of dry weight before and after HD and to analyze all methods in terms of fluid status variations induced by HD. An additional aim of this study was to establish the interoperator reproducibility of these methods.
Methods
Two nephrologists evaluated BIS, ULCs, IVCD during inspiration (min) and expiration (max), the inferior vena cava collapsibility index (IVCCI) as well as BNP before and after HD in 30 patients. The same operators measured BIS, ULCs and IVCD in 28 HD patients in a blinded fashion.
Results
There was a significant reduction in BIS, ULCs, IVCD and BNP after HD (p < 0.001), but a less significant reduction in IVCCI (p = 0.13). There was a significant correlation between BIS and ULCs, BNP and indexed IVCD (IVCDi)min (p < 0.05) before and after HD, and between BIS and IVCDimax only before HD.
Conclusion
All methods were able to describe hyperhydration before and after HD, except for IVCCI after HD. All techniques correlated with BIS before HD. After HD, ULCs correlated better with BIS than IVCD in terms of evaluation of fluid status. It could be expected that the ULCs can give a real-time evaluation of interstitial water. The reproducibility of the measurement of BIS, IVCD and ULCs between the two operators was high.
Key Words : Fluid assessment, Hemodialysis patients, Bioelectrical impedance analysis, Inferior vena cava diameter, Ultrasound lung comets, B-type natriuretic peptide
Introduction
Chronic volume overload is common in hemodialysis (HD) patients treated with standard thrice weekly treatment and contributes to the development of hypertension, left ventricular hypertrophy and heart failure [1]. The control of volume status can decrease cardiovascular morbidity and mortality [2]. The dry weight is largely determined empirically by clinical trials and can be considered the lowest tolerated post-dialysis weight at which there are minimal signs or symptoms of hypovolemia or hypervolemia [3]. Bedside clinical assessment of volume status is grossly inadequate [4].
Numerous attempts have been made in order to find alternative methods to better assess patients' volume status [5]. The gold standard is the measurement by radioimmunoassay, but this technique cannot be used in clinical practice [6]. Chest X-ray is limited due to the radiation exposure and the large interobserver variation [7]. Other technologies promise rapid and accurate evaluation of the hydration status (HS), including bioelectrical impedance analysis, biomarkers of volume overload such as natriuretic peptides (B-type natriuretic peptide, BNP), measurement of the inferior vena cava diameter (IVCD) by ultrasound (US) and detection of lung comets by chest US (ULC) [6].
Bioelectrical impedance analysis estimates body composition including total body, extracellular and intracellular water. Body bioimpedance spectroscopy (BIS) is a multifrequency bioimpedance [8,9]. BNP is a peptide synthesized and stored in cytoplasmatic vesicles of myocytes; its levels raise with ventricular stretch, caused by pressure or volume overload [10]. With regards to IVCD, it was reported that this parameter and blood volume decrease in parallel during HD sessions and increase 2 h after HD due to the refilling of the intravascular space, indicating that changes in IVCD reflect changes in blood volume [11]. ULC or B-lines are defined as multiple comet tails originating from the water-thickened interlobular septa. The score of ULC (ULCs) has a linear correlation with the extravascular lung water and provides useful information for the prognostic stratification of patients with dyspnea [12].
The aim of this study is to compare BIS with the other methods of fluid status assessment (ULCs, IVCD and BNP) for the estimation of dry weight before and after HD. Moreover, we analyze all methods in terms of fluid status variations induced by HD treatments. Another aim of this study is to establish the interoperator reproducibility of these methods (ULCs, IVCD and BIS) and their easy execution.
Methods
We performed a cross-sectional study of 32 patients undergoing chronic HD at the Department of Nephrology of our hospital. Two patients had a poor IVCD image and were therefore excluded, leaving 30 patients for analysis. Patients with interstitial lung disease were excluded because of pulmonary fibrosis that can modify ULCs independently of the state of hydration [13]. Moreover, we excluded patients in NYHA class IV because advanced chronic heart failure could cause pulmonary congestion that can be detected by echography as an increase in ULCs; we also excluded patients with acute complications within 3 months before the study. Patients gave their verbal consent because of the noninterventional nature of the study.
All patients were treated thrice weekly with standard bicarbonate dialysis using semisynthetic membranes. Fluid overload was considered as weight gain from the estimated dry weight based on clinical patient parameters such as weight, blood pressure, presence of edema and vascular congestion and previous BIS. HS is the actual measured weight of the patients minus the clinically determined dry weight. All patients were evaluated at the bedside for weight, HS by BIS, ULCs and IVCD by two different nephrologists trained in US.
BNP levels were measured immediately before and after HD. For each patient, we recorded episodes of intradialytic symptomatic hypotension in the previous 3 dialysis sessions. The device for BIS was the Body Composition Monitor (Fresenius Medical Care D, Bad Homburg, Germany) [8], and for US it was the Power Vision 6000 (SSA-370A, Toshiba, Tokyo, Japan) with a 3.75-Mhz probe. The patients' dry weight was considered the prescribed weight. We calculated the effective residual overload by the difference between HS (measured by BIS before HD) and weight loss during HD and classified the patients according to their residual overload after HD as overhydrated or non-overhydrated (residual overload >0.5 and ≤0.5 liters, respectively).
The number of B-lines was measured by US examination of the anterior and lateral chest walls on both sides [14]. The sum of ULC produced a score reflecting the extent of extravascular lung water accumulation (a limit of <8 lung comets was considered normal). On the basis of this score, we divided the patients into the following 3 categories of pulmonary congestion severity: mild pulmonary congestion with <14 lung comets, moderate pulmonary congestion with ≥14 lung comets, and severe pulmonary congestion with ≤30 lung comets [14].
The IVCD was explored within the subxiphoid window during inspiration (IVCDmin) and expiration (IVCDmax) within 2.5 cm of the IVC-right atrial junction. Indexed IVC size (IVCDi) was calculated by dividing IVCDmax and IVCDmin by the body surface area (in meters squared). The IVC collapsibility index (IVCCI) was calculated using the standard formula [(IVCDmax – IVCDmin)/IVCDmax × 100]. According to US criteria, patients were considered underhydrated if IVCDimax was <8 mm/m2, normohydrated if IVCDimax was between ≥8 and ≤11.5 mm/m2, and overhydrated if IVCDi was >11.5 mm/m2[3].
BNP was measured in our department's laboratory by using a electrochemiluminescence immunoassay system (Advia Centaur XP, Siemens, Germany). The limit of normal values for this method is <120 pg/ml. BNP levels >400 pg/ml were considered high.
Reproducibility Studies
The aforementioned parameters (IVCDimin, IVCDimax, ULCs and BIS) were measured by two nephrologists trained in US on 28 patients in a blinded fashion.
Statistical Analysis
Continuous variables are expressed as mean ± standard deviation. The relationships between the various parameters were evaluated using analysis of linear regression. The comparison of continuous independent variables across groups was calculated with a t test when variables could be assumed normally distributed. In nonnormally distributed variables, the Mann-Whitney U test was performed.
Correlations between the two observers were evaluated by Spearman's correlation coefficient, and the agreement between the two observers was calculated by the kappa test and Bland-Altman method. A p value of <0.05 was considered significant. Statistical analysis was performed using a commercially available software (SPSS 16.0).
Results
The demographic and clinical characteristics of our study population are shown in table 1. In terms of HS measurements according to BIS analysis, the average dry weight decreased to 0.9 kg below the clinically estimated dry weight. This post-dialysis weight was well tolerated and should therefore be a more accurate dry weight. Five patients had residual fluid overloads >0.5 liters probably because they did not tolerate more ultrafiltration, and therefore their dry weights were clinically estimated at inappropriately low values.
Table 1.
Baseline characteristics (mean ± SD)
| Number of patients | 30 |
| Age, years | 63.8 ± 16.2 |
| Female gender, % | 21.9 |
| BMI | 26 ± 5.4 |
| BSA | 1.8 ± 0.2 |
| Diabetes, % | 15.6 |
| Cardiovascular comorbidities, % | 75 ± 74.2 |
| Ejection fraction, % | 56.5 ± 8.1 |
| Patients treated with antihypertensive drugs, % | 77.4 |
| NYHA, % | |
| cl 1 | 19.4 |
| cl 2 | 16.1 |
| cl 3 | 19.4 |
| Dialysis duration, years | 5.2 ± 5.5 |
| Kt/V | 1.3683 ± 0.2 |
| Pre-HD weight, kg | 74.02 ± 14 |
| Post-HD weight, kg | 71.4 ± 13.6 |
| Dry weight, kg | 70.09 ± 13.05 |
| Weight loss, kg | 2.6 ± 0.9 |
| Systolic blood pressure, mm Hg (pre) | 143.3 ± 22.5 |
| Diastolic blood pressure, mm Hg (pre) | 76.3 ± 14 |
| Blood pressure, mm Hg (pre) | 109.8 ± 15.3 |
| Heart rate, beats/min (pre) | 69.6 ± 11.5 |
| Systolic blood pressure, mm Hg (post) | 139.6 ± 22.5 |
| Diastolic blood pressure, mm Hg (post) | 77.1 ± 11.9 |
| Blood pressure, mm Hg (post) | 108.3 ± 15.1 |
| Heart rate, beats/min (post) | 69.5 ± 8.9 |
| Hemoglobin, g/dl | 11.8 ± 1.1 |
Change in Fluid Status before and after HD
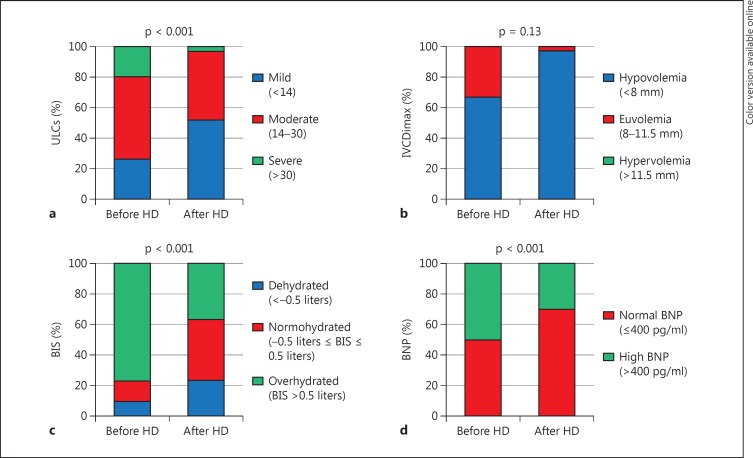
There was a significant reduction in the values of HS, ULCs, IVCDimax, IVCDimin and BNP after HD when compared to the values before HD (table 2). In contrast, IVCCI did not change. Figure 1 shows the same results analyzed according to the categories of each measurement method: in overhydrated patients, BIS, ULCs and BNP decreased significantly after HD (p < 0.001, p < 0.05 and p < 0.001, respectively). Using IVCD, none of the patients was hypervolemic either before or after HD.
Table 2.
Fluid status before and after HD (mean ± SD)
| Before HD | After HD | p | |
|---|---|---|---|
| OH by BIS, liters | 1.7 ± 1.8 | −0.05 ± 1.8 | <0.001 |
| Chest US | |||
| ULCs | 20 ± 11.5 | 12.7 ± 7.1 | <0.001 |
| IVCDi US | |||
| IVCDimin, mm | 5.4 ± 2.1 | 3.9 ± 1.9 | <0.001 |
| IVCDimax, mm | 6.8 ± 2.4 | 5.4 ± 2 | <0.001 |
| IVCCI, % | 21.1 ± 12.7 | 26 ± 17.7 | 0.13 |
| BNP (median/percentile) | 411/108.6 | 267/70.4 | <0.001 |
Fig. 1.
Fluid status by ULCs (a), IVCDimax (b), BIS (c) and BNP (d) before and after HD.
A total of 23/30 patients had moderate to severe pulmonary congestion as determined by the number of lung comets before HD. Considering this group, 8 patients had an improvement to mild pulmonary congestion, while 15 patients continued to have a significant pulmonary congestion after dialysis. Patients who have shown an improvement tended to have lower systolic blood pressure (147.5 ± 22.7 mm Hg) than the other group (127.8 ± 22.9 mm Hg) at the end of dialysis, without reaching statistical significance (p = 0.006). In contrast, there was no difference in the diastolic blood pressures between the two groups. As regards intradialytic hypotension episodes, no statistical difference was observed either (episodes 2 vs. 3, p = 0.59).
Comparison between Techniques
The correlation among the various methods is shown in table 3. It can be seen that before HD, BIS correlated with ULCs, IVCDimin, IVCDimax, IVCCI and BNP. However, after HD, BIS correlated with IVCDimin, IVCCI, ULCs and BNP, but not with IVCDimax.
Table 3.
Correlation between different techniques before and after HD
| ULCs | IVCDimin | IVCDimax | IVCCI | BNP | |
|---|---|---|---|---|---|
| Before HD | |||||
| BIS | 0.510** | 0.534** | 0.519** | 0.601** | 0.419* |
| ULCs | − | 0.514** | 0.390* | 0.444* | 0.201 |
| IVCDimin | − | − | 0.900** | 0.885** | 0.316 |
| IVCDimax | − | − | − | 0.964** | 0.279 |
| IVCCI | − | − | − | − | 0.223 |
| BNP | − | − | − | − | − |
| After HD | |||||
| BIS | 0.381* | 0.364* | 0.341* | 0.423* | 0.480* |
| ULCs | − | 0.375* | 0.312 | 0.358 | 0.220 |
| IVCDimin | − | − | 0.815** | 0.830** | 0.264 |
| IVCDimax | − | − | − | 0.971** | 0.308 |
| IVCCI | − | − | − | − | 0.264 |
| BNP | − | − | − | − | − |
0.001 < p < 0.05;
p < 0.001.
Between overhydrated (n = 5) and non-overhydrated (n = 25) patients, there was a significant difference in ULCs (p < 0.001), IVCDimin (p = 0.019) and IVCCI (p = 0.001); however, no differences were found for BNP and IVCDimax.
Reproducibility and Easy Use of the Techniques
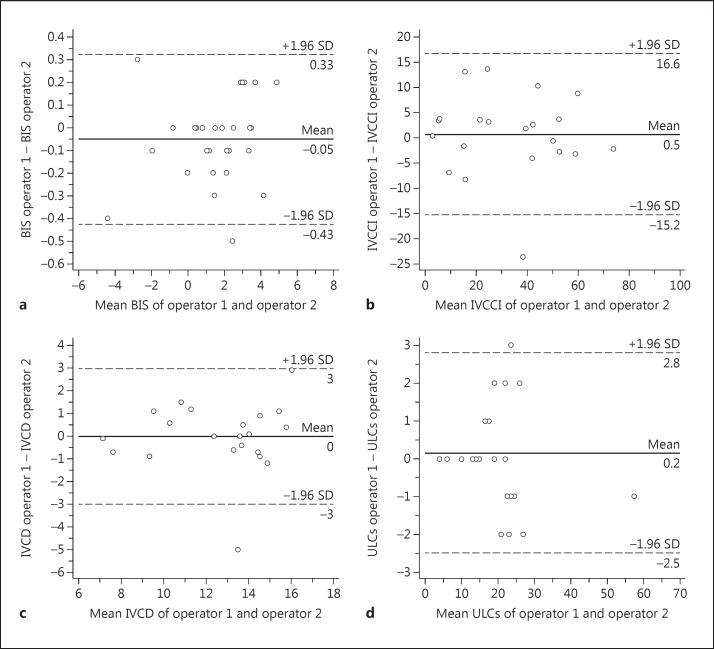
The r value for the interoperator variability for IVCDimax and IVCDimin was 0.847 (p < 0.001) and 0.948 (p < 0.001), respectively, while for IVCCI it was 0.926 (p < 0.001). The agreement for IVCDimax and IVCCI categories was high (k = 0.786, p < 0.001 and k = 0.783, p < 0.001), and the reproducibility of ULCs was very high (r = 0.991, p < 0.001). The agreement of the categories of ULCs was perfect (k = 1, p < 0.001). Finally, the correlation of BIS was very good (r = 0.996, p < 0.001) (fig. 1, 2).
Fig. 2.
Bland-Altman plots of BIS (a), IVCCI (b), IVCDmax (c) and ULCs (d).
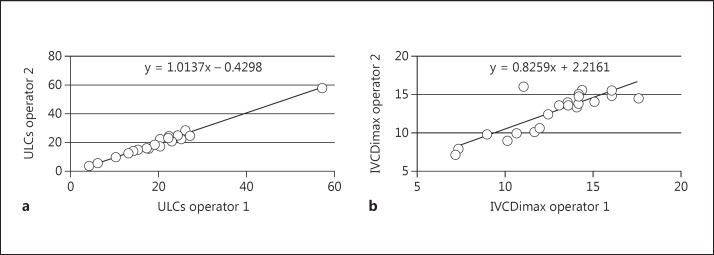
The measurements of ULCs and IVCDi were both easily executable. The mean execution time for the measurement of ULCs was 6.4 min (SD 2.6), and for the measurement of IVCDi it was 2.8 min (SD 1.3) Figure 3.
Fig. 3.
Scatter plots of ULCs (a) and IVCDimax (b) performed by the two operators.
Discussion
We performed a cross-sectional study to compare different techniques of volume status assessment in patients before and after HD. The following main results could be found: first, most techniques were able to show a reduction in overhydration after HD; second, there was a fair correlation between the different techniques; and third, the US techniques were reproducible and easy to perform.
The correct dry weight is difficult to estimate since physical examinations are not an accurate technique [4]. Newer alternative methods have significant theoretical and practical limitations. For example, severe cutaneous alterations, inadequate electrode adherence to the skin or erroneous positioning, electrical interferences and a status of severe obesity are limitations for BIS [6]. However, it was demonstrated that data obtained with BIS change 120 min after the end of HD [15]. BNP levels increase with volume overload, but also with other causes of myocardial stress [16]. Patients with chronic heart failure may have persistently elevated BNP levels at their baseline dry weight, so knowledge of the patients' medical history is necessary to appropriately interpret the BNP measurement [17]. US is the safest and less expensive technique. It is a simple, user-friendly, radiation-free technique that can be performed at patient bedside [18]; nevertheless, it is an operator-dependent technology. Supervised training is needed to ensure that the operator correctly interprets the sonographic findings [19]. The IVCD has been shown to reflect fluid status, even if it may have only modest sensitivity for detecting changes in fluid status after HD [20]: the re-equilibration of interstitial and intravascular compartments after HD takes some time, and the optimal timing for post-HD assessment is not clear [4]. In contrast, the B-lines have been correlated with extravascular water [18]. In patients whose ULCs decreased after dialysis, systolic blood pressure decreased without increasing the hypotensive episodes. However, statistical significance was not observed probably due to the low sample number. Moreover, the improvement of the systolic blood pressure emphasizes the ability of ULCs to detect the decrease in interstitial pulmonary congestion and body fluid volume.
In a study of 40 HD patients, Noble et al. [21] observed that B-line resolution appeared to occur in real time during the fluid removal from the body. If this is indeed the case, ULCs may be a more practical tool to use at patient bedside immediately after a HD session.
Limitations of our study include the small number of patients enrolled from one single dialysis center. Nevertheless, it is amount of effort and the results are statistically significant.
Conclusions
The determination of dry weight is a clinical estimation. All methods considered related more to the changes with HD rather than to the absolute pre- and post-dialysis values. In fact, all fluid assessment methods were able to describe changes of hyperhydration before and after HD, except for IVCCI after HD. Otherwise, after HD ULCs correlated better than IVCD with bioelectrical impedance analysis in terms of evaluation of fluid status. This can be explained by the equilibration of the interstitial compartment during dialysis. It could be expected that the ULCs can give a real-time evaluation of interstitial water. The reproducibility of BIS, IVCD and ULCs measurement between the two operators was high. All methods were fast and easy to perform.
Disclosure Statement
No author reports a conflict of interest.
References
- 1.Kuhlmann MK, Zhu F, Seibert E, Levin NW. Bioimpedance, dry weight and blood pressure control: new methods and consequences. Curr Opin Nephrol Hypertens. 2005;14:543–549. doi: 10.1097/01.mnh.0000185983.48319.00. [DOI] [PubMed] [Google Scholar]
- 2.Ozkahya M, Ok E, Toz H, Asci G, Duman S, Basci A, Kose T, Dorhout Mees EJ. Long-term survival rates in haemodialysis patients treated with strict volume control. Nephrol Dial Transplant. 2006;21:3506–3513. doi: 10.1093/ndt/gfl487. [DOI] [PubMed] [Google Scholar]
- 3.Brennan JM, Ronan A, Goonewardena S, Blair JE, Hammes M, Shah D, Vasaiwala S, Kirkpatrick JN, Spencer KT. Handcarried ultrasound measurement of the inferior vena cava for assessment of intravascular volume status in the outpatient hemodialysis clinic. Clin J Am Soc Nephrol. 2006;1:749–753. doi: 10.2215/CJN.00310106. [DOI] [PubMed] [Google Scholar]
- 4.Charra B. Fluid balance, dry weight, and blood pressure in dialysis. Hemodial Int. 2007;11:21–31. doi: 10.1111/j.1542-4758.2007.00148.x. [DOI] [PubMed] [Google Scholar]
- 5.Ishibe S, Peixoto AJ. Methods of assessment of volume status and intercompartmental fluid shifts in hemodialysis patients: Implications in clinical practice. Semin Dial. 2004;17:37–43. doi: 10.1111/j.1525-139x.2004.17112.x. [DOI] [PubMed] [Google Scholar]
- 6.Peacock WF, Soto KM. Current techniques of fluid status assessment. Contrib Nephrol. 2010;164:128–142. doi: 10.1159/000313726. [DOI] [PubMed] [Google Scholar]
- 7.Don C, Burns KD, Levine DZ. Body fluid volume status in hemodialysis patients: the value of the chest radiograph. Can Assoc Radiol J. 1990;41:123–126. [PubMed] [Google Scholar]
- 8.Voroneanu L, Cusai C, Hogas S, Ardeleanu S, Onofriescu M, Nistor I, Prisada O, Sascau R, Goldsmith D, Covic A. The relationship between chronic volume overload and elevated blood pressure in hemodialysis patients: use of bioimpedance provides a different perspective from echocardiography and biomarker methodologies. Int Urol Nephrol. 2010;42:789–797. doi: 10.1007/s11255-010-9767-y. [DOI] [PubMed] [Google Scholar]
- 9.Onofriescu M, Mardare NG, Segall L, Voroneanu L, Cusai C, Hogas S, Ardeleanu S, Nistor I, Prisada OV, Sascau R, Covic A. Randomized trial of bioelectrical impedance analysis versus clinical criteria for guiding ultrafiltration in hemodialysis patients: effects on blood pressure, hydration status, and arterial stiffness. Int Urol Nephrol. 2012;44:583–591. doi: 10.1007/s11255-011-0022-y. [DOI] [PubMed] [Google Scholar]
- 10.Dokainish H. Combining tissue Doppler echocardiography and B-type natriuretic peptide in the evaluation of left ventricular filling pressures: review of the literature and clinical recommendations. Can J Cardiol. 2007;23:983–989. doi: 10.1016/s0828-282x(07)70861-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katzarski KS, Randmaa I, Bergstrom J. Influence of hemodialysis on intravascular volume and vasoactive hormones. Clin Nephrol. 1999;52:304–311. [PubMed] [Google Scholar]
- 12.Frassi F, Gargani L, Tesorio P, Raciti M, Mottola G, Picano E. Prognostic value of extravascular lung water assessed with ultrasound lung comets by chest sonography in patients with dyspnea and/or chest pain. J Card Fail. 2007;13:830–835. doi: 10.1016/j.cardfail.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Gargani L, Doveri M, D'Errico L, Frassi F, Bazzichi ML, Delle Sedie A, Scali MC, Monti S, Mondillo S, Bombardieri S, Caramella D, Picano E. Ultrasound lung comets in systemic sclerosis: a chest sonography hallmark of pulmonary interstitial fibrosis. Rheumatology (Oxford) 2009;48:1382–1387. doi: 10.1093/rheumatology/kep263. [DOI] [PubMed] [Google Scholar]
- 14.Mallamaci F, Benedetto FA, Tripepi R, Rastelli S, Castellino P, Tripepi G, Picano E, Zoccali C. Detection of pulmonary congestion by chest ultrasound in dialysis patients. JACC Cardiovasc Imaging. 2010;3:586–594. doi: 10.1016/j.jcmg.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 15.Di Iorio BR, Scalfi L, Terracciano V, Bellizzi V. A systematic evaluation of bioelectrical impedance measurement after hemodialysis session. Kidney Int. 2004;65:2435–2440. doi: 10.1111/j.1523-1755.2004.00660.x. [DOI] [PubMed] [Google Scholar]
- 16.Coppola A, De Paola G, Suppa M, Maggi B, Giancaspro G, Colzi M, Arcieri A, Scarpellini G, Manetti E, Lacenere L, Gerratana G, Santulli M, Aguglia F. Variation in the plasma concentration of B-type natriuretic peptide in emergent paroxysmal atrial fibrillation, in acute pulmonary embolism, in acute coronary syndrome and in dilated cardiomyopathy (in Italian) Ann Ital Med Int. 2005;20:167–186. [PubMed] [Google Scholar]
- 17.Celik G, Silinou E, Vo-Van C, Jean G, Chazot C. Plasma BNP, a useful marker of fluid overload in hospitalized hemodialysis patients. Hemodial Int. 2012;16:47–52. doi: 10.1111/j.1542-4758.2011.00627.x. [DOI] [PubMed] [Google Scholar]
- 18.Trezzi M, Torzillo D, Ceriani E, Costantino G, Caruso S, Damavandi PT, Genderini A, Cicardi M, Montano N, Cogliati C. Lung ultrasonography for the assessment of rapid extravascular water variation: evidence from hemodialysis patients. Intern Emerg Med 2011, E-pub ahead of print. [DOI] [PubMed]
- 19.Mayo PH, Beaulieu Y, Doelken P, Feller-Kopman D, Harrod C, Kaplan A, Oropello J, Vieillard-Baron A, Axler O, Lichtenstein D, Maury E, Slama M, Vignon P. American College of Chest Physicians/La Société de Réanimation de Langue Française statement on competence in critical care ultrasonography. Chest. 2009;135:1050–1060. doi: 10.1378/chest.08-2305. [DOI] [PubMed] [Google Scholar]
- 20.Agarwal R, Bouldin JM, Light RP, Garg A. Inferior vena cava diameter and left atrial diameter measure volume but not dry weight. Clin J Am Soc Nephrol. 2011;6:1066–1072. doi: 10.2215/CJN.09321010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noble VE, Murray AF, Capp R, Sylvia-Reardon MH, Steele DJ, Liteplo A. Ultrasound assessment for extravascular lung water in patients undergoing hemodialysis. Time course for resolution. Chest. 2009;135:1433–1439. doi: 10.1378/chest.08-1811. [DOI] [PubMed] [Google Scholar]