Abstract
The publication of the psychomotor stimulant theory of addiction in 1987 and the finding that addictive drugs increase dopamine concentrations in the rat mesolimbic system in 1988 have led to a predominance of psychobiological theories that consider addiction to opiates and addiction to psychostimulants as essentially identical phenomena. Indeed, current theories of addiction — hedonic allostasis, incentive sensitization, aberrant learning and frontostriatal dysfunction — all argue for a unitary account of drug addiction. This view is challenged by behavioural, cognitive and neurobiological findings in laboratory animals and humans. Here, we argue that opiate addiction and psychostimulant addiction are behaviourally and neurobiologically distinct and that the differences have important implications for addiction treatment, addiction theories and future research.
In 1950, addiction experts in the World Health Organization proposed that drug addiction is fundamentally characterized by psychic dependence, independent of drug class1. Subsequently, early psychobiological theories identified common denominators of addiction in phenomena such as psychic tolerance (the presumed cause of escalating drug intake) and psychic withdrawal or abstinence agony (the presumed main obstacle to abstinence)2–4. In the 1970s and 1980s, building on the discovery that electrical stimulation of specific brain areas can induce reward5, investigators proposed that the mesotelencephalic dopamine system is the neurobiological substrate for the rewarding effects of both opiates (for example, heroin and morphine) and psychostimulants (for example, cocaine, amphetamine and metamphetamine)6,7. The same system was also implicated in the motivational effects of drug-associated cues8 and in the development of psychomotor sensitization to addictive drugs9. These neuropharmacological developments were the basis for the influential 1987 psychomotor stimulant theory of addiction10 (BOX 1), as well as for subsequent theories that emphasize shared psychobiological substrates for addiction, across drug classes: incentive sensitization11, aberrant learning12–14, frontostriatal dysfunction15–17 and hedonic allostasis18 (BOX 1). A unified view is at the core of current clinical definitions of drug addiction19.
Box 1 | Theories of addiction.
Over the past decades, several classes of addiction theories have been proposed by animal behaviour researchers and clinical researchers. Below, we describe some of these theories, which over the past two decades have had a substantial influence on the direction that drug addiction research has taken.
Aberrant-learning theories of addiction
These theories propose that repeated exposure to addictive drugs heightens Pavlovian and instrumental responsiveness to drug-associated cues through actions on neurons that control normal responses to non-drug conditioned cues; these actions may occur in the ventral striatum12, dorsal striatum14 or both13. A main theme of these theories is that the heightened responsiveness to drug cues is insensitive to outcome devaluation (for example, punishment), leading to continued drug use even when it has adverse consequences227,228. This aberrant learning process has been suggested to be mediated by a progressive dopamine-dependent ventral-to-dorsal striatal shift in control over drug seeking and drug taking227,228.
Frontostriatal-dysfunction theories of addiction
These theories propose that repeated exposure to addictive drugs causes deficits in top-down executive control over behaviour15–17, leading to loss of impulse control, impaired decision-making processes, exaggerated responsiveness to drug-associated cues and compulsive drug use despite adverse consequences. This idea was first advanced by Jentsch and Taylor15, who proposed that compulsive drug use is due to drug-induced alterations in cortical and limbic circuits, leading to exaggerated responses to drugs and drug-associated cues (owing to nucleus accumbens and amygdala dysfunction) and impaired inhibitory control (owing to medial prefrontal and orbitofrontal cortex dysfunction).
Hedonic-allostasis theory of addiction
A theory that is based on the opponent-process theory of motivation4; it proposes that although initial drug use is primarily controlled by the drug’s rewarding effects, chronic drug use leads to decreases in its rewarding effects and to recruitment of stress-related systems. This leads to a new emotional state, termed the ‘hedonic allostatic’ state, which represents a chronic change in the normal reward setpoint18. According to this theory, hedonic allostasis causes loss of control over drug use through cortico– striatal–thalamic circuits that are involved in compulsive behaviour.
Incentive-sensitization theory of addiction
This theory has three main components: first, the idea that addictive drugs increase mesocorticolimbic dopamine neurotransmission; second, the idea that one psychological function of this brain system is to attribute incentive salience to contexts, cues and other events that are associated with activation of this dopaminergic system; and third, the idea that repeated exposure to addictive drugs produces long-lasting adaptations in this neural system, rendering it hypersensitive to drugs and drug-associated cues11. Incentive salience is defined as a psychological process that increases the valence of reward-associated cues and makes them attractive incentive cues. Robinson and Berridge11 also argued that sensitization of neural systems that mediate incentive salience (drug ‘wanting’) occurs independently of changes in neural systems that control pleasurable effects of drugs (drug ‘liking’). They also suggested that motivation systems that control incentive salience are independent of those controlling drug withdrawal states.
Pschomotor-stimulant theory of addiction
This theory proposes that a common denominator of addictive drugs is their ability to cause psychomotor activation10. The theory is rooted in an earlier theory that all positive reinforcers activate a common biological mechanism that is associated with approach behaviours229. Wise and Bozarth10 put forward the idea that the major substrate of the approach system (and of psychomotor sensitization) is the mesocorticolimbic dopamine system. They also argued that drug withdrawal symptoms, which are drug-class dependent, do not play a major part in controlling compulsive drug use.
Unified theories of drug addiction have led to many important discoveries, some of which are described below, but they have also diverted investigators’ attention away from psychological and neurobiological processes that distinguish opiate addiction from psychostimulant addiction. For example, in the mid 1980s, studies using the intravenous drug self-administration (BOX 2) procedure in rats showed that dopamine-receptor blockade or lesions of the mesotelen-cephalic dopamine system decrease cocaine or amphetamine reward but not heroin or morphine reward20,21 (BOX 3; FIG. 1). The controversy that was stirred by these findings was quickly swept away by the tide of evidence (some of which is discussed below) that was used to support a unitary account of addiction.
Box 2 | Animal models of drug reward, subjective effects and relapse.
For many decades, investigators have used animal models to assess the behavioural effects of abused drugs that are potentially related to their effects in humans186,230–233. Below, we describe the main animal models that are currently used by addiction researchers to study the positive reinforcing (or rewarding) effects of drugs, the subjective effects of drugs and relapse to drug seeking.
Conditioned place preference (CCP) model
A Pavlovian (classical) conditioning model in which during the training phase one distinct context is paired with drug injections and another context is paired with vehicle injections. During the subsequent testing phase (which is drug-free), the animal’s preference for either context is determined by allowing the animal to move between the two contexts. An increase in preference for the drug-associated context serves as a measure of the drug’s Pavlovian reinforcing (or rewarding) effects.
Intravenous drug self-administration model
In this model, animals typically make a lever press or nose poke to receive contingent drug injections. The premise of this procedure is that drugs of abuse control behaviour by functioning as operant positive reinforcers.
Reinstatement model
An animal model of relapse to drug seeking. In the operant-conditioning version of this model, laboratory animals are first trained to self-administer drugs by making a lever press or nose poke, with drug injections typically paired with discrete cues (for example, a tone or a light). Subsequently, the animals undergo extinction training, during which lever presses (or nose pokes) are not reinforced with drug. Reinstatement of lever pressing (or nose pokes) under extinction conditions is then determined after manipulations such as non-contingent priming injections of the drug, exposure to discrete or contextual cues that are associated with drug intake, or exposure to stressors. In the classical-conditioning version of the model, CPP is induced by a drug, extinguished and then induced again by drug priming injections or stressors.
Runway model
In this operant-conditioning model, the speed with which a laboratory animal traverses a long, straight alley for a positive reinforcer (for example, food or a drug) provides an index of the animal’s motivation to seek the reinforcer. The dependent measure in this model is the run-time from a start box to the goal box in which the positive reinforcer (or the reward) is earned.
Drug-discrimination model
An animal model of the subjective effects of drugs. In this model, laboratory rodents or monkeys are trained to discriminate between a drug state and a non-drug state, or between different drug states. In a typical experiment, a food-restricted animal is trained in a two-lever operant chamber in which the food-reinforced lever differs as a function of whether drug or saline was administered before the session. After achieving a training criterion of correct responding, subjects are typically injected with various doses of the training drug to generate dose-response curves or injected with other drugs from the same or different drug classes to test for stimulus generalization.
Box 3| Does dopamine mediate opiate reward?
It is well established that the rewarding effects of psychostimulants are mediated by dopamine projections from the ventral tegmental area (VTA) to the nucleus accumbens (NAc); evidence for this is seen in both the self-administration and the conditioned place preference (CPP) models234–237. However, although addiction and neuroscience experts, the popular press and the public commonly assume that this dopamine projection is also crucial for the rewarding effects of opiates, the literature does not support this general conclusion.
On the one hand, there is evidence that opiate drugs activate VTA dopamine neurons47 and increase NAc dopamine release7. There is also evidence that fluctuations in NAc dopamine levels correlate with heroin self-administration behaviour238. In addition, morphine and other opiate agonists are self-administered directly into the VTA or the NAc and produce CPP following intracranial injections into either site239–243. Lastly, there is some evidence that morphine or heroin CPP is blocked by systemic or NAc injections of dopamine receptor antagonists236,244,245.
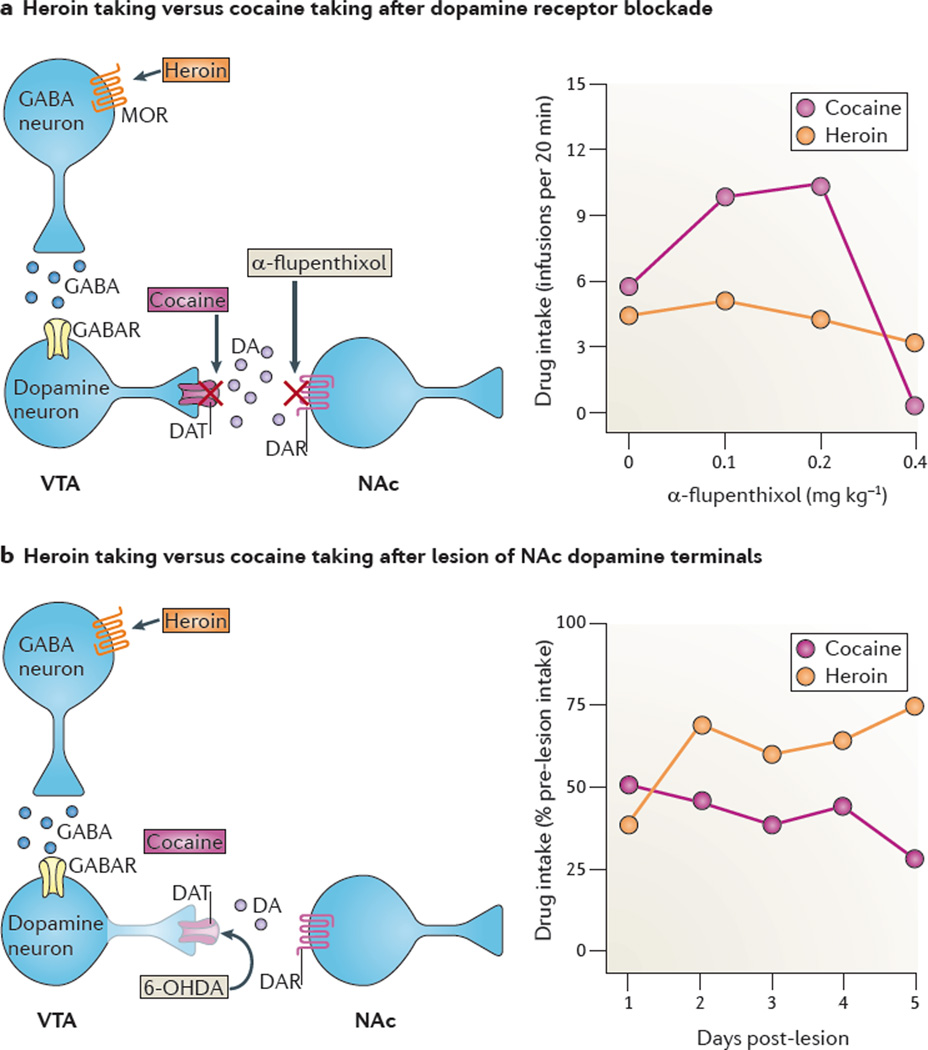
On the other hand, the CPP findings are not consistent across studies: 6-hydroxydopamine (6-OHDA) or excitotoxic lesions of the NAc seem to have no effect on CPP for morphine246,247, and dopamine receptor blockade decreases heroin CPP in heroin-dependent rats but not in non-dependent rats248,249. Self-administration data pose an even greater challenge to the theory of a unified, dopamine-based mechanism of drug reward. There is surprisingly little empirical evidence that dopamine transmission is crucial for self-administration of opiates. For example, systemic injections of dopamine receptor antagonists have minimal effect on self-administration of opiate agonists in rats and monkeys, unless the agonists are administered at doses that are high enough to be sedating20,213,250,251 (FIG. 1). In addition, self-administration of heroin or morphine is only minimally reduced by NAc disruptions such as 6-OHDA lesions or local injections of dopamine receptor antagonists250,252–254 (FIG. 1). Finally, chronic blockade of dopamine receptors with a-flupenthixol strongly potentiates, rather than inhibits, the rewarding effect of low heroin doses255.
In conclusion, as stated 15 years ago by Mello and Negus213, these data and related results “argue against a prominent role for dopamine in opioid self-administration.” Research that has been performed since then has not led to new empirical evidence that can be used to refute this conclusion.
Figure 1. Dopamine receptor blockade or lesions of the mesolimbic dopamine system decrease cocaine reward but not heroin reward.
a | The effect of dopamine receptor blockade: rats were trained to lever press for intravenous heroin (0.06 mg kg−1 per infusion) or cocaine (0.75 mg kg1 per infusion) on a fixed-ratio 1 (FR1) reinforcement schedule (each lever press was reinforced with drug infusion). After stable self-administration, the rats were injected on different days with different doses of the dopamine receptor antagonist a-flupenthixol (left part). Lower doses (0.1 or 0.2 mg kg−1) of a-flupenthixol increased cocaine intake but not heroin intake (right part); this effect presumably reflects a compensatory response to offset a decrease in the rewarding effects of cocaine but not heroin. A higher dose of a-flupenthixol (0.4 mg kg−1), which causes sedation, decreased both heroin and cocaine self-administration (right part). b | The effect of dopaminergic lesions: rats were trained to self-administer heroin or cocaine, as above. After stable self-administration, dopamine terminals in the nucleus accumbens (NAc) were lesioned with 6-hydroxydopamine (6-OHDA) (left part). Post-lesion responding for cocaine decreased over days, reflecting extinction of cocaine-reinforced responding. By contrast, post-lesion responding for heroin increased over days, reflecting recovery of the rewarding effects of heroin (right part). DA, dopamine; DAR, dopamine receptor; DAT, dopamine transporter; GABAR, GABA recptor; MOR, mu opioid receptor; VTA, ventral tegmental area. Part a is modified, with permission, from REF. 20 © (1982) Springer. Part b is modified, with permission, from REF 21 © (1984) Springer.
In this Perspective, our goal is to highlight differences between opiate and psycho-stimulant addictions, using behavioural, cognitive and neurobiological data from laboratory animals and humans. We first discuss differences in the cognitive and neurobiological effects of opiate and psychostimulant administration. We then review data from animal models of addiction showing behavioural and neurobiological differences between opiates and psychostimulants. Next, we consider selected studies in humans that also point to differences between opiate and psychostimulant addiction. We conclude by discussing how behavioural and neurobiological differences between opiates and psychostimulants may have implications for addiction treatment, addiction theories and future research on drug addiction. We restrict the discussion in this Perspective to differences between opiates and psycho-stimulants, but our argument that there are substantial differences in the neurobiological mechanisms of these two classes of drugs is also likely to apply to other classes of drugs of abuse, including nicotine, alcohol, cannabis, benzodiazepine and barbiturates.
Cognitive and neurobiological effects
Cognitive effects
Addiction is associated with impairments in prefrontal cortex (PFC)-dependent cognitive functions; it is thought that these impairments promote compulsive drug use and relapse15,17. Opiate addicts and psychostimulant addicts share some deficits in memory, cognitive flexibility and decision making22–25. Studies using laboratory animals have shown that repeated exposure to cocaine or heroin impairs spatial memory26,27 (however, see REF. 28 for different results) and causes transient deficits in attention29,30. These data suggest common neurobiological substrates for opiate- and psychostimulant-induced cognitive impairment. However, there is evidence that indicates that for some cognitive functions, particularly those related to impulsivity (a personality trait that is associated with drug addiction31,32), there are some fundamental differences between opiates and psychostimulants. For example, cocaine and amphetamine addicts are more impulsive and show more pronounced deficits in attention and cognitive flexibility than heroin addicts33–37. These behavioural differences resonate with observations that functional and structural abnormalities in the prefrontal cortex are less pronounced in heroin addicts than in cocaine addicts38.
It is unclear whether differences between heroin and cocaine addicts are due to drug use or pre-existing differences. Studies in laboratory animals, however, support the former possibility, and they specifically indicate that opiates and psychostimulants have different effects on impulsivity. Withdrawal from cocaine self-administration impaired inhibitory control in both rats39 and non- human primates40, whereas increased impulsivity was not observed in rats after withdrawal from heroin29. Furthermore, non-contingent experimenter-administered cocaine and amphetamine41 increased impulsivity in rats, whereas heroin did not42.
In conclusion, in both humans and laboratory animals, chronic exposure to psycho-stimulants seems to cause more pronounced deficits in impulse control and cognitive flexibility than chronic exposure to opiates.
Neurochemical and neurophysiological effects
Opiates and psychostimulants have very different pharmacodynamic profiles10,12,43 but they share the ability to increase dopamine levels in the nucleus accumbens (NAc)7 — one of the terminal regions of the mesocorticolimbic dopamine system — and this increase plays an important part in the rewarding effects of drugs and non-drug stimuli44,45. Psychostimulants do so by blocking dopamine reuptake or inverting dopamine transport43, whereas opiates indirectly activate dopaminergic neurons in the ventral tegmental area (VTA) — the cell-body region of the mesocorticolimbic dopamine system — through inhibition of GABAergic interneurons46,47.
Such similarities in the neurochemical effects of opiates and psychostimulants help to explain why these drugs produce similar effects on neuronal activity in the NAc and in the medial PFC (mPFC) — another terminal region of the mesocorticolimbic dopamine system that has been implicated in the behavioural and cognitive effects of addictive drugs31,48 and in relapse to drug use (see below). In vivo extracellular recording has been used to show that small populations of neurons in these regions are in fact either excited or inhibited during heroin or cocaine self-administration in the rat49,50. However, when neural activity was assessed using multiple-channel single-unit recordings in rats that consecutively self-administered heroin and cocaine (in the same session), only a small number of drug-responsive neurons (~20%) in the mPFC and NAc showed similar responses to both drugs51. Thus, the rewarding effects of heroin and cocaine seem to be encoded by distinct neuronal subpopulations.
Neuroadaptations
Since the early 1990s52,53, a central neurobiological framework for addiction research has been that compulsive drug use and relapse are due to drug-induced neuroadaptations in the mesocorticolimbic dopamine system and in the glutamatergic corticolimbic circuitry in which the dopamine projections are embedded54–56. An implicit assumption has been that the neuroadaptations are independent of drug class11,54, and indeed, some are. For example, both opiates and psychostimulants induce changes in intracellular signal transduction pathways in the mesocorticolimbic dopamine system54,57, induce long-term potentiation (LTP) of glutamatergic synapses in the VTA58–60 impair LTP in the bed nucleus of stria terminalis (BNST)61, and cause sensitization of dopamine and glutamate transmission in the terminal regions of the mesotelencephalic dopamine system52,62. In addition, withdrawal from both opiates and psychostimulants is associated with short-term decreases (a few days) in NAc dopamine levels63. However, there are also notable neurobiological differences, which are discussed below.
One difference concerns drug-induced synaptic plasticity. Studies using ex vivo whole-cell electrophysiology have shown that morphine and cocaine differ in their ability to induce LTP and long-term depression (LTD) at GABAergic synapses (LTP GABA and LTD GABA) on VTA dopamine neurons. Morphine exerts bidirectional control on such synapses: a single non-contingent injection of morphine in rats abolished both LTPGABA and LTDGABA64,65 in brain slices. By contrast, cocaine seems to downregulate the strength of such synapses: in rats, a single injection of cocaine had only modestly attenuating effects on LTPGABA65, and repeated injections occluded endocannabinoid-dependent LTD66, suggesting an induction of an LTD-like state by cocaine in vivo.
Additional differences in synaptic plasticity between opiates and psychostimulants are seen in the consequences of drug withdrawal on LTP in the mPFC. Facilitation of LTP in the mPFC of rats occurred after withdrawal from repeated cocaine exposure67,68. By contrast, withdrawal from heroin self-administration in rats had no effect on LTP as measured by the AMPA:NMDA ratio in the mPFC69. Furthermore, exposure to cues that had previously been associated with heroin intake reduced the AMPA:NMDA ratio in this area, suggesting decreased LTP69. These discrepancies should be interpreted with caution, however, as there were several important experimental differences between the studies, including the age of the rats, the route and type of drug administration (self-administration versus experimenter-delivered), the length of the withdrawal period, the electrophysiological end-points and the mPFC subregions. Nevertheless, the LTP results suggest that exposure to opiates and exposure to psychostimulants can cause qualitatively different changes in mPFC synaptic plasticity.
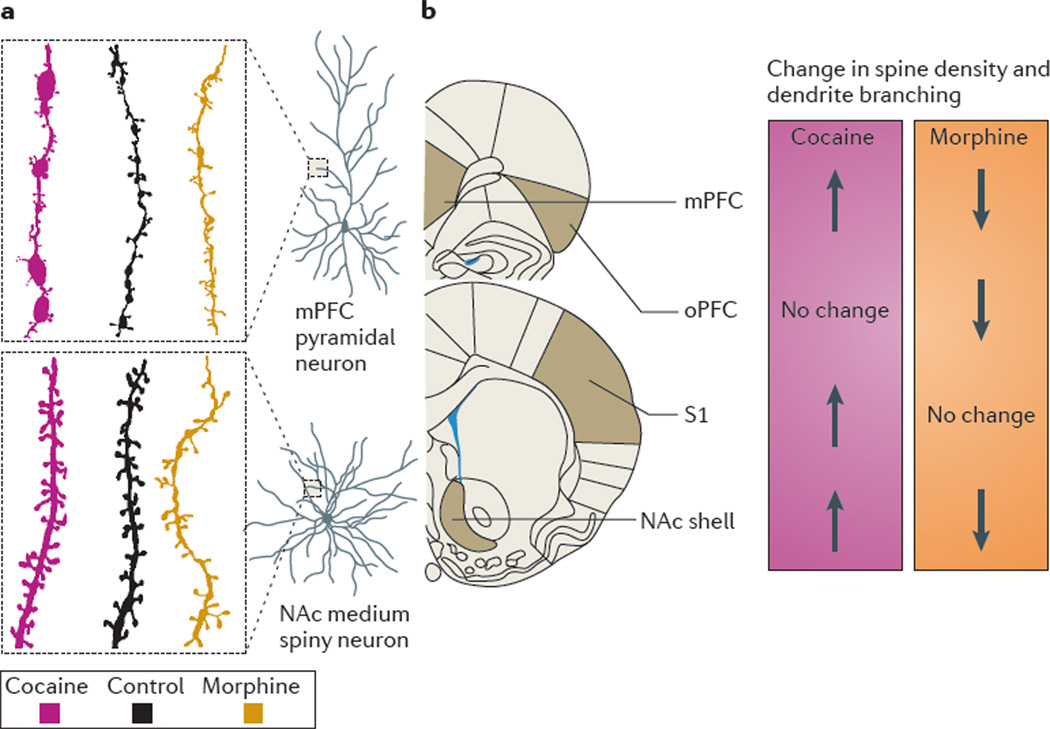
A second notable difference between opiates and psychostimulants concerns their effects on structural plasticity. In 1997, Robinson and Kolb70 found that repeated non-contingent injections of amphetamine in rats induce persistent increases in dendrite branching and spine density in NAc medium spiny neurons and mPFC layer III pyramidal neurons. These findings were extended to cocaine and amphetamine self-administration71,72. By contrast, morphine self-administration had the opposite effect; it causes long-lasting decreases in the complexity of dendritic branching and in the number of dendritic spines in NAc and mPFC71 (FIG. 2).
Figure 2. Morphine and cocaine have opposite effects on structural neuroplasticity in the NAc and mPFC.
a | Groups of rats were trained to self-administer morphine or cocaine intravenously for several weeks. The control groups were given daily intravenous infusions of vehicle for the same period of time. After 1 month of withdrawal from the drugs, the rats’ brains were processed using the Golgi staining procedure. Rats that were exposed to cocaine showed increased dendritic branching and increased spine density in both nucleus accumbens (NAc) medium spiny neurons and medial prefrontal cortex (mPFC) pyramidal neurons. By contrast, rats that were exposed to morphine had both reduced dendritic branching and reduced spine density in these brain regions. b | A summary of changes in spine density and dendritic branching that occur after exposure to cocaine or morphine relative to controls. A dissociation between the effects of cocaine and morphine was also observed in the orbital prefrontal cortex (oPFC) and in the primary somatosensory cortex (S1). Data from REF. 71.
These opposing effects might be explained by the differential engagement of the direct and indirect striatal pathways73, as indicated by changes in expression of immediate early genes such as FBJ osteosarcoma oncogene (Fos)74–76. In neurons of the direct pathway, both opiates and psychostimulants increase the expression of Fos74–76. In neurons of the indirect pathway, only psycho-stimulants increase Fos expression, whereas opiates (in this case, morphine) reduce it74–76. This differential regulation of Fos expression is potentially important because repeated drug-induced FOS (the protein product of the Fos gene) induction in the NAc leads to the formation of a more stable form of the FOS protein called ΔFOSB77, which plays a major part in drug-induced neuroadaptations in the striatum54, including the regulation of dendritic branches and spines78,79.
Lastly, a post-mortem study in cocaine and heroin addicts supports the idea that chronic exposure to these drugs leads to dissociable neuroadaptations: out of approximately 39,000 gene transcripts that were investigated in the NAc, only 25 genes showed changed expression in both cocaine and heroin abusers and in nearly half of these cases, the drugs had opposite effects on expression80. A question for future research is whether opposing cocaine- and heroin-induced changes in neuronal morphology and gene expression help to explain the drugs’ differing behavioural effects in animal models, which are described below.
Animal models
Drug addiction is not an automatic outcome of drug use. Only approximately 20% of people who use addictive drugs will switch from controlled to compulsive use81. Thus, one of the aims of modelling drug addiction in the laboratory is to identify the mechanisms that are responsible for the transition from one stage of the disorder to the next: from initial drug use to chronic drug use and then to compulsive, relapsing drug abuse. Vulnerable individuals often exhibit distinct personality traits or psychiatric profiles that are thought to facilitate this transition82. Not surprisingly, this vulnerability seems to be influenced not only by genes but also by environmental factors83, including adverse life experiences (especially in childhood), acute exposure to stressors, drug-associated contextual and discrete cues (acting as conditioned stimuli), and other, more subtle aspects of the environment84. Indeed, the behavioural and subjective effects of addictive drugs should be seen as the result of complex interactions among the drug, the user’s physiological and mental state (also referred to as ‘set’), and the circumstances of drug taking (referred to as ‘setting’)84–86. Current animal models of drug addiction can help to clarify how drug, set and setting interact to produce the transition from controlled to compulsive drug use84. Under certain conditions, these models reveal important differences between opiates and psychostimulants.
Vulnerability to initial drug use
There are several similarities between the initiation of opiate self-administration and the initiation of psychostimulant self-administration. At the most fundamental level, many studies that use intravenous drug self-administration (the gold-standard procedure for assessment of abuse liability) show that agonists of both drug classes are self-administered by rodents and monkeys87–89. In addition, food restriction strongly facilitates the acquisition of self-administration of both opiates and psychostimulants90, as do other environmental stressors, under certain conditions91,92. Furthermore, repeated non-contingent administration (which causes psychomotor sensitization) facilitates the acquisition of self-administration of both opiates and psychostimulants, as well as inducing conditioned place preference (CPP)93–95(BOX 2). Moreover, for both drug classes, the speed with which self-administration is acquired can be predicted by certain behavioural traits, including high preference for sweet solutions96 and high locomotor response to a novel environment (which is thought to model novelty seeking; a psychological trait that is associated with the initiation of drug use in humans)97–99. Furthermore, for both drugs, inter-individual differences in the propensity to acquire self-administration are associated with inter-individual differences in the extent to which drug-induced dopamine release in the NAc is modulated by the stress hormone corticosterone (through its actions on glucocorticoid receptors in the VTA and the NAc for opiates and psychostimulants, respectively)100,101.
However, there are several fundamental differences in the behavioural effects of opiates and psychostimulants. For example, in rats, exposure to cocaine causes an approach–avoidance conflict towards places that are associated with injection of the drug, whereas exposure to heroin does not. Studies that use a runway model (BOX 2) suggest that intravenous heroin induces an appetitive incentive motivational state that causes an approach behaviour, similar to that induced by palatable food in hungry rats102,103. By contrast, intravenous cocaine induces a motivational state with both an appetitive and an aversive component, leading to approach–avoidance behaviour similar to that caused by simultaneous exposure to food and shock in hungry rats104,105 (FIG. 3a). The mixed motivational state that is induced by cocaine is also observed in the CPP procedure: immediate intravenous cocaine administration (within the 5 min preceding a CPP training session) causes place preference, but delayed cocaine administration (15 min before a training session) causes place aversion106. These data from rats seem consistent with human epidemiological data that show an association between cocaine use and anxiety disorders107,108, and with human laboratory studies in which immediate self-reports of a cocaine high were followed by delayed (~8 min) negative affect-related self-reports of anxiety, paranoia, dysphoria or anhedonia109.
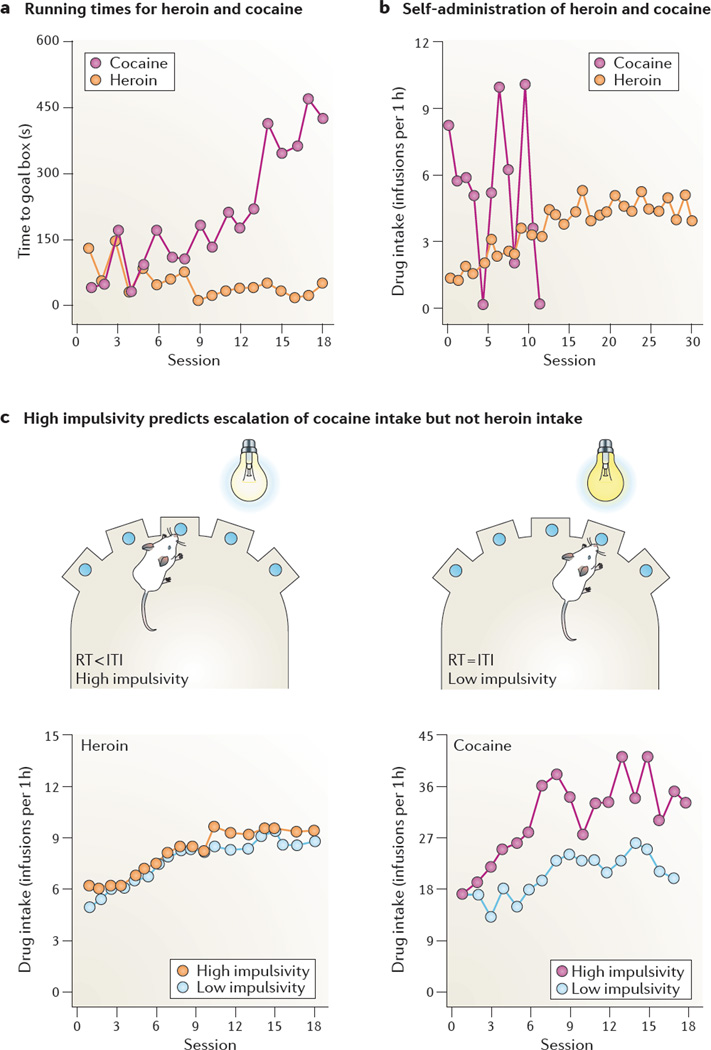
Figure 3. Initiation of drug use and transition to compulsive drug use.
a | In a runway procedure, two groups of rats were trained to traverse a straight alley to obtain either five heroin injections (0.06 mg kg−1 per infusion) or five cocaine injections (0.75 mg kg−1 per infusion) in the goal box. In heroin-trained rats, the running times of rats to the goal box to obtain heroin infusions decreased over days, indicating that heroin serves as an operant reinforcer (reward). In cocaine-trained rats, the time to obtain cocaine infusions increased over days, owing to repeated cycles of forward locomotion and retreats before reaching the goal box (a behavioural pattern that mimics the behaviour of hungry rats that receive both food and shock in the goal box). b | In the unlimited drug self-administration procedure, two groups of rats were trained to lever press for intravenous heroin (0.01 mg kg−1 per infusion) or cocaine (1.0 mg kg−1 per infusion) on a fixed-ratio 1 (FR1) reinforcement schedule for 24 hours per day. Heroin-trained rats gradually increased their drug intake over days and then maintained stable drug intake, whereas cocaine-trained rats rapidly lost control over cocaine intake and died of drug overdose within 12 days. cTo test the effect of impulsivity on self-administration, groups of naive rats were first assessed for trait impulsivity in the five-choice serial-reaction time test. Animals that showed premature responses — reaction times (RTs) were shorter than the inter-trial interval (ITI) — were classified as high impul-sivity, and animals that responded only when a stimulus appeared — reaction times were equal to the inter-trial interval — were classified as low impulsivity (top panels). They were then trained to lever press for either intravenous heroin (0.04 mg kg−1 per infusion) or cocaine (0.25 mg kg−1 per infusion). After 5 days of short access (1 h per d) to heroin or cocaine, the rats were given extended (6 h per d) access to the drugs for 18 consecutive days. High impulsivity predicted escalation of cocaine self-administration but not heroin self-administration (bottom panels). Part a is modified, with permission, from REF. 103 © (1993) Elsevier. Part b, data from REF. 117. Part c, left graph is modified, with permission, from REF. 29 © (2010) Springer. Part c, right graph is modified, with permission, from REF. 133 © (2007) American Association for the Advancement of Science.
A second example is that sex hormones seem to have different effects on the initiation of cocaine self-administration and the initiation of heroin self-administration. Female rats acquire cocaine self-administration faster than males; this sex difference is mediated by ovarian hormones110,111. For heroin, evidence for a sex difference is mixed. Two studies have shown that intact female rats acquire heroin self-administration faster than males96,112. By contrast, a study in which male rats were compared with intact females and with ovariectomized females that were given hormonal replacement found neither sex differences nor a role of ovarian hormones in the acquisition of heroin self-administration113. What might account for the mixed results? One issue to consider is that the rats in the ‘positive outcome’ studies received non-contingent injections of heroin before the daily sessions96,112, whereas the rats in the ‘negative outcome’ study did not113. Repeated non-contingent drug exposure is known to produce psychomotor sensitization and to facilitate the acquisition of drug self-administration94,95. As female rats develop faster morphine-induced psychomotor sensitization114, the observed sex differences in heroin intake in the ‘positive’ studies may simply be attributable to the experimental procedures.
Taken together, results from studies on the initiation of cocaine and heroin self-administration in rats suggest that initial cocaine exposure induces a mixed approach–avoidance motivational state that is not observed with heroin, and that sex differences and ovarian hormones may play a more prominent part in the initiation of cocaine self-administration than in the initiation of heroin self-administration.
Transition to compulsive drug use
Researchers have attempted to model in animals the loss of control over drug intake that characterizes addiction in humans19. One approach is to give rats prolonged (for example, 6 h per day)115,116 or unlimited daily access to the drug117–119. Under such conditions, most rats progressively escalate their drug intake, a phenomenon that is not observed in rats with limited drug access (for example, 1 h per day)115,116. Compulsive drug use has also been modelled in laboratory animals by imposing negative consequences on drug seeking and drug taking120. These include adding quinine (a bitter, aversive substance) to rewarding alcohol solutions121, administering shock to punish drug-taking or drug-seeking responses122–124, or exposing rats to fear-inducing conditioned cues that would normally inhibit operant responding125. Under these circumstances, some of the rats persist in drug seeking or drug taking.
These models show several similarities between heroin and cocaine, at least at the behavioural level29,115,121,126. There are, however, important differences. For example, in rats that are given prolonged drug access, escalation of heroin self-administration does not predict escalation of cocaine self-administration, and vice versa127. In addition, extended access to heroin self-administration is associated with increased resistance to extinction (that is, after extended access, animals show more persistent attempts to obtain the drug when it is no longer available), whereas this is not the case for cocaine self-administration115.
Another well-established difference is that rats that are given unlimited access to opiates gradually increase drug intake, whereas rats that are given unlimited access to psychostimulants cycle between days of binge intake and days of markedly reduced intake118,128. The consequences of this difference were shown in a study in which cocaine- and heroin-trained rats were given unlimited access to their drug: the heroin-trained rats gradually increased their intake over days and then maintained stable intake, whereas the cocaine-trained rats rapidly lost control over intake and, within 12 days, died of overdose117 (FIG. 3b).
A third difference is that social defeat promotes escalation of psychostimulant self-administration but not opiate self-administration. Social-defeat stress (intermittent exposure to a dominant male) promotes the development of psychomotor sensitization to both opiates and psycho-stimulants129; this effect is mediated by VTA NMDA receptors130, which control mesolimbic dopamine activity131. However, social-defeat stress facilitated the progression to binge-like drug intake in rats that had unlimited access to cocaine but not in those that had unlimited access to heroin132. This finding suggests a dissociation between psychomotor sensitization and drug-taking behaviour for opiates but not for psycho-stimulants. The difference between cocaine intake and heroin intake in the social-defeat paradigm might reflect the differential roles of the mesolimbic dopamine system in heroin self-administration versus cocaine self-administration20,21 (BOX 3).
Lastly, escalation of cocaine self-administration is predicted by high trait impulsivity, whereas escalation of heroin self-administration is not. As described earlier, cocaine and heroin have different effects on the expression of impulsive behaviours in rats. There is also evidence that trait impulsivity, which can be assessed before drug self-administration, predicts escalation of cocaine but not heroin intake. Thus, high trait impulsivity, reflected in premature responses in a five-choice serial reaction-time test, predicted escalation of cocaine self-administration but not heroin self-administration29,133 (FIG. 3c). In the cocaine study, trait impulsivity was also associated with low expression of D2 dopamine receptors in the NAc133. This indicates that the observed differences between the effects of cocaine and heroin may be yet another illustration of the differential role of the mesolimbic dopamine system in psycho-stimulant but not opiate self-administration (BOX 3).
Taken together, data from animal models indicate that when rats are given unlimited access to psychostimulants, they develop uncontrolled binge intake behaviour that is not seen in rats that are given unlimited access to opiates. In addition, prior exposure to social stress promotes escalation of cocaine self-administration but not heroin self-administration. Lastly, trait impulsivity predicts escalation of cocaine intake but not heroin intake.
Drug seeking and relapse
In humans, drug craving and relapse during abstinence are often triggered by acute re-exposure to the self-administered drug134, drug-associated cues135 or stress136,137. This clinical scenario can be modelled using a reinstatement procedure (BOX 2) in which laboratory animals are exposed to non-contingent injections of the self-administered drug or related drugs (a drug priming manipulation)138, drug cues139 or stress140. Cue-induced drug seeking and drug craving can also be modelled using second-order schedules of reinforcement141 and extinction142 procedures. The use of reinstatement and extinction procedures has led to the discovery of incubation of drug craving143, which seems relevant to human addiction144.
These animal models have not provided much evidence for a difference between cocaine and heroin relapse at the behavioural level, except for a finding that is discussed in the next section. Studies in rats have shown that after extinction, seeking of cocaine and heroin is reliably reinstated by acute injections of the drug, by different types of cues (discrete, discriminative or contextual) that are associated with the drug or by stressors such as intermittent footshock or yohimbine (a drug that induces stress-like responses in humans and non-human animals)145–148. Discrete cues that are paired with either cocaine or heroin injections also maintain robust drug seeking in second-order schedules of reinforcement141,149, and incubation of drug craving is equally robust for cocaine, methamphetamine and heroin150–152.
There is also evidence of similarities at the neurobiological level (FIG. 4b). Reinstatement of both heroin and cocaine seeking induced by drug priming and different cue types requires dopaminergic projections from the VTA to the NAc and mPFC147,153,154. Cue-induced seeking of both heroin and cocaine also seem to require the dorsolateral striatum155–157. In addition, drug priming-induced and discrete cue-induced reinstatement of both cocaine and heroin seeking require glutamatergic projections from the dorsal mPFC to the NAc core147,158,159. Lastly, reinstatement of both cocaine seeking and heroin seeking induced by intermittent-footshock stress requires activity in extrahypothalamic corticotropin releasing factor (CRF) and central noradren-aline systems160,161.
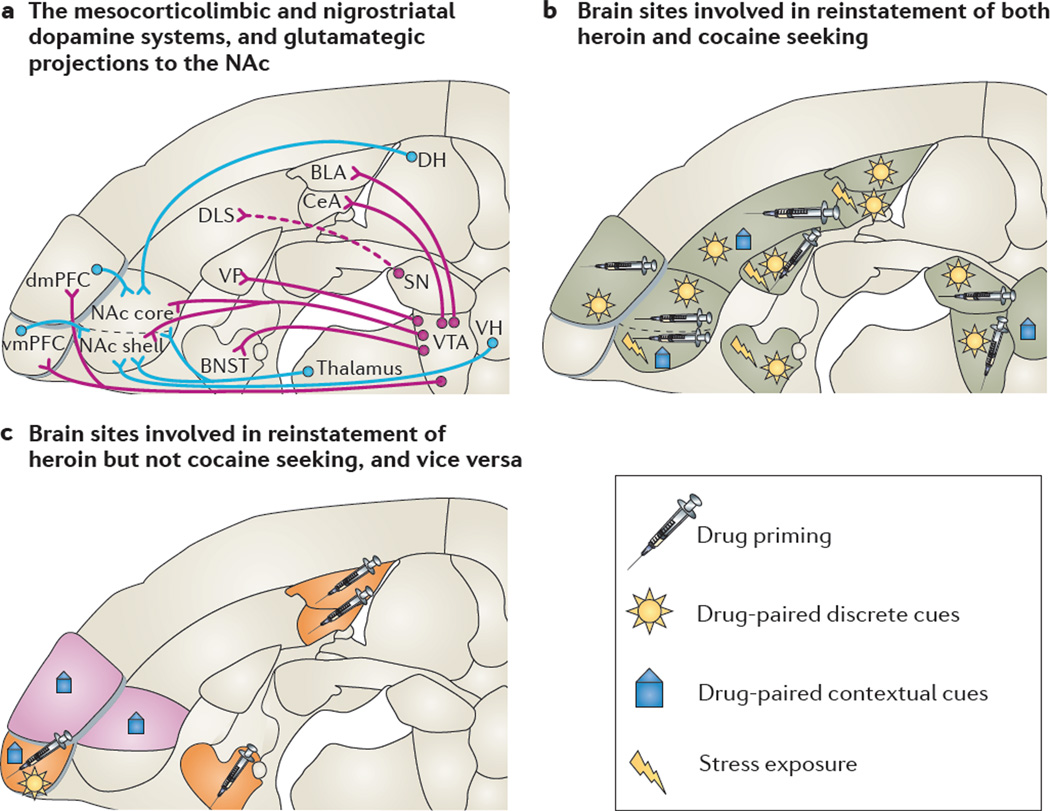
Figure 4. Similarities and differences in the brain sites controlling reinstatement of cocaine seeking and heroin seeking.
a | Horizontal section showing the mesocorticolimbic dopamine pro-jections (shown by purple lines) from the ventral tegmental area (VTA) and nigrostriatal dopamine projections (shown by dashed purple lines) from the substantia nigra (SN) to various brains areas, and the glutamatergic projections (shown by blue lines) to the nucleus accumbens (NAc). b | Several brain sites are implicated in the reinstatement of both heroin seeking and cocaine seeking. The brain areas that are involved depend on the way in which reinstatement is induced — by exposing animals to non-contingent injections of a drug (‘drug priming’), to drug-associated discrete or contextual cues, or to stress. c | Some brain sites are differentially implicated in heroin reinstatement (shown in orange) and cocaine reinstatement (shown in purple), depending on how reinstatement was induced. The basolateral and central nuclei of the amygdala (BLA and CeA, respectively), the bed nucleus of the stria terminalis (BNST) and the ventromedial prefrontal cortex (vmPFC) are differentially implicated in the reinstatement of heroin seeking induced by heroin priming. The vmPFC is also differentially involved in the reinstatement of heroin seeking that is induced by exposure to heroin-paired discrete cues or contextual cues. By contrast, the dorsomedial PFC (dmPFC) and the NAc core are differentially implicated in the reinstatement of cocaine seeking induced by exposure to cocaine-associated contextual cues. DH, dorsal hippocampus; DLS, dorsolateral striatum; VH, ventral hippocampus; VP ventral pallidum. Brain sections are modified, with permission, from REF. 256 © (2005) Elsevier.
However, there are also several differences (FIG. 4c). First, reinstatement of heroin seeking seems to involve more brain sites compared to reinstatement of cocaine seeking. Cocaine priming-induced reinstatement is attenuated by reversible inactivation of the VTA, dorsal mPFC, NAc core or ventral pallidum, but not of the ventral mPFC, NAc shell, substantia nigra, central and basolateral amygdala or mediodorsal thalamus159. By contrast, heroin priming-induced reinstatement is attenuated by reversible inactivation of any of the above brain areas, as well as the BNST162.
Second, pre-training excitotoxic (that is, permanent) lesions of the basolateral amygdala attenuated discrete cue-induced cocaine seeking but not discrete cue-induced heroin seeking in a second-order schedule of reinforcement163,164. No such dissociation was found after local reversible inactivation165,166. The discrepant results may reflect differences in the experimental procedures: although both second-order schedule and cue-induced reinstatement procedures assess the conditioned reinforcing effects of reward cues142, cue-induced reinstatement does not include drug delivery. Another possible explanation for the discrepancy is the use of different lesion and inactivation methods, and differences in the timing of their application (permanent cell-specific excitotoxic lesions before training163,164 versus acute reversible inactivation of both cell bodies and fibres of passage by tetrodotoxin165,166). A question for future research is whether reversible inactivation of the basolateral amygdala after training would decrease cue-induced heroin seeking in the second-order schedule, as it did with cue-induced heroin seeking in the reinstatement model165.
Third, context-induced reinstatement of cocaine seeking seems to involve subregions of mPFC and NAc that are functionally dissociable from those involved in context-induced reinstatement of heroin seeking. For cocaine, context-induced reinstatement is attenuated by reversible inactivation of the dorsal but not the ventral mPFC167, whereas the opposite is the case for heroin168. In addition, context-induced reinstatement of cocaine seeking is attenuated by reversible inactivation of either the NAc core or shell169, whereas context-induced reinstatement of heroin seeking is attenuated by manipulations of the NAcc shell and not by manipulations of the NAc core170,171. This possible difference between heroin and cocaine reinstatement should be interpreted with caution, because the manipulations that were used to test heroin reinstatement (dopamine-receptor blockade and inhibition of glutamate transmission) differed from those used to test cocaine reinstatement (muscimol in combination with baclofen), and these manipulations can have different effects on behaviour172. Furthermore, studies by Bossert et al.146,168, described above, indicate that reinstatement of heroin seeking requires activity in the ventral mPFC and NAc shell. By contrast, reinstatement of cocaine seeking is induced by reversible inactivation of the ventral mPFC (infralimbic area) or NAc shell169,173 and is attenuated by ventral mPFC AMPA receptor activation173. Functionally disconnecting these two brain regions by a unilateral inhibition of ventral mPFC and simultaneous unilateral inactivation of the NAc shell mimics the reinstatement of cocaine seeking induced by bilateral inactivation of either brain area173, suggesting that activation of projections from the ventral mPFC to the NAc shell inhibits cocaine seeking after extinction174.
Lastly, recent data suggest that incubation of psychostimulant craving and incubation of opiate craving have different underlying mechanisms143. In this regard, glial cell line-derived neurotrophic factor (GDNF) activity in the VTA is crucial for incubation of cocaine but not heroin craving175,176.
What might account for a divergence between the circuits that mediate the reinstatement of cocaine seeking versus heroin seeking? A possible explanation is the differences in the psychological states that are induced by cocaine versus heroin, and by extension, in the psychological states that are induced by cues associated with them. As mentioned above, runway studies by Ettenberg and colleagues102,177 (FIG. 3a) suggest that heroin induces seemingly pure approach behaviour, whereas cocaine induces approach–avoidance conflict behaviour. We speculate that the partial dissociation of brain circuits that control cocaine and heroin seeking (FIG. 4) may mirror this difference in motivational states. Given the evidence for a substantial aversive component in the response to cocaine177, it is perhaps not surprising that the circuits that control inhibition of cocaine seeking after extinction are more similar to the circuits that control fear inhibition174 than are those that control inhibition of heroin seeking.
In conclusion, although there are similarities between the brain circuits that control opiate and psychostimulant seeking in animal models of relapse, there are some notable differences with regard to drug-priming and context-induced reinstatement of drug seeking, and incubation of drug craving.
The setting of drug taking
It has been known for many years that environmental contexts or places in which drugs are taken play an important part in human addiction84–86,178. So far, animal research has focused mostly on the ability of the environmental context to alter drug seeking or drug taking by inducing stress or conditioned responses. In the previous section, we provided several examples of this. However, setting can also affect drug taking in ways that are not easily attributable to stress or conditioning. For example, the presence of novel objects can reduce intake of amphetamine179,180, and high temperatures can increase intake of 3,4-methylenedioxymethamphetamine181.
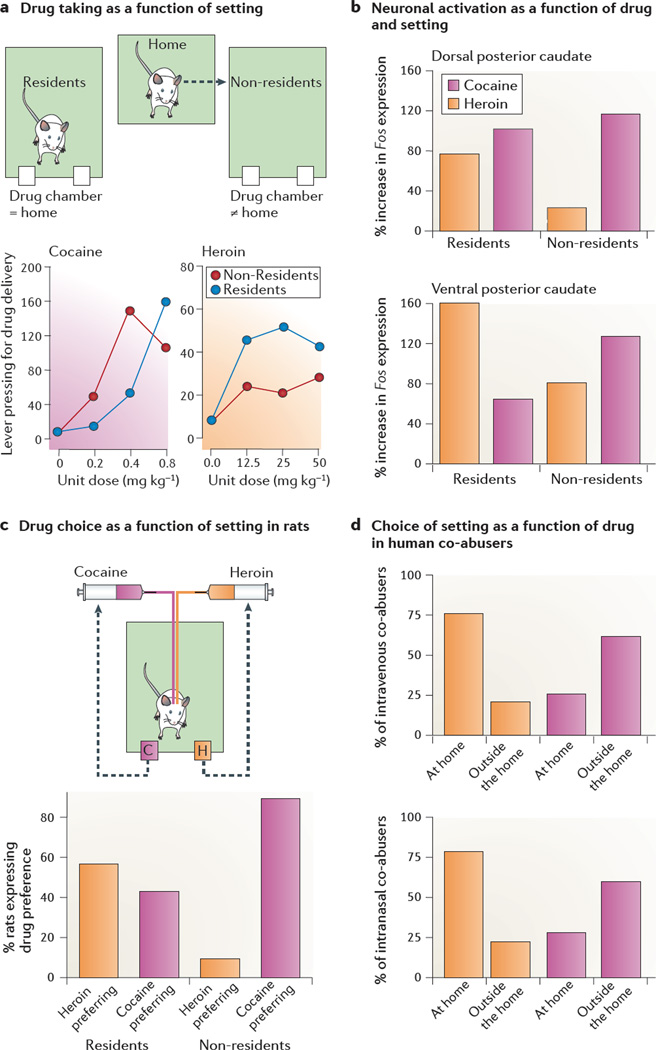
Even non-physical, seemingly negligible differences in setting can powerfully alter drug-taking behaviour, as indicated by a series of studies in which rats were trained to self-administer heroin or cocaine under two deceptively similar environmental conditions. Some rats were transferred to self-administration chambers immediately before experimental sessions (non-resident rats), a procedure commonly used in most self-administration studies. Other rats were kept in the self-administration chambers at all times (resident rats). Thus, the physical characteristics of the self-administration environment for resident versus non-resident rats were virtually identical, with all differences being purely a function of familiarity84 (FIG. 5). These studies yielded three major findings that challenge the prevailing view that environmental contexts influence opiate and psychostimulant drug taking and drug seeking in a similar way.
Figure 5. Setting differentially affects heroin and cocaine use in rats and humans.
a | In a study that examined drug taking as a function of setting, standard two-lever self-administration chambers were used (one lever was paired with drug infusions and the other lever was inactive). Some rats were transferred to the chambers immediately before the start of the sessions (referred to as non-resident rats), whereas other rats were kept in these chambers at all times (referred to as resident rats). Heroin was more rewarding in the resident rats than in the nonresidents rats (indicated by an upward shift in the dose-response curve). By contrast, cocaine was more rewarding in the non-resident rats than in the residents rats (indicated by a left shift in the dose-response curve of non-resident rats). b | In a study that examined drug-induced neural activity in the caudate nucleus as a function of setting, drug-naive resident and non-resident rats with intravenous catheters received a single ‘self-administration dose’ of either heroin (25 µg kg−1) or cocaine (400 µg kg−1), and their brains were processed 30 min later for in situ hybridization of Fos mRNA expression. Cocaine exposure induced greater increases in Fos expression in the dorsal and ventral parts of the caudate nucleus of non-resident rats than in that of resident rats, whereas heroin exposure induced greater increases in Fos expression in resident rats than non-resident rats. c | In a study that examined drug preference as a function of setting, resident and non-resident rats with double-lumen catheters were first trained to self-administer heroin and cocaine on alternate days, and were then given the opportunity to choose between cocaine and heroin within the same session. Most resident rats preferred heroin over cocaine, whereas most non-resident rats preferred cocaine over heroin. d | In a study that examined setting preferences as a function of drug in humans, most addicts reported using heroin at home and cocaine outside the home, regardless of whether the two drugs were injected (top panel) or snorted (bottom panel). Only a minority of addicts (<10%) indicated no clear preference for the setting of drug taking. Part a, left graph is modified, with permission, from REF. 183 © (2007) Springer. Part a, right graph, data from REF. 182. Part b is modified, with permission, from REF. 188 © (2009) Springer. Part c is modified, with permission, from REF. 184. © (2009) Elsevier. Part d, data from REFS. 184, 207.
First, cocaine and amphetamine self-administrations were greater and seemed to be more rewarding (when tested in a progressive-ratio schedule) in non-resident rats than in resident rats (FIG. 5). By contrast, heroin self-administration was greater and more rewarding in resident rats than in non-resident rats182,183. Second, when rats with double-lumen catheters were permitted to self-administer either cocaine or heroin within the same session, most resident rats preferred heroin over cocaine, whereas most non-resident rats preferred cocaine over heroin184 (FIG. 5). Third, preliminary data suggest that resident and non-resident rats show differential reinstatement of drug seeking after cocaine or heroin priming185. Resident and non-resident rats were first trained to self-administer heroin and cocaine on alternate days. After extinction, heroin priming had a stronger effect on reinstatement in the resident rats than in the non-resident rats. By contrast, cocaine priming had a stronger effect on reinstatement in the non-resident rats than in the resident rats.
Additional experiments using the drug-discrimination procedure186 suggest that opiates and psychostimulants produce interoceptive cues of different strength in resident rats and non-resident rats. Non-resident rats discriminated amphetamine or cocaine from saline more readily than resident rats, whereas resident rats discriminated heroin from saline more readily than non-resident rats84,187. Thus, the setting of drug exposure seems to modulate the effects of opiates and psychostimulants in opposite directions with regard to two major attributes of addictive drugs: the degree of reward and the strength of subjective effects186.
Why do the environmental settings differentially affect heroin and cocaine taking, as well as the propensity to relapse to drug seeking? At a proximal, neurobiological level, there is evidence that the initial exposure to low doses of intravenous heroin and cocaine (such as those used in self-administration experiments) differentially activate dorsal striatum neurons in resident and non-resident rats, respectively188 (FIG. 5). The functional significance of this differential neuronal activation is a subject for future research.
At a more distal level, it is tempting to view the setting as an ecological backdrop against which drug effects are appraised as adaptive or maladaptive184. Thus, the sedative effects of heroin may facilitate introspection in a safe, non-challenging home environment, but may be appraised as performance impairing in a potentially unsafe non-home environment. By contrast, the arousing and activating effects of cocaine may be appraised as performance enhancing in a challenging non-home environment, whereas the same state may be appraised as mainly anxiogenic in the home environment. This hypothesis requires rigorous testing but initial evidence in support of this idea comes from studies showing that ketamine — which, like cocaine, has activating and sympathomimetic effects — is more readily self-administered by rats in the nonresident environment189. By contrast, alcohol — which, like heroin, initially causes drowsiness and sedation — is more readily self-administered in the resident environment190.
The ability of setting to differentially affect heroin and cocaine reward may have important implications for the incentive sensitization theory of addiction. Earlier studies have shown in fact that repeated administrations of heroin or morphine produce greater psychomotor sensitization in non-resident than in resident rats191,192. Thus, it appears that psychomotor sensitization and drug reward can be modulated in opposite directions, at least under certain circumstances. This discrepancy goes beyond the reports of mere dissociation between psychomotor sensitization and drug reward193,194.
In conclusion, in laboratory rats, the setting of drug availability affects heroin taking and seeking, and cocaine taking and seeking in a different way. Below, we discuss results that suggest that this is also the case in humans.
Epidemiological and clinical aspects
At the epidemiological level, heroin and smoked cocaine seem similar in terms of the severity and type of harm that they are likely to cause to drug users195, although the proportion of users who become addicted is somewhat higher for heroin (~23%) than for cocaine (~17%)196. Users of these drugs also show similar likelihoods of relapse in the year following treatment197. Non-pharmacological treatments, such as contingency management or cognitive behavioural therapy, are moderately effective in both types of addiction198,199. Lastly, attempts to identify trait determinants of the ‘drug of choice’, in terms of self-medication hypotheses200, have not fared well201–203, and it has even been suggested that an addicted person’s choice of drug is largely determined by chance204.
Nevertheless, there is ample evidence that psychostimulant addiction and opiate addiction have distinct profiles, and in the following sections we briefly discuss selected studies that point to fundamental differences between these two drug classes in human addicts.
Genetic and environmental factors in the vulnerability to opiate and psychostimulant use
There is some evidence that shared genetic and environmental influences contribute to a generalized vulnerability to drug abuse and dependence83,205. However, there is also evidence that unique genetic and environmental factors underlie a differential vulnerability to heroin versus cocaine use.
Data from the Vietnam Era Twin Registry, for example, suggest that vulnerability to heroin use is more strongly influenced by unique genetic factors compared to vulnerability to other drugs, including cocaine83. This finding was not replicated in a cohort study based on the Virginia Twin Registry205, suggesting that it may have been specific to the all-military Vietnam-era cohort. However, small-scale linkage studies and genome-wide association studies also support the idea that specific genetic variants are differentially associated with opiate and psychostimulant use206.
Lastly, both the Vietnam Era Twin Registry and the Virginia Twin Registry cohort studies indicate that a sizeable portion of the variability in the susceptibility to drug use is due to environmental influences that are unique to opiates versus psychostimulants83,205. A host of environmental factors are thought to influence the initiation and maintenance of drug addiction, including price and availability of drug and non-drug rewards, peer pressure, aversive life experiences and occurrence of negative consequences. What is not clear is which of these environmental factors can differentially influence opiate and psychostimulant use. In the next section, we examine at least one way in which environmental context has been shown to interact differently with heroin and cocaine taking in humans.
Patterns of heroin or cocaine use in drug addicts
As discussed above, studies conducted using rats have shown differential preferences for heroin and cocaine as a function of the environmental setting or context184,188. These preclinical findings are supported by a study in human addicts (outpatients at an addiction clinic), who were heroin and cocaine co-abusers. Retrospective self-reports184 and written diaries207 showed that the respondents used cocaine in different settings from those in which they used heroin. In most cases, heroin was used at home, whereas cocaine was used outside the home; respondents said that these choices of location reflected real preferences rather than social or practical constraints. The route of drug taking did not play a major part in these findings, as comparable results were obtained when the analysis was limited to subgroups of respondents who injected or snorted heroin and cocaine separately, often on the same day (FIG. 5). The within-subject design of this study makes the findings especially compelling, because the difference in preferred settings for heroin use compared to cocaine use cannot readily be attributed to differences in drug availability, peer influence or other socio-demographic factors.
Other studies conducted with heroin and cocaine co-abusers208, using real-time electronic diary reports, have examined the predictive value of potential triggers of craving and relapse, such as negative moods, positive moods and exposure to drug-associated cues209,210. Episodes of cocaine use, but not craving, were reliably predicted by any of these triggers on a timescale of 5 hours. For heroin, the results were nearly the opposite: episodes of craving, but not use, were reliably predicted by increases in triggers that induced negative mood. These findings suggest that heroin and cocaine differ in terms of the factors that induce craving and in terms of their use in daily life. A caveat to this interpretation is that the participants were all in methadone maintenance therapy, which may have partly decoupled heroin craving from heroin use.
In summary, the human data reviewed here suggest that there are differences between aspects of heroin and cocaine use, abuse and craving. Indeed, there are no pharmacological treatments that are similarly effective for heroin addiction and cocaine addiction. For example, the effectiveness of agonist maintenance therapy (for example, methadone treatment) for heroin addiction211 has no clear parallel in cocaine addiction, although efforts to find such a therapy continue212.
Conclusions and future directions
Opiate addiction, psychostimulant addiction and other types of addiction are often seen as mere variants of the same disorder. Indeed, current diagnostic criteria for addiction cut across drug classes19, and influential theories of addiction emphasize the shared psychological processes and neurobiological substrates of different types of drug addiction (BOX 1). Here, we have attempted to offer a different perspective. We argue that although there are commonalities in the ways in which opiates and psychostimulants affect brain and behaviour, much can be learned from considering the distinctive features of each type of addiction. The human and animal studies reviewed here suggest that there are substantial differences in the neurobiological and behavioural mechanisms underlying opiate addiction and psychostimulant addiction (Supplementary information S1 (box)).
At the neurobiological level, the most fundamental difference is that mesocor-ticolimbic dopamine transmission seems to be crucial for psychostimulant self-administration but not opiate self-administration (BOX 3). By contrast, the mu opioid receptor is crucial in mediating the effects of intravenous opiate self-administration but plays only a minor part in psychostimulant self-administration20,213. Other notable differences include the distinct populations of mPFC and NAc neurons that are associated with heroin self-administration compared to cocaine self-administration51, the opposite synaptic and structural plasticity changes in the PFC after withdrawal from opiates and psychostimulants67–69,71,72, the opposite regulation of immediate-early gene expression in the striatal neurons of the indirect pathway by opiates and psychostimulants74–76, and the differential roles of mPFC subregions in context-induced relapse to heroin seeking and cocaine seeking167,168. Lastly, human studies suggest that there is minimal overlap between the genes that are associated with opiate and psychostimulant addiction83,206,214, as well as minimal overlap between the profiles of post-mortem gene-expression changes in the striatum of opiate and psychostimulant users80.
At the behavioural and psychological levels, perhaps the two most fundamental differences between cocaine and heroin (and by implication other psychostimulants and opiates) were revealed in rat models: cocaine exposure leads to a mixed motivational state characterized by approach–avoidance conflict towards drug-associated places, and unlimited cocaine access leads to complete loss of control over drug intake. By contrast, heroin exposure leads to a classical approach behaviour similar to that observed in hungry rats seeking food, and unlimited heroin access does not lead to loss of control over drug intake102,117 (FIG. 3). There are also fundamental differences in the way in which the environment interacts with opiate and psychostimulant reward: in both rats and humans, the preferred setting for opiate use is the home environment, whereas the preferred setting for psychostimulant use is outside of the home environment182–184 (FIG. 5). These findings may help to account for data from population studies that suggest that there are unique environmental influences on opiate addiction and psychostimulant addic-tion83,214. Furthermore, in rats, escalation of cocaine self-administration is predicted by high trait impulsivity, whereas escalation of heroin self-administration is not29,133. Lastly , in humans, psychostimulants cause more pronounced deficits in impulse control and cognitive flexibility than do opiates33–37.
The neurobiological, behavioural and psychological differences between opiates and psychostimulants have implications for addiction treatment. These differences may account for the fact that no known medication effectively treats both opiate and psychostimulant addiction. For example, approved treatments for opiate addiction, such as methadone and buprenorphine, have shown limited efficacy in decreasing cocaine use in concurrent users of heroin and cocaine215–218 (however, see REF. 219 for different results). In addition, the realization that drug choice is crucially dependent on setting has implications for cognitive behavioural therapies in which addicts learn to identify and respond appropriately to use-provoking risk factors.
The data reviewed here also have implications for addiction theories (BOX 1), which, as mentioned above, have attempted to provide a unitary account of addiction across drug classes. It is beyond the scope of this Perspective to analyse how each of the neurobiological or behavioural differences discussed above can or cannot be accounted for by current theories. However, we argue that these theories would struggle to explain the opposite modulatory role of the environment on opiate and psychostimulant reward and choice, the finding that escalation of cocaine intake does not predict escalation of heroin use (and vice versa), the finding that opiate self-administration is mostly independent of mesocorticolimbic dopamine transmission, and the opposite structural and synaptic changes in PFC that are induced by exposure to opiates and psychostimulants.
Lastly, the data reviewed here have implications for future neuroscience research on drug addiction. Since the late 1980s and the early 1990s52,53,220, such research has focused on a search for drug-induced neuroadaptations that can account for compulsive drug use and relapse across drug classes. In the vast majority of these studies, cocaine has been used as the prototypical, presumably representative drug of abuse221,222. Based on the differences across drug classes that we have discussed here, we believe that generalizations from cocaine to other drugs of abuse should be made with extreme caution, and that the field would benefit from more systematic comparisons of the roles of different signalling molecules and synaptic-plasticity mechanisms in reward and relapse across drug classes.
It is beyond the scope of this Perspective to compare and contrast similarities and differences in the behavioural and neuro-biological mechanisms of addiction across all drug classes. However, our concerns about differences between opiates and psychostimulants probably also apply to other addictive drugs. For example, to our knowledge, it has not been established in animal models or human studies that the mesocorticolimbic dopamine system plays a part in addiction to benzodiazepines or barbiturates. Even for nicotine and alcohol, empirical data raise questions about the centrality of the mesocorticolimbic dopa-mine system in the rewarding effects of these drugs223–226.
We hope that this Perspective will serve as a starting point for more balanced future research that will avoid the Scylla of rigidly unified models and the Charybdis of excessively compartmentalized ones. In particular, we believe that it is crucial for models of drug addiction to be formulated and validated on the basis of empirical results from comparative studies that include several classes of addictive drugs.
Supplementary Material
Acknowledgements
This Perspective was written with financial support from the Ricerche di Università Program of the Sapienza University of Rome, Italy (A.B.), the Institut National de la Santé et de la Recherche Médicale (INSERM) (D.B.) and the Intramural Research Program of the US National Institutes of Health (NIH) National Institute on Drug Abuse (NIDA) (D.C., D.E. and Y.S.). We thank R. See for sharing with us unpublished data that are included in the summary diagram in FIG. 4, A. Ettenberg for sharing historical data with us, and M. Heilig and E. Koya for very helpful comments.
Glossary
- AMPA:NMDA ratio
A measure of postsynaptic changes in synaptic strength. It is defined as the peak synaptic AMPA receptor current relative to the peak synaptic NMDA receptor current
- Craving
An affective state that can be induced in human drug users by exposure to the drug itself, drug-associated cues or stress. In laboratory animals, craving is often inferred from the subjects’ behavioural response (for example, lever-pressing) to drugs, drug-associated cues or stress
- Direct and indirect striatal pathways
The two efferent pathways in the basal ganglia. The direct pathway connects the striatum with the substantia nigra pars reticulata and entopeduncular nucleus. The indirect pathway connects the striatum with the globus pallidus and ventral pallidum
- Extinction
The decrease in the frequency or intensity of learned responses after the removal of the unconditioned stimulus (for example, food or a drug) that has reinforced the learning
- Incentive motivational state
A motivational state that is induced by exposure to unconditioned aversive or appetitive stimuli or cues that become associated with these stimuli
- Incubation of drug craving
A hypothetical motivational process that is inferred from findings of time-dependent increases in cue-induced drug seeking after withdrawal from drug self-administration in rats
- In vivo extracellular recording
A set of methods in which bundles of microwires are targeted to specific areas of the brain to allow measurement of extracellular currents (action potentials) of single cells
- Long-term depression
(LTD). A form of synaptic plasticity that is defined by a persistent weakening of synaptic strength
- Long-term potentiation
(LTP). A form of synaptic plasticity that is defined by a persistent increase in synaptic strength
- LTDGABA
A form of long-term depression (LTD) that is observed in dopaminergic neurons in the ventral tegmental area and that reduces synaptic efficacy between presynaptic GABAergic neurons and postsynaptic dopaminergic neurons
- LTPGABA
A form of long-term potentiation (LTP) that is observed in dopaminergic neurons in the ventral tegmental area. It results in increased GABA release and in the strengthening of inhibitory synapses
- Mesotelencephalic dopamine system
Also known as the mesocorticolimbic dopamine system. A major ascending dopaminergic pathway that originates in the ventral tegmental area and projects to, among other regions, the nucleus accumbens, the bed nucleus of the stria terminalis, the amygdala, the olfactory tubercle and the medial prefrontal cortex
- Psychic (or psychological) dependence
A concept, established early in the addiction field, that refers to a compulsion that requires periodic or continuous intake of an abused drug to produce psychological pleasure or to avoid psychological distress, regardless of whether physical dependence is also present
- Psychomotor sensitization
A progressive increase in locomotor or activity or stereotypy with repeated drug (for example, cocaine) administration
- Relapse
The resumption of drug-taking behaviour after self-imposed or forced abstinence in humans with a history of abuse or dependence
- Second-order schedule of reinforcement
A complex reinforcement schedule in which the completion of the response requirement of one schedule is treated as a unitary response that is reinforced according to another schedule
- Stress
n animal models, stress typically refers to forced exposure to events or conditions that the animal would normally avoid. In humans, stress often refers to a condition in which the environmental demands exceed the coping abilities of the individual
- Synaptic plasticity
Activity-dependent direct or indirect modifications of the strength of synaptic transmission at pre-existing synapses.;
Footnotes
Competing interests statement
The authors declare no competing financial interests.
FURTHER INFORMATION
Yavin Shaham’s homepage: http://irp.drugabuse.gov/BNRB.php
SUPPLEMENTARY INFORMATION
See online article: S1 (box)
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
Contributor Information
Aldo Badiani, Department of Physiology and Pharmacology Vittorio Erspamer, Sapienza University of Rome, Rome, Italy, and at the Drug Addiction and Clinical Pharmacology Unit, University Hospital Umberto I, Sapienza University of Rome, 00185 Rome, Italy..
David Belin, AVENIR team ‘Psychobiology of Compulsive Disorders’ within the Institut National de la Santé et de la Recherche Médicale (INSERM) Experimental and Clinical Neurosciences Laboratory, University of Poitiers, 86000 Poitiers, France..
David Epstein, Intramural Research Program, National Institute on Drug Abuse (NIDA), National Institutes of Health (NIH), 251 Bayview Boulevard, Baltimore, Maryland 21224, USA..
Donna Calu, Intramural Research Program, National Institute on Drug Abuse (NIDA), National Institutes of Health (NIH), 251 Bayview Boulevard, Baltimore, Maryland 21224, USA..
Yavin Shaham, Intramural Research Program, National Institute on Drug Abuse (NIDA), National Institutes of Health (NIH), 251 Bayview Boulevard, Baltimore, Maryland 21224, USA..
References
- 1.Eddy NB, Isbell H. Addiction liability and narcotics control. Public Health Rep. 1959;74:755–763. [PMC free article] [PubMed] [Google Scholar]
- 2.Collier HO. Supersensitivity and dependence. Nature. 1968;220:228–231. doi: 10.1038/220228a0. [DOI] [PubMed] [Google Scholar]
- 3.Collier HO. Supersensitivity and dependence on cocaine. Nature. 1968;220:1327–1328. doi: 10.1038/2201327a0. [DOI] [PubMed] [Google Scholar]
- 4.Solomon RL, Corbit JD. An opponent-process theory of motivation. II. Cigarette addiction. J. Abnorm. Psychol. 1973;81:158–171. doi: 10.1037/h0034534. [DOI] [PubMed] [Google Scholar]
- 5.Olds J, Milner PM. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J. Comp. Physiol. Psychol. 1954;47:419–427. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- 6.Wise RA. Catecholamine theories of reward: a critical review. Brain Res. 1978;152:215–247. doi: 10.1016/0006-8993(78)90253-6. [DOI] [PubMed] [Google Scholar]
- 7.Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc. Natl Acad. Sci. USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stewart J, de Wit H, Eikelboom R. Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychol. Rev. 1984;91:251–268. [PubMed] [Google Scholar]
- 9.Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res. 1986;396:157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- 10.Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol. Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- 11.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res. Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 12.Di Chiara G. Drug addiction as dopamine-dependent associative learning disorder. Eur. J. Pharmacol. 1999;375:13–30. doi: 10.1016/s0014-2999(99)00372-6. [DOI] [PubMed] [Google Scholar]
- 13.Everitt BJ, et al. Associative processes in addiction and reward. The role of amygdala–ventral striatal subsystems. Ann. NY Acad. Sci. 1999;877:412–438. doi: 10.1111/j.1749-6632.1999.tb09280.x. [DOI] [PubMed] [Google Scholar]
- 14.White NM. Addictive drugs as reinforcers: multiple partial actions on memory systems. Addiction. 1996;91:921–949. [PubMed] [Google Scholar]
- 15.Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharamacology. 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- 16.Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45:647–650. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb. Cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- 18.Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- 19.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th edn. Washington DC: American Psychiatric Association; 2000. [Google Scholar]
- 20.Ettenberg A, Pettit HO, Bloom FE, Koob GF. Heroin and cocaine intravenous self-administration in rats: mediation by separate neural systems. Psychopharmacology. 1982;78:204–209. doi: 10.1007/BF00428151. [DOI] [PubMed] [Google Scholar]
- 21.Pettit HO, Ettenberg A, Bloom FE, Koob GF. Destruction of dopamine in the nucleus accumbens selectively attenuates cocaine but not heroin self-administration. Psychopharmacology. 1984;84:167–173. doi: 10.1007/BF00427441. [DOI] [PubMed] [Google Scholar]
- 22.Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nature Neurosci. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- 23.Fu LP, et al. Impaired response inhibition function in abstinent heroin dependents: an fMRI study. Neurosci. Lett. 2008;438:322–326. doi: 10.1016/j.neulet.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 24.Fernandez-Serrano MJ, Perez-Garcia M, Schmidt Rio-Valle J, Verdejo-Garcia A. Neuropsychological consequences of alcohol and drug abuse on different components of executive functions. J. Psychopharmacol. 2010;24:1317–1332. doi: 10.1177/0269881109349841. [DOI] [PubMed] [Google Scholar]
- 25.Ornstein TJ, et al. Profiles of cognitive dysfunction in chronic amphetamine and heroin abusers. Neuropsychopharmacology. 2000;23:113–126. doi: 10.1016/S0893-133X(00)00097-X. [DOI] [PubMed] [Google Scholar]
- 26.Muriach M, et al. Cocaine causes memory and learning impairments in rats: involvement of nuclear factor κB and oxidative stress, and prevention by topiramate. J. Neurochem. 2010;114:675–684. doi: 10.1111/j.1471-4159.2010.06794.x. [DOI] [PubMed] [Google Scholar]
- 27.Tramullas M, Martinez-Cue C, Hurle MA. Chronic administration of heroin to mice produces up-regulation of brain apoptosis-related proteins and impairs spatial learning and memory. Neuropharmacology. 2008;54:640–652. doi: 10.1016/j.neuropharm.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 28.Fole A, et al. Effects of chronic cocaine administration on spatial learning and hippocampal spine density in two genetically different strains of rats. Neurobiol. Learn. Mem. 2011;95:491–497. doi: 10.1016/j.nlm.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 29.McNamara R, Dalley JW, Robbins TW, Everitt BJ, Belin D. Trait-like impulsivity does not predict escalation of heroin self-administration in the rat. Psychopharmacology. 2010;212:453–464. doi: 10.1007/s00213-010-1974-9. [DOI] [PubMed] [Google Scholar]
- 30.Dalley JW, et al. Attentional and motivational deficits in rats withdrawn from intravenous self-administration of cocaine or heroin. Psychopharmacology. 2005;182:579–587. doi: 10.1007/s00213-005-0107-3. [DOI] [PubMed] [Google Scholar]
- 31.Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69:680–694. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 32.de Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addiction Biol. 2009;14:22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ersche KD, Clark L, London M, Robbins TW, Sahakian BJ. Profile of executive and memory function associated with amphetamine and opiate dependence. Neuropsychopharmacology. 2006;31:1036–1047. doi: 10.1038/sj.npp.1300889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ersche KD, et al. Abnormal frontal activations related to decision-making in current and former amphetamine and opiate dependent individuals. Psychopharmacology. 2005;180:612–623. doi: 10.1007/s00213-005-2205-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ersche KD, Roiser JP, Robbins TW, Sahakian BJ. Chronic cocaine but not chronic amphetamine use is associated with perseverative responding in humans. Psychopharmacology. 2008;197:421–431. doi: 10.1007/s00213-007-1051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.London ED, et al. Cerebral metabolic dysfunction and impaired vigilance in recently abstinent methamphetamine abusers. Biol. Psychiatry. 2005;58:770–778. doi: 10.1016/j.biopsych.2005.04.039. [DOI] [PubMed] [Google Scholar]
- 37.Lundqvist T. Cognitive consequences of cannabis use: comparison with abuse of stimulants and heroin with regard to attention, memory and executive functions. Pharmacol. Biochem. Behav. 2005;81:319–330. doi: 10.1016/j.pbb.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 38.Verdejo-Garcia A, Perez-Garcia M, Sanchez-Barrera M, Rodriguez-Fernandez A, Gomez-Rio M. Neuroimagen y drogodependencias: correlatos neuroanatómicos del consumo de cocaína, opiáceos, cannabis y éxtasis. Rev. Neurol. 2007;44:432–439. (in Spanish) [PubMed] [Google Scholar]
- 39.Winstanley CA, et al. Increased impulsivity during withdrawal from cocaine self-administration: role for DeltaFosB in the orbitofrontal cortex. Cereb. Cortex. 2009;19:435–444. doi: 10.1093/cercor/bhn094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu S, Heitz RP, Bradberry CW. A touch screen based Stop Signal Response Task in rhesus monkeys for studying impulsivity associated with chronic cocaine self-administration. J. Neurosci. Meth. 2009;177:67–72. doi: 10.1016/j.jneumeth.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fletcher PJ, Rizos Z, Noble K, Higgins GA. Impulsive action induced by amphetamine, cocaine and MK801 is reduced by 5HT(2C) receptor stimulation and 5HT(2A) receptor blockade. Neuropharmacology. 2011;61:468–477. doi: 10.1016/j.neuropharm.2011.02.025. [DOI] [PubMed] [Google Scholar]
- 42.Harty SC, Whaley JE, Halperin JM, Ranaldi R. Impulsive choice, as measured in a delay discounting paradigm, remains stable after chronic heroin administration. Pharmacol. Biochem. Behav. 2011;98:337–340. doi: 10.1016/j.pbb.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 43.Harris JE, Baldessarini RJ. Uptake of (3H)-catecholamines by homogenates of rat corpus striatum and cerebral cortex: effects of amphetamine analogues. Neuropharmacology. 1973;12:669–679. doi: 10.1016/0028-3908(73)90120-2. [DOI] [PubMed] [Google Scholar]
- 44.Wise RA. Dopamine, learning and motivation. Nature Rev. Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 45.Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J. Neurosci. 2002;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gysling K, Wang RY. Morphine-induced activation of A10 dopamine neurons in the rat. Brain Res. 1983;277:119–127. doi: 10.1016/0006-8993(83)90913-7. [DOI] [PubMed] [Google Scholar]
- 47.Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J. Neurosci. 1992;12:483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am. J. Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 49.Carelli RM, King VC, Hampson RE, Deadwyler SA. Firing patterns of nucleus accumbens neurons during cocaine self-administration in rats. Brain Res. 1993;626:14–22. doi: 10.1016/0006-8993(93)90557-4. [DOI] [PubMed] [Google Scholar]
- 50.Chang JY, Zhang L, Janak PH, Woodward DJ. Neuronal responses in prefrontal cortex and nucleus accumbens during heroin self-administration in freely moving rats. Brain Res. 1997;754:12–20. doi: 10.1016/s0006-8993(97)00012-7. [DOI] [PubMed] [Google Scholar]
- 51.Chang JY, Janak PH, Woodward DJ. Comparison of mesocorticolimbic neuronal responses during cocaine and heroin self-administration in freely moving rats. J. Neurosci. 1998;18:3098–3115. doi: 10.1523/JNEUROSCI.18-08-03098.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res. Rev. 1991;16:223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- 53.Nestler EJ. Molecular mechanisms of drug addiction. J. Neurosci. 1992;12:2439–2450. doi: 10.1523/JNEUROSCI.12-07-02439.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nature Rev. Neurosci. 2001;2:119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- 55.Thomas MJ, Kalivas PW, Shaham Y. Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. Br. J. Pharmacol. 2008;154:327–342. doi: 10.1038/bjp.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wolf ME, Sun X, Mangiavacchi S, Chao SZ. Psychomotor stimulants and neuronal plasticity. Neuropharmacology. 2004;47:61–79. doi: 10.1016/j.neuropharm.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 57.Girault JA, Valjent E, Caboche J, Herve D. D. ERK2: a logical AND gate critical for drug-induced plasticity? Curr. Opin. Pharmacol. 2007;7:77–85. doi: 10.1016/j.coph.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 58.Bonci A, Williams JT. A common mechanism mediates long-term changes in synaptic transmission after chronic cocaine and morphine. Neuron. 1996;16:631–639. doi: 10.1016/s0896-6273(00)80082-3. [DOI] [PubMed] [Google Scholar]
- 59.Bowers MS, Chen BT, Bonci A. AMPA receptor synaptic plasticity induced by psychostimulants: the past, present, and therapeutic future. Neuron. 2010;67:11–24. doi: 10.1016/j.neuron.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- 61.Francesconi W, et al. Protracted withdrawal from alcohol and drugs of abuse impairs long-term potentiation of intrinsic excitability in the juxtacapsular bed nucleus of the stria terminalis. J. Neurosci. 2009;29:5389–5401. doi: 10.1523/JNEUROSCI.5129-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology. 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- 63.Rossetti ZL, Hmaidan Y, Gessa GL. Marked inhibition of mesolimbic dopamine release: a common feature of ethanol, morphine, cocaine and amphetamine abstinence in rats. Eur. J. Pharmacol. 1992;221:227–234. doi: 10.1016/0014-2999(92)90706-a. [DOI] [PubMed] [Google Scholar]
- 64.Dacher M, Nugent FS. Morphine-induced modulation of LTD at GABAergic synapses in the ventral tegmental area. Neuropharmacology. 2010 Dec 1; doi: 10.1016/j.neuropharm.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 65.Niehaus JL, Murali M, Kauer JA. Drugs of abuse and stress impair LTP at inhibitory synapses in the ventral tegmental area. Eur. J. Neurosci. 2010;32:108–117. doi: 10.1111/j.1460-9568.2010.07256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pan B, Hillard CJ, Liu QS. Endocannabinoid signaling mediates cocaine-induced inhibitory synaptic plasticity in midbrain dopamine neurons. J. Neurosci. 2008;28:1385–1397. doi: 10.1523/JNEUROSCI.4033-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang CC, Lin HJ, Hsu KS. Repeated cocaine administration promotes long-term potentiation induction in rat medial prefrontal cortex. Cereb. Cortex. 2007;17:1877–1888. doi: 10.1093/cercor/bhl096. [DOI] [PubMed] [Google Scholar]
- 68.Lu H, Cheng PL, Lim BK, Khoshnevisrad N, Poo MM. Elevated BDNF after cocaine withdrawal facilitates LTP in medial prefrontal cortex by suppressing GABA inhibition. Neuron. 2010;67:821–833. doi: 10.1016/j.neuron.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Van den Oever MC, et al. Prefrontal cortex AMPA receptor plasticity is crucial for cue-induced relapse to heroin-seeking. Nature Neurosci. 2008;11:1053–1058. doi: 10.1038/nn.2165. [DOI] [PubMed] [Google Scholar]
- 70.Robinson TE, Kolb B. Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine. J. Neurosci. 1997;17:8491–8497. doi: 10.1523/JNEUROSCI.17-21-08491.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47:33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 72.Russo SJ, et al. The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends Neurosci. 2010;33:267–276. doi: 10.1016/j.tins.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gerfen CR. The neostriatal mosaic: multiple levels of compartmental organization. Trends Neurosci. 1992;15:133–139. doi: 10.1016/0166-2236(92)90355-c. [DOI] [PubMed] [Google Scholar]
- 74.Badiani A, et al. Environmental modulation of amphetamine-induced cfos expression in D1 versus D2 striatal neurons. Behav. Brain Res. 1999;103:203–209. doi: 10.1016/s0166-4328(99)00041-8. [DOI] [PubMed] [Google Scholar]
- 75.Ferguson SM, Robinson TE. Amphetamine-evoked gene expression in striatopallidal neurons: regulation by corticostriatal afferents and the ERK/ MAPK signaling cascade. J. Neurochem. 2004;91:337–348. doi: 10.1111/j.1471-4159.2004.02712.x. [DOI] [PubMed] [Google Scholar]
- 76.Uslaner J, et al. Amphetamine and cocaine induce different patterns of cfos mRNA expression in the striatum and subthalamic nucleus depending on environmental context. Eur. J. Neurosci. 2001;13:1977–1983. doi: 10.1046/j.0953-816x.2001.01574.x. [DOI] [PubMed] [Google Scholar]
- 77.Hope BT, et al. Induction of a long-lasting AP1 complex composed of altered Fos-like proteins in brain by chronic cocaine and other chronic treatments. Neuron. 1994;13:1235–1244. doi: 10.1016/0896-6273(94)90061-2. [DOI] [PubMed] [Google Scholar]
- 78.Maze I, et al. Essential role of the histone methyltransferase G9a in cocaine-induced plasticity. Science. 2010;327:213–216. doi: 10.1126/science.1179438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee KW, et al. Cocaine-induced dendritic spine formation in D1 and D2 dopamine receptor-containing medium spiny neurons in nucleus accumbens. Proc. Natl Acad. Sci. USA. 2006;103:3399–3404. doi: 10.1073/pnas.0511244103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Albertson DN, Schmidt CJ, Kapatos G, Bannon MJ. Distinctive profiles of gene expression in the human nucleus accumbens associated with cocaine and heroin abuse. Neuropsychopharmacology. 2006;31:2304–2312. doi: 10.1038/sj.npp.1301089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Warner LA, Kessler RC, Hughes M, Anthony JC, Nelson CB. Prevalence and correlates of drug use and dependence in the United States. Results from the National Comorbidity Survey. Arch. Gen. Psychiatry. 1995;52:219–229. doi: 10.1001/archpsyc.1995.03950150051010. [DOI] [PubMed] [Google Scholar]
- 82.Glantz MD, Pickens RW. Vulnerability to Drug Abuse. Washington DC: American Psychological Association; 1992. [Google Scholar]
- 83.Tsuang MT, et al. Co-occurrence of abuse of different drugs in men: the role of drug-specific and shared vulnerabilities. Arch. Gen. Psychiatry. 1998;55:967–972. doi: 10.1001/archpsyc.55.11.967. [DOI] [PubMed] [Google Scholar]
- 84.Caprioli D, Celentano M, Paolone G, Badiani A. Modeling the role of environment in addiction. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2007;31:1639–1653. doi: 10.1016/j.pnpbp.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 85.Harding WM, Zinberg NE. Controlling intoxicant use. J. Psychoactive Drugs. 1984;16:101–106. doi: 10.1080/02791072.1984.10471811. [DOI] [PubMed] [Google Scholar]
- 86.Robins LN, Davis DH, Goodwin DW. Drug use by U. S. Army enlisted men in Vietnam: a follow-up on their return home. Am. J. Epidemiol. 1974;99:235–249. doi: 10.1093/oxfordjournals.aje.a121608. [DOI] [PubMed] [Google Scholar]
- 87.Brady JV. Animal models for assessing drugs of abuse. Neurosci. Biobehav. Rev. 1991;15:35–43. doi: 10.1016/s0149-7634(05)80089-2. [DOI] [PubMed] [Google Scholar]
- 88.Schuster CR, Thompson T. Self administration of and behavioral dependence on drugs. Annu. Rev. Pharmacol. 1969;9:483–502. doi: 10.1146/annurev.pa.09.040169.002411. [DOI] [PubMed] [Google Scholar]
- 89.Thomsen M, Caine SB. Intravenous drug self-administration in mice: practical considerations. Behav. Genet. 2007;37:101–118. doi: 10.1007/s10519-006-9097-0. [DOI] [PubMed] [Google Scholar]
- 90.Carroll ME, Meisch ME. In: Advances in Behavioral Pharmacology. Thompson T, Dews PB, Barrett JE, editors. New York: Academic Press; 1984. pp. 47–88. [Google Scholar]
- 91.Lu L, Shepard JD, Scott Hall F, Shaham Y. Effect of environmental stressors on opiate and psychostimulant reinforcement, reinstatement and discrimination in rats: a review. Neurosci. Biobehav. Rev. 2003;27:457–491. doi: 10.1016/s0149-7634(03)00073-3. [DOI] [PubMed] [Google Scholar]
- 92.Piazza PV, Le Moal M. The role of stress in drug self-administration. Trends Pharmacol. Sci. 1998;19:67–74. doi: 10.1016/s0165-6147(97)01115-2. [DOI] [PubMed] [Google Scholar]
- 93.Lett BT. Repeated exposures intensify rather than diminish the rewarding effects of amphetamine, morphine, and cocaine. Psychopharmacology. 1989;98:357–362. doi: 10.1007/BF00451687. [DOI] [PubMed] [Google Scholar]
- 94.Shippenberg TS, Elmer GI. The neurobiology of opiate reinforcement. Crit. Rev. Neurobiol. 1998;12:267–303. doi: 10.1615/critrevneurobiol.v12.i4.10. [DOI] [PubMed] [Google Scholar]
- 95.Vezina P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychostimulant drugs. Neurosci. Biobehav. Rev. 2004;27:827–839. doi: 10.1016/j.neubiorev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 96.Carroll ME, Morgan AD, Lynch WJ, Campbell UC, Dess NK. Intravenous cocaine and heroin self-administration in rats selectively bred for differential saccharin intake: phenotype and sex differences. Psychopharmacology. 2002;161:304–313. doi: 10.1007/s00213-002-1030-5. [DOI] [PubMed] [Google Scholar]
- 97.Wills TA, Vaccaro D, McNamara G. Novelty seeking, risk taking, and related constructs as predictors of adolescent substance use: an application of Cloninger’s theory. J. Subst. Abuse. 1994;6:1–20. doi: 10.1016/s0899-3289(94)90039-6. [DOI] [PubMed] [Google Scholar]
- 98.Piazza PV, Le Moal M. Pathophysiological basis of vulnerability to drug abuse: interaction between stress, glucocorticoids, and dopaminergic neurons. Ann. Rev. Pharmacol. Toxicol. 1996;36:359–378. doi: 10.1146/annurev.pa.36.040196.002043. [DOI] [PubMed] [Google Scholar]
- 99.Marinelli M, Piazza PV. Interaction between glucocorticoid hormones, stress and psychostimulant drugs. Eur. J. Neurosci. 2002;16:387–394. doi: 10.1046/j.1460-9568.2002.02089.x. [DOI] [PubMed] [Google Scholar]
- 100.Marinelli M, Aouizerat B, Barrot M, Le Moal M, Piazza PV. Dopamine-dependent responses to morphine depend on glucocorticoid receptors. Proc. Natl Acad. Sci. USA. 1998;95:7742–7747. doi: 10.1073/pnas.95.13.7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ambroggi F, et al. Stress and addiction: glucocorticoid receptor in dopaminoceptive neurons facilitates cocaine seeking. Nature Neurosci. 2009;12:247–249. doi: 10.1038/nn.2282. [DOI] [PubMed] [Google Scholar]
- 102.Ettenberg A, et al. Opponent process properties of self-administered cocaine. Neurosci. Biobehav. Rev. 2004;27:721–728. doi: 10.1016/j.neubiorev.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 103.Ettenberg A, Geist TD. Qualitative and quantitative differences in the operant runway behavior of rats working for cocaine and heroin reinforcement. Pharmacol. Biochem. Behav. 1993;44:191–198. doi: 10.1016/0091-3057(93)90298-8. [DOI] [PubMed] [Google Scholar]
- 104.Ettenberg A, Raven MA, Danluck DA, Necessary BD. Evidence for opponent-process actions of intravenous cocaine. Pharmacol. Biochem. Behav. 1999;64:507–512. doi: 10.1016/s0091-3057(99)00109-4. [DOI] [PubMed] [Google Scholar]
- 105.Geist TD, Ettenberg A. Concurrent positive and negative goalbox events produce runway behaviors comparable to those of cocaine-reinforced rats. Pharmacol. Biochem. Behav. 1997;57:145–150. doi: 10.1016/s0091-3057(96)00300-0. [DOI] [PubMed] [Google Scholar]
- 106.Knackstedt LA, Samimi MM, Ettenberg A. Evidence for opponent-process actions of intravenous cocaine and cocaethylene. Pharmacol. Biochem. Behav. 2002;72:931–936. doi: 10.1016/s0091-3057(02)00764-5. [DOI] [PubMed] [Google Scholar]
- 107.Anthony JC, Tien AY, Petronis KR. Epidemiologic evidence on cocaine use and panic attacks. Am. J. Epidemiol. 1989;129:543–549. doi: 10.1093/oxfordjournals.aje.a115166. [DOI] [PubMed] [Google Scholar]
- 108.Geracioti TD, Jr, Post RM. Onset of panic disorder associated with rare use of cocaine. Biol. Psychiatry. 1991;29:403–406. doi: 10.1016/0006-3223(91)90227-d. [DOI] [PubMed] [Google Scholar]
- 109.Breiter HC, et al. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19:591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- 110.Becker JB, Hu M. Sex differences in drug abuse. Frontiers Neuroendocrinol. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Roth ME, Cosgrove KP, Carroll ME. Sex differences in the vulnerability to drug abuse: a review of preclinical studies. Neurosci. Biobehav. Rev. 2004;28:533–546. doi: 10.1016/j.neubiorev.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 112.Lynch WJ, Carroll ME. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology. 1999;144:77–82. doi: 10.1007/s002130050979. [DOI] [PubMed] [Google Scholar]
- 113.Stewart J, Woodside BC, Shaham Y. Changes in ovarian hormones do not affect the initiation of intravenous self-administration of heroin in the female rat. Psychobiology. 1996;24:154–159. [Google Scholar]
- 114.Stewart J, Rodaros D. The effects of gonadal hormones on the development and expression of the stimulant effects of morphine in male and female rats. Behav. Brain Res. 1999;102:89–98. doi: 10.1016/s0166-4328(99)00002-9. [DOI] [PubMed] [Google Scholar]
- 115.Ahmed SH. Escalation of drug use. Neuromethods. 2011;53:267–292. [Google Scholar]
- 116.Ahmed SH, Koob GF. Transition to drug addiction: a negative reinforcement model based on an allostatic decrease in reward function. Psychopharmacology. 2005;180:473–490. doi: 10.1007/s00213-005-2180-z. [DOI] [PubMed] [Google Scholar]
- 117.Bozarth MA, Wise RA. Toxicity associated with long-term intravenous heroin and cocaine self-administration in the rat. J. Am. Med. Assoc. 1985;254:81–83. [PubMed] [Google Scholar]
- 118.Pickens R, Harris WC. Self-administration of damphetamine by rats. Psychopharmacologia. 1968;12:158–163. doi: 10.1007/BF00401545. [DOI] [PubMed] [Google Scholar]
- 119.Covington HE, 3rd, Miczek KA. Repeated social-defeat stress cocaine or morphine Effects on behavioral sensitization and intravenous cocaine self-administration “binges”. Psychopharmacology. 2001;158:388–398. doi: 10.1007/s002130100858. [DOI] [PubMed] [Google Scholar]
- 120.Belin D, Economidou D, Pelloux Y, Everitt BJ. Habit formation and compulsion. Neuromethods. 2011;53:337–378. [Google Scholar]
- 121.Wolffgramm J, Heyne A. From controlled drug intake to loss of control: the irreversible development of drug addiction in the rat. Behav. Brain Res. 1995;70:77–94. doi: 10.1016/0166-4328(95)00131-c. [DOI] [PubMed] [Google Scholar]
- 122.Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305:1014–1017. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- 123.Pelloux Y, Everitt BJ, Dickinson A. Compulsive drug seeking by rats under punishment: effects of drug taking history. Psychopharmacology. 2007;194:127–137. doi: 10.1007/s00213-007-0805-0. [DOI] [PubMed] [Google Scholar]
- 124.Cooper A, Barnea-Ygael N, Levy D, Shaham Y, Zangen A. A conflict rat model of cue-induced relapse to cocaine seeking. Psychopharmacology. 2007;194:117–125. doi: 10.1007/s00213-007-0827-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vanderschuren LJ, Everitt BJ. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science. 2004;305:1017–1019. doi: 10.1126/science.1098975. [DOI] [PubMed] [Google Scholar]
- 126.Heyne A, Wolffgramm J. The development of addiction to damphetamine in an animal model: same principles as for alcohol and opiate. Psychopharmacology. 1998;140:510–518. doi: 10.1007/s002130050796. [DOI] [PubMed] [Google Scholar]
- 127.Lenoir M, Guillem K, Koob GF, Ahmed SH. Drug specificity in extended access cocaine and heroin self-administration. Addiction Biol. in the press doi: 10.1111/j.1369-1600.2011.00385.x. [DOI] [PubMed] [Google Scholar]
- 128.Weeks JR, Collins J. Patterns of intravenous self-injection by morphine-addicted rats. Res. Publ. Assoc. Res. Nerv. Ment. Dis. 1968;46:288–298. [PubMed] [Google Scholar]
- 129.Miczek KA, Yap JJ, Covington HE. Social stress, therapeutics and drug abuse: preclinical models of escalated and depressed intake. Pharmacol. Ther. 2008;120:102–128. doi: 10.1016/j.pharmthera.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Covington HE, 3rd, Tropea TF, Rajadhyaksha AM, Kosofsky BE, Miczek KA. NMDA receptors in the rat VTA: a critical site for social stress to intensify cocaine taking. Psychopharmacology. 2008;197:203–216. doi: 10.1007/s00213-007-1024-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Westerink BH, Kwint HF, deVries JB. The pharmacology of mesolimbic dopamine neurons: a dual-probe microdialysis study in the ventral tegmental area and nucleus accumbens of the rat brain. J. Neurosci. 1996;16:2605–2611. doi: 10.1523/JNEUROSCI.16-08-02605.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cruz FC, Quadros IM, Hogenelst K, Planeta CS, Miczek KA. Social defeat stress in rats: escalation of cocaine and “speedball” binge self-administration, but not heroin. Psychopharmacology. 2011;215:165–175. doi: 10.1007/s00213-010-2139-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dalley JW, et al. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.de Wit H. Priming effects with drugs and other reinforcers. Exp. Clin. Psychopharmacol. 1996;4:5–10. [Google Scholar]
- 135.Childress AR, et al. Cue reactivity and cue reactivity interventions in drug dependence. NIDA Res. Monogr. 1993;137:73–95. [PubMed] [Google Scholar]
- 136.Sinha R. How does stress increase risk of drug abuse and relapse. Psychopharmacology. 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- 137.Heilig M, et al. Translating the neuroscience of alcoholism into clinical treatments: from blocking the buzz to curing the blues. Neurosci. Biobehav. Rev. 2010;35:334–344. doi: 10.1016/j.neubiorev.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.de Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology. 1981;75:134–143. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- 139.Meil WM, See RE. Conditioned cued recovery of responding following prolonged withdrawal from self-administered cocaine in rats: an animal model of relapse. Behav. Pharmacol. 1996;7:754–763. [PubMed] [Google Scholar]
- 140.Shaham Y, Stewart J. Stress reinstates heroin self-administration behavior in drug-free animals: an effect mimicking heroin, not withdrawal. Psychopharmacology. 1995;119:334–341. doi: 10.1007/BF02246300. [DOI] [PubMed] [Google Scholar]
- 141.Everitt BJ, Robbins TW. Second-order schedules of drug reinforcement in rats and monkeys: measurement of reinforcing efficacy and drug-seeking behaviour. Psychopharmacology. 2000;153:17–30. doi: 10.1007/s002130000566. [DOI] [PubMed] [Google Scholar]
- 142.Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol. Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- 143.Pickens CL, et al. Neurobiology of incubation of drug craving. Trends Neurosci. 2011;34:411–420. doi: 10.1016/j.tins.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bedi G, et al. Incubation of cue-induced cigarette craving during abstinence in human smokers. Biol. Psychiatry. 2011;69:708–711. doi: 10.1016/j.biopsych.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Shaham Y, Erb S, Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res. Brain Res. Rev. 2000;33:13–33. doi: 10.1016/s0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- 146.Crombag H, Bossert JM, Koya E, Shaham Y. Context-induced relapse to drug seeking: a review. Trans. R. Soc. Lond. B. 2008;363:3233–3243. doi: 10.1098/rstb.2008.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Feltenstein MW, See RE. The neurocircuitry of addiction: an overview. Br. J. Pharmacol. 2008;154:261–274. doi: 10.1038/bjp.2008.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Weiss F. Neurobiology of craving, conditioned reward and relapse. Curr. Opin. Pharmacol. 2005;5:9–19. doi: 10.1016/j.coph.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 149.Schindler CW, Panlilio LV, Goldberg SR. Second-order schedules of drug self-administration in animals. Psychopharmacology. 2002;163:327–344. doi: 10.1007/s00213-002-1157-4. [DOI] [PubMed] [Google Scholar]
- 150.Grimm JW, Hope BT, Wise RA, Shaham Y. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Shalev U, Morales M, Hope B, Yap J, Shaham Y. Time-dependent changes in extinction behavior and stress-induced reinstatement of drug seeking following withdrawal from heroin in rats. Psychopharmacology. 2001;156:98–107. doi: 10.1007/s002130100748. [DOI] [PubMed] [Google Scholar]
- 152.Shepard JD, Bossert JM, Liu SY, Shaham Y. The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol. Psychiatry. 2004;55:1082–1089. doi: 10.1016/j.biopsych.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 153.Schmidt HD, Anderson SM, Famous KR, Kumaresan V, Pierce RC. Anatomy and pharmacology of cocaine priming-induced reinstatement of drug seeking. Eur. J. Pharmacol. 2005;526:65–76. doi: 10.1016/j.ejphar.2005.09.068. [DOI] [PubMed] [Google Scholar]
- 154.Self DW, et al. Involvement of cAMP-dependent protein kinase in the nucleus accumbens in cocaine self administration and relapse of cocaine-seeking behavior. J. Neurosci. 1998;18:1848–1859. doi: 10.1523/JNEUROSCI.18-05-01848.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bossert JM, Wihbey KA, Pickens CL, Nair SG, Shaham Y. Role of dopamine D1-family receptors in dorsolateral striatum in context-induced reinstatement of heroin seeking in rats. Psychopharmacology. 2009;206:51–60. doi: 10.1007/s00213-009-1580-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Fuchs RA, Branham RK, See RE. Different neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate-putamen. J. Neurosci. 2006;26:3584–3588. doi: 10.1523/JNEUROSCI.5146-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Vanderschuren LJ, Di Ciano P, Everitt BJ. Involvement of the dorsal striatum in cue-controlled cocaine seeking. J. Neurosci. 2005;25:8665–8870. doi: 10.1523/JNEUROSCI.0925-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.LaLumiere RT, Kalivas PW. Glutamate release in the nucleus accumbens core is necessary for heroin seeking. J. Neurosci. 2008;28:3170–3177. doi: 10.1523/JNEUROSCI.5129-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J. Neurosci. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Shaham Y, Highfield D, Delfs JM, Leung S, Stewart J. Clonidine blocks stress-induced reinstatement of heroin seeking in rats: an effect independent of the locus coeruleus noradrenergic neurons. Eur. J. Neurosci. 2000;12:292–302. doi: 10.1046/j.1460-9568.2000.00899.x. [DOI] [PubMed] [Google Scholar]
- 161.Shalev U, Erb S, Shaham Y. Role of CRF and other neuropeptides in stress-induced reinstatement of drug seeking. Brain Res. 2010;1314:15–28. doi: 10.1016/j.brainres.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Rogers JL, Ghee S, See RE. The neural circuitry underlying reinstatement of heroin-seeking behavior in an animal model of relapse. Neuroscience. 2008;151:579–588. doi: 10.1016/j.neuroscience.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Alderson HL, Robbins TW, Everitt BJ. The effects of excitotoxic lesions of the basolateral amygdala on the acquisition of heroin-seeking behaviour in rats. Psychopharmacology. 2000;153:111–119. doi: 10.1007/s002130000527. [DOI] [PubMed] [Google Scholar]
- 164.Whitelaw RB, Markou A, Robbins TW, Everitt BJ. Excitotoxic lesions of the basolateral amygdala impair the acquisition of cocaine-seeking behaviour under a second-order schedule of reinforcement. Psychopharmacology. 1996;127:213–224. [PubMed] [Google Scholar]
- 165.Fuchs RA, See RE. Basolateral amygdala inactivation abolishes conditioned stimulus- and heroin-induced reinstatement of extinguished heroin-seeking behavior in rats. Psychopharmacology. 2002;160:425–433. doi: 10.1007/s00213-001-0997-7. [DOI] [PubMed] [Google Scholar]
- 166.McLaughlin J, See RE. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology. 2003;168:57–65. doi: 10.1007/s00213-002-1196-x. [DOI] [PubMed] [Google Scholar]
- 167.Fuchs RA, et al. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology. 2005;30:296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- 168.Bossert JM, et al. Ventral medial prefrontal cortex neuronal ensembles mediate context-induced relapse to heroin. Nature Neurosci. 2011;14:420–422. doi: 10.1038/nn.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Fuchs RA, Ramirez DR, Bell GH. Nucleus accumbens shell and core involvement in drug context-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2008;200:545–556. doi: 10.1007/s00213-008-1234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bossert JM, Poles GC, Sheffler-Collins SI, Ghitza UE. The mGluR2/3 agonist LY379268 attenuates context- and discrete cue-induced reinstatement of sucrose seeking but not sucrose self-administration in rats. Behav. Brain Res. 2006;173:148–152. doi: 10.1016/j.bbr.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 171.Bossert JM, Poles GC, Wihbey KA, Koya E, Shaham Y. Differential effects of blockade of dopamine D1-family receptors in nucleus accumbens core or shell on reinstatement of heroin seeking induced by contextual and discrete cues. J. Neurosci. 2007;27:12655–12663. doi: 10.1523/JNEUROSCI.3926-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bossert JM, Ghitza UE, Lu L, Epstein DH, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: an update and clinical implications. Eur. J. Pharmacol. 2005;526:36–50. doi: 10.1016/j.ejphar.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 173.Peters J, LaLumiere RT, Kalivas PW. Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J. Neurosci. 2008;28:6046–6053. doi: 10.1523/JNEUROSCI.1045-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn. Mem. 2009;16:279–288. doi: 10.1101/lm.1041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lu L, et al. Role of ventral tegmental area glial cell line-derived neurotrophic factor in incubation of cocaine craving. Biol. Psychiatry. 2009;66:137–145. doi: 10.1016/j.biopsych.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Airavaara M, et al. Endogenous GDNF in ventral tegmental area and nucleus accumbens does not play a role in the incubation of heroin craving. Addiction Biol. 2011;16:261–272. doi: 10.1111/j.1369-1600.2010.00281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ettenberg A. The runway model of drug self-administration. Pharmacol. Biochem. Behav. 2009;91:271–277. doi: 10.1016/j.pbb.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wikler A. Dynamics of drug dependence, implication of a conditioning theory for research and treatment. Arch. Gen. Psychiatry. 1973;28:611–616. doi: 10.1001/archpsyc.1973.01750350005001. [DOI] [PubMed] [Google Scholar]
- 179.Cain ME, Smith CM, Bardo MT. The effect of novelty on amphetamine self-administration in rats classified as high and low responders. Psychopharmacology. 2004;176:129–138. doi: 10.1007/s00213-004-1870-2. [DOI] [PubMed] [Google Scholar]
- 180.Klebaur JE, Phillips SB, Kelly TH, Bardo MT. Exposure to novel environmental stimuli decreases amphetamine self-administration in rats. Exp. Clin. Psychopharmacol. 2001;9:372–379. [PubMed] [Google Scholar]
- 181.Cornish JL, et al. Heat increases 3,4-methylenedioxymethamphetamine self-administration and social effects in rats. Eur. J. Pharmacol. 2003;482:339–341. doi: 10.1016/j.ejphar.2003.09.060. [DOI] [PubMed] [Google Scholar]
- 182.Caprioli D, et al. Opposite environmental regulation of heroin and amphetamine self-administration in the rat. Psychopharmacology. 2008;198:395–404. doi: 10.1007/s00213-008-1154-3. [DOI] [PubMed] [Google Scholar]
- 183.Caprioli D, et al. Environmental modulation of cocaine self-administration in the rat. Psychopharmacology. 2007;192:397–406. doi: 10.1007/s00213-007-0717-z. [DOI] [PubMed] [Google Scholar]
- 184.Caprioli D, et al. Ambience and drug choice: cocaine-and heroin-taking as a function of environmental context in humans and rats. Biol. Psychiatry. 2009;65:893–899. doi: 10.1016/j.biopsych.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 185.Montanari CL, De Luca MT, Meringolo M, Contu L, Badiani A. Environmental modulation of drug-induced reinstatement of cocaine versus heroin drug seeking in rats trained to self-administer both drugs. Behav. Pharmacol. 2011;22(e-suppl. A):e21. [Google Scholar]
- 186.Stolerman I. Drugs of abuse: behavioural principles, methods and terms. Trends Pharmacol. Sci. 1992;13:170–176. doi: 10.1016/0165-6147(92)90059-f. [DOI] [PubMed] [Google Scholar]
- 187.Paolone G, Palopoli M, Marrone MC, Nencini P, Badiani A. Environmental modulation of the interoceptive effects of amphetamine in the rat. Behav Brain Res. 2004;152:149–155. doi: 10.1016/j.bbr.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 188.Celentano M, et al. Drug context differently regulates cocaine versus heroin self-administration and cocaine- versus heroin-induced Fos mRNA expression in the rat. Psychopharmacology. 2009;204:349–360. doi: 10.1007/s00213-009-1467-x. [DOI] [PubMed] [Google Scholar]
- 189.De Luca MT, Badiani A. Ketamine self-administration in the rat: evidence for a critical role of setting. Psychopharmacology. 2011;214:549–556. doi: 10.1007/s00213-010-2062-x. [DOI] [PubMed] [Google Scholar]
- 190.Testa A, Nencini P, Badiani A. The role of setting in the oral self-administration of alcohol in the rat. Psychopharmacology. 2011;215:749–760. doi: 10.1007/s00213-011-2176-9. [DOI] [PubMed] [Google Scholar]
- 191.Badiani A, Robinson TE. Drug-induced neurobehavioral plasticity: the role of environmental context. Behav. Pharmacol. 2004;15:327–339. doi: 10.1097/00008877-200409000-00004. [DOI] [PubMed] [Google Scholar]
- 192.Paolone G, et al. Modulatory effect of environmental context and drug history on heroin-induced psychomotor activity and fos protein expression in the rat brain. Neuropsychopharmacology. 2007;32:2611–2623. doi: 10.1038/sj.npp.1301388. [DOI] [PubMed] [Google Scholar]
- 193.Lenoir M, Ahmed SH. Heroin-induced reinstatement is specific to compulsive heroin use and dissociable from heroin reward and sensitization. Neuropsychopharmacology. 2007;32:616–624. doi: 10.1038/sj.npp.1301083. [DOI] [PubMed] [Google Scholar]
- 194.Ahmed SH, Cador M. Dissociation of psychomotor sensitization from compulsive cocaine consumption. Neuropsychopharmacology. 2006;31:563–571. doi: 10.1038/sj.npp.1300834. [DOI] [PubMed] [Google Scholar]
- 195.Nutt DJ, King LA, Phillips LD. Drug harms in the UK: a multicriteria decision analysis. Lancet. 2010;376:1558–1565. doi: 10.1016/S0140-6736(10)61462-6. [DOI] [PubMed] [Google Scholar]
- 196.Anthony JC, Warner LA, Kessler RC. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: basic findings from the National Comorbidity Survey. Drug Alcohol Depend. 1994;2:244–268. [Google Scholar]
- 197.Hubbard RL, Marsden ME. In: NIDA Research Monograph 72: Relapse and Recovery in Drug Abuse. Tims FM, Leukefeld CG, editors. Rockville, Maryland: Department of Health and Human Services; 1986. pp. 157–166. [Google Scholar]
- 198.Dutra L, et al. A meta-analytic review of psychosocial interventions for substance use disorders. Am. J. Psychiatry. 2008;165:179–187. doi: 10.1176/appi.ajp.2007.06111851. [DOI] [PubMed] [Google Scholar]
- 199.Higgins ST, Heil SH, Lussier JP. Clinical implications of reinforcement as a determinant of substance use disorders. Annu. Rev. Psychol. 2004;55:431–461. doi: 10.1146/annurev.psych.55.090902.142033. [DOI] [PubMed] [Google Scholar]
- 200.Khantzian EJ. The self-medication hypothesis of addictive disorders: focus on heroin and cocaine dependence. Am. J. Psychiatry. 1985;142:1259–1264. doi: 10.1176/ajp.142.11.1259. [DOI] [PubMed] [Google Scholar]
- 201.Craig RJ, Olson RE. MCMI comparisons of cocaine abusers and heroin addicts. J. Clin. Psychol. 1990;46:230–237. doi: 10.1002/1097-4679(199003)46:2<230::aid-jclp2270460217>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 202.O’Connor L, Berry JW. The drugofchoice phenomenon: why addicts start using their preferred drug. J. Psychoactive Drugs. 1990;22:305–311. doi: 10.1080/02791072.1990.10472553. [DOI] [PubMed] [Google Scholar]
- 203.Suh JJ, Robins CE, Ruffins S, Albanese MJ, Khantzian EJ. Self-medication hypothesis: connecting affective experience and drug choice. Psychoanal. Psychol. 2008;25:518–532. [Google Scholar]
- 204.Tarter RE, Mezzich AC. In: Vulnerability to Drug Abuse. Glantz M, Pickens R, editors. Washington DC: American Psychological Association; 1992. pp. 149–178. [Google Scholar]
- 205.Kendler KS, Jacobson KC, Prescott CA, Neale MC. Specificity of genetic and environmental risk factors for use and abuse/dependence of cannabis, cocaine, hallucinogens, sedatives, stimulants, and opiates in male twins. Am. J. Psychiatry. 2003;160:687–695. doi: 10.1176/appi.ajp.160.4.687. [DOI] [PubMed] [Google Scholar]
- 206.Yuferov V, Levran O, Proudnikov D, Nielsen DA, Kreek MJ. Search for genetic markers and functional variants involved in the development of opiate and cocaine addiction and treatment. Ann. NY Acad. Sci. 2010;1187:184–207. doi: 10.1111/j.1749-6632.2009.05275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Spagnolo PA, Celentano M, Dubla A, Badiani A. Setting preferences for heroin versus cocaine taking in human co-abusers: role of environmental variables in drug use and relapse. Behav. Pharmacol. 2011;22(e-suppl. A):e21. [Google Scholar]
- 208.Epstein DH, et al. Real-time electronic diary reports of cue exposure and mood in the hours before cocaine and heroin craving and use. Arch. Gen. Psychiatry. 2009;66:88–94. doi: 10.1001/archgenpsychiatry.2008.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Heather N, Stallard A, Tebbutt J. Importance of substance cues in relapse among heroin users: comparison of two methods of investigation. Addictive Behav. 1991;16:41–49. doi: 10.1016/0306-4603(91)90038-j. [DOI] [PubMed] [Google Scholar]
- 210.Marlatt GA, Gordon JRE. Relapse Prevention: Maintenance Strategies in the Treatment of Addictive Behaviors. Guilford, New York: 1985. [Google Scholar]
- 211.Dole VP, Nyswander ME, Kreek MJ. Narcotic blockade. Arch. Intern. Med. 1966;118:304–309. [PubMed] [Google Scholar]
- 212.Shearer J. The principles of agonist pharmacotherapy for psychostimulant dependence. Drug Alcohol Rev. 2008;27:301–308. doi: 10.1080/09595230801927372. [DOI] [PubMed] [Google Scholar]
- 213.Mello NK, Negus SS. Preclinical evaluation of pharmacotherapies for treatment of cocaine and opioid abuse using drug self-administration procedures. Neuropsychopharmacology. 1996;14:375–424. doi: 10.1016/0893-133X(95)00274-H. [DOI] [PubMed] [Google Scholar]
- 214.Nielsen DA, et al. Genome-wide association study identifies genes that may contribute to risk for developing heroin addiction. Psychiatr. Genet. 2010;20:207–214. doi: 10.1097/YPG.0b013e32833a2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Compton PA, Ling W, Charuvastra VC, Wesson DR. Buprenorphine as a pharmacotherapy for cocaine abuse: a review of the evidence. J. Addictive Dis. 1995;14:97–114. doi: 10.1300/J069v14n03_07. [DOI] [PubMed] [Google Scholar]
- 216.Schottenfeld RS, Pakes JR, Oliveto A, Ziedonis D, Kosten TR. Buprenorphine vs methadone maintenance treatment for concurrent opioid dependence and cocaine abuse. Arch. Gen. Psychiatry. 1997;54:713–720. doi: 10.1001/archpsyc.1997.01830200041006. [DOI] [PubMed] [Google Scholar]
- 217.Epstein DH, et al. Promoting abstinence from cocaine and heroin with a methadone dose increase and a novel contingency. Drug Alcohol Depend. 2009;101:92–100. doi: 10.1016/j.drugalcdep.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kosten TR, Rounsaville BJ, Kleber HDA. 2.5-year follow-up of cocaine use among treated opioid addicts. Have our treatments helped? Arch. Gen. Psychiatry. 1987;44:281–284. doi: 10.1001/archpsyc.1987.01800150101012. [DOI] [PubMed] [Google Scholar]
- 219.Montoya ID, et al. Randomized trial of buprenorphine for treatment of concurrent opiate and cocaine dependence. Clin. Pharmacol. Ther. 2004;75:34–48. doi: 10.1016/j.clpt.2003.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Wolf ME. The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Prog. Neurobiol. 1998;54:679–720. doi: 10.1016/s0301-0082(97)00090-7. [DOI] [PubMed] [Google Scholar]
- 221.Hyman SE, Malenka RC, Nestler EJ. Neuronal mechanisms of addiction: the role of reward-related learning and memory. Annu. Rev. Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 222.Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nature Rev. Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- 223.Laviolette SR, van der Kooy D. Blockade of mesolimbic dopamine transmission dramatically increases sensitivity to the rewarding effects of nicotine in the ventral tegmental area. Mol. Psychiatry. 2003;8:50–59. doi: 10.1038/sj.mp.4001197. [DOI] [PubMed] [Google Scholar]
- 224.Laviolette SR, van der Kooy D. The neurobiology of nicotine addiction: bridging the gap from molecules to behaviour. Nature Rev. Neurosci. 2004;5:55–65. doi: 10.1038/nrn1298. [DOI] [PubMed] [Google Scholar]
- 225.Rassnick S, Stinus L, Koob GF. The effects of 6hydroxydopamine lesions of the nucleus accumbens and the mesolimbic dopamine system on oral self-administration of ethanol in the rat. Brain Res. 1993;623:16–24. doi: 10.1016/0006-8993(93)90004-7. [DOI] [PubMed] [Google Scholar]
- 226.Amit Z, Brown ZW. Actions of drugs of abuse on brain reward systems: a reconsideration with specific attention to alcohol. Pharmacol. Biochem. Behav. 1982;17:233–238. doi: 10.1016/0091-3057(82)90075-2. [DOI] [PubMed] [Google Scholar]
- 227.Belin D, Jonkman S, Dickinson A, Robbins TW, Everitt BJ. Parallel and interactive learning processes within the basal ganglia: relevance for the understanding of addiction. Behav. Brain Res. 2009;199:89–102. doi: 10.1016/j.bbr.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 228.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nature Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 229.Glickman SE, Schiff BB. A biological theory of reinforcement. Psychol. Rev. 1967;74:81–109. doi: 10.1037/h0024290. [DOI] [PubMed] [Google Scholar]
- 230.Beach HD. Morphine addiction in rats. Can. J. Psychol. 1957;11:104–112. doi: 10.1037/h0083703. [DOI] [PubMed] [Google Scholar]
- 231.Weeks JR. Experimental morphine addiction: method for automatic intravenous injections in unrestrained rats. Science. 1962;143:143–144. doi: 10.1126/science.138.3537.143. [DOI] [PubMed] [Google Scholar]
- 232.Stretch R, Gerber GJ. Drug-induced reinstatement of amphetamine self-administration behaviour in monkeys. Can. J. Psychol. 1973;27:168–177. doi: 10.1037/h0082466. [DOI] [PubMed] [Google Scholar]
- 233.Colpaert FC, Lal H, Niemegeers CJ, Janssen PA. Investigations on drug produced and subjectively experienced discriminative stimuli. I. The fentanyl cue, a tool to investigate subjectively experience narcotic drug actions. Life Sci. 1975;16:705–715. doi: 10.1016/0024-3205(75)90347-1. [DOI] [PubMed] [Google Scholar]
- 234.Wise RA, Rompre PP. Brain dopamine and reward. Annu. Rev. Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- 235.Pierce RC, Kumaresan V. The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neurosci. Biobehav. Rev. 2006;30:215–238. doi: 10.1016/j.neubiorev.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 236.Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog. Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- 237.Roberts DC, Koob GF, Klonoff P, Fibiger HC. Extinction and recovery of cocaine self-administration following 6hydroxydopamine lesions of the nucleus accumbens. Pharmacol. Biochem. Behav. 1980;12:781–787. doi: 10.1016/0091-3057(80)90166-5. [DOI] [PubMed] [Google Scholar]
- 238.Wise RA, Leone P, Rivest R, Leeb K. Elevations of nucleus accumbens dopamine and DOPAC levels during intravenous heroin self-administration. Synapse. 1995;21:140–148. doi: 10.1002/syn.890210207. [DOI] [PubMed] [Google Scholar]
- 239.Bozarth MA, Wise RA. Intracranial self-administration of morphine into the ventral tegmental area in rats. Life Sci. 1981;28:551–555. doi: 10.1016/0024-3205(81)90148-x. [DOI] [PubMed] [Google Scholar]
- 240.Phillips AG, LePiane FG. Reinforcing effects of morphine microinjection into the ventral tegmental area. Pharmacol. Biochem. Behav. 1980;12:965–968. doi: 10.1016/0091-3057(80)90460-8. [DOI] [PubMed] [Google Scholar]
- 241.Olds ME. Reinforcing effects of morhpine in the nucleus accumbens. Brain Res. 1982;237:429–440. doi: 10.1016/0006-8993(82)90454-1. [DOI] [PubMed] [Google Scholar]
- 242.van der Kooy D, Mucha RF, O’Shaughnessy M, Bucenieks P. Reinforcing effects of brain microinjections of morphine revealed by conditioned place preference. Brain Res. 1982;243:107–117. doi: 10.1016/0006-8993(82)91124-6. [DOI] [PubMed] [Google Scholar]
- 243.McBride WJ, Murphy JM, Ikemoto S. Localization of brain reinforcement mechanisms: intracranial self- administration and intracranial place-conditioning studies. Behav. Brain Res. 1999;101:129–152. doi: 10.1016/s0166-4328(99)00022-4. [DOI] [PubMed] [Google Scholar]
- 244.Shippenberg TS, Bals-Kubik R, Herz A. Examination of the neurochemical substrates mediating the motivational effects of opioids: role of the mesolimbic dopamine system and D1 vs. D2 dopamine receptors. J. Pharmacol. Exp. Ther. 1993;265:53–59. [PubMed] [Google Scholar]
- 245.Bechara A, Nader K, van der Kooy D. A twoseparatemotivational-systems hypothesis of opioid addiction. Pharmacol. Biochem. Behav. 1998;59:1–17. doi: 10.1016/s0091-3057(97)00047-6. [DOI] [PubMed] [Google Scholar]
- 246.Sellings LH, Clarke PB. Segregation of amphetamine reward and locomotor stimulation between nucleus accumbens medial shell and core. J. Neurosci. 2003;23:6295–6303. doi: 10.1523/JNEUROSCI.23-15-06295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Olmstead MC, Franklin KB. The development of a conditioned place preference to morphine: effects of lesions of various CNS sites. Behav. Neurosci. 1997;111:1313–1323. doi: 10.1037//0735-7044.111.6.1313. [DOI] [PubMed] [Google Scholar]
- 248.Mackey WB, van der Kooy D. Neuroleptics block the positive reinforcing effects of amphetamine but not of morphine as measured by place conditioning. Pharmacol. Biochem. Behav. 1985;22:101–105. doi: 10.1016/0091-3057(85)90492-7. [DOI] [PubMed] [Google Scholar]
- 249.Nader K, Bechara A, Roberts DC, van der Kooy D. Neuroleptics block high- but not low-dose heroin place preferences: further evidence for a two-system model of motivation. Behav. Neurosci. 1994;108:1128–1138. doi: 10.1037//0735-7044.108.6.1128. [DOI] [PubMed] [Google Scholar]
- 250.Van Ree JM, Ramsey N. The dopamine hypothesis of opiate reward challenged. Eur. J. Pharmacol. 1987;134:239–243. doi: 10.1016/0014-2999(87)90172-5. [DOI] [PubMed] [Google Scholar]
- 251.Winger G. Dopamine antagonist effects on behavior maintained by cocaine and alfentanil in rhesus monkeys. Behav. Pharmacol. 1994;5:141–152. doi: 10.1097/00008877-199404000-00005. [DOI] [PubMed] [Google Scholar]
- 252.Gerrits MAFM, Ramsey NF, Wolterink G, Van Ree JM. Lack of evidence for an involvement of nucleus accumbens dopamine D1 receptors in the initiation of heroin self-administration. Psychopharmacology. 1994;114:486–494. doi: 10.1007/BF02249340. [DOI] [PubMed] [Google Scholar]
- 253.Gerrits MA, Van Ree JM. Effect of nucleus accumbens dopamine depletion on motivational aspects involved in initiation of cocaine and heroin self-administration in rats. Brain Res. 1996;713:114–124. doi: 10.1016/0006-8993(95)01491-8. [DOI] [PubMed] [Google Scholar]
- 254.Dworkin SI, Guerin GF, Co C, Goeders NE, Smith JE. Lack of an effect of 6hydroxydopamine lesions of the nucleus accumbens on intravenous morphine self-administration. Pharmacol. Biochem. Behav. 1988;30:1051–1057. doi: 10.1016/0091-3057(88)90138-4. [DOI] [PubMed] [Google Scholar]
- 255.Stinus L, et al. Chronic flupenthixol treatment potentiates the reinforcing properties of systemic heroin administration. Biol. Psychiatry. 1989;26:363–371. doi: 10.1016/0006-3223(89)90052-8. [DOI] [PubMed] [Google Scholar]
- 256.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Amsterdam: Elsevier Academic Press; 2005. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.