Abstract
Purpose/Objectives
To test the feasibility and acceptability of a strength-training intervention in patients receiving hematopoietic stem cell transplantation (HSCT).
Design
One-group prospective, repeated-measures design.
Setting
Academic medical center in the midwestern United States.
Sample
Convenience sample of 10 patients receiving HSCT.
Methods
The strength-training intervention consisted of a comprehensive program of progressive resistance to strengthen the upper body, lower body, and abdominal muscles using elastic resistance bands. Instruction and low-intensity training began while the patients were hospitalized and progressed to a moderate level immediately following discharge from the hospital. Training continued for six weeks following hospital discharge.
Main Research Variables
Acceptability of the strength-training intervention was evaluated via subjective assessment and by determining the patient’s ability to perform the exercises. Feasibility was evaluated by determining the number of patients who were able to complete the prescribed strength intervention and whether the patients used elastic resistance bands.
Findings
The strength-training intervention was refined from an unsupervised, home-based program to a combination supervised and unsupervised program with weekly clinic visits. Patients reported that the exercises were very acceptable, although some started out at a very low intensity.
Conclusions
This pilot study demonstrates the feasibility and acceptability of the strength-training intervention. The level of supervision required for the strength-training intervention was higher than expected.
Implications for Nursing
Strength training may be an effective intervention to alleviate problems with decreased physical activity, reduced muscle mass, and fatigue in HSCT recipients. Additional research is needed.
People with hematologic malignancies such as acute myeloid leukemia and lymphoma receive high-dose chemotherapy followed by hematopoietic stem cell transplantation (HSCT) as curative therapy (Hahn et al., 2001, 2003, 2006; Oliansky et al., 2008, 2009; Rowe et al., 1994). HSCT recipients frequently experience considerable deterioration of their health status as a result of dose-intensive therapy, particularly during the immediate post-transplantation period. This deterioration in health status has the potential to affect all aspects of life, and the impact may be felt for years following treatment. A marked reduction in physical activity immediately following high-dose chemotherapy and HSCT has been documented (Hacker et al., 2006). Although the specific cause is not understood, the physical inactivity may be protracted and sufficient to cause physical deconditioning, loss of muscle mass, and decreased strength and endurance (Coleman et al., 2003; Cunningham et al., 1986). Aerobic and strength-training exercises have been used successfully to increase levels of physical activity. Aerobic exercise improves cardiorespiratory conditioning, whereas strength training is more effective in minimizing skeletal muscle wasting associated with prolonged physical inactivity (American College of Sports Medicine Position Stand, 2009). A strength-training intervention that minimizes muscle wasting in patients undergoing HSCT is particularly attractive if the end result is an enhanced ability to perform activities of daily living and improved health status perceptions and quality of life.
Although the beneficial effects of exercise are well documented, few exercise studies have been conducted in the HSCT population and only three have incorporated strength training (Coleman et al., 2003; Cunningham et al., 1986; Hayes, Davies, Parker, & Bashford, 2003). Most focused on aerobic training (Carlson, Smith, Russell, Fibich, & Whittaker, 2006; Dimeo et al., 1996; Dimeo, Fetscher, Lange, Mertelsmann, & Keul, 1997) or a combination of aerobic and strength training (Coleman et al., 2003; Hayes et al., 2003). When aerobic and strength training are combined, determining the individual effects of either one alone is difficult, and that information is an important consideration when trying to minimize the burden associated with exercise training. Only one study employed strength training alone; however, the intervention lasted only 35 days following the transplantation while the patient was hospitalized, and the intensity of the strength training was not described (Cunningham et al., 1986). No studies found in the literature examined the impact of strength training on physical activity, muscle strength, fatigue, general health perceptions, and quality of life in HSCT recipients.
This pilot study (phase I) was part of a two-phase study. The overall study purpose was to determine the effects of a strength-training exercise program on physical activity, muscle strength, fatigue, health status perceptions, and quality of life in patients following high-dose chemotherapy and HSCT. The purpose of phase I was to pilot test the exercise intervention to determine the acceptability and feasibility of the exercise intervention with respect to the appropriate time for initiating the intervention and the appropriate intensity of the exercise intervention. A secondary purpose was to determine whether the data collection schedule, procedures, and measurements were appropriate. Revisions to the strength-training intervention and methods were to be made in phase I prior to implementation in a two-group, randomized, clinical trial (phase II). This article focuses on the findings from feasibility and acceptability of the strength-training intervention (phase I). The research methods are presented here as originally planned, and required modifications are presented in the results section.
Methods
Design
This pilot study used a one-group prospective, repeated-measures design to evaluate the feasibility and acceptability of a strength-training intervention following high-dose chemotherapy and HSCT. Institutional review board approval was obtained prior to implementation. Instruction began while the patients were hospitalized. Patients began moderate-intensity training immediately following discharge from the hospital, and training continued for six weeks. Dependent variables included physical activity, muscle strength, fatigue, perceived health status, and quality of life. Physical activity was measured using wrist actigraphy. Tests of muscle strength were performed by laboratory assessment (isokinetic strength of the knee flexors and extensors and hand grip dynamometer) and functional assessment (30-second chair-stand test and time needed to stand up from bed-rest examination). Fatigue was measured with a one-item fatigue intensity scale, using computerized ecologic momentary assessment (real-time assessment) and the fatigue subscale of the European Organisation for Research and Treatment of Cancer, Quality-of-Life Questionnaire–Core 30, version 3.0 (EORTC QLQ-C30) (Aaronson et al., 1993). General health perceptions and quality of life were measured with the EORTC QLQ-C30 and the Quality of Life Index (QLI), respectively (Ferrans, 1990; Ferrans & Powers, 1985). Variables were measured three times: prior to admission to the hospital for the HSCT (time 1), day 8 following transplantation (time 2), and six weeks following discharge from the hospital (time 3). The full battery of tests was conducted at times 1 and 3. A limited number of tests were conducted at time 2 because researchers expected that patients would be experiencing profound neutropenia and other side effects from the high-dose chemotherapy.
Sample and Setting
Adult patients electing to undergo HSCT at an academic medical center in the midwestern United States were invited to participate. Initial eligibility criteria included patients scheduled to receive a transplantation, who were able to speak English and comprehend the purpose of the study, and who did not have a history of a psychiatric illness. Patients eligible to receive HSCT undergo extensive medical workup prior to transplantation; therefore, all of the pretesting procedures are standards of care and were not considered part of the research. Pretesting included a history and physical; multigated acquisition scans to assess heart function; pulmonary function tests to assess pulmonary function; various blood tests to assess exposure to viruses and kidney, liver, and blood cell function; chest x-rays; urinalysis; and a dental examination. The treating physicians reviewed all of the pretests and provided approval for patients to participate in this study. All patients received HSCT for treatment of an underlying malignancy.
After enrolling five patients undergoing autologous HSCT, the eligibility criteria were expanded to include patients undergoing allogeneic transplantation. Fifteen patients were eligible to participate. Eleven enrolled in the study and 10 completed the research activities. One did not receive a stem cell transplantation and was no longer eligible to participate. The four patients who declined to participate stated that they were too busy or already had “too much on their plate.” Patients enrolled in the study over a 13-month period.
Strength-Training Intervention
The strength-training intervention consisted of a comprehensive program of progressive resistance to strengthen the upper body, lower body, and abdominal muscles using elastic resistance bands and body weight for resistance. Instruction began while the patients were hospitalized for the HSCT. Patients began moderate-intensity training immediately following discharge from the hospital. Training continued for six weeks. The exercise prescription was tailored to the individual’s capabilities. The Borg (1998) rating of perceived exertion, a 20-point scale, was used to estimate the intensity of the resistance. The moderate-intensity exercise prescription was based on a rating of “somewhat hard” (Borg scale 13). Instruction and training were conducted by the principal investigator or one trained member of the research team.
The strength-training intervention consisted of 11 preselected exercises with concentric and eccentric muscle contractions: eight exercises using elastic resistance bands (chest fly, biceps curl, triceps extension, shoulder shrug, shoulder upright row, shoulder lateral raise, knee flexion, and knee extension) and three exercises that used body weight as resistance (wall push-ups, squats, and bed sit-ups). The exercise prescription was tailored to the patient’s capabilities. Patients were prescribed as many exercises as they could perform, starting with the easiest exercises and advancing to the most difficult. Patients were expected to exercise three times per week. Progression of the exercise prescription was structured to first increase the number of sets from one to two sets of 8–10 repetitions and then to increase the resistance level of elastic tubing. Patients provided return demonstrations of the exercises to ensure proper form and reduce the chance of injury. When discharged from the hospital, patients were scheduled to receive one home visit to supervise the exercises followed by weekly phone calls to determine adherence and discuss tolerance of the exercise prescription. Patients documented completion of each individual exercise, including information about the number of repetitions and sets on preprinted exercise logs.
Instrumentation
All of the instruments used in this pilot study have established reliability and validity. Because the focus of this article is feasibility and acceptability of the strength-training intervention and study design, the measurement of each variable will be briefly discussed.
Muscle strength was evaluated by laboratory assessment (isokinetic strength of the knee flexors and extensors and hand grip dynamometer) and functional assessment (30-second chair-stand test and time needed to stand up from a bed-rest examination). Isokinetic strength of the knee flexors and extensors and hand grip strength were used as proxy variables to reflect general skeletal muscle strength and have established reliability and validity (Balogun, Akomolafe, & Amusa, 1991; Frontera, Hughes, Dallal, & Evans, 1993; Giles, Henke, Edmonds, & McNeil, 1990; Kues, Rothstein, & Lamb, 1992; Reddon, Stefanyk, Gill, & Renney, 1985; Schaubert & Bohannon, 2005; Watanabe et al., 2005). The two functional assessment tests evaluated physical performance; both have established reliability and validity (Jones, Rikli, & Beam, 1999; Maeda, Yuasa, Nakamura, Higuchi, & Motohashi, 2000).
Fatigue was measured in real-time using a one-item fatigue intensity scale and the fatigue subscale of the EORTC QLQ-C30. Real-time fatigue assessment has been used successfully in patients with cancer, including HSCT recipients (Curran, Beacham, & Andrykowski, 2004; Hacker & Ferrans, 2007). The fatigue subscale of the EORTC QLQ-C30 has been used extensively in patients with cancer and has established reliability and validity (Aaronson et al., 1993; Knobel et al., 2003).
The EORTC QLQ-C30 was used to measure patients’ perceptions of health status in terms of deviations from an optimal state of functioning. The EORTC QLQ-C30 is a well-established instrument, and the psychometric properties have been reported previously (Aaronson et al., 1993; Aaronson, Cull, Kaasa, & Sprangers, 1996; Bjordal & Kaasa, 1992; Hacker & Ferrans, 2003; Osoba, Aaronson, Zee, Sprangers, & te Velde, 1997; Osoba, Zee, Warr, Kaizer, & Latreille, 1994).
The QLI, used to measure life satisfaction (Ferrans, 1990; Ferrans & Powers, 1985), is a well-established tool that has been used internationally in more than 200 published studies in a variety of populations, including patients with cancer (Anderson & Ferrans, 1997; Belec, 1992; Ferrans & Powers, 1985; Gupta, Lis, & Grutsch, 2007; Hacker et al., 2006) and in large clinical trials (Jenkins et al., 2005).
Data Collection Schedule and Procedures
Wrist actigraphs were placed on a patient’s nondominant hand prior to hospitalization for HSCT (time 1) to measure physical activity. The patients were instructed to leave the device in place for the next five days. Because of the expected variability in time associated with placing and removing the wrist actigraph on the first and fifth day, only the 72 hours of complete, continuous data were analyzed. Patients were instructed to carry on with normal activities, including bathing and showering. Fatigue was rated three times during the course of the day (10 am, 2 pm, and 6 pm) on an intensity scale ranging from 1 (no fatigue) to 10 (worst fatigue). An alarm on the wrist actigraph sounded at these times to remind patients to complete the data. Patients were instructed to enter the rating directly into the subjective event marker of the wrist actigraph. At the end of the five-day period, patients completed the tests for muscle strength, general health perceptions, and quality of life. The EORTC QLQ-C30 and QLI were administered via interview. Patients were given a copy of the questionnaire to view while answering the questions to facilitate the interview process. The muscle strength tests took approximately 20 minutes to complete, and the questionnaires took approximately 15 minutes. All data collection procedures were conducted by the principal investigator or one trained member of the research team.
The procedures for placing and removing the wrist actigraph during hospitalization for HSCT (days 4–8) (time 2) were the same as described for time 1. The device was placed on day 4 following the transplantation and removed on day 8. Patients rated the intensity of fatigue three times per day as described for time 1. The tests for general health perceptions and quality of life were administered via interview on day 8 following the transplantation. In addition, patients completed two tests for muscle strength.
Six weeks following hospital discharge (time 3), the procedures for data collection were the same as described in time 1 prior to hospitalization.
Data Analysis
The acceptability of the 11 exercises was evaluated by soliciting feedback from patients regarding suitability of using elastic resistance bands for strength training, tolerability of specific strength-training exercises, and determining the patients’ ability to physically perform the exercises. The feasibility of the exercise intervention was evaluated by determining the number of patients who were able to complete the prescribed strength intervention on a weekly basis and whether elastic resistance bands were used. Exercise prescriptions were modified as described in the results section of this pilot study.
Results
Sample
The participants ranged in age from 48–71 (, SD = 7.56) and were racially diverse: African American (n = 5), Caucasian (n = 4), and Hispanic (n = 1). Six men and four women participated in the study. The majority were married (n = 6) and attained a high school degree as their highest level of education (n = 7). Five reported income levels less than $20,000. Patients received a transplantation for a variety of hematologic malignancies. All were outpatients at time 1, inpatients at time 2, and outpatients again at time 3.
Study Adjustments
One muscle strength test was revised. Isokinetic measurement of knee flexors and extensor muscle strength using the Cybex 340 Dynamometer (Cybex International) was replaced with a timed stair climb. The isokinetic measurement of muscle strength requires maximal effort on the part of patients. The first two patients had great difficulty exerting maximal effort at times 1 and 3. Neither study participant was able to generate enough force to register a measure of strength on the Cybex 340 Dynamometer. The timed stair climb has excellent reliability, and the results for timed stair climbs have been significantly correlated with isokinetic strength measurement of knee flexors and extensors (Salem, Wang, Young, Marion, & Greendale, 2000). In addition, stair climbing is a common activity in daily life, and people who are able to climb stairs generally know how to perform this activity, avoiding the need for practice sessions.
Two major refinements to the strength-training intervention occurred during the feasibility and acceptability testing. The strength-training intervention was changed from an unsupervised, home-based program to a combination supervised and unsupervised program with weekly clinic visits. The first two patients required frequent adjustments in the exercise prescriptions that could not be explained adequately over the telephone, necessitating an additional home visit or visit in the clinic. Based on this experience, the intervention was modified so that all patients were seen once a week during their regularly scheduled clinic visit to exercise under supervision. The strength-training program was revised so that patients who were not strong enough to use elastic resistance bands for strength training participated in the study by performing the exercises (concentric and eccentric contractions of muscles) without bands.
Acceptability of the Exercises
Patients evaluated the acceptability of the exercise intervention. All who used the bands reported that they were easy to use and very portable, thus facilitating participation in the strength-training intervention. Patients reported that most of the exercises were acceptable and within their capabilities even if they were not able to perform all of the exercises at once. The ability to stand for an extended period of time (more than 20 minutes) was not a requirement for the strength-training intervention. Many of the exercises (biceps curl, triceps extension, shoulder shrug, shoulder upright row, shoulder lateral raise, knee flexion, and knee extension) could be performed standing or sitting in a chair. Two patients found the knee squats with elastic resistance bands to be difficult and expressed concern about losing their balance. This exercise was modified by eliminating the use of elastic resistance bands and performing knee squats while holding on to a counter or railing for balance. Other patients found this modification acceptable. Over the six-week study period, most were able to add more exercises to the exercise prescription. Table 1 provides a detailed list of the maximum number of exercises that were performed by each patient during a single session on a week-to-week basis.
Table 1.
Maximum Number of Exercises Performed in a Single Session
| Week |
||||||
|---|---|---|---|---|---|---|
| Patient | 1 | 2 | 3 | 4 | 5 | 6 |
| Aa,b | – | – | – | – | – | – |
| B | 5 | – | 7 | 7 | 7 | 7 |
| Cc | 3 | 8 | 8 | – | – | – |
| D | 7 | – | 7 | 7 | 7 | 7 |
| E | – | 6 | 7 | – | – | 6 |
| Fd | – | – | – | – | 9 | 10 |
| G | 7 | – | 6 | 6 | 8 | 8 |
| Hb,d | – | 6 | 7 | 8 | 8 | 8 |
| I | – | – | – | – | – | 4 |
| Jb,d | 4 | 4 | 8 | 10 | 10 | 10 |
Unable to participate in the strength-training intervention because of low platelet counts
Did not use elastic resistance bands
Unable to continue the strength-training intervention because of an unrelated medical complication
Received allogeneic stem cell transplantation
Feasibility of the Exercise Program
The feasibility of the exercise intervention was evaluated by determining the week-to-week exercise frequency of participants (see Figure 1). At discharge, few patients were able to complete the exercise prescription three times per week. As time progressed, more were able to exercise and, by week 6 of the intervention, 6 of the 10 patients were exercising three times per week and another 2 exercised once or twice a week. The reasons for not completing the prescribed exercises three times per week were evaluated. One patient’s platelet count never rose above the required 50,000/mm3 to start exercising. Another initially exercised in the clinic with the research staff but did not exercise at home. This patient stated that he or she had “never been much of an exerciser” and did not like to exercise at home although he or she enjoyed the supervised exercise sessions in the clinic. Two patients felt “too ill” to exercise until week 5 or 6.
Figure 1.
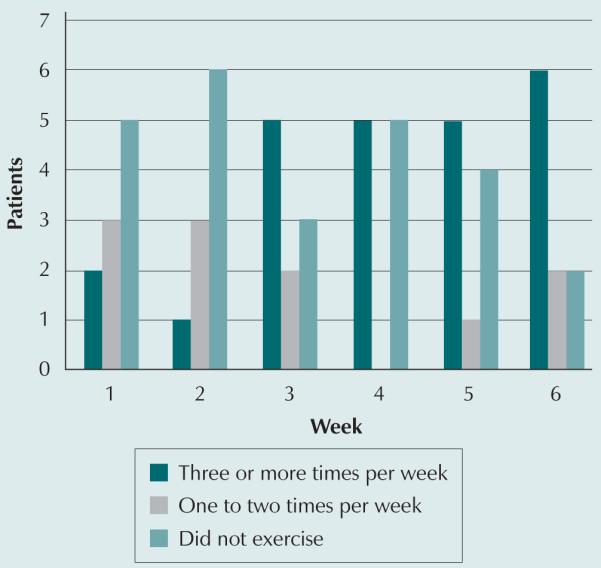
Exercise Frequency Following Discharge From the Hospital After Hematopoietic Stem Cell Transplantation
The feasibility of the exercise intervention also was assessed by determining the number of patients who were defined as exercisers (exercised at least two times per week for a minimum of three weeks) and the number of patients who were able to progress to using elastic resistance bands. In this study, six patients were exercising three times per week and, therefore, were defined as exercisers. Seven of the 10 patients were able to progress to using elastic resistance bands. The patients receiving an allogeneic transplantation experienced more difficulty with the elastic resistance bands. Two of the three allogeneic transplantation recipients felt too weak during the six-week posthospital discharge period to use elastic resistance bands. Instead, these patients performed the same exercise motions without the use of elastic bands. Table 2 provides information regarding ability to exercise and use of bands further delineated by gender, age, and type of transplantation.
Table 2.
Strength Training by Gender, Age, and Type of Transplantation
| Exercisea |
Resistance Bandsb |
|||
|---|---|---|---|---|
| Variable | Yes | No | Yes | No |
| Gender | ||||
| Male (n = 7) | 4 | 3 | 6 | 1 |
| Female (n = 3) | 2 | 1 | 1 | 2 |
| Age | ||||
| Younger than 50 years (n = 1) | 1 | – | – | 1 |
| 50–69 years (n = 5) | 3 | 2 | 4 | 1 |
| 70 years or older (n = 4) | 2 | 2 | 3 | 1 |
| Type of transplantation | ||||
| Autologous (n = 7) | 4 | 3 | 6 | 1 |
| Allogeneic (n = 3) | 2 | 1 | 1 | 2 |
At least twice a week for three weeks
Patients progressed to using elastic resistance bands.
General Health Perceptions, Quality of Life, Physical Activity, and Hand Grip Strength
Mean scores and standard deviations for general health perceptions (EORTC QLQ-C30), quality of life, physical activity, and hand grip strength are presented in Table 3. These results are reported for descriptive purposes only as the sample size is too small to make any conclusions regarding the effectiveness of the strength-training intervention.
Table 3.
General Health Perceptions, Quality of Life, Physical Activity, and Hand Grip Strength
| Time 1 |
Time 2 |
Time 3 |
||||
|---|---|---|---|---|---|---|
| Variable | SD | SD | SD | |||
| General Health Perceptions | ||||||
|
| ||||||
| Functional scales | ||||||
| Cognitive | 91.67 | 11.76 | 60 | 25.09 | 83.33 | 13.61 |
| Emotional | 83.33 | 20.03 | 65.83 | 19.82 | 80 | 15.32 |
| Physical | 75.33 | 18.07 | 52 | 20.56 | 73.33 | 12.57 |
| Role | 80 | 23.31 | 31.67 | 34.65 | 65 | 22.84 |
| Social | 83.33 | 17.57 | 43.33 | 45.27 | 71.67 | 17.66 |
| Global quality of life | 79.17 | 13.75 | 50 | 19.24 | 67.5 | 20.95 |
| Symptom scales | ||||||
| Fatigue | 33.33 | 33.54 | 75.56 | 22.1 | 37.78 | 17.53 |
| Pain | 30 | 32.2 | 58.33 | 38.69 | 15 | 22.84 |
| Nausea and vomiting | 3.33 | 7.02 | 46.67 | 15.32 | 13.33 | 13.15 |
| Single items | ||||||
| Appetite loss | 10 | 16.1 | 73.33 | 30.61 | 16.67 | 17.57 |
| Constipation | 3.33 | 10.54 | 13.33 | 32.2 | 20 | 23.31 |
| Diarrhea | 10 | 22.5 | 70 | 33.15 | 13.33 | 23.31 |
| Dyspnea | 20 | 23.31 | 30 | 24.6 | 23.33 | 22.5 |
| Financial impact | 16.67 | 32.39 | 33.33 | 35.14 | 20 | 32.2 |
| Sleep disturbances | 10 | 16.1 | 56.67 | 41.72 | 30 | 18.92 |
|
| ||||||
| Quality of Life | ||||||
|
| ||||||
| Overall quality of life | 23.51 | 3.65 | 23.44 | 3.13 | 24 | 3.88 |
| Health and functioning | 21.17 | 4.43 | 20.35 | 3.94 | 21.88 | 4.35 |
| Social and economic | 23.84 | 4.66 | 24.94 | 4.46 | 24.95 | 3.65 |
| Psychological and spiritual | 25.58 | 3.72 | 25.7 | 3.85 | 25.49 | 4.55 |
| Family | 26.65 | 3.86 | 26.55 | 3.81 | 26.29 | 4.355 |
| Physical activity countsa | 171.4 | 66.21 | 80.8 | 48.84 | 112.3 | 48.83 |
|
| ||||||
| Muscle Strength | ||||||
|
| ||||||
| Hand grip strength (kilograms of force) |
32.66 | 12.34 | – | – | 28.18 | 12.42 |
| Time needed to stand up from bed rest (seconds)b |
4.63 | 1.65 | 6.28 | 3.62 | 4.05 | 1.5 |
| 30-second chair-stand testc | 7.6 | 3.1 | 3.2 | 3.71 | 8.22 | 3.42 |
N = 10
Average physical activity counts for one-minute epochs. One patient removed the accelerometer for more than eight hours during time 3 (n = 9).
Two patients were unable to complete the intervention because of poor health status at time 2 (n = 8) and one subject was unable to complete the intervention because of equipment availability issues at time 3 (n = 9).
One patient was unable to complete the intervention (time 3; n = 9) because of an unrelated medical condition.
Discussion
This pilot study demonstrates that implementing a strength-training intervention in patients receiving high-dose chemotherapy followed by HSCT is feasible, specifically during the first six weeks following discharge from the hospital after treatment. In this study, the implementation of the strength-training intervention required a tailored approach with more frequent in-person follow-up. The eight exercises using elastic resistance bands and three using body weight as resistance were considered acceptable, although none of the patients was able to perform all of the exercises at once when first beginning to exercise.
The strength-training intervention originally was designed to be home-based with weekly telephone calls. Very early during the course of the study, a determination was made that patients required more frequent in-person follow-up, particularly when initiating the exercise program following discharge from the hospital because of their changing health status. Patients enrolled earlier in the study required frequent adjustments in the exercise prescriptions that could not be explained adequately over the telephone, necessitating an additional home visit or visit in the clinic. In addition, none of the initial patients was able to perform all of the exercises when starting the strength-training program. As their health status improved, patients were able to increase the number of exercises performed. Based on these initial experiences, the intervention was modified so that patients were seen once or twice a week during their regularly scheduled clinic visit to exercise under supervision; this facilitated progression with the exercise intervention. Patients exercised in the clinic examination rooms during their regularly scheduled clinic appointments with the principal investigator or other research staff present. Changes to the exercise prescription, mostly advancements, were made at that time. Patients were encouraged to exercise on their own one or two more times during the week for a total of three times per week. This refinement changed the strength-training intervention from a home-based, unsupervised program to a combined supervised and unsupervised program. Although the strength-training intervention was more time and labor intensive than originally planned, 8 of the 10 patients were exercising two to three times per week by week 6, suggesting that the tailored approach enhances feasibility and acceptability.
The second revision to the strength-training intervention that enhanced feasibility and acceptability was to allow patients to perform the exercises (concentric and eccentric contractions of muscles) without elastic resistance bands if they were not strong enough to use the bands for strength training. During the initial stages of the study’s implementation, patients were only given an exercise prescription if they were able to exercise with the elastic resistance bands. Although many were able to perform at least some of the exercises with bands immediately after hospital discharge, some patients, particularly those undergoing allogeneic transplantation, experienced more difficulty using bands when first discharged from the hospital. The majority were able to eventually progress to using elastic resistance bands by week 6. Of the three who did not, two had received allogeneic transplantations and one was not able to exercise because of low platelet counts.
The decision to include patients undergoing allogeneic HSCT was made only after implementing the strength-training intervention in five patients undergoing autologous transplantation. Although the risks and benefits related to the strength-training intervention are the same for autologous and allogeneic, enrolling patients undergoing allogeneic transplantation had the potential to introduce additional challenges related to increased acuity or prolonged hospitalizations. Based on this experience, the authors believe that this method of strength training is feasible for patients with allogeneic transplantations and warrants additional investigation. However, the progression of training may be slower.
This strength-training intervention was more labor-intensive than initially anticipated. Originally designed to minimize the staff time required for implementation, thereby minimizing the cost of the intervention, extra staff time was required when the need for direct supervision became apparent. However, costly home visits were avoided and may be justified if future research demonstrates that strength training improves functioning and quality of life.
Nursing Implications
The connections between exercise and improved physical and psychological health have been well established in patients with cancer (Courneya & Friedenreich, 1999; Galvao & Newton, 2005; Knols, Aaronson, Uebelhart, Fransen, & Aufdemkampe, 2005; McNeely et al., 2006; Schmitz et al., 2005; Stevinson, Lawlor, & Fox, 2004). Questions still remain, however, about the type of exercise that is best for achieving specific outcomes in various subgroups. Deciding on an appropriate exercise intervention requires detailed knowledge of the cancer treatment and expected recovery period. In this study, strength training was chosen as a means to maintain or enhance strength to facilitate recovery from the high-dose chemotherapy and subsequent transplantation. A strength-training program using elastic resistance bands was chosen because of the bands’ portability, ease of use, and potential ability to easily translate the strength-training program into clinical practice should the intervention prove effective in the second phase of the study.
Conclusion
Pilot study work, such as the work detailed here, must transpire before embarking on a large-scale study to improve the latter study’s quality and efficiency while avoiding costly mistakes. This study demonstrates that conducting a strength-training intervention in patients receiving intensive cancer therapy is feasible. The patients found the strength-training intervention very acceptable, although some started out at a very low intensity. Those that exercised made progress. The entire process had to be kept as simple as possible to avoid undue patient burden. The level of supervision required for the strength-training intervention was higher than expected, making the strength-training intervention more time, labor, and cost intensive than originally anticipated. Additional research is needed to determine the effects of the strength training on physical activity, muscle strength, fatigue, general health perceptions, and quality of life following HSCT.
Acknowledgments
This article was supported with funding from the National Institutes of Health, National Institute of Nursing Research (principal investigator: E. Hacker, K01 NR009375). Elastic resistance bands were provided by Hygenic Corporation.
Contributor Information
Eileen Danaher Hacker, Department of Biobehavioral Health Science in the College of Nursing at the University of Illinois in Chicago.
Janet L. Larson, Division of Acute, Critical, and Long-Term Care in the School of Nursing at the University of Michigan in Ann Arbor.
David Peace, College of Medicine at the University of Illinois in Chicago..
References
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, de Haes JC. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality of life instrument for use in international clinical trials in oncology. Journal of the National Cancer Institute. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- Aaronson NK, Cull AN, Kaasa S, Sprangers MA. Quality of life and pharmacoeconomics. 2nd ed Lippincott-Raven; Philadelphia, PA: 1996. [Google Scholar]
- American College of Sports Medicine Position Stand Progression models in resistance training for healthy adults. Medicine and Science in Sports and Exercise. 2009;41:687–708. doi: 10.1249/MSS.0b013e3181915670. [DOI] [PubMed] [Google Scholar]
- Anderson JS, Ferrans CE. The quality of life of persons with chronic fatigue syndrome. Journal of Nervous and Mental Disease. 1997;185:359–367. doi: 10.1097/00005053-199706000-00001. doi: 10.1097/00005053-199706000-00001. [DOI] [PubMed] [Google Scholar]
- Balogun JA, Akomolafe CT, Amusa LO. Grip strength: Effects of testing posture and elbow position. Archives of Physical Medicine and Rehabilitation. 1991;72:280–283. [PubMed] [Google Scholar]
- Belec RH. Quality of life: Perceptions of long-term survivors of bone marrow transplantation. Oncology Nursing Forum. 1992;19:31–37. [PubMed] [Google Scholar]
- Berger AM. Patterns of fatigue and activity and rest during adjuvant breast cancer chemotherapy. Oncology Nursing Forum. 1998;25:51–62. [PubMed] [Google Scholar]
- Berger AM, Higginbotham P. Correlates of fatigue during and following adjuvant breast cancer chemotherapy: A pilot study. Oncology Nursing Forum. 2000;27:1443–1448. [PubMed] [Google Scholar]
- Bjordal K, Kaasa S. Psychometric validation of the EORTC core quality of life questionnaire, 30 item version and a diagnosis-specific module for head and neck cancer patients. Acta Oncologica. 1992;31:311–321. doi: 10.3109/02841869209108178. doi: 10.3109/02841869209108178. [DOI] [PubMed] [Google Scholar]
- Borg G. Borg’s perceived exertion and pain scales. Human Kinetics; Stockholm, Sweden: 1998. [Google Scholar]
- Carlson LE, Smith D, Russell J, Fibich C, Whittaker T. Individualized exercise program for the treatment of severe fatigue in patients after allogeneic hematopoietic stem-cell transplant: A pilot study. Bone Marrow Transplantation. 2006;37:945–954. doi: 10.1038/sj.bmt.1705343. [DOI] [PubMed] [Google Scholar]
- Coleman EA, Coon S, Hall-Barrow J, Richards K, Gaylor D, Stewart B. Feasibility of exercise during treatment for multiple myeloma. Cancer Nursing. 2003;26:410–419. doi: 10.1097/00002820-200310000-00012. [DOI] [PubMed] [Google Scholar]
- Courneya KS, Friedenreich CM. Physical exercise and quality of life following cancer diagnosis: A literature review. Annals of Behavorial Medicine. 1999;21:171–179. doi: 10.1007/BF02908298. [DOI] [PubMed] [Google Scholar]
- Cunningham BA, Morris G, Cheney CL, Buergel N, Aker SN, Lenssen P. Effects of resistive exercise on skeletal muscle in marrow transplant recipients receiving total parenteral nutrition. Journal of Parenteral and Enteral Nutrition. 1986;10:558–563. doi: 10.1177/0148607186010006558. [DOI] [PubMed] [Google Scholar]
- Curran SL, Beacham AO, Andrykowski MA. Ecological momentary assessment of fatigue following breast cancer treatment. Journal of Behavioral Medicine. 2004;27:425–444. doi: 10.1023/b:jobm.0000047608.03692.0c. doi: 10.1023/B:JOBM.0000047608.03692.0c. [DOI] [PubMed] [Google Scholar]
- Dimeo F, Bertz H, Finke J, Fetscher S, Mertelsmann R, Keul J. An aerobic exercise program for patients with haematological malignancies after bone marrow transplantation. Bone Marrow Transplantation. 1996;18:1157–1160. [PubMed] [Google Scholar]
- Dimeo F, Fetscher S, Lange W, Mertelsmann R, Keul J. Effects of aerobic exercise on the physical performance and incidence of treatment-related complications after high-dose chemotherapy. Blood. 1997;90:3390–3394. [PubMed] [Google Scholar]
- Ferrans C. Development of a quality of life index for patients with cancer. Oncology Nursing Forum. 1990;17(3, Suppl.):15–19. [PubMed] [Google Scholar]
- Ferrans C, Powers M. Quality of Life Index: Development and psychometric properties. Advances in Nursing Science. 1985;8:15–24. doi: 10.1097/00012272-198510000-00005. [DOI] [PubMed] [Google Scholar]
- Frontera WR, Hughes VA, Dallal GE, Evans WJ. Reliability of isokinetic muscle strength testing in 45- to 78-year-old men and women. Archives of Physical Medicine and Rehabilitation. 1993;74:1181–1185. [PubMed] [Google Scholar]
- Galvao DA, Newton RU. Review of exercise intervention studies in cancer patients. Journal of Clinical Oncology. 2005;23:899–909. doi: 10.1200/JCO.2005.06.085. [DOI] [PubMed] [Google Scholar]
- Giles B, Henke P, Edmonds J, McNeil D. Reproducibility of isokinetic muscle strength measurements in normal and arthritic individuals. Scandinavian Journal of Rehabilitation Medicine. 1990;22(2):93–99. [PubMed] [Google Scholar]
- Gupta D, Lis CG, Grutsch JF. The relationship between cancer-related fatigue and patient satisfaction with quality of life in cancer. Journal of Pain and Symptom Management. 2007;34:40–47. doi: 10.1016/j.jpainsymman.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Hacker ED, Ferrans C, Verlen E, Ravandi F, van Besien K, Gelms J, Dieterle N. Fatigue and physical activity in patients undergoing hematopoietic stem cell transplant. Oncology Nursing Forum. 2006;33:614–624. doi: 10.1188/06.ONF.614-624. doi: 10.1188/06.ONF.614-624. [DOI] [PubMed] [Google Scholar]
- Hacker ED, Ferrans CE. Quality of life immediately after peripheral blood stem cell transplantation. Cancer Nursing. 2003;26:312–322. doi: 10.1097/00002820-200308000-00010. [DOI] [PubMed] [Google Scholar]
- Hacker ED, Ferrans CE. Ecological momentary assessment of fatigue in patients receiving intensive cancer therapy. Journal of Pain and Symptom Management. 2007;33:267–275. doi: 10.1016/j.jpainsymman.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Hahn T, Wall D, Camitta B, Davies S, Dillon H, Gaynon P, McCarthy PL., Jr. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the therapy of acute lymphoblastic leukemia in adults: An evidence-based review. Biology of Blood and Marrow Transplantation. 2006;12:1–30. doi: 10.1016/j.bbmt.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Hahn T, Wingard JR, Anderson KC, Bensinger WI, Berenson JR, Brozeit G, McCarthy PL., Jr. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the therapy of multiple myeloma: An evidence-based review. Biology of Blood and Marrow Transplantation. 2003;9:4–37. doi: 10.1053/bbmt.2003.50002. doi: 10.1053/bbmt.2003.50002. [DOI] [PubMed] [Google Scholar]
- Hahn T, Wolff SN, Czuczman M, Fisher RI, Lazarus HM, Vose J, McCarthy PL., Jr. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the therapy of diffuse large cell B-cell non-Hodgkin’s lymphoma: An evidence-based review. Biology of Blood and Marrow Transplantation. 2001;7:308–331. doi: 10.1016/s1083-8791(01)80003-3. [DOI] [PubMed] [Google Scholar]
- Hayes S, Davies PS, Parker T, Bashford J. Total energy expenditure and body composition changes following peripheral blood stem cell transplantation and participation in an exercise programme. Bone Marrow Transplantation. 2003;31:331–338. doi: 10.1038/sj.bmt.1703867. [DOI] [PubMed] [Google Scholar]
- Jenkins LS, Brodsky M, Schron E, Chung M, Rocco T, Jr., Lader E, Shemanski L. Quality of life in atrial fibrillation: The Atrial Fibrillation Follow-up Investigation of rhythm management (AFFIRM) study. American Heart Journal. 2005;149:112–120. doi: 10.1016/j.ahj.2004.03.065. [DOI] [PubMed] [Google Scholar]
- Jones CJ, Rikli RE, Beam WC. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Research Quarterly for Exercise and Sport. 1999;70(2):113–119. doi: 10.1080/02701367.1999.10608028. [DOI] [PubMed] [Google Scholar]
- Knobel H, Loge JH, Brenne E, Fayers P, Hjermstad MJ, Kaasa S. The validity of EORTC QLQ-C30 fatigue scale in advanced cancer patients and cancer survivors. Palliative Medicine. 2003;17:664–672. doi: 10.1191/0269216303pm841oa. [DOI] [PubMed] [Google Scholar]
- Knols R, Aaronson NK, Uebelhart D, Fransen J, Aufdemkampe G. Physical exercise in cancer patients during and after medical treatment: A systematic review of randomized and controlled clinical trials. Journal of Clinical Oncology. 2005;23:3830–3842. doi: 10.1200/JCO.2005.02.148. [DOI] [PubMed] [Google Scholar]
- Kues JM, Rothstein JM, Lamb RL. Obtaining reliable measurements of knee extensor torque produced during maximal voluntary contractions: An experimental investigation. Physical Therapy. 1992;72:492–501. doi: 10.1093/ptj/72.7.492. [DOI] [PubMed] [Google Scholar]
- Maeda A, Yuasa T, Nakamura K, Higuchi S, Motohashi Y. Physical performance tests after stroke: Reliability and validity. American Journal of Physical Medicine and Rehabilitation. 2000;79:519–525. doi: 10.1097/00002060-200011000-00008. [DOI] [PubMed] [Google Scholar]
- McNeely ML, Campbell KL, Rowe BH, Klassen TP, Mackey JR, Courneya KS. Effects of exercise on breast cancer patients and survivors: A systematic review and meta-analysis. Canadian Medical Association Journal. 2006;175:34–41. doi: 10.1503/cmaj.051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miaskowski C, Lee KA. Pain, fatigue, and sleep disturbances in oncology outpatients receiving radiation therapy for bone metastasis: A pilot study. Journal of Pain and Symptom Management. 1999;17:320–332. doi: 10.1016/s0885-3924(99)00008-1. doi: 10.1016/S0885-3924(99)00008-1. [DOI] [PubMed] [Google Scholar]
- Oliansky DM, Antin JH, Bennett JM, Deeg HJ, Engelhardt C, Heptinstall KV, Hahn T. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the therapy of myelodysplastic syndromes: An evidence-based review. Biology of Blood and Marrow Transplantation. 2009;15:137–172. doi: 10.1016/j.bbmt.2008.12.003. doi: 10.1016/j.bbmt.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Oliansky DM, Appelbaum F, Cassileth PA, Keating A, Kerr J, Nieto Y, Hahn T. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the therapy of acute myelogenous leukemia in adults: An evidence-based review. Biology of Blood and Marrow Transplantation. 2008;14:137–180. doi: 10.1016/j.bbmt.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Osoba D, Aaronson N, Zee B, Sprangers M, te Velde A. Modification of the EORTC QLQ-C30 (version 2.0) based on content validity and reliability testing in large samples of patients with cancer. Quality of Life Research. 1997;6:103–108. doi: 10.1023/a:1026429831234. doi: 10.1023/A:1026429831234. [DOI] [PubMed] [Google Scholar]
- Osoba D, Zee B, Warr D, Kaizer L, Latreille J. Psychometric properties and responsiveness of the EORTC quality of life questionnaire (QLQ-C30) in patients with breast, ovarian and lung cancer. Quality of Life Research. 1994;3:353–364. doi: 10.1007/BF00451727. doi: 10.1007/BF00451727. [DOI] [PubMed] [Google Scholar]
- Reddon JR, Stefanyk WO, Gill DM, Renney C. Hand dynamometer: Effects of trials and sessions. Perceptual and Motor Skills. 1985;61(3, Pt. 2):1195–1198. doi: 10.2466/pms.1985.61.3f.1195. [DOI] [PubMed] [Google Scholar]
- Rowe JM, Ciobanu N, Ascensao J, Stadtmauer EA, Weiner RS, Schenkein DP, Lazarus HM. Recommended guidelines for the management of autologous and allogeneic bone marrow transplantation. Annals of Internal Medicine. 1994;120:143–158. doi: 10.7326/0003-4819-120-2-199401150-00008. [DOI] [PubMed] [Google Scholar]
- Salem GJ, Wang MY, Young JT, Marion M, Greendale GA. Knee strength and lower- and higher-intensity functional performance in older adults. Medicine and Science in Sports and Exercise. 2000;32:1679–1684. doi: 10.1097/00005768-200010000-00003. doi: 10.1097/00005768-200010000-00003. [DOI] [PubMed] [Google Scholar]
- Sarna L, Conde F. Physical activity and fatigue during radiation therapy: A pilot study using actigraph monitors. Oncology Nursing Forum. 2001;28:1043–1046. [PubMed] [Google Scholar]
- Schaubert KL, Bohannon RW. Reliability and validity of three strength measures obtained from community-dwelling elderly persons. Journal of Strength and Conditioning Research. 2005;19:717–720. doi: 10.1519/R-15954.1. [DOI] [PubMed] [Google Scholar]
- Schmitz KH, Holtzman J, Courneya KS, Masse LC, Duval S, Kane R. Controlled physical activity trials in cancer survivors: A systematic review and meta-analysis. Cancer Epidemiology, Biomarkers and Prevention. 2005;14:1588–1595. doi: 10.1158/1055-9965.EPI-04-0703. [DOI] [PubMed] [Google Scholar]
- Stevinson C, Lawlor DA, Fox KR. Exercise interventions for cancer patients: Systematic review of controlled trials. Cancer Causes and Control. 2004;15:1035–1056. doi: 10.1007/s10552-004-1325-4. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Owashi K, Kanauchi Y, Mura N, Takahara M, Ogino T. The short-term reliability of grip strength measurement and the effects of posture and grip span. Journal of Hand Surgery. 2005;30:603–609. doi: 10.1016/j.jhsa.2004.12.007. doi: 10.1016/j.jhsa.2004.12.007. [DOI] [PubMed] [Google Scholar]


