Abstract
The products of the maternal-effect genes, nanos (nos) and oskar (osk), are important for the development of germ cells in insects. Furthermore, these genes have been proposed as candidates for donating functional DNA regulatory sequences for use in gene drive systems to control transmission of mosquito-borne pathogens. The nos and osk genes of the cosmopolitan vector mosquito, Culex quinquefasciatus, encode proteins with domains common to orthologues found in other mosquitoes. Expression analyses support the conclusion that the role of these genes is conserved generally among members of the nematocera. Hybridization in situ analyses reveal differences in mRNA distribution in early embryos in comparison with the cyclorraphan, Drosophila melanogaster, highlighting a possible feature in the divergence of the clades each insect represents.
Keywords: gene expression, osk, nos, mosquito, germ-line, pole cells, hybridization in situ.
Introduction
Mosquitoes are important vectors of parasitic and viral pathogens and serious nuisance pests to humans and animals. Therefore the management of mosquito populations and control of mosquito-borne disease transmission are of prime importance to public health and welfare. Among the most widely-used mosquito control strategies at present are source reduction to eliminate mosquito breeding sites, biocontrol agents such as Gambusia ssp., Bacillus sphaericus and Bacillus thuringiensis, and various formulations of chemical insecticides to kill larvae and adults (Lacey, 2007; Walton, 2007). However, these tools are not always efficient, applicable, or available. Source reduction is not possible in areas where it would impact wetland resources and associated endangered species. Increased resistance of mosquitoes to chemical and biologically-derived insecticides and the cost of these interventions make them prohibitive in many settings (Hemingway et al., 2006; Federici et al., 2007; Nauen, 2007).
A hypothesis was proposed that genetically-modified mosquitoes may provide supplementary tools for controlling mosquito populations and mosquito-borne diseases, and a series of research objectives was identified to test it (Anonymous, 1991). Much progress has been made in laboratory-based research in the methodology and creation of genetically-modified mosquitoes that exhibit impaired vector competence for dengue viruses (Franz et al., 2006) and malaria parasites (Ito et al., 2002; Jasinskiene et al., 2007; Yoshida et al., 2007). In addition, insects carrying a repressible dominant lethal genetic mechanism also have been considered to substitute for conventional mutagen-induced sterile insect technique control programs (Heinrich & Scott, 2000; Thomas et al., 2000). Mosquitoes with such a lethal gene have been engineered and their applicability for vector population control has been validated theoretically (Phuc et al., 2007). Further research is required to make these technologies available for field use.
Gene drive systems are needed for some population reduction and replacement strategies to spread rapidly an engineered gene in a target mosquito population (Curtis & Graves, 1988; James, 2005). Transposable elements have been proposed as a mechanistic basis for the development of one such a system (Calvo et al., 2005), and there also is strong interest in a meiotic drive mechanism called MEDEA, or ‘maternal effect dominant embryonic arrest’ (Beeman et al., 1992). Recent work with Drosophila melanogaster (Chen et al., 2007), shows promise for the efficacy of artificial MEDEA-like constructs, but work needs to be done to identify suitable genes and promoters for similar applications in mosquitoes.
One of the technical challenges to developing efficient gene drive systems for population replacement involves restricting the activity of the gene driver to where it is needed, in the germline of the target species. This challenge can be met partially by putting the gene drive agent under the control of DNA derived from developmentally-regulated genes to direct sex-, tissue-, and stage-specific transposition. (Adelman et al., 2007; Chen et al., 2007). The primary structure and expression profiles of the nanos (nos) and oskar (osk) genes in fruit flies and mosquitoes have been analyzed. Moreover, nos and osk cis-regulatory sequences can direct germline-restricted expression of transgenes and genetic elements (Kim-Ha et al., 1991; Gavis & Lehmann, 1994; Gavis et al., 1996; Adelman et al., 2007; Bischof et al., 2007; Chen et al., 2007). The expression profiles of these genes qualify them as candidates for use in developing gene drive systems.
The peridomestic mosquito Culex quinquefasciatus is a significant vector of the filarial nematode, Wuchereria bancrofti, and can transmit under favorable conditions a variety of encephalitis-causing viruses, including West Nile Virus (Hardstone et al., 2007; Vaidyanathan & Scott, 2007; Hamer et al., 2008). It also is a significant nuisance pest to humans, having a persistent and nocturnal biting behavior (Vaughan & Hemingway, 1995; Mahanta et al., 1999). It is therefore relevant to identify and characterize genes that may be used to develop genetic control strategies for this mosquito and its associated pathogens. Towards this goal, transgenesis technologies were established for this insect (Allen et al., 2001) and a whole-genome sequencing project is nearing completion (http://cpipiens.vectorbase.org/index.php). We characterized the sequences, gene structures, expression and transcript accumulation patterns of the nos and osk orthologues of Cx. quinquefasciatus. These data are consistent with the expression profiles of the genes in the yellow fever mosquito, Aedes aegypti, and the human malaria vector, Anopheles gambiae, and highlight potentially significant differences between the nematocera and cyclorrhaphan diptera (Goltsev et al., 2004; Juhn & James, 2006). In addition, these results support the conclusion that these genes are candidates for donating regulatory sequences for the development of gene drive systems in this mosquito species.
Results
Identification of Cx. quinquefasciatus nos and osk genes
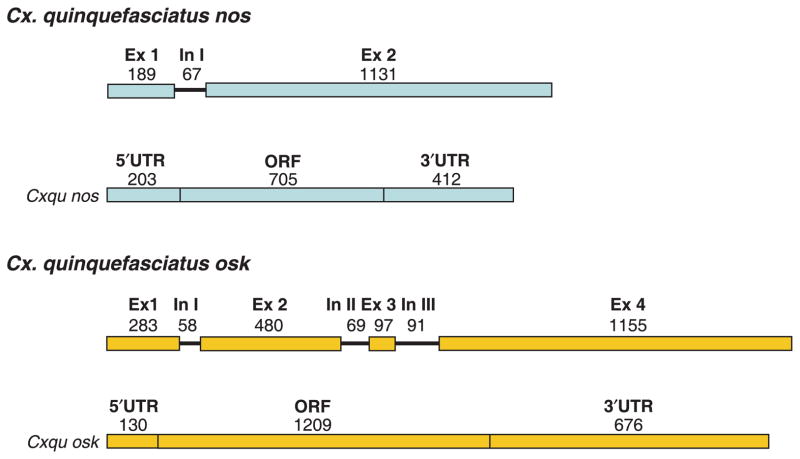
Cx. quinquefasciatus nos (Cxqu nos) was isolated with gene amplification procedures using cDNA made from total RNA isolated from ovaries, and degenerate oligonucleotide primers complementary to the genomic DNA that flanks the region encoding a conserved zinc-finger binding domain (Calvo et al., 2005). The complete cDNA sequence was determined by standard RT-PCR and RACE amplification techniques. The Cxqu nos cDNA is 1320 nucleotides (nt) in length with a 5′-end untranslated region (UTR), open-reading frame (ORF) and 3′-end UTR of 203, 705, and 412 nt in length, respectively (Fig. 1). The gene structure was established by alignment of the sequences of cloned genomic amplification products and cDNAs. Cxqu nos comprises two exons and a single, small intron (67 nt) located near the 5′-end of the coding region, and this structure aligns significantly with the Vector-Base gene annotation CPIJ011551 (Culex quinquefasciatus Johannesburg strain gene set CpipJ1.1; http://vectorbase.org; data not shown). However, discrepancies exist between the annotation of the predicted transcript (CPIJ011551-RA) and the one obtained here. Sequence comparisons of Cxqu nos and genomic amplification products indicate that the single intron is located in the 5′-end UTR of the gene. In contrast, the database predicts an intron at the 3′-end of the transcript, overlapping the translational stop codon. This annotation is not supported by our findings. cDNA sequence data derived independently by the Broad Institute (accession number EV298811) support further our conclusion that an intron does not exist in the 3′-end of Cxqu nos. Unlike Cxqu nos, the Ae. aegypti and An. gambiae orthologues have two introns (Calvo et al., 2005). The introns located in 5′-ends of these genes have relative positions similar but not identical to the intron in Cxqu nos. Both Ae. aegypti and An. gambiae have a second intron in the ORF near the 3′-end. The Anopheles stephensi nos gene has a single intron located near the center of its ORF.
Figure 1.
Schematic representation of the gene and cDNA structures of Culex quinquefasciatus nos and osk genes. Arabic-numbered exons (Ex) are indicated as colored boxes. Exon length in nucleotides appears above each box. Roman-enumerated introns (In) are represented by lines with lengths in nucleotides listed above. Below each gene schematic is a representation of the cDNA product (Cxqu nos; Cxqu osk). The 5′-UTR, ORF and 3′-UTR of each cDNA structure are indicated by colored boxes with the corresponding nucleotide length listed above each. Abbreviations: Ex, exon; In, intron; ORF, open reading frame; UTR, untranslated region. GenBank accession numbers: Cx. quinquefasciatus nos gene, EU517696; Cx. quinquefasciatus osk gene, EU517695.
Conceptual translation of the Cxqu nos ORF yields a protein 235 amino acids (aa) in length that compares favorably with the sizes predicted for Ae. aegypti, An. gambiae and An. stephensi (246, 260 and 255 aa, respectively; Calvo et al., 2005). Comparisons of the predicted amino acid sequences with those of other mosquito Nos proteins show low overall amino acid identity (23–35%; data not shown), and these values are consistent with pair-wise comparisons among the other three mosquitoes (Calvo et al., 2005). In contrast, the zinc-finger domains characteristic of the carboxyl-terminal regions of this family of proteins have higher sequence similarity (59–70%), with absolute conservation of a series of cysteine and histidine residues (Fig. 2). The inconsistencies between the Cxqu nos cDNA determined in this study and that of the predicted transcript CPIJ011551-RA result in only minor differences in the translation products and do not affect the sequence integrity of the zinc-finger domain essential for Nos function in vivo. These data support the conclusion that we have identified the nos orthologous gene of Cx. quinquefasciatus.
Figure 2.

Conservation of mosquito and other dipteran Nos and Osk protein domains. (A) Alignment of the amino acids composing the zinc-finger domain (pfam05741, zf-nanos, Nanos RNA binding domain) of insect Nos proteins. Conserved cysteine and histidine amino acid residues that form two zinc-finger motifs are indicated by asterisks. Amino acids conserved among all sequences are shaded in black. Amino acid positions are indicated with numerals. Abbreviations (Genbank accession numbers are referenced in parentheses): AngaNos, Anopheles gambiae Nanos protein (AAS93544); AnstNos, Anopheles stephensi Nanos protein (AAS93543); AeaeNos, Aedes aegypti Nanos protein (AAS93542); CxquNos, Culex quinquefasciatus Nanos protein (EU517696); DmelNos, Drosophila melanogaster Nanos protein (AAA28715); DsimNos, Drosophila simulans Nanos protein (AAF68506); MdomNos, Musca domestica Nanos protein (AAA87461); CsamNos, Chironomus samoensis Nanos protein (AAA87459). (B) Sequence alignment of the C-terminal regions of predicted mosquito and Drosophila Osk proteins. Amino acids conserved in sequence in all, five-of-six and four-of-six species are shaded in black, dark grey and light grey, respectively. Amino acid positions are indicated with numerals. Abbreviations (Genbank accession numbers are referenced in parentheses): AngaOsk, An. gambiae Oskar protein (ABC54566); AeaeOsk, Ae. agypti Oskar protein (ABC41128); Cxqu Osk, Cx. quinquefasciatus Oskar protein (EU517695); DmelOsk, D. melanogaster Oskar isoform A protein (AAF54306); DvirOsk, Drosophila virilis Oskar protein (AAA28426); DimmOsk, Drosophila immigrans Oskar protein (ABH12272).
The Cxqu osk gene was identified by first conducting discontinuous Mega BLAST searches of the Cx. quinquefasciatus WGS TRACE file archive (ncbi.nlm.nih.gov/blast) using the cDNA of Ae. aegypti osk (Juhn & James, 2006) as a query sequence. TRACE files matching the query sequence were assembled to produce a contiguous consensus sequence. Oligonucleotide primers based on the genomic sequence and standard RACE amplification procedures with RNA templates were used to synthesize a complete cDNA sequence 2015 nt in length that begins with the transcription initiation nucleotide and comprises the 5′-end UTR (130 nt), ORF (1209 nt) and 3′-UTR (676 nt) (Fig. 1). The gene structure of Cxqu osk was determined readily by alignment of the genomic sequence scaffold and the complete cDNA sequence, and is similar to Ae. aegypti and An. gambiae in the number and relative location of the exons and introns (Juhn & James, 2006). The four Cxqu osk exons are similar in size to those of the Ae. aegypti osk (Aeae osk), while the corresponding introns are smaller. The small size of the third intron of Cxqu osk, relative to that of Aeae osk, and limited sequencing of the latter (data not shown), support the conclusion that the large intron in Ae. aegypti arose from insertions of mobile DNA elements (Juhn & James, 2006). The Cxqu osk cDNA matches exactly the predicted transcript CPIJ007471-RA in VectorBase, except for the presence of eight additional nucleotides at the 5′-end and absence of three nucleotides at the 3′-end. These differences result likely from discrepancies in the VectorBase-generated annotations of the exact site of transcription initiation and termination.
Unlike the other characterized mosquito osk cDNA sequences (Juhn & James, 2006), Cxqu osk has three potential translation start codons. Variations in the lengths of the 5′-end UTRs determined for other mosquito osk cDNAs prevent an accurate assignment of the start codon based on sequence alignment comparisons alone. However, the first Cxqu osk AUG fits best the consensus translation initiation codon (Kozak, 1987) and begins a 1209 nt ORF capable of encoding a predicted protein of 403 aa. This conceptual translation product is intermediate in size when compared to Ae. aegypti and An. gambiae Osk proteins (394 and 408 aa, respectively, Juhn & James, 2006). Comparisons of the amino acid sequences of all mosquito Osk proteins show an overall identity of 36–55%, with the greatest similarity shared by Cxqu Osk and Aeae Osk. Furthermore, the C-terminal half of Cxqu Osk contains tracks of amino acids that are conserved highly among all insects and are presumed to represent functional protein domains, although their predicted functions are unknown (Fig. 2). Analysis of Cxqu Osk using protein domain databases predicted a dispersed motif in the C-terminus that also is found in the SGNH hydrolase subfamily (Pssmid 58505; InterPro: IPR013830). Further analyses show that similar predicted protein motifs exist in the C-termini of other mosquito and Drosophila Osk proteins (data not shown). These combined sequence data support the conclusion that the gene described here is the osk orthologue of Cx. quinquefasciatus.
Cxqu nos and Cxqu osk mRNA expression and accumulation are restricted to adult female ovaries and embryos
RT-PCR amplification procedures and total RNA isolated from both sexes at various stages of development were used to determine mRNA expression and accumulation profiles for Cxqu nos and Cxqu osk. Diagnostic amplification products indicate that the corresponding mRNAs are present in ovaries dissected from sugar- and blood-fed adult females (48–72 h post blood meal [PBM]) and in embryos (Fig. 3). No amplified fragments were detected in RNA isolated from larvae, pupae, adult males or adult female carcasses (whole bodies without ovaries). The developmental accumulation patterns of Cxqu osk and Cxqu nos are similar to those of the other mosquito orthologous gene products (Calvo et al., 2005; Juhn & James, 2006), and are consistent with those expected of germline-associated, posterior group, maternal-effect genes (Goltsev et al., 2004).
Figure 3.
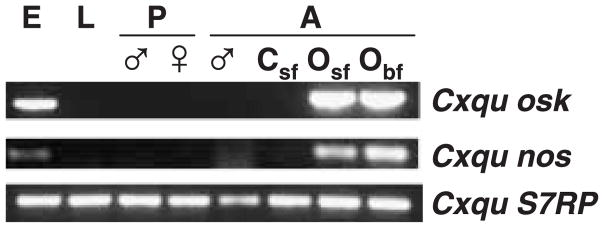
Stage-, sex-, and tissue-specific expression profiles of Culex quinquefasciatus nos and osk genes. Gene amplification procedures with oligonucleotide primers specific to the Cxqu nos and Cxqu osk ORFs and control S7 ribosomal protein (S7RP) genes were used to detect DNA targets of diagnostic lengths. Abbreviations: E, embryos; L, larvae; ♂, males; ♀, females; P, pupae; A, adults; Csf, carcasses (whole bodies of sugar-fed females with ovaries removed); Osf, Obf, ovaries from sugar- and 72 h blood-fed adult females, respectively.
Cxqu nos and Cxqu osk mRNAs localize in nurse cells, the posterior pole of oocytes, and in the pole cells of developing embryos
Mosquito ovarioles are composed of an oocyte and attendant nurse cells, all of which are surrounded by follicle cells (Fig. 4). The developing Cx. quinquefasciatus oocyte has an intermediate shape when compared to the elliptical egg follicle of An. gambiae and ovoid follicle of Ae. aegypti (Juhn & James, 2006). In addition, the oocyte has a bulb-like anterior end, which remains a characteristic feature of the developing embryo (Figs 5 and 6).
Figure 4.
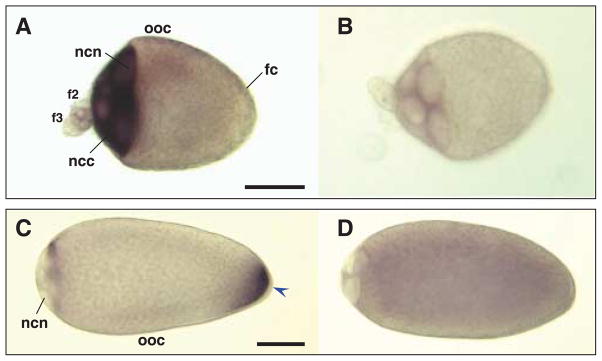
Hybridizations in situ of Culex quinquefasciatus nos and osk antisense and sense RNA probes to whole-mount oocytes and nurse cells. All primary follicles are orientated with the anterior on the left of the figure. Primary oocyte (ooc), with attached secondary (f2) and tertiary follicles (f3) dissected from ovaries of bloodfed Cx. quinquefasciatus females. Follicle cells (fc) surround the primary oocyte. Samples are hybridized with Cxqu nos antisense (A) and sense RNA (B) probes, or Cxqu osk antisense (C) and sense (D) RNA probes. (A) Strong staining is evident in the nurse cell cytoplasm (ncc) at stage IIIa and large nurse cell nuclei (ncn) are recognizable. (C) In stage IVa, oocytes appear elongated with a larger, bulb-shaped anterior end and the nurse cell nuclei occupy ~10% of the follicle length. Hybridization with Cxqu osk antisense RNA probes result in strong staining at the posterior end (blue arrowhead). (B, D) Stage IIIa and IVa oocytes hybridized with sense probes show no specific staining. Bar = 50 μM.
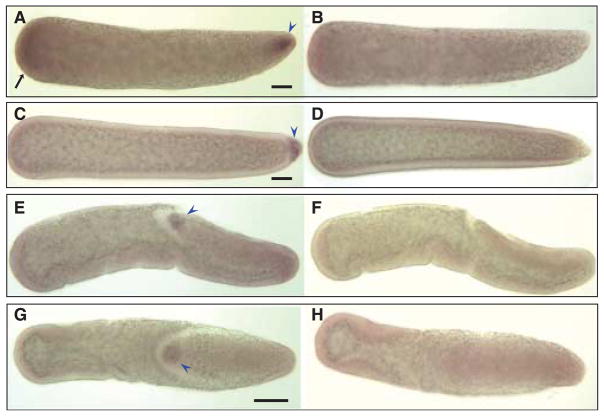
Figure 5.
Hybridizations in situ of Cxqu nos RNA probes to whole-mount Culex quinquefasciatus embryos. All embryos are orientated with anterior on the left of the figure. (A) A preblastoderm embryo hybridized with an antisense Cxqu nos RNA probe shows probe-specific staining at the anterior (black arrow) and posterior end (blue arrowhead), compared to a embryo hybridized with sense RNA probe (B). (C) Anterior staining appears to be transient and disappears after cellularization of the embryo; at this stage pole cells are stained strongly (blue arrowhead) when compared to a sense-probed embryo (D). Gastrulation stage embryos show specific staining of pole cells (blue arrowhead) in both lateral (E) and dorsal (G) views. F and H are the corresponding control embryos hybridized with sense-strand probes. Bar = 50 μM.
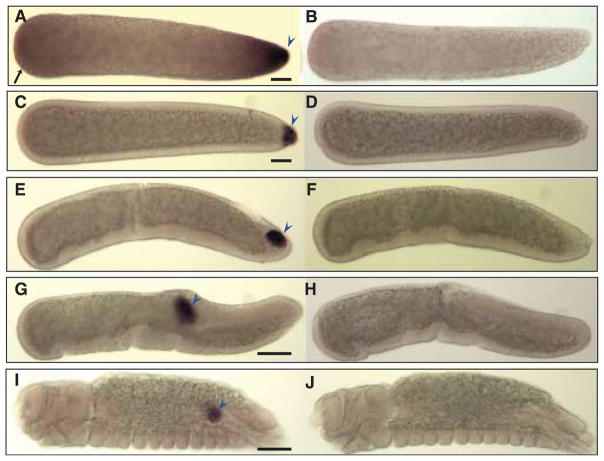
Figure 6.
Hybridizations in situ of Cxqu osk RNA probes to whole-mount Culex quinquefasciatus embryos. All embryos are orientated as in Figure 3. (A) A preblastoderm embryo hybridized with an antisense Cxqu osk RNA probe shows strong staining at the posterior end (blue arrowhead) and faint anterior (black arrow) staining when compared to a sense-probed embryo (B). (C) Transient anterior staining diminishes at blastoderm cellularization, and pole cells are stained strongly at this stage (blue arrowhead). (E) At the onset of gastrulation, pole cells are observed to lie on a cell plate and migrate dorsally (G) as germband elongation occurs. (I) In the final stages of embryonic development, pole cells have coalesced to the future gonad (blue arrowhead). Embryos hybridized with Cxqu osk sense probes exhibit no specific staining patterns (B, D, F, H, and J). Bar = 50 μM.
Hybridizations in situ of digoxigenin-labelled anti-sense RNA to whole-mount ovaries dissected from females at 24 and 32 h PBM, ~Stage IIIb and IVa oocytes, respectively (Clements, 1992) show that Cxqu nos mRNA is localized in the cytoplasm of nurse cells in the primary and secondary follicles (Fig. 4A). Cxqu osk mRNA is localized in the cytoplasm of apoptotic nurse cells (oocyte anterior), and accumulates in the posterior of the stage IVa oocyte (Fig. 4B). These findings are consistent with nos and osk mRNA localization in the developing egg chambers of other diptera (Kim-Ha et al., 1991; Forrest & Gavis, 2003; Calvo et al., 2005; Juhn & James, 2006). The developing oocytes also show faint punctate staining in the cytoplasm, as was previously observed for hybridization in oocytes of An. gambiae and Ae. aegypti (Juhn & James, 2006). This observation most likely reflects the occlusion of signal by densely-packed yolk spheres that fill the cytoplasmic space of the developing oocyte. Whole-mount ovary preparations hybridized with control sense-strand nos RNA probes show a faint signal in the cytoplasm of early stage nurse cells (Fig. 4B). This staining pattern also was observed in sense-strand hybridized nurse cells of the primary and secondary follicles of An. gambiae, and therefore is thought to be the result of physical interactions of the labeled probe with the contents of the nurse cell cytoplasm. The faint signal observed in the interior of oocytes hybridized with sense-strand osk RNA probes likely results from over-development of the substrate reaction (Fig. 4D).
Hybridizations in situ also showed that Cxqu nos and Cxqu osk mRNAs localize to the posterior pole plasm of pre-blastoderm embryos and within the pole cells following cellularization of the blastoderm (Figs 5 and 6). Transient, diffuse localization of both gene products is observed in the anterior poles of embryos prior to cellularization of the blastoderm. This also was observed for An. gambiae osk (Anga osk) and may be typical of nematoceran development (Goltsev et al., 2004; Juhn & James, 2006). Following cellularization of the blastoderm (Figs 5C and 6C), and concurrent with gastrulation and germ-band elongation, the pole cells located outside the posterior blastoderm are moved anteriorly along the dorsal surface of the embryo (Fig. 5E). Subsequently, the pole cells are internalized (Figs 5G and 6G) and later found to separate into two clusters that localize within the primordial gonads (Fig. 6I).
Discussion
Among the germline associated, posterior group genes, only nos and osk have been characterized both in Drosophila species and vector mosquitoes (Lehmann & Nusslein-Volhard, 1986; Kim-Ha et al., 1991; Gavis & Lehmann, 1994; Webster et al., 1994; Gavis et al., 1996; Calvo et al., 2005; Juhn & James, 2006). The analysis of Cxqu nos and Cxqu osk provides support for the conclusion that the expression profiles and roles of these genes in early development are conserved generally among the Diptera. The significance of the transient localization of Anga osk and Cxqu osk mRNA in the anterior of the mosquito embryos is unknown, although Goltsev et al. (2004) suggest a possible role in anterior pole development.
Variations in exon/intron length and location are observed in all mosquito nos and osk orthologues, and there is low overall conservation of the respective ORFs outside the known and proposed functional domains. The mosquito nos orthologues differ markedly in their primary gene structures. These differences may reflect positive selection for variation, but analyzing this requires experimental verification of the significance of the intron number, placement and length, and the role and extent of the functional domains in the proteins.
Although the cellular events during pole-cell migration have been described in detail in D. melanogaster, we lacked direct evidence of how these events occurred in mosquitoes. The morphological changes observed here for Cx. quinquefasciatus embryos are similar to those seen for early gastrulation stages in D. melanogaster and supplement previously-reported illustrations and images of Culicine embryonic development (Davis, 1967; Raminani & Cupp, 1975; Clements, 1992). Methods using cross-sectional and whole-mount confocal and electron microscopy were used to visualize the development of mosquito embryos (Monnerat et al., 2002). However, the whole-mount embryo preparations (with exochorion removal) provide higher resolution images and enable visualization of embryonic development in greater detail than observed previously.
Comparative gene expression and morphological analyses of early development among the Diptera provide a basis for parallel development of genetic and molecular genetic approaches for vector control. Specifically, the data showing localization of the mRNAs of Cx. quinquefasciatus nos and osk genes indicate that their regulatory sequences could serve a role in controlling the expression of trans-genes in the germline. Therefore, these genes would be useful in the development of drive systems for spreading desirable traits through populations of Cx. quinquefasciatus.
Methods
Mosquito rearing
Culex quinquefasciatus were reared at 28 °C, 80% humidity with a photoperiod of 18 h light: 6 h dark. Mosquitoes were provided raisins (sugar source) and water, and mated females were fed on anesthetized rabbits or chickens for egg production.
Tissue dissection and RNA isolation
Mosquito ovaries were dissected in 1X phosphate-buffered saline (PBS) from blood-fed (72 h PBM) adult Cx. quinquefasciatus mosquitoes. Dissected ovaries, carcasses (female bodies without ovaries), whole males, larvae, and pupae were flash-frozen in liquid N2 and stored at −80 °C prior to use. Embryos were collected from gravid females by placing a cup of water into the mosquito rearing cage for 1 h. Embryos were removed by rinsing with water onto a fine mesh, and transferring them subsequently with a fine paintbrush into a microfuge tube containing distilled-deionized (dd) H2O. Excess water was removed, the embryos flash-frozen and stored at −80 °C prior to RNA extraction. Total RNA was extracted from frozen samples with Trizol (Invitrogen, Carlsbad, CA) and resuspended in DEPC-treated ddH2O. Total RNA was treated for 30 min at 37 °C with RQ1 DNAse (Promega, Madison, WI). DNAse was inactivated by using EDTA-containing RQ1 stop solution and heating at 65 °C for 10 min.
RT-PCR and cloning
The One-Step RT-PCR kit (Qiagen, Valencia, CA) was used for all cDNA amplification reactions. Degenerate primers DegN4F (5′-CAYTGYGTNTTYTGYKWNAAYAAY-3′) and DegN2R (5′-ACNGGYTTNWDNGGRCARTAYTT-3′) were used to amplify a short Cxqu nos cDNA. Primer pairs used for the amplification of Cxqu osk cDNA were designed to span regions encoding conserved amino acids: CxoskF1 (5′-GGCAAATGATCGGAGACGACTTTTTC-3′) and CxoskR1 (5′-GGAATTTGATGATACGCTGGCGTCC-3′). RT-PCR amplifications were done using these primers to obtain partial cDNA sequences. Primers (5′-CTGGAGATG AACTGGACCT-3′) and (5′-CTTGTACACCGACGTGAAGG-3′) were used for amplification of an internal control mRNA for ribosomal protein S7. The reaction mixtures were incubated at 50 °C for 30 min and 15 min at 95 °C. Amplification conditions were 2 min at 94 °C followed by 30 cycles of 45 s at 94 °C, 45 s at 60 °C, and 1 min at 72 °C. RT-PCR products were cloned into the TOPO-TA PCR4 cloning vector (Invitrogen) for sequencing and probe production.
Cloning and analysis of cDNAs
Standard gene amplification protocols were used for cloning of the Cxqu nos and Cxqu osk cDNAs. 5′- and 3′-end RACE were performed using the Smart cDNA RACE kit (Clontech, Mountain View, CA). Gene-specific oligonucleotide primers complementary to the Cxqu nanos cDNA sequences, Cxnos-5′R (5′-CCCGGCACACATGGCTCAT-GTACACC-3′) and Cxnos-3′F (5′-GGACGAGCGCGGCCAGGTG-3′), and Cxqu osk cDNA sequences, Cxosk5′R (5′-GATTGTCAAGCCGGCCCTGCAAAGG-3′) and Cxosk3′F (5′-GCAACCAACCCCATGTTCTGTGGAA-3′), were used for the first round of amplifications to obtain the 5′- and 3′-end untranslated regions (UTR) sequences, respectively. The amplification reactions were performed as follows: 1 cycle of 95 °C for 5 min, 30 cycles of 94 °C for 30 s, 65 °C for 30 s, and 72 °C for 2 min. The final step was extended for 10 min at 72 °C. The amplification products were ligated into the TOPO-TA PCR4 plasmids (Invitrogen) and used for transformation of Escherichia coli strain Top10 (Invitrogen). Several clones were sequenced in both antisense and sense directions. The resulting sequences were used to create contiguous scaffolds for both Cxqu nos and Cxqu osk cDNAs.
Gene reconstruction and sequence analysis
The Mega BLAST search tool was used to obtain several genomic TRACE file sequences, to generate a genomic scaffold representing putative Cxqu nos and osk genes. Alignment comparisons of the complete cDNA sequences and the respective genomic sequences were done to define intronexon boundaries. Translation of the cDNA sequences was performed using the SeqBuilder program in the Laser-gene software package (DNAstar Inc., Madison, WI). Subsequent analyses of the predicted amino acid sequence were done using the NCBI Conserved Protein Domain Database (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi).
Ovary and embryo fixation and hybridization in situ
Digoxygenin (DIG)-labeled sense and antisense nos and osk RNA probes were synthesized in vitro using T3 or T7 RNA polymerases (Ambion, Austin, TX). Dissected ovaries (24–36 h PBM), and embryos at various stages of development (5–24 h post-oviposition) were prepared, probed and hybridization signals detected as previously described (Juhn & James, 2006).
Acknowledgments
The authors thank Lynn Olson for help in preparing the manuscript. This work was supported in part by a grant from the National Institutes of Health (AI44238).
References
- Adelman ZN, Jasinskiene N, Onal S, Juhn J, Ashikyan A, Salampessy M, et al. nanos gene control DNA mediates developmentally regulated transposition in the yellow fever mosquito Aedes aegypti. Proc Natl Acad Sci USA. 2007;104:9970–9975. doi: 10.1073/pnas.0701515104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen ML, O’Brochta DA, Atkinson PW, Levesque CS. Stable, germ-line transformation of Culex quinquefasciatus (Diptera: Culicidae) J Med Entomol. 2001;38:701–710. doi: 10.1603/0022-2585-38.5.701. [DOI] [PubMed] [Google Scholar]
- Anonymous. World Health Organization-Special Program for Research and Training in Tropical Diseases TDR/BCV/MAL-ENT/91.3. 1991. Report of meeting: prospects for malaria control by genetic manipulation of its vectors. [Google Scholar]
- Beeman RW, Friesen KS, Denell RE. Maternal-effect selfish genes in flour beetles. Science. 1992;256:89–92. doi: 10.1126/science.1566060. [DOI] [PubMed] [Google Scholar]
- Bischof J, Maeda RK, Hediger M, Karch F, Basler K. An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc Natl Acad Sci USA. 2007;104:3312–3317. doi: 10.1073/pnas.0611511104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo E, Walter M, Adelman ZN, Jimenez A, Onal S, Marinotti O, et al. Nanos (nos) genes of the vector mosquitoes, Anopheles gambiae, Anopheles stephensi and Aedes aegypti. Insect Biochem Mol Biol. 2005;35:789–798. doi: 10.1016/j.ibmb.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Chen CH, Huang H, Ward CM, Su JT, Schaeffer LV, Guo M, et al. A synthetic maternal-effect selfish genetic element drives population replacement in Drosophila. Science. 2007;316:597–600. doi: 10.1126/science.1138595. [DOI] [PubMed] [Google Scholar]
- Clements AN. The Biology of Mosquitoes. Chapman and Hall; New York: 1992. [Google Scholar]
- Curtis CF, Graves PM. Methods for replacement of malaria vector populations. J Trop Med Hyg. 1988;91:43–48. [PubMed] [Google Scholar]
- Davis CWC. A comparative study of larval embryogenesis in the mosquito Culex fatigans Wiedemann (Diptera: Culicidae) and the sheep-fly Lucilla sericata Meigen (Diptera: Calliphoridae) Aust J Zool. 1967;15:547–579. [Google Scholar]
- Federici BA, Park HW, Bideshi DK, Wirth MC, Johnson JJ, Sakano Y, et al. Developing recombinant bacteria for control of mosquito larvae. J Am Mosquito Control Assoc. 2007;23:164–175. doi: 10.2987/8756-971X(2007)23[164:DRBFCO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Forrest KM, Gavis ER. Live imaging of endogenous RNA reveals a diffusion and entrapment mechanism for nanos mRNA localization in Drosophila. Curr Biol. 2003;13:1159–1168. doi: 10.1016/s0960-9822(03)00451-2. [DOI] [PubMed] [Google Scholar]
- Franz AW, Sanchez-Vargas I, Adelman ZN, Blair CD, Beaty BJ, James AA, et al. Engineering RNA interference-based resistance to dengue virus type 2 in genetically-modified Aedes aegypti. Proc Natl Acad Sci USA. 2006;103:4198–4203. doi: 10.1073/pnas.0600479103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavis ER, Lehmann R. Translational regulation of nanos by RNA localization. Nature. 1994;369:315–318. doi: 10.1038/369315a0. [DOI] [PubMed] [Google Scholar]
- Gavis ER, Curtis D, Lehmann R. Identification of cis-acting sequences that control nanos RNA localization. Develop Biol. 1996;176:36–50. doi: 10.1006/dbio.1996.9996. [DOI] [PubMed] [Google Scholar]
- Goltsev Y, Hsiong W, Lanzaro G, Levine M. Different combinations of gap repressors for common stripes in Anopheles and Drosophila embryos. Develop Biol. 2004;275:435–446. doi: 10.1016/j.ydbio.2004.08.021. [DOI] [PubMed] [Google Scholar]
- Hamer GL, Kitron UD, Brawn JD, Loss SR, Ruiz MO, Goldberg TL, et al. Culex pipiens (Diptera: Culicidae): a bridge vector of West Nile virus to humans. J Med Entomol. 2008;45:125–128. doi: 10.1603/0022-2585(2008)45[125:cpdcab]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Hardstone MC, Leichter C, Harrington LC, Kasai S, Tomita T, Scott JG. Cytochrome P450 monooxygenase-mediated permethrin resistance confers limited and larval specific cross-resistance in the southern house mosquito, Culex pipiens quinquefasciatus. Pesticide Biochem Physiol. 2007;89:3. [Google Scholar]
- Heinrich JC, Scott MJ. A repressible female-specific lethal genetic system for making transgenic insect strains suitable for a sterile-release program. Proc Natl Acad Sci USA. 2000;97:8229–8232. doi: 10.1073/pnas.140142697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemingway J, Beaty BJ, Rowland M, Scott TW, Sharp BL. The Innovative Vector Control Consortium: improved control of mosquito-borne diseases. Trends Parasitol. 2006;22:308–312. doi: 10.1016/j.pt.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Ito J, Ghosh A, Moreira LA, Wimmer EA, Jacobs-Lorena M. Transgenic anopheline mosquitoes impaired in transmission of a malaria parasite. Nature. 2002;417:452–455. doi: 10.1038/417452a. [DOI] [PubMed] [Google Scholar]
- James AA. Gene drive systems in mosquitoes: rules of the road. Trends Parasitol. 2005;21:64–67. doi: 10.1016/j.pt.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Jasinskiene N, Coleman J, Ashikyan A, Salampessy M, Marinotti O, James AA. Genetic control of malaria parasite transmission: threshold levels for infection in an avian model system. Am J Trop Med Hyg. 2007;76:1072–1078. [PubMed] [Google Scholar]
- Juhn J, James AA. oskar gene expression in the vector mosquitoes, Anopheles gambiae and Aedes aegypti. Insect Mol Biol. 2006;15:363–372. doi: 10.1111/j.1365-2583.2006.00655.x. [DOI] [PubMed] [Google Scholar]
- Kim-Ha J, Smith JL, Macdonald PM. oskar mRNA is localized to the posterior pole of the Drosophila oocyte. Cell. 1991;66:23–35. doi: 10.1016/0092-8674(91)90136-m. [DOI] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5′-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987;15:8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey LA. Bacillus thuringiensis serovariety israelensis and Bacillus sphaericus for mosquito control. J Am Mosquito Control Assoc. 2007;23:133–163. doi: 10.2987/8756-971X(2007)23[133:BTSIAB]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Lehmann R, Nusslein-Volhard C. Abdominal segmentation, pole cell formation, and embryonic polarity require the localized activity of oskar, a maternal gene in Drosophila. Cell. 1986;47:141–152. doi: 10.1016/0092-8674(86)90375-2. [DOI] [PubMed] [Google Scholar]
- Mahanta B, Handique R, Dutta P, Narain K, Mahanta J. Temporal variations in biting density and rhythm of Culex quinquefasciatus in tea agro-ecosystem of Assam, India. Southeast Asian J Trop Med Public Health. 1999;30:804–809. [PubMed] [Google Scholar]
- Monnerat AT, Machado MP, Vale BS, Soares MJ, Lima JB, Lenzi HL, et al. Anopheles albitarsis embryogenesis: morphological identification of major events. Memorias do Instituto Oswaldo Cruz. 2002;97:589–596. doi: 10.1590/s0074-02762002000400026. [DOI] [PubMed] [Google Scholar]
- Nauen R. Insecticide resistance in disease vectors of public health importance. Pest management Sci. 2007;63:628–633. doi: 10.1002/ps.1406. [DOI] [PubMed] [Google Scholar]
- Phuc HK, Andreasen MH, Burton RS, Vass C, Epton MJ, Pape G, et al. Late-acting dominant lethal genetic systems and mosquito control. BMC Biol. 2007;5:11. doi: 10.1186/1741-7007-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raminani LN, Cupp EW. Early Embryology of Aedes aegypti (L.) (Diptera: Culicidae) Int J Insect Morphol Embryol. 1975;4:517–528. [Google Scholar]
- Thomas DD, Donnelly CA, Wood RJ, Alphey LS. Insect population control using a dominant, repressible, lethal genetic system. Science. 2000;287:2474–2476. doi: 10.1126/science.287.5462.2474. [DOI] [PubMed] [Google Scholar]
- Vaidyanathan R, Scott TW. Geographic variation in vector competence for West Nile virus in the Culex pipiens (Diptera: Culicidae) complex in California. Vector borne zoonotic Dis. 2007;7:193–198. doi: 10.1089/vbz.2006.0589. [DOI] [PubMed] [Google Scholar]
- Vaughan A, Hemingway J. Mosquito carboxylesterase Est alpha 2(1) (A2). Cloning and sequence of the full-length cDNA for a major insecticide resistance gene worldwide in the mosquito Culex quinquefasciatus. J Biol Chem. 1995;270:17044–17049. doi: 10.1074/jbc.270.28.17044. [DOI] [PubMed] [Google Scholar]
- Walton WE. Larvivorous fish including Gambusia. J Am Mosquito Control Assoc. 2007;23:184–220. doi: 10.2987/8756-971X(2007)23[184:LFIG]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Webster PJ, Suen J, Macdonald PM. Drosophila virilis oskar transgenes direct body patterning but not pole cell formation or maintenance of mRNA localization in D. melanogaster. Development. 1994;120:2027–2037. doi: 10.1242/dev.120.7.2027. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Shimada Y, Kondoh D, Kouzuma Y, Ghosh AK, Jacobs-Lorena M, et al. Hemolytic C-Type Lectin CEL-III from Sea Cucumber Expressed in Transgenic Mosquitoes Impairs Malaria Parasite Development. PLoS Pathog. 2007;3:e192. doi: 10.1371/journal.ppat.0030192. [DOI] [PMC free article] [PubMed] [Google Scholar]