Abstract
This review is an updated and expanded version of the three prior reviews that were published in this journal in 1997, 2003 and 2007. In the case of all approved therapeutic agents, the time frame has been extended to cover the 30 years from January 1st 1981 to December 31st 2010 for all diseases world-wide, and from 1950 (earliest so far identified) to December 2010 for all approved antitumor drugs world-wide. We have continued to utilize our secondary subdivision of a “natural product mimic” or “NM” to join the original primary divisions, and have added a new designation “natural product botanical” or “NB” to cover those botanical “defined mixtures” that have now been recognized as drug entities by the FDA and similar organizations. From the data presented, the utility of natural products as sources of novel structures, but not necessarily the final drug entity, is still alive and well. Thus, in the area of cancer, over the time frame from around the 1940s to date, of the 175 small molecules, 131 or 74.8% are other than “S” (synthetic), with 85 or 48.6% actually being either natural products or directly derived there from. In other areas, the influence of natural product structures is quite marked with, as expected from prior information, the anti-infective area being dependent on natural products and their structures. Although combinatorial chemistry techniques have succeeded as methods of optimizing structures, and have been used very successfully in the optimization of many recently approved agents, we are only able to identify only one de novo combinatorial compound approved as a drug in this 30-year time frame. We wish to draw the attention of readers to the rapidly evolving recognition that a significant number of natural product drugs/leads are actually produced by microbes and/or microbial interactions with the “host from whence it was isolated”, and therefore we consider that this area of natural product research should be expanded significantly.
Introduction
It is fourteen years since the publication of our first,1 eight years since the second,2 and four years3 since our last full analysis of the sources of new and approved drugs for the treatment of human diseases, although there have been intermediate reports in specific areas such as cancer,4, 5 and anti-infectives,6 together with a more general discussion on natural products as leads to potential drugs.7 All of these articles demonstrated that natural product and/or natural product structures continued to play a highly significant role in the drug discovery and development process.
That Nature in one guise or another has continued to influence design of small molecules is shown by inspection of the information given below, where with the advantage of now 30 years of data, the system has been able to be refined. We have eliminated some duplicated entries that crept into the original datasets and have revised a few source designations as newer information has been obtained from diverse sources. In particular, as behooves authors from the National Cancer Institute (NCI), in the specific case of cancer treatments, we have continued to consult the records of the FDA, and added comments from investigators who have informed us of compounds that may have been approved in other countries and that were not captured in our earlier searches. As was done previously, the cancer data will be presented as a stand-alone section from the beginning of formal chemotherapy in the very late 1930s or early 1940s to the present, but information from the last 30 years will be included in the datasets used in the overall discussion.
A trend mentioned in our 2003 review2 in that though the development of high-throughput screens based on molecular targets had led to a demand for the generation of large libraries of compounds, the shift away from large combinatorial libraries that was becoming obvious at that time has continued, with the emphasis now being on small focused (100-∼3000 plus) collections that contain much of the “structural aspects” of natural products. Various names have been given to this process, including “diversity oriented syntheses”,8-12 but we prefer to simply refer to “more natural product-like”, in terms of their combinations of heteroatoms and significant numbers of chiral centers within a single molecule,13 or even”natural product mimics” if they happen to be direct competitive inhibitors of the natural substrate. It should also be pointed out that Lipinski's fifth rule effectively states that the first four rules do not apply to natural products nor to any molecule that is recognized by an active transport system when considering “druggable chemical entities”.14-16 Recent commentaries on the “industrial perspective in regard to drug sources17 and high throughput screening18 have been published by the GSK group and can be accessed by interested readers.
Although combinatorial chemistry in one or more of its manifestations has now been used as a discovery source for approximately 70% of the time covered by this review, to date, we still can only find one de novo new chemical entity (NCE) reported in the public domain as resulting from this method of chemical discovery and approved for drug use anywhere. This is the antitumor compound known as sorafenib (Nexavar®, 1) from Bayer, approved by the FDA in 2005 for treatment of renal cell carcinoma, and then in 2007, another approval was given for treatment of hepatocellular carcinoma. It was known during development as BAY-43-9006 and is a multi-kinase inhibitor, targeting several serine/threonine and receptor tyrosine kinases (RAF kinase, VEGFR-2, VEGFR-3, PDGFR-beta, KIT and FLT-3). It has been approved in Switzerland, the European Union and the People's Republic of China, with additional filings in other countries. Currently, it is still in multiple clinical trials in both combination and single agent therapies, a common practice once a drug is approved for an initial class of cancer treatment.
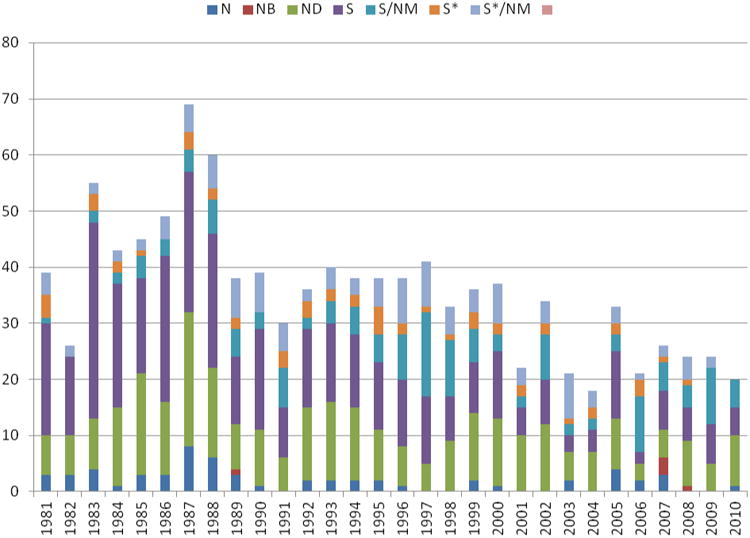
As mentioned by the present authors and others in prior reviews on this topic, the developmental capability of combinatorial chemistry as a means for structural optimization, once an active skeleton has been identified, is without par. An expected surge in productivity however, has not materialized. Thus, the number of new active substances (NASs) from our dataset, also known as New Chemical Entities (NCEs), which we consider to encompass all molecules, including biologics and vaccines, hit a 24-year low of 25 in 2004 (although 28% of these were assigned to the ND category), leading to a rebound to 54 in 2005, with 24% being N or ND and 37% being biologics (B) or vaccines (V), as we discuss subsequently. The trend to small numbers of approvals continues to this day as can be seen by inspection of Figures 2 and 4 (see Discussion section below).
Figure 2. All New Approved Drugs by Source/Year.
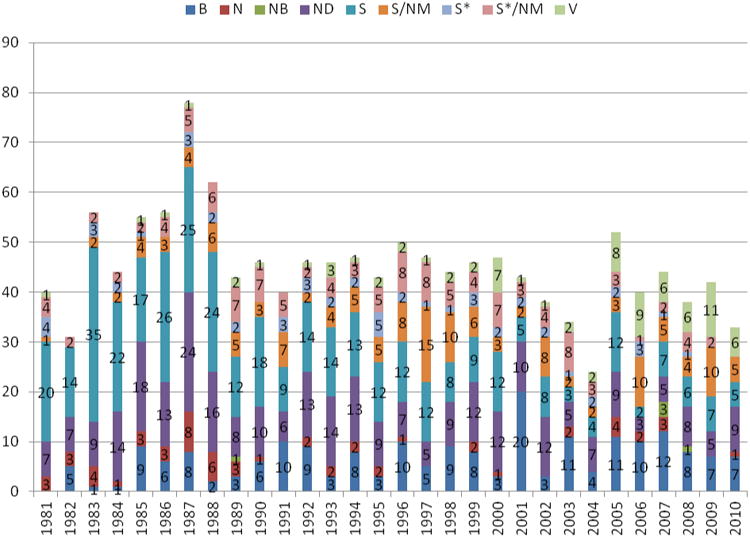
Figure 4. Sources of Small Molecule NCEs by Source/Year.
Fortunately, however, research being conducted by groups such as Danishefsky's, Ganesan's, Nicolaou's, Porco's, Quinn's, Schreiber's, Shair's, Tan's, Waldmann's, and Wipf's, together with those of other synthetic chemists, is continuing the modification of active natural product skeletons as leads to novel agents. This was recently exemplified by the groups of Quinn19 and Danishefsky20 or the utilization of the “lessons learned” from studying such agents as reported by the groups of Tan21, 22 and Kombarov23 to just some of the some recent publications. Thus, in due course, the numbers of materials developed by linking Mother Nature to combinatorial synthetic techniques should increase. These aspects, plus the potential contributions from the utilization of genetic analyses of microbes will be discussed at the end of this review.
Against this backdrop, we now present an updated analysis of the role of natural products in the drug discovery and development process, dating from 01/1981 through 12/2010. As in our earlier analyses,1-3 we have consulted the Annual Reports of Medicinal Chemistry in this case from 1984-2010,24-50 and have produced a more comprehensive coverage of the 1981-2010 time frame through addition of data from the publication, Drug News and Perspective,51-71 and searches of the Prous (now Thomson-Reuter's Integrity™) database, as well as by including information from individual investigators. As in the last review, biologics data prior to 2005 were updated using information culled from disparate sources that culminated in a 2005 review on biopharmaceutical drugs.72 We have also attempted to capture vaccine data in the last few years, but this area of the database is not as complete as we would hope.
We have also included relevant references in a condensed form in Tables 2-5 and 8-10. If we were to provide the full citations, the numbers of references cited in the present review would become overwhelming. In these tables, “ARMC ##” refers to the volume of Annual Reports in Medicinal Chemistry together with the page on which the structure(s) and commentary can be found. Similarly, “DNP ##” refers to the volume of Drug News and Perspective and the corresponding page(s), though this journal has now ceased publication as of the 2010 volume, and an “I######” is the accession number in the Prous (now Thomson-Reuters, Integrity™) database. Finally, we have used “Boyd” to refer to a review article73 on clinical antitumor agents and “M'dale” to refer to Martindale74 with the relevant page noted.
Table 2. Antibacterial Drugs from 01.01.81 to 12.31.10 Organized Alphabetically by Generic Name within Source.
| generic name | trade name | year introduced | volume | page | source |
|---|---|---|---|---|---|
| carumonam | Amasulin | 1988 | ARMC 24 | 298 | N |
| daptomycin | Cubicin | 2003 | ARMC 39 | 347 | N |
| fosfomycin trometamol | Monuril | 1988 | I 112334 | N | |
| isepamicin | Isepacin | 1988 | ARMC 24 | 305 | N |
| micronomicin sulfate | Sagamicin | 1982 | P091082 | N | |
| miokamycin | Miocamycin | 1985 | ARMC 21 | 329 | N |
| mupirocin | Bactroban | 1985 | ARMC 21 | 330 | N |
| netilimicin sulfate | Netromicine | 1981 | I 070366 | N | |
| RV-11 | Zalig | 1989 | ARMC 25 | 318 | N |
| teicoplanin | Targocid | 1988 | ARMC 24 | 311 | N |
| apalcillin sodium | Lumota | 1982 | I 091130 | ND | |
| arbekacin | Habekacin | 1990 | ARMC 26 | 298 | ND |
| aspoxicillin | Doyle | 1987 | ARMC 23 | 328 | ND |
| astromycin sulfate | Fortimicin | 1985 | ARMC 21 | 324 | ND |
| azithromycin | Sunamed | 1988 | ARMC 24 | 298 | ND |
| aztreonam | Azactam | 1984 | ARMC 20 | 315 | ND |
| biapenem | Omegacin | 2002 | ARMC 38 | 351 | ND |
| cefbuperazone sodium | Tomiporan | 1985 | ARMC 21 | 325 | ND |
| cefcapene pivoxil | Flomox | 1997 | ARMC 33 | 330 | ND |
| cefdinir | Cefzon | 1991 | ARMC 27 | 323 | ND |
| cefditoren pivoxil | Meiact | 1994 | ARMC 30 | 297 | ND |
| cefepime | Maxipime | 1993 | ARMC 29 | 334 | ND |
| cefetamet pivoxil HCl | Globocef | 1992 | ARMC 28 | 327 | ND |
| cefixime | Cefspan | 1987 | ARMC 23 | 329 | ND |
| cefmenoxime HCl | Tacef | 1983 | ARMC 19 | 316 | ND |
| cefminox sodium | Meicelin | 1987 | ARMC 23 | 330 | ND |
| cefodizime sodium | Neucef | 1990 | ARMC 26 | 300 | ND |
| cefonicid sodium | Monocid | 1984 | ARMC 20 | 316 | ND |
| cefoperazone sodium | Cefobis | 1981 | I 127130 | ND | |
| ceforanide | Precef | 1984 | ARMC 20 | 317 | ND |
| cefoselis | Wincef | 1998 | ARMC 34 | 319 | ND |
| cefotetan disodium | Yamatetan | 1984 | ARMC 20 | 317 | ND |
| cefotiam HCl | Pansporin | 1981 | I 091106 | ND | |
| cefozopran HCl | Firstcin | 1995 | ARMC 31 | 339 | ND |
| cefpimizole | Ajicef | 1987 | ARMC 23 | 330 | ND |
| cefpiramide sodium | Sepatren | 1985 | ARMC 21 | 325 | ND |
| cefpirome sulfate | Cefrom | 1992 | ARMC 28 | 328 | ND |
| cefpodoxime proxetil | Banan | 1989 | ARMC 25 | 310 | ND |
| cefprozil | Cefzil | 1992 | ARMC 28 | 328 | ND |
| cefsoludin sodium | Takesulin | 1981 | I 091108 | ND | |
| ceftazidime | Fortam | 1983 | ARMC 19 | 316 | ND |
| cefteram pivoxil | Tomiron | 1987 | ARMC 23 | 330 | ND |
| ceftibuten | Seftem | 1992 | ARMC 28 | 329 | ND |
| ceftizoxime sodium | Epocelin | 1982 | I 070260 | ND | |
| ceftobiprole medocaril | Zeftera | 2008 | ARMC 44 | 589 | ND |
| ceftriaxone sodium | Rocephin | 1982 | I 091136 | ND | |
| cefuroxime axetil | Zinnat | 1987 | ARMC 23 | 331 | ND |
| cefuzonam sodium | Cosmosin | 1987 | ARMC 23 | 331 | ND |
| clarithromycin | Klaricid | 1990 | ARMC 26 | 302 | ND |
| dalfopristin | Synercid | 1999 | ARMC 35 | 338 | ND |
| dirithromycin | Nortron | 1993 | ARMC 29 | 336 | ND |
| doripenem | Finibax | 2005 | DNP 19 | 42 | ND |
| ertapenem sodium | Invanz | 2002 | ARMC 38 | 353 | ND |
| erythromycin acistrate | Erasis | 1988 | ARMC 24 | 301 | ND |
| flomoxef sodium | Flumarin | 1988 | ARMC 24 | 302 | ND |
| flurithromycin ethylsuccinate | Ritro | 1997 | ARMC 33 | 333 | ND |
| fropenam | Farom | 1997 | ARMC 33 | 334 | ND |
| imipenem/cilastatin | Zienam | 1985 | ARMC 21 | 328 | ND |
| lenampicillin HCI | Varacillin | 1987 | ARMC 23 | 336 | ND |
| loracarbef | Lorabid | 1992 | ARMC 28 | 333 | ND |
| meropenem | Merrem | 1994 | ARMC 30 | 303 | ND |
| moxalactam disodium | Shiomarin | 1982 | I 070301 | ND | |
| panipenem/betamipron | Carbenin | 1994 | ARMC 30 | 305 | ND |
| quinupristin | Synercid | 1999 | ARMC 35 | 338 | ND |
| retapamulin | Altabax | 2007 | ARMC 43 | 486 | ND |
| rifabutin | Mycobutin | 1992 | ARMC 28 | 335 | ND |
| rifamixin | Normix | 1987 | ARMC 23 | 341 | ND |
| rifapentine | Rifampin | 1988 | ARMC 24 | 310 | ND |
| rifaximin | Rifacol | 1985 | ARMC 21 | 332 | ND |
| rokitamycin | Ricamycin | 1986 | ARMC 22 | 325 | ND |
| roxithromycin | Rulid | 1987 | ARMC 23 | 342 | ND |
| sultamycillin tosylate | Unasyn | 1987 | ARMC 23 | 343 | ND |
| tazobactam sodium | Tazocillin | 1992 | ARMC 28 | 336 | ND |
| telavancin HCl | Vibativ | 2009 | DNP 23 | 15 | ND |
| telithromycin | Ketek | 2001 | DNP 15 | 35 | ND |
| temocillin disodium | Temopen | 1984 | ARMC 20 | 323 | ND |
| tigecycline | Tygacil | 2005 | DNP 19 | 42 | ND |
| balafloxacin | Q-Roxin | 2002 | ARMC 38 | 351 | S |
| besifloxacin | Besivance | 2009 | DNP 23 | 20 | S |
| ciprofloxacin | Ciprobay | 1986 | ARMC 22 | 318 | S |
| enoxacin | Flumark | 1986 | ARMC 22 | 320 | S |
| fleroxacin | Quinodis | 1992 | ARMC 28 | 331 | S |
| garenoxacin | Geninax | 2007 | ARMC 43 | 471 | S |
| gatilfloxacin | Tequin | 1999 | ARMC 35 | 340 | S |
| gemifloxacin mesilate | Factive | 2003 | ARMC 40 | 458 | S |
| grepafloxacin | Vaxor | 1997 | DNP 11 | 23 | S |
| levofloxacin | Floxacin | 1993 | ARMC 29 | 340 | S |
| linezolid | Zyvox | 2000 | DNP 14 | 21 | S |
| lomefloxacin | Uniquin | 1989 | ARMC 25 | 315 | S |
| moxifloxacin HCl | Avelox | 1999 | ARMC 35 | 343 | S |
| nadifloxacin | Acuatim | 1993 | ARMC 29 | 340 | S |
| norfloxacin | Noroxin | 1983 | ARMC 19 | 322 | S |
| ofloxacin | Tarivid | 1985 | ARMC 21 | 331 | S |
| pazufloxacin | Pasil | 2002 | ARMC 38 | 364 | S |
| pefloxacin mesylate | Perflacine | 1985 | ARMC 21 | 331 | S |
| prulifloxacin | Sword | 2002 | ARMC 38 | 366 | S |
| rufloxacin hydrochloride | Qari | 1992 | ARMC 28 | 335 | S |
| sitafloxacin hydrate | Gracevit | 2008 | DNP 22 | 15 | S |
| sparfloxacin | Spara | 1993 | ARMC 29 | 345 | S |
| taurolidine | Taurolin | 1988 | I 107771 | S | |
| temafloxacin hydrochloride | Temac | 1991 | ARMC 27 | 334 | S |
| tosufloxacin | Ozex | 1990 | ARMC 26 | 310 | S |
| trovafloxacin mesylate | Trovan | 1998 | ARMC 34 | 332 | S |
| brodimoprin | Hyprim | 1993 | ARMC 29 | 333 | S*/NM |
| ACWY meningoccal PS vaccine | Mencevax | 1981 | I 420128 | V | |
| DTPw-HepB-Hib | Quinvaxem | 2006 | DNP 20 | 26 | V |
| H. influenzae b vaccine | Hibtitek | 1989 | DNP 03 | 24 | V |
| H. influenzae b vaccine | Prohibit | 1989 | DNP 03 | 24 | V |
| MCV-4 | Menactra | 2005 | DNP 19 | 43 | V |
| menACWY-CRM | Menveo | 2010 | I 341212 | V | |
| meningitis b vaccine | MeNZB | 2004 | DNP 18 | 29 | V |
| meningococcal vaccine | Menigetec | 1999 | DNP 14 | 22 | V |
| meningococcal vaccine | NeisVac-C | 2000 | DNP 14 | 22 | V |
| meningococcal vaccine | Menjugate | 2000 | DNP 14 | 22 | V |
| oral cholera vaccine | Orochol | 1994 | DNP 08 | 30 | V |
| pneumococcal vaccine | Prevnar | 2000 | DNP 14 | 22 | V |
| PsA-TT | MenAfriVac | 2010 | I 437718 | V | |
| vi polysaccharide typhoid vacc | Typherix | 1998 | DNP 12 | 35 | V |
Table 5. Antiparasitic Drugs from 01.01.81 to 12.01.10 Organized Alphabetically by Generic Name within Source.
| generic name | trade name | year introduced | volume | page | source |
|---|---|---|---|---|---|
| artemisinin | Artemisin | 1987 | ARMC 23 | 327 | N |
| ivermectin | Mectizan | 1987 | ARMC 23 | 336 | N |
| arteether | Artemotil | 2000 | DNP 14 | 22 | ND |
| artemether | Artemetheri | 1987 | I 90712 | ND | |
| artesunate | Arinate | 1987 | I 91299 | ND | |
| eflornithine HCl | Ornidyl | 1990 | DNP 04 | 104 | ND |
| mefloquine HCI | Fansimef | 1985 | ARMC 21 | 329 | ND |
| albendazole | Eskazole | 1982 | I 129625 | S | |
| halofantrine | Halfan | 1988 | ARMC 24 | 304 | S |
| lumefantrine | ? | 1987 | I 269095 | S | |
| quinfamide | Amenox | 1984 | ARMC 20 | 322 | S |
| atovaquone | Mepron | 1992 | ARMC 28 | 326 | S* |
| bulaquine/chloroquine | Aablaquin | 2000 | DNP 14 | 22 | S* |
| trichomonas vaccine | Gynatren | 1986 | I 125543 | V |
Table 8. Anticancer Drugs from 01.01.81 to 12.31.10 Organized Alphabetically by Generic Name within Source.
| generic name | trade name | year introduced | volume | page | source |
|---|---|---|---|---|---|
| Rexin-G | 2007 | I 346431 | B | ||
| 131I-chTNT | 2007 | I 393351 | B | ||
| alemtuzumab | Campath | 2001 | DNP 15 | 38 | B |
| bevacizumab | Avastin | 2004 | ARMC 40 | 450 | B |
| catumaxomab | Removab | 2009 | DNP 23 | 18 | B |
| celmoleukin | Celeuk | 1992 | DNP 06 | 102 | B |
| cetuximab | Erbitux | 2003 | ARMC 39 | 346 | B |
| denileukin diftitox | Ontak | 1999 | ARMC 35 | 338 | B |
| H-101 | 2005 | DNP 19 | 46 | B | |
| ibritumomab | Zevalin | 2002 | ARMC 38 | 359 | B |
| interferon α-2a | Roferon-A | 1986 | I 204503 | B | |
| interferon, γ-1a | Biogamma | 1992 | ARMC 28 | 332 | B |
| interleukin-2 | Proleukin | 1989 | ARMC 25 | 314 | B |
| mobenakin | Octin | 1999 | ARMC 35 | 345 | B |
| BIOMAb | |||||
| nimotuzumab | EFGR | 2006 | DNP 20 | 29 | B |
| ofatumumab | Arzerra | 2009 | DNP 23 | 18 | B |
| panitumumab | Vectibix | 2006 | DNP 20 | 28 | B |
| pegaspargase | Oncaspar | 1994 | ARMC 30 | 306 | B |
| rituximab | Rituxan | 1997 | DNP 11 | 25 | B |
| sipuleucel-T | Provenge | 2010 | I 259673 | B | |
| tasonermin | Beromun | 1999 | ARMC 35 | 349 | B |
| teceleukin | Imumace | 1992 | DNP 06 | 102 | B |
| tositumomab | Bexxar | 2003 | ARMC 39 | 364 | B |
| trastuzumab | Herceptin | 1998 | DNP 12 | 35 | B |
| aclarubicin | Aclacin | 1981 | P090013 | N | |
| angiotensin II | Delivert | 1994 | ARMC 30 | 296 | N |
| arglabin | ? | 1999 | ARMC 35 | 335 | N |
| masoprocol | Actinex | 1992 | ARMC 28 | 333 | N |
| paclitaxel | Taxol | 1993 | ARMC 29 | 342 | N |
| paclitaxel nanoparticles | Abraxane | 2005 | DNP 19 | 45 | N |
| paclitaxel nanoparticles | Nanoxel | 2007 | I 422122 | N | |
| pentostatin | Nipent | 1992 | ARMC 28 | 334 | N |
| peplomycin | Pepleo | 1981 | P090889 | N | |
| romidepsin | Istodax | 2010 | DNP 23 | 18 | N |
| trabectedin | Yondelis | 2007 | ARMC 43 | 492 | N |
| solamargines | Curaderm | 1989 | DNP 03 | 25 | NB |
| alitretinoin | Panretin | 1999 | ARMC 35 | 333 | ND |
| amrubicin HCl | Calsed | 2002 | ARMC 38 | 349 | ND |
| belotecan hydrochloride | Camtobell | 2004 | ARMC 40 | 449 | ND |
| cabazitaxel | Jevtana | 2010 | I 287186 | ND | |
| cladribine | Leustatin | 1993 | ARMC 29 | 335 | ND |
| cytarabine ocfosfate | Starsaid | 1993 | ARMC 29 | 335 | ND |
| docetaxel | Taxotere | 1995 | ARMC 31 | 341 | ND |
| elliptinium acetate | Celiptium | 1983 | P091123 | ND | |
| epirubicin HCI | Farmorubicin | 1984 | ARMC 20 | 318 | ND |
| eribulin | Halaven | 2010 | I 287199 | ND | |
| etoposide phosphate | Etopophos | 1996 | DNP 10 | 13 | ND |
| exemestane | Aromasin | 1999 | DNP 13 | 46 | ND |
| formestane | Lentaron | 1993 | ARMC 29 | 337 | ND |
| fulvestrant | Faslodex | 2002 | ARMC 38 | 357 | ND |
| gemtuzumab | |||||
| ozogamicin | Mylotarg | 2000 | DNP 14 | 23 | ND |
| hexyl aminolevulinate | Hexvix | 2004 | I 300211 | ND | |
| idarubicin hydrochloride | Zavedos | 1990 | ARMC 26 | 303 | ND |
| irinotecan hydrochloride | Campto | 1994 | ARMC 30 | 301 | ND |
| ixabepilone | Ixempra | 2007 | ARMC 43 | 473 | ND |
| mifamurtide | Junovan | 2010 | DNP 23 | 18 | ND |
| miltefosine | Miltex | 1993 | ARMC 29 | 340 | ND |
| pirarubicin | Pinorubicin | 1988 | ARMC 24 | 309 | ND |
| pralatrexate | Folotyn | 2009 | DNP 23 | 18 | ND |
| talaporfin sodium | Laserphyrin | 2004 | ARMC 40 | 469 | ND |
| temsirolimus | Toricel | 2007 | ARMC 43 | 490 | ND |
| topotecan HCl | Hycamptin | 1996 | ARMC 32 | 320 | ND |
| triptorelin | Decapeptyl | 1986 | I 090485 | ND | |
| valrubicin | Valstar | 1999 | ARMC 35 | 350 | ND |
| vapreotide acetate | Docrised | 2004 | I 135014 | ND | |
| vinflunine | Javlor | 2010 | I 219585 | ND | |
| vinorelbine | Navelbine | 1989 | ARMC 25 | 320 | ND |
| zinostatin stimalamer | Smancs | 1994 | ARMC 30 | 313 | ND |
| aminoglutethimide | Cytadren | 1981 | I 070408 | S | |
| amsacrine | Amsakrin | 1987 | ARMC 23 | 327 | S |
| arsenic trioxide | Trisenox | 2000 | DNP 14 | 23 | S |
| bisantrene hydrochloride | Zantrene | 1990 | ARMC 26 | 300 | S |
| carboplatin | Paraplatin | 1986 | ARMC 22 | 318 | S |
| flutamide | Drogenil | 1983 | ARMC 19 | 318 | S |
| fotemustine | Muphoran | 1989 | ARMC 25 | 313 | S |
| heptaplatin/SK-2053R | Sunpla | 1999 | ARMC 35 | 348 | S |
| lobaplatin | Lobaplatin | 1998 | DNP 12 | 35 | S |
| lonidamine | Doridamina | 1987 | ARMC 23 | 337 | S |
| miriplatin hydrate | Miripla | 2010 | DNP 23 | 17 | S |
| nedaplatin | Aqupla | 1995 | ARMC 31 | 347 | S |
| nilutamide | Anadron | 1987 | ARMC 23 | 338 | S |
| oxaliplatin | Eloxatin | 1996 | ARMC 32 | 313 | S |
| plerixafor hydrochloride | Mozobil | 2009 | DNP 22 | 17 | S |
| porfimer sodium | Photofrin | 1993 | ARMC 29 | 343 | S |
| ranimustine | Cymerine | 1987 | ARMC 23 | 341 | S |
| sobuzoxane | Parazolin | 1994 | ARMC 30 | 310 | S |
| sorafenib | Nexavar | 2005 | DNP 19 | 45 | S |
| anastrozole | Arimidex | 1995 | ARMC 31 | 338 | S/NM |
| bicalutamide | Casodex | 1995 | ARMC 31 | 338 | S/NM |
| bortezomib | Velcade | 2003 | ARMC 39 | 345 | S/NM |
| camostat mesylate | Foipan | 1985 | ARMC 21 | 325 | S/NM |
| dasatinib | Sprycel | 2006 | DNP 20 | 27 | S/NM |
| erlotinib hydrochloride | Tarceva | 2004 | ARMC 40 | 454 | S/NM |
| fadrozole HCl | Afema | 1995 | ARMC 31 | 342 | S/NM |
| gefitinib | Iressa | 2002 | ARMC 38 | 358 | S/NM |
| imatinib mesilate | Gleevec | 2001 | DNP 15 | 38 | S/NM |
| lapatinib ditosylate | Tykerb | 2007 | ARMC 43 | 475 | S/NM |
| letrazole | Femara | 1996 | ARMC 32 | 311 | S/NM |
| nilotinib hydrochloride | Tasigna | 2007 | ARMC 43 | 480 | S/NM |
| pazopanib | Votrient | 2009 | DNP 23 | 18 | S/NM |
| sunitinib malate | Sutent | 2006 | DNP 20 | 27 | S/NM |
| temoporfin | Foscan | 2002 | I 158118 | S/NM | |
| toremifene | Fareston | 1989 | ARMC 25 | 319 | S/NM |
| zoledronic acid | Zometa | 2000 | DNP 14 | 24 | S |
| azacytidine | Vidaza | 2004 | ARMC 40 | 447 | S* |
| capecitabine | Xeloda | 1998 | ARMC 34 | 319 | S* |
| carmofur | Mifurol | 1981 | I 091100 | S* | |
| clofarabine | Clolar | 2005 | DNP 19 | 44 | S* |
| decitabine | Dacogen | 2006 | DNP 20 | 27 | S* |
| doxifluridine | Furtulon | 1987 | ARMC 23 | 332 | S* |
| enocitabine | Sunrabin | 1983 | ARMC 19 | 318 | S* |
| fludarabine phosphate | Fludara | 1991 | ARMC 27 | 327 | S* |
| gemcitabine HCl | Gemzar | 1995 | ARMC 31 | 344 | S* |
| mitoxantrone HCI | Novantrone | 1984 | ARMC 20 | 321 | S* |
| nelarabine | Arranon | 2006 | ARMC 42 | 528 | S* |
| abarelix | Plenaxis | 2004 | ARMC 40 | 446 | S*/NM |
| bexarotene | Targretine | 2000 | DNP 14 | 23 | S*/NM |
| degarelix | Firmagon | 2009 | DNP 22 | 16 | S*/NM |
| pemetrexed disodium | Alimta | 2004 | ARMC 40 | 463 | S*/NM |
| raltitrexed | Tomudex | 1996 | ARMC 32 | 315 | S*/NM |
| tamibarotene | Amnoid | 2005 | DNP 19 | 45 | S*/NM |
| temozolomide | Temodal | 1999 | ARMC 35 | 350 | S*/NM |
| vorinostat | Zolinza | 2006 | DNP 20 | 27 | S*/NM |
| Cervarix | 2007 | I 309201 | V | ||
| autologous tumor cell-BCG | OncoVAX | 2008 | DNP 22 | 17 | V |
| bcg live | TheraCys | 1990 | DNP 04 | 104 | V |
| melanoma theraccine | Melacine | 2001 | DNP 15 | 38 | V |
| vitespen | Oncophage | 2008 | DNP 22 | 17 | V |
Table 10. Antidiabetic Drugs from 01.01.1981 to 12.31.2010 Organized Alphabetically by Generic Name within Source.
| generic name | trade name | year introduced | volume | page | source | |
|---|---|---|---|---|---|---|
| biphasic porcine insulin | Pork Mixtard 30 | 1982 | I 303034 | B | ||
| hu neutral insulin | Insuman | 1992 | I 255451 | B | ||
| hu insulin zinc suspension | Humulin Zn | 1985 | I 091584 | B | ||
| human insulin Zn suspension | Humulin L | 1985 | I 302828 | B | ||
| human neutral insulin | Novolin R | 1991 | I 182551 | B | ||
| insulin aspart | NovoRapid | 1999 | DNP 13 | 41 | B | |
| insulin aspart/IA protamine | NovoMix 30 | 2001 | DNP 15 | 34 | B | |
| insulin determir | Levemir | 2004 | DNP 18 | 27 | B | |
| insulin glargine | Lantus | 2000 | DNP 14 | 19 | B | |
| insulin glulisine | Apidra | 2005 | DNP 19 | 39 | B | |
| insulin lispro | Humalog | 1996 | ARMC 32 | 310 | B | |
| isophane insulin | Humulin N | 1982 | I 091583 | B | ||
| mecasermin | Somazon | 1994 | DNP 08 | 28 | B | |
| oral insulin | Oral-lyn | 2005 | DNP 19 | 39 | B | |
| porcine isophane insulin | Pork Insulatard | 1982 | I 302757 | B | ||
| porcine neutral insulin | Pork Actrapid | 1998 | I 302749 | B | ||
| pulmonary insulin | Exubera | 2005 | DNP 20 | 23 | B | |
| soluble insulin | Velosulin BR | 1986 | I 091581 | B | ||
| voglibose | Basen | 1994 | ARMC 30 | 313 | N | |
| acarbose | Glucobay | 1990 | DNP 03 | 23 | ND | |
| extenatide | Byetta | 2005 | DNP 19 | 40 | ND | |
| liraglutide | Victoza | 2009 | DNP 23 | 13 | ND | |
| miglitol | Diastabol | 1998 | ARMC 34 | 325 | ND | |
| triproamylin acetate | Normylin | 2005 | DNP 19 | 40 | ND | |
| glimepiride | Amaryl | 1995 | ARMC 31 | 344 | S | |
| mitiglinide calcium hydrate | Glufast | 2004 | ARMC 40 | 460 | S | |
| pioglitazone NCl | Actos | 1999 | ARMC 35 | 346 | S | |
| repaglinide | Prandin | 1998 | ARMC 34 | 329 | S | |
| alogliptin benzoate | Nesina | 2010 | I 405286 | S/NM | ||
| epalrestat | Kinedak | 1992 | ARMC 28 | 330 | S/NM | |
| rosiglitazone maleate | Avandia | 1999 | ARMC 35 | 348 | S/NM | |
| saxagliptin | Onglyza | 2009 | DNP 23 | 13 | S/NM | |
| sitagliptin | Januvia | 2006 | DNP 20 | 23 | S/NM | |
| tolrestat | Alredase | 1989 | ARMC 25 | 319 | S/NM | |
| troglitazone | Rezulin | 1997 | ARMC 33 | 344 | S/NM | |
| vildagliptin | Galvus | 2007 | ARMC 43 | 494 | S/NM | |
| nateglinide | Starsis | 1999 | ARMC 35 | 344 | S* | |
It should be noted that the “Year” header in all tables is equivalent to the “Year of Introduction” of the drug. In a number of cases over the years, there are discrepancies between sources as to the actual year due to differences in definitions. Some reports will use the year of approval (registration by non-USA/FDA organizations) while others will use the first recorded sales. We have generally taken the earliest year in the absence of further information.
Results
As in previous reviews, we have only covered New Chemical Entities (NCEs) in the present analysis. As mentioned in the earlier reviews, if one reads the FDA and PhRMA web sites, the numbers of NDA approvals are in the high ten to low hundred numbers for the last few years. If, however, combinations of older drugs and old drugs with new indications, and/or improved delivery systems are removed, then the number of true NCEs has ranged between the 20s to just over 50 per year since 1989. If one now removes biologicals and vaccines thus noting only “small molecules”, then the figures show that over the same time frame, the numbers have ranged from close to 40 for most of the 1989 to 2000 time frame, dropping to 20 or less from 2001 to 2010 with the exception of 2002 and 2004 when the figures climbed above 30 (cf., Figures 2 and 4 below).
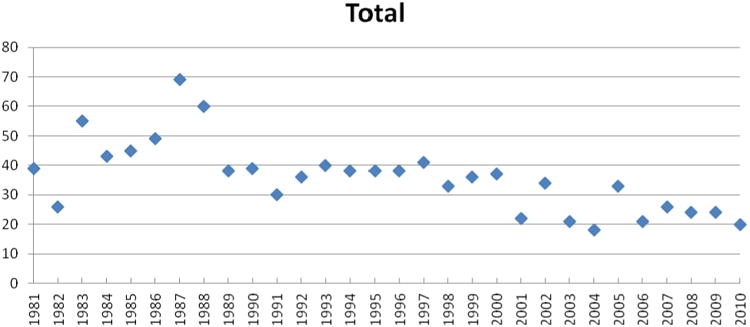
For the first time, now with 30 years of data to analyze, it was decided to add two other graphs to the listings, of which one might be of significant interest to the natural products community. In Figure 5 the percentage of approved NCEs have been plotted per year from 1981 to 2010 where the designation is basically an “N” or a subdivision (“NB” or “ND”) with the total numbers of small molecules approved by year as a point chart in Figure 6. Thus, we have deliberately not included any designations that could be considered as “inspired by a natural product structure”, although from the data provided either in the tables or from the supporting information, any reader who so desires, may calculate their own particular variation(s) on Figure 5.
Figure 5. Percent N/NB/ND by Year, 1981 – 2010.
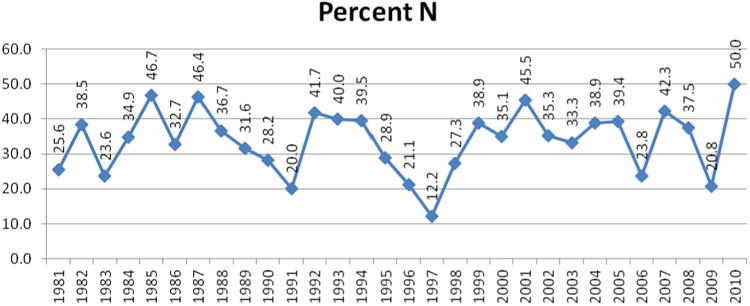
Figure 6. Total Small Molecules by Year, 1981 – 2010.
As in our earlier reviews,1-3 the data have been analyzed in terms of numbers and classified according to their origin using the previous major categories and their subdivisions.
Major Categories of Sources
The major categories used are as follows:
“B” Biological; usually a large (>45 residues) peptide or protein either isolated from an organism/cell line or produced by biotechnological means in a surrogate host.
“N” Natural product.
“NB” Natural product “Botanical” (in general these have been recently approved).
“ND” Derived from a natural product and is usually a semi-synthetic modification.
“S” Totally synthetic drug, often found by random screening/modification of an existing agent.
“S*” Made by total synthesis, but the pharmacophore is/was from a natural product.
“V” Vaccine.
Sub-category
“NM” Natural Product Mimic (see rationale and examples below) (For amplification as to the rationales used for categorizing using the above subdivisions, the reader should consult the earlier reviews.1-3)
In the field of anticancer therapy, the advent in 2001 of Gleevec®, a protein tyrosine kinase inhibitor, was justly heralded as a breakthrough in the treatment of leukemia. This compound was classified as an “/NM” on the basis of its competitive displacement of the natural substrate, ATP, in which the intracellular concentrations can approach 5 mM. We have continued to classify PTK and other kinase inhibitors that are approved as drugs under the “/NM” category for exactly the same reasons as elaborated in the 2003 review,2 and have continued to extend it to cover other direct inhibitors/antagonists of the natural substrate/receptor interaction whether obtained by direct experiment or by in silico studies followed by direct assay in the relevant system.
Similarly, a number of new peptidic drug entities, although formally synthetic in nature, are simply produced by synthetic methods rather than by the use of fermentation or extraction. In some cases, an end group might have been changed for ease of recovery. In addition, a number of compounds produced totally by synthesis, are in fact isosteres of the peptidic substrate and are thus “natural product mimics” in the truest sense of the term. For further information on this area, interested readers should consult the excellent earlier review by Hruby,75 his 2009 “Perspective” review,76 and very recent work in the same area by Audie and Boyd 77 and VanHee et al.78 in order to fully appreciate the potential of such (bio)chemistry.
As an example of what can be found by studies around relatively simple peptidomimics of the angiotensin II structure, the paper of Wan et al.79 demonstrating the modification of the known but non-selective AT1/AT2 agonist, L-162313 (2, itself related to the sartans), into the highly selective AT2 agonist 3 (a peptidomimetic structure), led to the identification of short pseudopeptides exemplified by 4, which is equipotent (binding affinity = 500 pM) with angiotensin II and has a better than 20,000-fold selectivity versus AT1, whereas angiotensin II has only a five-fold binding selectivity in the same assay,80 as reported in our 2007 review. The chemistry leading to these compounds was reported in 2007 in greater detail by Georgsson et al.81 with a thorough discussion of the role of AT2 receptors in a multiplicity of disease states being published in 2008.82 To date, we have not found any clinical trials reported on these materials.
In the area of modifications of natural products by combinatorial methods to produce entirely different compounds that may bear little if any resemblance to the original, but are legitimately assignable to the “/NM” category, citations are given in previous reviews.8, 83-90 In addition, one should consult the reports from Waldmann's group91,92 and those by Ganesan,93,94 Shang and Tan,95 Bauer et al.21 Constantino and Barlocco,96 Bade et al.97 and Violette et al.98 demonstrating the use of privileged structures as a source of molecular skeletons around which one may build libraries. Another paper of interest in this regard is the editorial by Macarron from GSK,15 as this may be the first time where data from industry on the results of HTS screens of combichem libraries versus potential targets was reported with a discussion of lead discovery rates. In this paper, Macarron re-emphasizes the fifth Lipinski rule, which is often ignored; “natural products do not obey the other four”.
Overview of Results
The data we have analyzed in a variety of ways are presented as a series of bar graphs and pie charts and two major tables in order to establish the overall picture, and then are further subdivided into some major therapeutic areas using a tabular format. The time frame covered is the 30 years from 01/01/1981 - 12/31/2010:
| •New Approved Drugs: | With all source categories (Figure 1) |
| •New Approved Drugs: | By source/year (Figure 2) |
| •Sources of all NCEs: | Where four or more drugs were approved per medical indication (Table 1), with listings of diseases with < 3 approved drugs |
| •Sources of Small-Molecule NCEs: | All subdivisions (Figure 3) |
| •Sources of Small-Molecule NCEs: | By source/year (Figure 4) |
| •Percent N/NB/ND: | By year (Figure 5) |
| •Total Small Molecules: | By year (Figure 6) |
| •Antibacterial Drugs: | Generic and trade names, year, reference and source (Table 2) |
| •Antifungal Drugs | Generic and trade names, year, reference and source (Table 3) |
| •Antiviral Drugs | Generic and trade names, year, reference and source (Table 4) |
| •Antiparasitic Drugs | Generic and trade names, year, reference and source (Table 5) |
| •Antiinfective Drugs | All molecules, source and numbers (Table 6) |
| •Antiinfective Drugs | Small molecules, source and numbers (Table 7) |
| •Anticancer Drugs | Generic and trade names, year, reference and source (Table 8; Figure 7) |
| •All Anticancer Drugs (very late 1930s-12/2010) | Generic and trade names, year, reference and source Table 9; Figures 8, 9) |
| •Antidiabetic Drugs | Generic and trade names, year, reference and source (Table 10) |
Figure 1. All New Approved Drugs; n = 1355.
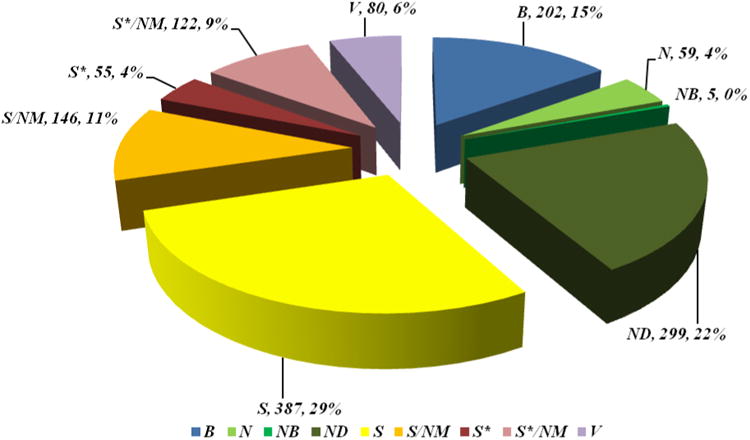
Table 1. New Chemical Entities and Medical Indications by Source of Compound 01.01.81-12.31.2010a.
| indication | total | B | N | NB | ND | S | S/NM | S* | S*/NM | V |
|---|---|---|---|---|---|---|---|---|---|---|
| COPD | 4 | 1 | 3 | |||||||
| analgesic | 17 | 1 | 11 | 3 | 2 | |||||
| anesthetic | 5 | 5 | ||||||||
| anti-Alzheimer | 4 | 1 | 3 | |||||||
| anti-Parkinsonian | 12 | 2 | 1 | 5 | 4 | |||||
| antiallergic | 17 | 1 | 1 | 4 | 11 | |||||
| antianginal | 5 | 5 | ||||||||
| antiarrhythmic | 17 | 1 | 14 | 2 | ||||||
| antiarthritic | 17 | 6 | 1 | 1 | 3 | 6 | ||||
| antiasthmatic | 14 | 1 | 3 | 2 | 6 | 2 | ||||
| antibacterial | 118 | 10 | 67 | 26 | 1 | 14 | ||||
| anticancer | 128 | 24 | 11 | 1 | 32 | 20 | 16 | 11 | 8 | 5 |
| anticoagulant | 19 | 5 | 13 | 1 | ||||||
| antidepressant | 23 | 7 | 14 | 2 | ||||||
| antidiabetic | 37 | 18 | 1 | 5 | 4 | 8 | 1 | |||
| antiemetic | 11 | 1 | 2 | 8 | ||||||
| antiepileptic | 15 | 2 | 9 | 2 | 2 | |||||
| antifungal | 29 | 1 | 3 | 22 | 3 | |||||
| antiglaucoma | 14 | 5 | 5 | 1 | 3 | |||||
| antihistamine | 13 | 13 | ||||||||
| antihyperprolactinemia | 4 | 4 | ||||||||
| antihypertensive | 79 | 2 | 28 | 14 | 2 | 33 | ||||
| antiinflammatory | 51 | 1 | 13 | 37 | ||||||
| antimigraine | 10 | 2 | 1 | 7 | ||||||
| antiobesity | 4 | 1 | 3 | |||||||
| antiparasitic | 14 | 2 | 5 | 4 | 2 | 1 | ||||
| antipsoriatic | 9 | 3 | 1 | 3 | 1 | 1 | ||||
| antipsychotic | 10 | 3 | 5 | 2 | ||||||
| antithrombotic | 29 | 13 | 1 | 5 | 2 | 6 | 2 | |||
| antiulcer | 34 | 1 | 1 | 12 | 20 | |||||
| antiviral | 110 | 14 | 4 | 9 | 2 | 23 | 10 | 48 | ||
| anxiolytic | 10 | 8 | 2 | |||||||
| benign prostatic | ||||||||||
| hypertrophy | 4 | 1 | 1 | 1 | 1 | |||||
| bronchodilator | 8 | 2 | 6 | |||||||
| calcium metabolism | 20 | 8 | 9 | 3 | ||||||
| cardiotonic | 13 | 3 | 2 | 3 | 5 | |||||
| chelator | 4 | 4 | ||||||||
| contraception | 9 | 8 | 1 | |||||||
| diuretic | 6 | 4 | 2 | |||||||
| erythropoiesis | 5 | 5 | ||||||||
| gastroprokinetic | 4 | 1 | 2 | 1 | ||||||
| hematopoiesis | 6 | 6 | ||||||||
| hemophilia | 12 | 12 | ||||||||
| hormone | 22 | 12 | 10 | |||||||
| hormone replacement therapy | 8 | 8 | ||||||||
| hypnotic | 12 | 12 | ||||||||
| hypocholesterolemic | 13 | 4 | 1 | 2 | 1 | 5 | ||||
| hypolipidemic | 8 | 1 | 7 | |||||||
| immunomodulator | 4 | 2 | 1 | 1 | ||||||
| immunostimulant | 11 | 5 | 3 | 2 | 1 | |||||
| immunosuppressant | 12 | 4 | 5 | 3 | ||||||
| irritable bowel syndrome | 4 | 1 | 3 | |||||||
| male sexual dysfunction | 4 | 4 | ||||||||
| multiple sclerosis | 6 | 3 | 1 | 1 | 1 | |||||
| muscle relaxant | 10 | 4 | 2 | 1 | 3 | |||||
| neuroleptic | 9 | 1 | 6 | 2 | ||||||
| nootropic | 8 | 3 | 5 | |||||||
| osteoporosis | 5 | 3 | 1 | 1 | ||||||
| platelet aggregation inhibitor | 4 | 3 | 1 | |||||||
| respiratory distress syndrome | 6 | 3 | 1 | 1 | 1 | |||||
| urinary incontinence | 5 | 2 | 3 | |||||||
| vulnerary | 5 | 2 | 2 | 1 | ||||||
| Grand Total | 1130 | 144 | 47 | 3 | 247 | 325 | 130 | 50 | 116 | 68 |
Diseases where ≤ 3 drugs approved 1981 – 2010; 225 drugs fall into this category and are subdivided as follows: B, 58; N, 12; NB, 2; ND, 52; S, 62, S/NM. 16; S*, 5; S*/NM, 6; V, 12. The diseases covered the following; 5 α-reductase inhibitor, ADHD, CAPS, CHF, CNS Stimulant, Crohn's disease, DVT, Fabry's disease, Gaucher's disease, Hunter syndrome, Japanese encephalitis, Lambert-Eaton Myasthenic Syndrome, Lyme disease, MI, acute, MMRC, PAH, PCP/Toxoplasmosis, PNH, Pompe's disease, Turner Syndrome, abortifacient, acromelagy, actinic keratoses, adjuvant/colorectal cancer, alcohol deterrent, allergic rhinitis, anabolic metabolism, analeptic, anemia, anti sickle cell anemia, anti-smoking, antiacne, antiathersclerotic, anticonvulsant, antidiarrheal, antidote, antiemphysemic, antihyperuricemia, antihypotensive, antinarcolepsy, antinarcotic, antinauseant, antiperistaltic, antipneumococcal, antiprogestogenic, antirheumatic, antisecretory, antisepsis, antiseptic, antispasmodic, antispastic, antitussive, antityrosinaemia, antixerostomia, atrial fibrillation, benzodiazepine antagonist, β-lactamase inhibitor, blepharospasm, bone disorders, bone morphogenesis, bowel evacuant, cardioprotective, cardiovascular disease, cartilage disorders, cervical dystonia, choleretic, chronic idiopathic constipation, cognition enhancer, congestive heart failure, constipation, cystic fibrosis, cytoprotective, dementia (Alzheimer's), diabetic foot ulcers, diabetic neuropathies, digoxin toxicity, dpt, dry eye syndrome, dyslipidemia, dysuria, endometriosis, enzyme, expectorant, fertility inducer, gastroprotectant, genital warts, hematological, hemorrhage, hemostasis, hemostatic, hepatoprotectant, hereditary angioedema, homocystinuria, hyperammonemia, hyperparathyroidism, hyperphenylalaninemia, hyperphosphatemia, hyperuricemia, hypoammonuric, hypocalciuric, hypogonadism, hyponatremia, idiopathic pulmonary fibrosis, idiopathic thrombocytopenia, immediate allergy, infertility (female), inflammatory bowel disease, insomnia, joint lubricant, lipoprotein disorders, macular degeneration, mucolytic, mucopolysaccharidosis, mucositis, myleodysplasia, narcolepsy, nasal decongestant, neuropathic pain, neuroprotective, ocular inflammation, opiate detoxification, osteoarthritis, overactive bladder, ovulation, pancreatic disorders, pancreatitis, pertussis, photosensitizer, pituitary disorders, porphyria, premature birth, premature ejaculation, progestogen, psychostimulant, pulmonary arterial hypertension, purpura fulminans, rattlesnake antivenom, reproduction, restenosis, schizophrenia, sclerosant, secondary hyperthryoidism, sedative, skin photodamage, strabismus, subarachnoid hemorrhage, thrombocytopenia, treatment of GH deficiency, ulcerative colitis, urea cycle disorders, uremic pruritis, urolithiasis, vaccinia complications, varicella (chicken pox), vasodilator, vasodilator (cerebral), vasodilator (coronary), vasoprotective, venous thromboembolism
Figure 3. Source of Small Molecule Approved Drugs; n = 1073.
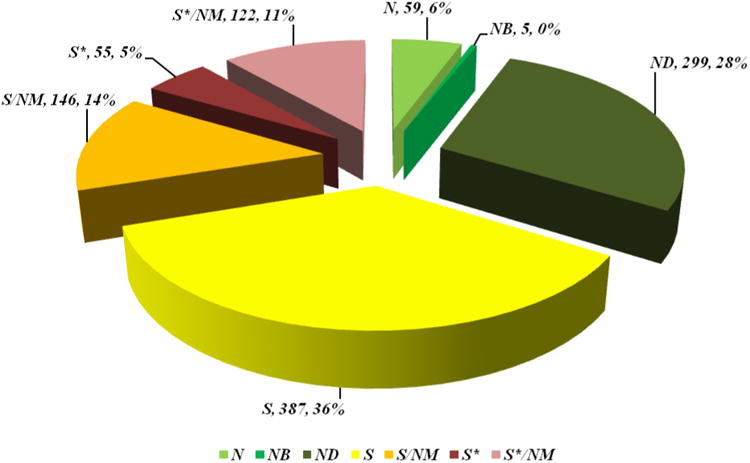
Table 3. Antifungal Drugs from 01.01.81 to 12.31.10 Organized Alphabetically by Generic Name within Source.
| generic name | trade name | year introduced | volume | page | source |
|---|---|---|---|---|---|
| interferon γ-n1 | OGamma100 | 1996 | DNP 10 | 13 | B |
| anidulafungin | Eraxis | 2006 | DNP 20 | 24 | ND |
| caspofungin acetate | Cancidas | 2001 | DNP 15 | 36 | ND |
| micafungin sodium | Fungard | 2002 | ARMC 38 | 360 | ND |
| amorolfine hydrochloride | Loceryl | 1991 | ARMC 27 | 322 | S |
| butoconazole | Femstat | 1986 | ARMC 22 | 318 | S |
| ciclopirox olamine | Loprox | 1982 | I 070449 | S | |
| cloconazole HCI | Pilzcin | 1986 | ARMC 22 | 318 | S |
| eberconazole | Ebernet | 2005 | DNP 19 | 42 | S |
| fenticonazole nitrate | Lomexin | 1987 | ARMC 23 | 334 | S |
| fluconazole | Diflucan | 1988 | ARMC 24 | 303 | S |
| flutrimazole | Micetal | 1995 | ARMC 31 | 343 | S |
| fosfluconazole | Prodif | 2003 | DNP 17 | 49 | S |
| itraconazole | Sporanox | 1988 | ARMC 24 | 305 | S |
| ketoconazole | Nizoral | 1981 | I 116505 | S | |
| lanoconazole | Astat | 1994 | ARMC 30 | 302 | S |
| luliconazole | Lulicon | 2005 | DNP 19 | 42 | S |
| naftifine HCI | Exoderil | 1984 | ARMC 20 | 321 | S |
| neticonazole HCI | Atolant | 1993 | ARMC 29 | 341 | S |
| oxiconazole nitrate | Oceral | 1983 | ARMC 19 | 322 | S |
| posaconazole | Noxafil | 2005 | DNP 19 | 42 | S |
| sertaconazole nitrate | Dermofix | 1992 | ARMC 28 | 336 | S |
| sulconazole nitrate | Exelderm | 1985 | ARMC 21 | 332 | S |
| terconazole | Gyno-Terazol | 1983 | ARMC 19 | 324 | S |
| tioconazole | Trosyl | 1983 | ARMC 19 | 324 | S |
| voriconazole | Vfend | 2002 | ARMC 38 | 370 | S |
| butenafine hydrochloride | Mentax | 1992 | ARMC 28 | 327 | S/NM |
| liranaftate | Zefnart | 2000 | DNP 14 | 21 | S/NM |
| terbinafine hydrochloride | Lamisil | 1991 | ARMC 27 | 334 | S/NM |
Table 4. Antiviral Drugs from 01.01.81 to 12.31.10 Organized Alphabetically by Generic Name within Source.
| generic name | trade name | year introduced | volume | page | source |
|---|---|---|---|---|---|
| interferon α | Alfaferone | 1987 | I 215443 | B | |
| interferon α-n3 | Alferon N | 1990 | DNP 04 | 104 | B |
| interferon β | Frone | 1985 | I115091 | B | |
| immunoglobulin | |||||
| intravenous | Gammagard Liquid | 2005 | I 231564 | B | |
| interferon alfacon-1 | Infergen | 1997 | ARMC 33 | 336 | B |
| IGIV-HB | Niuliva | 2009 | DNP 23 | 16 | B |
| Oralgen | 2007 | I 415378 | B | ||
| peginterferon α-2a | Pegasys | 2001 | DNP 15 | 34 | B |
| peginterferon α-2b | Pegintron | 2000 | DNP 14 | 18 | B |
| resp syncytial virus IG | RespiGam | 1996 | DNP 10 | 11 | B |
| palivizumab | Synagis | 1998 | DNP 12 | 33 | B |
| interferon α-2b | Viraferon | 1985 | I 165805 | B | |
| interferon α-n1 | Wellferon | 1986 | I 125561 | B | |
| thymalfasin | Zadaxin | 1996 | DNP 10 | 11 | B |
| enfuvirtide | Fuzeon | 2003 | ARMC 39 | 350 | ND |
| laninamivir octanoate | Inavir | 2010 | I 340894 | ND | |
| peramivir | PeramiFlu | 2010 | I 273549 | ND | |
| zanamivir | Relenza | 1999 | ARMC 35 | 352 | ND |
| imiquimod | Aldara | 1997 | ARMC 33 | 335 | S |
| maraviroc | Celsentri | 2007 | ARMC 43 | 478 | S |
| foscarnet sodium | Foscavir | 1989 | ARMC 25 | 313 | S |
| raltegravir potassium | Isentress | 2007 | ARMC 43 | 484 | S |
| delavirdine mesylate | Rescriptor | 1997 | ARMC 33 | 331 | S |
| rimantadine HCI | Roflual | 1987 | ARMC 23 | 342 | S |
| propagermanium | Serosion | 1994 | ARMC 30 | 308 | S |
| efavirenz | Sustiva | 1998 | ARMC 34 | 321 | S |
| nevirapine | Viramune | 1996 | ARMC 32 | 313 | S |
| darunavir | Prezista | 2006 | DNP 20 | 25 | S/NM |
| oseltamivir | Tamiflu | 1999 | ARMC 35 | 346 | S/NM |
| entecavir | Baraclude | 2005 | DNP 19 | 39 | S* |
| ganciclovir | Cymevene | 1988 | ARMC 24 | 303 | S* |
| emtricitabine | Emtriva | 2003 | ARMC 39 | 350 | S* |
| lamivudine | Epivir | 1995 | ARMC 31 | 345 | S* |
| famciclovir | Famvir | 1994 | ARMC 30 | 300 | S* |
| adefovir dipivoxil | Hepsera | 2002 | ARMC 38 | 348 | S* |
| epervudine | Hevizos | 1988 | I 157373 | S* | |
| zalcitabine | Hivid | 1992 | ARMC 28 | 338 | S* |
| inosine pranobex | Imunovir | 1981 | I 277341 | S* | |
| etravirine | Intelence | 2008 | DNP 22 | 15 | S* |
| clevudine | Levovir | 2007 | ARMC 43 | 466 | S* |
| zidovudine | Retrovir | 1987 | ARMC 23 | 345 | S* |
| telbividine | Sebivo | 2006 | DNP 20 | 22 | S* |
| sorivudine | Usevir | 1993 | ARMC 29 | 345 | S* |
| valganciclovir | Valcyte | 2001 | DNP 15 | 36 | S* |
| valaciclovir HCl | Valtrex | 1995 | ARMC 31 | 352 | S* |
| penciclovir | Vectavir | 1996 | ARMC 32 | 314 | S* |
| didanosine | Videx | 1991 | ARMC 27 | 326 | S* |
| tenofovir disoproxil | |||||
| fumarate | Viread | 2001 | DNP 15 | 37 | S* |
| cidofovir | Vistide | 1996 | ARMC 32 | 306 | S* |
| stavudine | Zerit | 1994 | ARMC 30 | 311 | S* |
| abacavir sulfate | Ziagen | 1999 | ARMC 35 | 333 | S* |
| acyclovir | Zovirax | 1981 | I 091119 | S* | |
| amprenavir | Agenerase | 1999 | ARMC 35 | 334 | S*/NM |
| tipranavir | Aptivus | 2005 | DNP 19 | 42 | S*/NM |
| indinavir sulfate | Crixivan | 1996 | ARMC 32 | 310 | S*/NM |
| saquinavir mesylate | Invirase | 1995 | ARMC 31 | 349 | S*/NM |
| lopinavir | Kaletra | 2000 | ARMC 36 | 310 | S*/NM |
| fosamprenevir | Lexiva | 2003 | ARMC 39 | 353 | S*/NM |
| ritonavir | Norvir | 1996 | ARMC 32 | 317 | S*/NM |
| atazanavir | Reyataz | 2003 | ARMC 39 | 342 | S*/NM |
| neflinavir mesylate | Viracept | 1997 | ARMC 33 | 340 | S*/NM |
| fomivirsen sodium | Vitravene | 1998 | ARMC 34 | 323 | S*/NM |
| H5N1 avian flu vaccine | 2007 | I 440743 | V | ||
| Influenza A(H1N1) | |||||
| monovalent | 2010 | I 678265 | V | ||
| ACAM-2000 | 2007 | I 328985 | V | ||
| influenza virus vaccine | Afluria | 2007 | I 449226 | V | |
| hepatitis A vaccine | Aimmugen | 1995 | DNP 09 | 23 | V |
| hepatitis A and B vaccine | Ambirix | 2003 | I 334416 | V | |
| split influenza vaccine | Anflu | 2006 | DNP 20 | 26 | V |
| inact hepatitis A vaccine | Avaxim | 1996 | DNP 10 | 12 | V |
| hepatitis B vaccine | Biken-HB | 1993 | DNP 07 | 31 | V |
| Bilive | 2005 | DNP 19 | 43 | V | |
| hepatitis B vaccine | Bio-Hep B | 2000 | DNP 14 | 22 | V |
| Celtura | 2009 | DNP 23 | 17 | V | |
| Celvapan | 2009 | DNP 23 | 17 | V | |
| Daronix | 2007 | I 427024 | V | ||
| hepatitis B vaccine | Engerix B | 1987 | I 137797 | V | |
| rubella vaccine | Ervevax | 1985 | I 115078 | V | |
| hepatitis B vaccine | Fendrix | 2005 | DNP 19 | 43 | V |
| influenza virus (live) | FluMist | 2003 | ARMC 39 | 353 | V |
| Fluval P | 2009 | DNP 23 | 17 | V | |
| Focetria | 2009 | DNP 23 | 17 | V | |
| hpv vaccine | Gardasil | 2006 | DNP 20 | 26 | V |
| Grippol Neo | 2009 | DNP 23 | 16 | V | |
| hepatitis a vaccine | Havrix | 1992 | DNP 06 | 99 | V |
| hepatitis b vaccine | Hepacure | 2000 | DNP 14 | 22 | V |
| anti-Hep B | |||||
| immunoglobulin | HepaGam B | 2006 | DNP 20 | 27 | V |
| HN-VAC | HNVAC | 2010 | I 684608 | V | |
| influenza vaccine | Invivac | 2004 | I 391186 | V | |
| MR vaccine | Mearubik | 2005 | DNP 19 | 44 | V |
| hepatitis b vaccine | Meinyu | 1997 | DNP 11 | 24 | V |
| attenuated chicken pox | Merieux Varicella | ||||
| vaccine | Vaccine | 1993 | DNP 07 | 31 | V |
| Optaflu | 2007 | I 410266 | V | ||
| influenza vaccine | Optaflu | 2008 | DNP 22 | 16 | V |
| Pandremix | 2009 | DNP 23 | 17 | V | |
| Panenza | 2009 | DNP 23 | 17 | V | |
| Panflu | 2008 | DNP 22 | 16 | V | |
| VCIV | PreFluCel | 2010 | I 444826 | V | |
| GSK-1562902A | Prepandrix | 2008 | DNP 22 | 16 | V |
| antirabies vaccine | Rabirix | 2006 | DNP 20 | 27 | V |
| rotavirus vaccine | Rotarix | 2005 | DNP 18 | 29 | V |
| rotavirus vaccine | Rota-Shield | 1998 | DNP 12 | 35 | V |
| rotavirus vaccine | Rotateq | 2006 | DNP 20 | 26 | V |
| rec hepatitis B vaccine | Supervax | 2006 | DNP 20 | 27 | V |
| hepatitis a vaccine | Vaqta | 1996 | DNP 10 | 11 | V |
| varicella virus vaccine | Varivax | 1995 | DNP 09 | 25 | V |
| VariZIG | 2005 | I 230590 | V | ||
| Vaxiflu-S | 2010 | I 698015 | V | ||
| zoster vaccine live | Zostavax | 2006 | DNP 20 | 26 | V |
Table 6. All Antiinfective (Bacterial, Fungal, Parasitic, and Viral) Drugs (n = 270).
| indication | total | B | N | ND | S | S/NM | S* | S*/NM | V |
|---|---|---|---|---|---|---|---|---|---|
| Antibacterial | 118 | 10 | 67 | 26 | 1 | 14 | |||
| Antifungal | 29 | 1 | 3 | 22 | 3 | ||||
| Antiparasitic | 14 | 2 | 5 | 4 | 2 | 1 | |||
| Antiviral | 109 | 14 | 4 | 9 | 2 | 23 | 10 | 47 | |
| total | 270 | 15 | 12 | 79 | 61 | 5 | 25 | 11 | 62 |
| percentage | 100 | 5.6 | 4.4 | 29.3 | 22.6 | 1.8 | 9.3 | 4 | 23 |
Table 7. Small Molecule Antiinfective (Bacterial, Fungal, Parasitic, and Viral) Drugs (n = 193).
| indication | total | N | ND | S | S/NM | S* | S*/NM |
|---|---|---|---|---|---|---|---|
| Antibacterial | 104 | 10 | 67 | 26 | 1 | ||
| Antifungal | 28 | 3 | 22 | 3 | |||
| Antiparasitic | 13 | 2 | 5 | 4 | 2 | ||
| Antiviral | 48 | 4 | 9 | 2 | 23 | 10 | |
| total | 193 | 12 | 79 | 61 | 5 | 25 | 11 |
| percentage | 100 | 6.2 | 40.9 | 31.6 | 2.6 | 13 | 5.7 |
Figure 7. All Anticancer Drugs, 1981 – 2010.
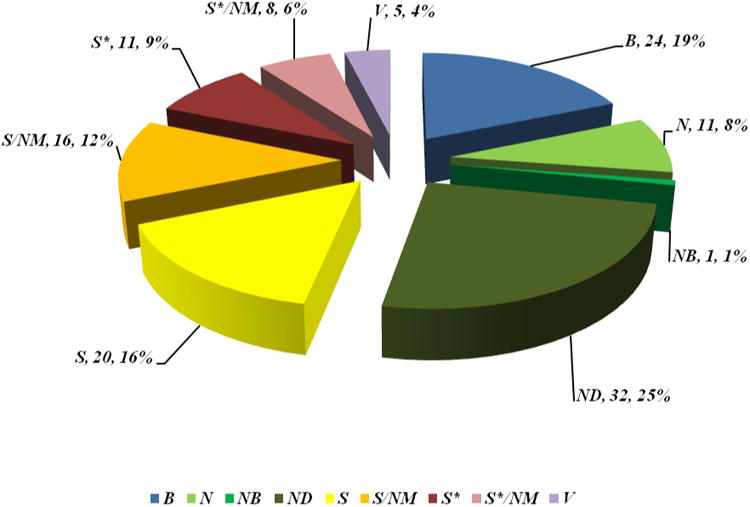
Table 9. All Anticancer Drugs (1940s to 12.31.10) Organized Alphabetically by Generic Name within Sourcea.
| generic name | year introduced | reference | page | source |
|---|---|---|---|---|
| 131I-chTNT | 2007 | I 393351 | B | |
| alemtuzumab | 2001 | DNP 15 | 38 | B |
| aldesleukin | 1992 | ARMC 25 | 314 | B |
| bevacizumab | 2004 | ARMC 40 | 450 | B |
| catumaxomab | 2009 | DNP 23 | 18 | B |
| celmoleukin | 1992 | DNP 06 | 102 | B |
| cetuximab | 2003 | ARMC 39 | 346 | B |
| denileukin diftitox | 1999 | ARMC 35 | 338 | B |
| H-101 | 2005 | DNP 19 | 46 | B |
| ibritumomab | 2002 | ARMC 38 | 359 | B |
| interferon alfa2a | 1986 | I 204503 | B | |
| interferon alfa2b | 1986 | I 165805 | B | |
| interferon, gamma-1a | 1992 | ARMC 28 | 332 | B |
| interleukin-2 | 1989 | ARMC 25 | 314 | B |
| mobenakin | 1999 | ARMC 35 | 345 | B |
| nimotuzumab | 2006 | DNP 20 | 29 | B |
| ofatumumab | 2009 | DNP 23 | 18 | B |
| panitumumab | 2006 | DNP 20 | 28 | B |
| pegaspargase | 1994 | ARMC 30 | 306 | B |
| Rexin-G (Trade name) | 2007 | I 346431 | B | |
| rituximab | 1997 | DNP 11 | 25 | B |
| sipuleucel-T | 2010 | I 259673 | B | |
| tasonermin | 1999 | ARMC 35 | 349 | B |
| teceleukin | 1992 | DNP 06 | 102 | B |
| tositumomab | 2003 | ARMC 39 | 364 | B |
| trastuzumab | 1998 | DNP 12 | 35 | B |
| aclarubicin | 1981 | I 090013 | N | |
| actinomycin D | 1964 | FDA | N | |
| angiotensin II | 1994 | ARMC 30 | 296 | N |
| arglabin | 1999 | ARMC 35 | 335 | N |
| asparaginase | 1969 | FDA | N | |
| bleomycin | 1966 | FDA | N | |
| carzinophilin | 1954 | Japan Antibiotics | N | |
| chromomycin A3 | 1961 | Japan Antibiotics | N | |
| daunomycin | 1967 | FDA | N | |
| doxorubicin | 1966 | FDA | N | |
| leucovorin | 1950 | FDA | N | |
| masoprocol | 1992 | ARMC 28 | 333 | N |
| mithramycin | 1961 | FDA | N | |
| mitomycin C | 1956 | FDA | N | |
| neocarzinostatin | 1976 | Japan Antibiotics | N | |
| paclitaxel | 1993 | ARMC 29 | 342 | N |
| paclitaxel nanoparticles (Abraxane) | 2005 | DNP 19 | 45 | N |
| paclitaxel nanoparticles (Nanoxel) | 2007 | I 422122 | N | |
| pentostatin | 1992 | ARMC 28 | 334 | N |
| peplomycin | 1981 | I 090889 | N | |
| romidepsin | 2010 | DNP 23 | 18 | N |
| sarkomycin | 1954 | FDA | N | |
| streptozocin | pre-1977 | Carter | N | |
| testosterone | pre-1970 | Cole | N | |
| trabectedin | 2007 | ARMC 43 | 492 | N |
| vinblastine | 1965 | FDA | N | |
| vincristine | 1963 | FDA | N | |
| solamargines | 1989 | DNP 03 | 25 | NB |
| alitretinoin | 1999 | ARMC 35 | 333 | ND |
| amrubicin HCl | 2002 | ARMC 38 | 349 | ND |
| belotecan hydrochloride | 2004 | ARMC 40 | 449 | ND |
| cabazitaxel | 2010 | I 287186 | ND | |
| calusterone | 1973 | FDA | ND | |
| cladribine | 1993 | ARMC 29 | 335 | ND |
| cytarabine ocfosfate | 1993 | ARMC 29 | 335 | ND |
| dexamethasone | 1958 | FDA | ND | |
| docetaxel | 1995 | ARMC 31 | 341 | ND |
| dromostanolone | 1961 | FDA | ND | |
| elliptinium acetate | 1983 | P091123 | ND | |
| epirubicin HCI | 1984 | ARMC 20 | 318 | ND |
| eribulin | 2010 | I 287199 | ND | |
| estramustine | 1980 | FDA | ND | |
| ethinyl estradiol | pre-1970 | Cole | ND | |
| etoposide | 1980 | FDA | ND | |
| etoposide phosphate | 1996 | DNP 10 | 13 | ND |
| exemestane | 1999 | DNP 13 | 46 | ND |
| fluoxymesterone | pre-1970 | Cole | ND | |
| formestane | 1993 | ARMC 29 | 337 | ND |
| fosfestrol | pre-1977 | Carter | ND | |
| fulvestrant | 2002 | ARMC 38 | 357 | ND |
| gemtuzumab ozogamicin | 2000 | DNP 14 | 23 | ND |
| goserelin acetate | 1987 | ARMC 23 | 336 | ND |
| hexyl aminolevulinate | 2004 | I 300211 | ND | |
| histrelin | 2004 | I 109865 | ND | |
| hydroxyprogesterone | pre-1970 | Cole | ND | |
| idarubicin hydrochloride | 1990 | ARMC 26 | 303 | ND |
| irinotecan hydrochloride | 1994 | ARMC 30 | 301 | ND |
| ixabepilone | 2007 | ARMC 43 | 473 | ND |
| leuprolide | 1984 | ARMC 20 | 319 | ND |
| medroxyprogesterone acetate | 1958 | FDA | ND | |
| megesterol acetate | 1971 | FDA | ND | |
| methylprednisolone | 1955 | FDA | ND | |
| methyltestosterone | 1974 | FDA | ND | |
| mifamurtide | 2010 | DNP 23 | 18 | ND |
| miltefosine | 1993 | ARMC 29 | 340 | ND |
| mitobronitol | 1979 | FDA | ND | |
| nadrolone phenylpropionate | 1959 | FDA | ND | |
| norethindrone acetate | pre-1977 | Carter | ND | |
| pirarubicin | 1988 | ARMC 24 | 309 | ND |
| pralatrexate | 2009 | DNP 23 | 18 | ND |
| prednisolone | pre-1977 | Carter | ND | |
| prednisone | pre-1970 | Cole | ND | |
| talaporfin sodium | 2004 | ARMC 40 | 469 | ND |
| temsirolimus | 2007 | ARMC 43 | 490 | ND |
| teniposide | 1967 | FDA | ND | |
| testolactone | 1969 | FDA | ND | |
| topotecan HCl | 1996 | ARMC 32 | 320 | ND |
| triamcinolone | 1958 | FDA | ND | |
| triptorelin | 1986 | I 090485 | ND | |
| valrubicin | 1999 | ARMC 35 | 350 | ND |
| vapreotide acetate | 2004 | I 135014 | ND | |
| vindesine | 1979 | FDA | ND | |
| vinflunine | 2010 | I 219585 | ND | |
| vinorelbine | 1989 | ARMC 25 | 320 | ND |
| zinostatin stimalamer | 1994 | ARMC 30 | 313 | ND |
| amsacrine | 1987 | ARMC 23 | 327 | S |
| arsenic trioxide | 2000 | DNP 14 | 23 | S |
| bisantrene hydrochloride | 1990 | ARMC 26 | 300 | S |
| busulfan | 1954 | FDA | S | |
| carboplatin | 1986 | ARMC 22 | 318 | S |
| carmustine (BCNU) | 1977 | FDA | S | |
| chlorambucil | 1956 | FDA | S | |
| chlortrianisene | pre-1981 | Boyd | S | |
| cis-diamminedichloroplatinum | 1979 | FDA | S | |
| cyclophosphamide | 1957 | FDA | S | |
| dacarbazine | 1975 | FDA | S | |
| diethylstilbestrol | pre-1970 | Cole | S | |
| flutamide | 1983 | ARMC 19 | 318 | S |
| fotemustine | 1989 | ARMC 25 | 313 | S |
| heptaplatin/SK-2053R | 1999 | ARMC 35 | 348 | S |
| hexamethylmelamine | 1979 | FDA | S | |
| hydroxyurea | 1968 | FDA | S | |
| ifosfamide | 1976 | FDA | S | |
| lenalidomide | 2005 | DNP 19 | 45 | S |
| levamisole | pre-1981 | Boyd | S | |
| lobaplatin | 1998 | DNP 12 | 35 | S |
| lomustine (CCNU) | 1976 | FDA | S | |
| lonidamine | 1987 | ARMC 23 | 337 | S |
| mechlorethanamine | 1958 | FDA | S | |
| melphalan | 1961 | FDA | S | |
| miriplatin hydrate | 2010 | DNP 23 | 17 | S |
| mitotane | 1970 | FDA | S | |
| nedaplatin | 1995 | ARMC 31 | 347 | S |
| nilutamide | 1987 | ARMC 23 | 338 | S |
| nimustine hydrochloride | pre-1981 | Boyd | S | |
| oxaliplatin | 1996 | ARMC 32 | 313 | S |
| pamidronate | 1987 | ARMC 23 | 326 | S |
| pipobroman | 1966 | FDA | S | |
| plerixafor hydrochloride | 2009 | DNP 22 | 17 | S |
| porfimer sodium | 1993 | ARMC 29 | 343 | S |
| procarbazine | 1969 | FDA | S | |
| ranimustine | 1987 | ARMC 23 | 341 | S |
| razoxane | pre-1977 | Carter | S | |
| semustine (MCCNU) | pre-1977 | Carter | S | |
| sobuzoxane | 1994 | ARMC 30 | 310 | S |
| sorafenib | 2005 | DNP 19 | 45 | S |
| thiotepa | 1959 | FDA | S | |
| triethylenemelamine | pre-1981 | Boyd | S | |
| zoledronic acid | 2000 | DNP 14 | 24 | S |
| anastrozole | 1995 | ARMC 31 | 338 | S/NM |
| bicalutamide | 1995 | ARMC 31 | 338 | S/NM |
| bortezomib | 2003 | ARMC 39 | 345 | S/NM |
| camostat mesylate | 1985 | ARMC 21 | 325 | S/NM |
| dasatinib | 2006 | DNP 20 | 27 | S/NM |
| erlotinib hydrochloride | 2004 | ARMC 40 | 454 | S/NM |
| fadrozole HCl | 1995 | ARMC 31 | 342 | S/NM |
| gefitinib | 2002 | ARMC 38 | 358 | S/NM |
| imatinib mesilate | 2001 | DNP 15 | 38 | S/NM |
| lapatinib ditosylate | 2007 | ARMC 43 | 475 | S/NM |
| letrazole | 1996 | ARMC 32 | 311 | S/NM |
| nafoxidine | pre-1977 | Carter | S/NM | |
| nilotinib hydrochloride | 2007 | ARMC 43 | 480 | S/NM |
| pazopanib | 2009 | DNP 23 | 18 | S/NM |
| sunitinib malate | 2006 | DNP 20 | 27 | S/NM |
| tamoxifen | 1973 | FDA | S/NM | |
| temoporfin | 2002 | I 158118 | S/NM | |
| toremifene | 1989 | ARMC 25 | 319 | S/NM |
| aminoglutethimide | 1981 | FDA | S* | |
| azacytidine | 2004 | ARMC 40 | 447 | S* |
| capecitabine | 1998 | ARMC 34 | 319 | S* |
| carmofur | 1981 | I 091100 | S* | |
| clofarabine | 2005 | DNP 19 | 44 | S* |
| cytosine arabinoside | 1969 | FDA | S* | |
| decitabine | 2006 | DNP 20 | 27 | S* |
| doxifluridine | 1987 | ARMC 23 | 332 | S* |
| enocitabine | 1983 | ARMC 19 | 318 | S* |
| floxuridine | 1971 | FDA | S* | |
| fludarabine phosphate | 1991 | ARMC 27 | 327 | S* |
| fluorouracil | 1962 | FDA | S* | |
| ftorafur | 1972 | FDA | S* | |
| gemcitabine HCl | 1995 | ARMC 31 | 344 | S* |
| mercaptopurine | 1953 | FDA | S* | |
| methotrexate | 1954 | FDA | S* | |
| mitoxantrone HCI | 1984 | ARMC 20 | 321 | S* |
| nelarabine | 2006 | ARMC 42 | 528 | S* |
| thioguanine | 1966 | FDA | S* | |
| uracil mustard | 1966 | FDA | S* | |
| abarelix | 2004 | ARMC 40 | 446 | S*/NM |
| bexarotene | 2000 | DNP 14 | 23 | S*/NM |
| degarelix | 2009 | DNP 22 | 16 | S*/NM |
| pemetrexed disodium | 2004 | ARMC 40 | 463 | S*/NM |
| raltitrexed | 1996 | ARMC 32 | 315 | S*/NM |
| tamibarotene | 2005 | DNP 19 | 45 | S*/NM |
| temozolomide | 1999 | ARMC 35 | 350 | S*/NM |
| vorinostat | 2006 | DNP 20 | 27 | S*/NM |
| autologous tumor cell-BCG | 2008 | DNP 22 | 17 | V |
| bcg live | 1990 | DNP 04 | 104 | V |
| Cervarix (Trade name) | 2007 | I 309201 | V | |
| melanoma theraccine | 2001 | DNP 15 | 38 | V |
| vitespen | 2008 | DNP 22 | 17 | V |
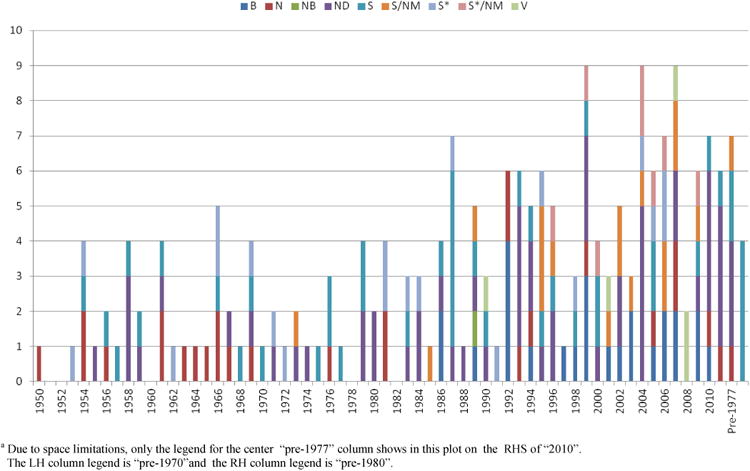
Note that in Figure 9 there are three vertical bars corresponding to the drugs noted in the “year introduced” column above as “pre-1970”, “pre-1977” and “pre-1981”. The entries under these three categories are not repeated the other two, as the drugs are individually distinct entries, but their actual dates cannot be determined.
Figure 8. All Anticancer Drugs 1940s – 2010 by Source.
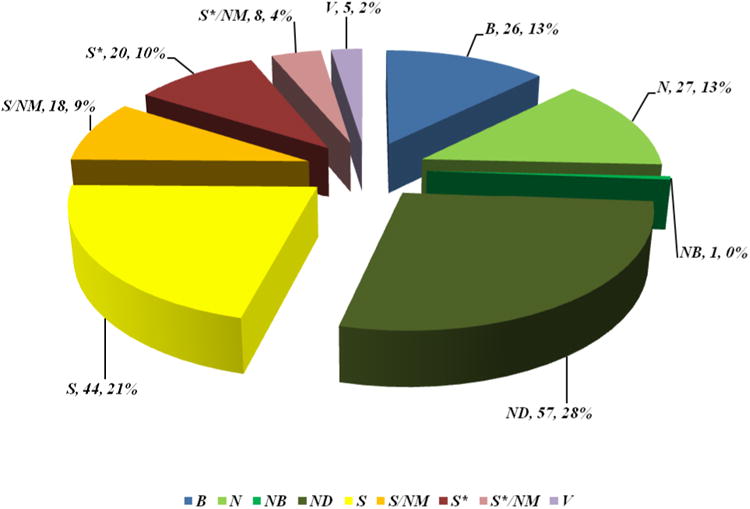
Figure 9. All Anticancer Drugs 1940s – 2010 by Year/Source.
The extensive datasets shown in the figures and tables referred to above highlight the continuing role that natural products and structures derived from or related to natural products from all sources have played, and continue to play, in the development of the current therapeutic armamentarium of the physician. Inspection of the data shows the continued important role for natural products in spite of the current greatly reduced level of natural products-based drug discovery programs in major pharmaceutical houses.
Inspection of the rate of NCE approvals as shown in Figures 2, and 4 - 6 demonstrates that even in 2010, the natural products field is still producing or is involved in ca. 50% of all small molecules in the years 2000 – 2010. This is readily demonstrated in Figures 5 and 6 where the percentage of just the “N” linked materials is shown, with figures ranging from a low of 20.8% in 2009, to a high of 50% in 2010, with the mean and standard deviation for those 11 years being 36.5 + 8.6, without including any of the natural product inspired classifications (S*, S*/NM and S/NM). What is quite fascinating is that in 2010, fully half of the 20 approved small molecule NCEs fell into the “N” categories, including the majority of the antitumor agents (cf., Tables 2 – 4; 8).
As was shown in the 2007 review, a significant number of all NCEs still fall into the categories of biological (“B”) or vaccines (“V”), with 282 of 1355 or (20.8%) over the full 30-year period, and it is to be admitted that not all of the vaccines approved in these 30 years have been identified, although in the last 10 or 11 years probably a great majority have been captured. Thus, the proportion of approved vaccines may well be higher over the longer time frame. Inspection of Figure 2 shows the significant proportion that these two categories hold in the number of approved drugs from 2000, where, in some years, these categories accounted for ca. 50% of all approvals. If the three “N” categories are included then the proportions of nonsynthetics are even higher for these years. This is so in spite of many years of work by the pharmaceutical industry devoted to high-throughput screening of predominately combinatorial chemistry products, and this time period should have provided a sufficient time span for combinatorial chemistry work from the late 1980s onwards to have produced a number of approved NCEs.
Overall, of the 1355 NCEs covering all diseases/countries/sources in the years 01/1981-12/2010, and using the “NM” classifications introduced in our 2003 review,2 29% were synthetic in origin, thus demonstrating the influence of “other than formal synthetics” on drug discovery and approval (Figure 1). In the 2007 review, the corresponding figure was 30%.3
Inspection of Table 1 demonstrates that overall, the major disease areas that have been investigated (in terms of numbers of drugs approved) in the pharmaceutical industry continue to be infectious diseases (microbial, parasitic and viral), cancer, hypertension, and inflammation, all with over 50 approved drug therapies. It should be noted however, that numbers of approved drugs/disease do not correlate with the “value” as measured by sales. For example, the best selling drug of all is atorvastatin (Lipitor®), a hypocholesterolemic descended directly from a microbial natural product, which sold over $(U.S.) 11 billion in 2004, and, if one includes sales by Pfizer and Astellas Pharma over the 2004 to 2010 time frames, sales have hovered between $(U.S.) 12-14 billion depending upon the year. The first US patent for this drug expired in March 2010 and Ranbaxy, the Indian generics company launched the generic version in the U.S.A. in December 2011, following FDA approval on the last day of the Pfizer patent, November 30th, 2011.
The major category by far is that of antiinfectives including antiviral vaccines, with 270 (23.9%) of the total (1130 for indications ≥4) falling into this one major human disease area. On further analysis (Tables 6 and 7), the influence of biologicals and vaccines in this disease complex is such that only 22.6% are synthetic in origin (Table 6). If one only considers small molecules (reducing the total by 77 to 193; Table 7), then the synthetic figure goes up to 31.6%, marginally greater than in our previous report.3 As reported previously,1-3 these synthetic drugs tend to be of two basic chemotypes, the azole-based antifungals and the quinolone-based antibacterials,
Six small-molecule drugs were approved in the antibacterial area from 01/2006 to 12/2010. Three were classified as ND, with the first retapamulin (5) being a semisynthetic modification of the well known pleuromutilin structure by GSK in 2007, the second being ceftobiprole medocaril, a cephalosporin prodrug (6) from the Roche spin-off company Basilea in 2008 in Switzerland and Canada. The compound was later withdrawn as of September 2010 by Basilea/Janssen-Cilag (J&J) and it is currently back in Phase III trials, with Johnson and Johnson having terminated their license. The third agent was the modified vancomycin, telavancin (7) by Astellas Pharma in conjunction with Theravance in 2009. The three synthetic antibacterials in this time frame were the fluoroquinolones, garenoxacin (8) from Astellas Pharma in 2007, sitafloxacin from Daiichi (9) in 2008, and besifloxacin (10) from Bausch and Lomb in 2009. Overall, in the antibacterial area, as shown in Table 7, small molecules account for 104 agents, with “N” and “ND” compounds accounting for just under 75% of the approved agents.
In the antifungal area, only one drug was approved in the 2006 to 2010 time frame. This was the echinocandin derivative, anidulafungin (ND; 11) approved for use in the USA in early 2006 and was covered in the 2007 review but without a structure. As is the case with a significant number of compounds, the final company was not the originator. This molecule was first synthesized by Lilly under the code number LY-303366, then licensed to Versicor in 1999; Versicor became Vicuron in 2003 and Pfizer purchased Vicuron in 2005.
In contrast to the antibacterial case, in the antifungal area, as shown in Table 7, small molecules account for 28 agents, but in the 30 years of coverage, only three agents fall into the “ND” category, accounting for just over 10% of the approved drugs. This can be seen in the treatment regimens that still use agents such as amphotericin and griseofulvin, which are both listed in the Integrity™ database as being launched in 1958.
In the antiviral area, a very significant number of the agents are vaccines, as mentioned earlier, predominately directed against various serotypes of influenza, as would be expected from the avian flu outbreaks. In the time frame 2006 to 2010, and looking at small molecules, seven drugs were approved for a variety of viral diseases. In contrast to the previous reviews,1-3 the number of anti-HIV drugs decreased with only three being reported in the four years since the previous report. These were darunavir (S/NM, 12) in 2006 from Tibotec/Janssen, an HIV protease inhibitor, the first HIV attachment inhibitor, maraviroc (S, 13), in 2007, from the joint venture between Pfizer and GSK on anti-HIV therapies, and, in the same year the first integrase inhibitor, raltegravir (S, 14) by Merck. Of definite import during the last five years, however, is the approval of two new drugs for the treatment of hepatitis B in 2006, The first, telbivudine, a simple thymine analogue that is a DNA-polymerase inhibitor with a 2-deoxyribose derivative as the sugar moiety (S*, 15), was licensed in from Idenix by Novartis. The second, clevudine (S*, 16), with the same mechanism of action, is also a thymine derivative, but, in this case, the sugar moiety is further substituted by a fluorine atom on the sugar compared to telbivudine. This compound was originally identified at Yale University and the University of Georgia, then was licensed by the Korean company Bukwang, who then sub-licensed it to Eisai for further development.
The last two compounds, both of which were approved in 2010, are small-molecule inhibitors of the influenza virus.99 The first, peramivir (S/NM, 17) can be considered as a successful in silico derivative as it was modeled into the sialidase crystal structure by BioCryst (Birmingham, AL) who subsequently licensed it to Green Cross and then Shionogi in Japan for treatment of influenza A and B. The second molecule, laninamivir (ND, 18), is basically similar in structure to both zanamivir (1999, ND, 19) and oseltamivir (1999, ND, 20), both modeled on N-acetyl-neuraminic acid (21, the substrate of the sialidases), and for which synthetic routes can come from either quinic acid (22) or shikimic acid (23),100 with the latter compound being produced from the star anise plant, Illicium anisatum,101 or via fermentation of genetically modified E. coli strains.102, 103
In contrast to the antibacterial and antifungal areas, in the antiviral case, as shown in Table 7, small molecules account for 48 drugs, with only four (or 8%) in the 30 years of coverage falling into the “ND” category. However, consistently we have placed modified nucleosides and peptidomimetics, etc., as falling into the “S*” or “S*/NM” categories. If these are added to the four drugs listed above, then the other than synthetic molecules account for 37 or 57% overall.
As reported in our earlier analyses,1-3 there are still significant therapeutic classes where the available drugs are totally synthetic at the present time. These include antihistamines, diuretics, and hypnotics for indications with four or more approved drugs (cf., Table 1), and, as found previously, there are still a substantial number of indications in which there are three or less approved drugs that are also totally synthetic. As mentioned in our earlier reviews,2,3 due to the introduction of the “NM” subcategory, indications such as antidepressants, bronchodilators and cardiotonics now have substantial numbers that, although formally “S” or “S*”, fall into the “S/NM” or “S*/NM) subcategories, as the information in the literature points to their interactions at active sites as competitive inhibitors.
With anticancer drugs (Table 8), where in the time frame covered (01/1981-12/2010) there were 128 NCEs in toto, with the number of non-biologicals aka small molecules being 99 (77%), a slightly lower percentage compared to the last review's value of 81%.3 Using the total of 99 as being equal to 100%, the breakdown was as follows, with the values from the last review inserted for comparison: N (11, 11.1% {9, 11.1%}), NB (1, 1% {none}), ND (32, 32.3% {25; 30.9%}), S (20, 20.2% {18, 22.2%}), S/NM (16, 16.2% {12, 14.8%}), S* (11, 11.1% {11, 13.6%}) and S*/NM (8, 8.1% {6, 7.4%}). Thus, using our criteria, only 20.2% of the total number of small-molecule anticancer drugs was classifiable into the S (synthetic) category. Expressed as a proportion of the non-biologicals/vaccines, then 79 of 99 (79.8%) were either natural products per se or were based thereon, or mimicked natural products in one form or another.
In this current review, we have continued as in our previous contribution (2007)3 to reassess the influence of natural products and their mimics as leads to anticancer drugs from the beginnings of antitumor chemotherapy in the very late 1930s to early 1940s. By using data from the FDA listings of antitumor drugs, coupled to our previous data sources and with help from Japanese colleagues, we have been able to specify the years in which all but 18 of the 206 drugs listed in Table 9 were approved. We then identified these other 18 agents by inspection of three time-relevant textbooks on antitumor treatment,73, 104, 105 and these were added to the overall listings using the lead authors' names as the source citation.
Inspection of Figure 9 and Table 9 shows that, over the whole category of anticancer drugs approved world-wide, the 206 approved agents can be categorized as follows: B (26; 13%), N (27; 13%), NB (1; 0.5%), ND (57; 28%), S (44; 21%), S/NM (18; 9%), S* (20; 10%), S*/NM (8; 4%) and V (5; 2%). If one then removes the high molecular weight materials (biologicals and vaccines), reducing the overall number to 175 (100%), the number of naturally inspired agents (i.e., N, ND, S/NM, S*, S*/NM) is 131 (74.9%). Etoposide phosphate and various nanopaticle formulations of Taxol® have been included for the sake of completeness.
There are at least two points of definitive interest to natural products scientists in these figures over the last few years, in particular in the last four (2006-2010), when the sources of approved antitumor drugs are considered. Thus, the first antitumor agent that is a “botanical” (or NB), polyphenon E, was approved by the FDA in 2007 for treatment of genital warts linked to human papilloma viruses (HPV),106 though one can argue from a chemical aspect that Curaderm®, which is a mixture of solamargines and was approved in 1989, was the first of these. We have now listed it as an “NB” rather than an “N” in Table 8. Polyphenon E is currently in a number of trials against various cancers as both a preventative and as a direct agent against chronic lymphocytic leukemia, bladder and lung cancers at the Phase II level, and in breast cancer at Phase I level, with a number of trials being sponsored by NCI.
What is perhaps of equal or perhaps higher significance, is that if one looks at the seven antitumor agents approved in 2010, roughly 20 years after the move away from natural product-based discovery programs by big pharmaceutical companies, then one, romidepsin (24) an histone deacetylase inhibitor (HDAC) is a microbial natural product107-110 without any modification, and, although it has been synthesized, this compound is still produced by fermentation. Of the remaining six, four are derived from natural products, with three, vinflunine (25), cabazitaxel (26) and the totally synthetic halichondrin B-derived eribulin (27), being tubulin-interactive agents, but all binding to different sites on tubulin. Although the vinca and taxane sites are reasonably well described, eribulin appears to bind to site(s) that are different from these.111, 112 The remaining one in this category, mifamurtide (28), is a derivatized muramyl dipeptide approved for the treatment of osteosarcoma.113 The remaining small molecule, miriplatin hydrate (29) is totally synthetic, and is a new member of a very old class, the platinates, although its structure is dissimilar to others in the class in having what might be described as myristyl ester linkages to the platinum atom, giving it significant lipid solubility.114
In our earlier papers, the number of non-synthetic antitumor agents approximated 60% for other than biological/vaccines, without using the “NM” subcategory. The corresponding figure obtained by removing the NM subcategory in this analysis is 60%. Thus, the proportion has remained similar in spite of some reassignments of sources and the continued use of combinatorial chemistry as a source of test substances.
In the case of the antidiabetic drugs, both for diabetes I and II, the numbers since our last review have increased by five from 32 to 37 (Table 10), with one of the five falling into the “ND” category (cf., discussion on liragultide below). However, one biologic for which much was expected, being the first inhaled product, Exubera®, was approved in 2005 by the FDA and then withdrawn in 2008. We have, however, still included it in the tabulation. Four of the other five fall into the S/NM category, but the remaining one, liraglutide,115 is a very interesting derivative of the glucagon-like peptide-1 (GLP-1) and can best be described as [Nε-[(Nα-hexadecanoyl)-γ-L-Glu]-L-Lys26,L-Arg34]-GLP-1(7-37), where two amino acids have been changed in the 7 to 37 portion of the sequence, followed by addition of lipid “tails”. Further information on the utility of GLP-1 agonists can be found in the very recent review by Marre and Penformis.116
Discussion
As alluded to in our last two reviews,2,3 the decline or leveling of the output of the R&D programs of the pharmaceutical companies has continued, with the number of drugs of all types dropping in 2006 to 40 NCEs launched, of which 19 (48%) were classified in the “other than small molecules” or B/V categories. The corresponding figures for the next four years (2007-2010) are as follows. In 2007 there were 44 NCEs launched with 18 (41%) classified as B/V. In 2008, 38 NCEs were launched with 14 (37%) classified as B/V. In 2009, 42 NCEs were launched with 18 (43%) classified as B/V. Then in the last year of this analysis, 2010, there were 33 NCEs launched with 13 (39%) classified as B/V. Thus, one can see that an average of 42% of all NCEs in this five year time frame were biologicals or vaccines, and as mentioned earlier, the numbers of vaccines during this time period may have been underestimated.
As mentioned in the discussion of the antitumor agents and the dramatic influence of natural product structures in the approvals in 2010, we would be remiss if comment was not made on one other very important compound also approved that year. The compound in question is fingolimod (30, Gilenya®), the first orally active compound for once-a-day treatment of patients with relapsing forms of multiple sclerosis. The details of the derivation of this compound from an old fungal metabolite known as myriocin (31) and the many years of modifications required to produce the drug, have been told in detail in two recent reviews.117, 118 What is also of significance is the recent report that fingolimod (30) also might have activity as a radio-sensitizing agent in treatment of prostate cancer.119
Although combinatorial chemistry continues to play a major role in the drug development process, as mentioned earlier, it is noteworthy that the trend toward the synthesis of complex natural product-like libraries has continued. Even including these newer methodologies, we still cannot find another de novo combinatorial compound approved anywhere in the world, although reliable data are not on hand on approvals in Russia and the People's Republic of China at this time. We think that it is appropriate to re-echo the comments by Danishefsky that was used in the 2007 review: “In summary, we have presented several happy experiences in the course of our program directed toward bringing to bear nature's treasures of small molecule natural products on the momentous challenge of human neurodegenerative diseases. While biological results are now being accumulated for systematic disclosure, it is already clear that there is considerable potential in compounds obtained through plowing in the landscape of natural products. Particularly impressive are those compounds that are obtained through diverted total synthesis, i.e., through methodology, which was redirected from the original (and realized) goal of total synthesis, to encompass otherwise unavailable congeners. We are confident that the program will lead, minimally, to compounds that are deserving of serious preclinical follow-up. At the broader level, we note that this program will confirm once again (if further confirmation is, indeed, necessary) the extraordinary advantages of small molecule natural products as sources of agents, which interject themselves in a helpful way in various physiological processes.
We close with the hope and expectation that enterprising and hearty organic chemists will not pass up the unique head start that natural products provide in the quest for new agents and new directions in medicinal discovery. We would chance to predict that even as the currently fashionable “telephone directory” mode of research is subjected to much overdue scrutiny and performance-based assessment, organic chemists in concert with biologists and even clinicians will be enjoying as well as exploiting the rich troves provided by nature's small molecules”.120
A rapid analysis of the entities approved from 2006 to 2010 indicated that there were significant numbers of antitumor, antibacterial, and antifungal agents approved as mentioned above, with the unexpected showing, as exemplified in Figures 5 and 6, that in 2010, of the 20 small molecules approved, the second lowest number in the 30 years of analysis covered in this review, fully half were natural products or directly derived there from, with the majority of these being in the antitumor area, ten years after the approval of the first protein tyrosine kinase inhibitor, Gleevec®, in 2001. Included in the 2010 antitumor approvals was eribulin (27), to our knowledge the most complex drug yet approved made totally by synthesis.
It is highly probable that in the near future, totally synthetic variations on complex natural products will be part of the arsenal of physicians. One has only to look at the extremely elegant syntheses of complex natural products reported recently by Baran and his co-workers to visualize the potential of coupling very active and interesting natural products with the skills of synthetic chemists in academia and industry.121-124 Also of great significance are the modeling of reactions based on Nature such as those described recently by Furst and Stephenson.125 Further examples of where selective modification via synthesis of very active peptidic-based molecules can also be seen from the recent paper by Luesch's group on improvements of the in vivo antitumor activity of the apratoxins, molecules produced by cyanobacteria.126
It is often not appreciated that the major hurdle in bringing a totally synthetic complex molecule to market, is not the basic synthesis but the immense problems faced by process chemists in translating research laboratory discoveries to commercial items.127,128 In the case of eribulin, the process chemistry group utilized selective crystallization steps rather than chromatography in order to provide the intermediates and the final product itself.
In this review, as we stated in 2003 and 2007,2,3 we have yet again demonstrated that natural products play a dominant role in the discovery of leads for the development of drugs for the treatment of human diseases. As we mentioned in earlier articles, some of our colleagues argued (though not in press, only in personal conversations at various forums) that the introduction of categories such as S/NM and S*/NM is an overstatement of the role played by natural products in the drug discovery process. On the contrary, we would still argue that these further serve to illustrate the inspiration provided by Nature to receptive organic chemists in devising ingenious syntheses of structural mimics to compete with Mother Nature's longstanding substrates. Even if we discount these categories, the continuing and overwhelming contribution of natural products to the expansion of the chemotherapeutic armamentarium is clearly evident as demonstrated in Figures 5 and 6, and as we stated in our earlier papers, much of Nature's “treasure trove of small molecules” remains to be explored, particularly from the marine and microbial environments.
From the perspective of microbes and their role(s) as sources of novel bioactive entities, it is now becoming quite evident that there are molecules for which the production depends upon the interaction among organisms from similar and also at times, widely different taxa.129 Recent examples include activation of silent gene clusters in fungi,130 or the activations of natural product biosyntheses in Streptomyces by mycolic acid-containing bacteria,131 and the production of marine natural products via interactions between sponges and their associated microbes.132
Over the last few years, some data have been published indicating, but not as yet fully proving, that a number of fungi isolated from a significant number of different terrestrial plants may contain the full biosynthetic cluster for Taxol® production.133 The one piece missing in the biosynthetic process, the presence of the gene for taxadiene synthetase was identified but the production of the metabolite was not fully confirmed in the view of some.134,135 The possibilities relating to the production of this agent via fungi have been discussed recently by Flores-Bustamente et al.136 and recently further evidence of production from a Taxus globosa source was reported.137
A point emphasized in the review by Flores-Bustamente et al,136 is effectively the same as those made following the reports a few years ago of multiple unexpected (silent) gene clusters in Aspergillus nidulans by Bok et al.138 That work demonstrated that one has to be able to find the “genetic on-switch” to be able to obtain expression of such clusters outside of the host, as exemplified by further work from the Wisconsin group.139 Similarly, as recently demonstrated by the group from the Leibnitz Institute in Jena following full genomic analyses of interactions between Aspergillus nidulans and Streptomyces rapamycinicus, the majority of biosynthetic clusters are “silent” under normal laboratory growth conditions. The interaction between these two microbes switched on a previously unrecognized PKS cluster that encoded the production of orsellinic acid, its derivative lecanoric acid, and the cathepsin K inhibitors F-9775A and F-9775B.140 In addition to these papers, the reader's attention is also drawn to the excellent review article by Gunatilaka141 on this subject, which, since its publication in 2006, has been cited over 100 times to date with reports showing materials isolated from plant endophytes. As a result, investigators need to consider all possible routes to novel agents.
To us, a multidisciplinary approach to drug discovery, involving the generation of truly novel molecular diversity from natural product sources, combined with total and combinatorial synthetic methodologies, and including the manipulation of biosynthetic pathways, will continue to provide the best solution to the current productivity crisis facing the scientific community engaged in drug discovery and development.
Once more, as we stated in our 2003 and 2007 reviews,2,3 we strongly advocate expanding, not decreasing, the exploration of Nature as a source of novel active agents which may serve as the leads and scaffolds for elaboration into desperately needed efficacious drugs for a multitude of disease indications. A very recent commentary by Carter in the review journal, Natural Products Reports shows that such a realization might be closer than one may think.142
Supplementary Material
Footnotes
Dedicated to Dr. Gordon M. Cragg, Chief of the NCI's Natural Products Branch from 1989 to 2004, for his pioneering work on bioactive natural products and on a more personal note, for his advice, support and friendship to me (DJN) over the last twenty-plus years. May his advice and help continue for a long time into the future.
Supplementary Information Available. An Excel 2003 workbook with the full data sets is available free-of-charge via the Internet at http://pubs.acs.org
The opinions discussed in this review are those of the authors and are not necessarily those of the U.S. Government
References
- 1.Cragg GM, Newman DJ, Snader KM. J Nat Prod. 1997;60:52–60. doi: 10.1021/np9604893. [DOI] [PubMed] [Google Scholar]
- 2.Newman DJ, Cragg GM, Snader KM. J Nat Prod. 2003;66:1022–1037. doi: 10.1021/np030096l. [DOI] [PubMed] [Google Scholar]
- 3.Newman DJ, Cragg GM. J Nat Prod. 2007;70:461–477. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- 4.Cragg GM, Grothaus PG, Newman DJ. Chem Rev. 2009;109:3012–3043. doi: 10.1021/cr900019j. [DOI] [PubMed] [Google Scholar]
- 5.Newman DJ, Cragg GM. In: Functional Molecules from Natural Sources. Wrigley SK, Thomas R, Nicholson N, Bedford C, editors. RSC Publications; Cambridge, UK: 2010. pp. 3–36. [Google Scholar]
- 6.Newman DJ, Cragg GM. In: RSC Biomolecular Sciences No 18; Natural Product Chemistry for Drug Discovery. Buss AD, Butler MS, editors. Royal Society of Chemistry; Cambridge, UK: 2010. pp. 3–27. [Google Scholar]
- 7.Newman DJ. J Med Chem. 2008;51:2589–2599. doi: 10.1021/jm0704090. [DOI] [PubMed] [Google Scholar]
- 8.Pelish HE, Westwood NJ, Feng Y, Kirchausen T, Shair MD. J Am Chem Soc. 2001;123:6740–6741. doi: 10.1021/ja016093h. [DOI] [PubMed] [Google Scholar]
- 9.Spring DR. Org Biomol Chem. 2003;1:3867–3870. doi: 10.1039/b310752n. [DOI] [PubMed] [Google Scholar]
- 10.Burke MD, Schreiber SL. Angew Chem Int Ed. 2004;43:46–58. doi: 10.1002/anie.200300626. [DOI] [PubMed] [Google Scholar]
- 11.Zhonghong G, Reddy PT, Quevillion S, Couve-Bonnaire S, Ayra PA. Angew Chem Int Ed. 2005;44:1366–1368. doi: 10.1002/anie.200462298. [DOI] [PubMed] [Google Scholar]
- 12.Dandapani S, Marcaurelle LA. Curr Opin Chem Biol. 2010;14:362–370. doi: 10.1016/j.cbpa.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 13.Reayi A, Arya P. Curr Opin Chem Biol. 2005;9:240–247. doi: 10.1016/j.cbpa.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 14.Lipinski CA. Drug Discov Today: Technol. 2004;1:337–341. doi: 10.1016/j.ddtec.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Macarron R. Drug Discov Today. 2006;11:277–279. doi: 10.1016/j.drudis.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Keller TH, Pichota A, Yin Z. Curr Opin Chem Biol. 2006;10:357–361. doi: 10.1016/j.cbpa.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 17.Drewry DH, Macarron R. Curr Opin Chem Biol. 2010;14:289–298. doi: 10.1016/j.cbpa.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 18.Macarron R, Banks MN, Bojanic D, Burns DJ, Cirovic DA, Garyantes T, Green DVS, Hertzberg RP, Janzen WP, Paslay JW, Schopfer U, Sittampalam GS. Nat Rev Drug Discov. 2011;10:188–195. doi: 10.1038/nrd3368. [DOI] [PubMed] [Google Scholar]
- 19.Jenkins ID, Lacrampe F, Ripper J, Alcaraz L, Le PV, Nikolakopoulos G, de Almeida Leone P, White RH, Quinn RJ. J Org Chem. 2009;74:1304–1313. doi: 10.1021/jo802456w. [DOI] [PubMed] [Google Scholar]
- 20.Danishefsky S. Nat Prod Rep. 2010;27:1114–1116. doi: 10.1039/c003211p. [DOI] [PubMed] [Google Scholar]
- 21.Bauer RA, Wurst JM, Tan DS. Curr Opin Chem Biol. 2010;14:308–314. doi: 10.1016/j.cbpa.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moura-Letts G, DiBlasi CM, Bauer RA, Tan DS. Proc Nat Acad Sci USA. 2011;108:6745–6750. doi: 10.1073/pnas.1015268108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kombarov R, Altieri A, Genis D, Kirpichenok M, Kochubey V, Rakitina N, Titarenko Z. Mol Divers. 2010;14:193–200. doi: 10.1007/s11030-009-9157-5. [DOI] [PubMed] [Google Scholar]
- 24.Allen RC. In: Annual Reports in Medicinal Chemistry. Bailey DM, editor. Vol. 19. Academic Press; Orlando: 1984. pp. 313–326. [Google Scholar]
- 25.Allen RC. In: Annual Reports in Medicinal Chemistry. Bailey DM, editor. Vol. 20. Academic Press; Orlando: 1985. pp. 315–325. [Google Scholar]
- 26.Allen RC. In: Annual Reports in Medicinal Chemistry. Bailey DM, editor. Vol. 21. Academic Press; Orlando: 1986. pp. 323–335. [Google Scholar]
- 27.Allen RC. In: Annual Reports in Medicinal Chemistry. Bailey DM, editor. Vol. 22. Academic Press; Orlando: 1987. pp. 315–330. [Google Scholar]
- 28.Ong HH, Allen RC. In: Annual Reports in Medicinal Chemistry. Allen RC, editor. Vol. 23. Academic Press; San Diego: 1988. pp. 325–348. [Google Scholar]
- 29.Ong HH, Allen RC. In: Annual Reports in Medicinal Chemistry. Allen RC, editor. Vol. 24. Academic Press; San Diego: 1989. pp. 295–315. [Google Scholar]
- 30.Ong HH, Allen RC. In: Annual Reports in Medicinal Chemistry. Bristol JA, editor. Vol. 25. Academic Press; San Diego: 1990. pp. 309–322. [Google Scholar]
- 31.Strupczewski JD, Ellis DB, Allen RC. In: Annual Reports in Medicinal Chemistry. Bristol JA, editor. Vol. 26. Academic Press; San Diego: 1991. pp. 297–313. [Google Scholar]
- 32.Strupczewski JD, Ellis DB. In: Annual Reports in Medicinal Chemistry. Bristol JA, editor. Vol. 27. Academic Press; San Diego: 1992. pp. 321–337. [Google Scholar]
- 33.Strupczewski JD, Ellis DB. In: Annual Reports in Medicinal Chemistry. Bristol JA, editor. Vol. 28. Academic Press; San Diego: 1993. pp. 325–341. [Google Scholar]
- 34.Cheng XM. In: Annual Reports in Medicinal Chemistry. Bristol JA, editor. Vol. 29. Academic Press; San Diego: 1994. pp. 331–354. [Google Scholar]
- 35.Cheng XM. In: Annual Reports in Medicinal Chemistry. Bristol JA, editor. Vol. 30. Academic Press; San Diego: 1995. pp. 295–317. [Google Scholar]
- 36.Cheng XM. In: Annual Reports in Medicinal Chemistry. Bristol JA, editor. Vol. 31. Academic Press; San Diego: 1996. pp. 337–355. [Google Scholar]
- 37.Galatsis P. In: Annual reports in Medicinal Chemistry. Bristol JA, editor. Vol. 32. Academic Press; San Diego: 1997. pp. 305–326. [Google Scholar]
- 38.Galatsis P. In: Annual Reports in Medicinal Chemistry. Bristol JA, editor. Vol. 33. Academic Press; San Diego: 1998. pp. 327–353. [Google Scholar]
- 39.Gaudilliere B. In: Annual Reports in Medicinal Chemistry. Doherty AM, editor. Vol. 34. Academic Press; San Diego: 1999. pp. 317–338. [Google Scholar]
- 40.Gaudilliere B, Berna P. In: Annual Reports in Medicinal Chemistry. Doherty AM, editor. Vol. 35. Academic Press; San Diego: 2000. pp. 331–355. [Google Scholar]
- 41.Gaudilliere B, Bernardelli P, Berna P. In: Annual Reports in Medicinal Chemistry. Doherty AM, editor. Vol. 36. Academic Press; San Diego: 2001. pp. 293–318. [Google Scholar]
- 42.Bernardelli P, Gaudilliere B, Vergne F. In: Annual Reports in Medicinal Chemistry. Doherty AM, editor. Vol. 37. Academic Press; Amsterdam: 2002. pp. 257–277. [Google Scholar]
- 43.Boyer-Joubert C, Lorthiois E, Moreau F. In: Annual Reports in Medicinal Chemistry. Doherty AM, editor. Vol. 38. Academic Press; Amsterdam: 2003. pp. 347–374. [Google Scholar]
- 44.Hegde S, Carter J. In: Annual Reports in Medicinal Chemistry. Doherty AM, editor. Vol. 39. Academic Press; Amsterdam: 2004. pp. 337–368. [Google Scholar]
- 45.Hegde S, Schmidt M. In: Annual Reports in Medicinal Chemistry. Doherty AM, editor. Vol. 40. Academic Press; Amsterdam: 2005. pp. 443–473. [Google Scholar]
- 46.Hegde S, Schmidt M. In: Annual Reports in Medicinal Chemistry. Wood A, editor. Vol. 40. Academic Press; Amsterdam: 2006. pp. 439–477. [Google Scholar]
- 47.Hegde S, Schmidt M. In: Annual Reports in Medicinal Chemistry. Macor JE, editor. Vol. 42. Academic Press; Amsterdam: 2007. pp. 505–554. [Google Scholar]
- 48.Hegde S, Schmidt M. In: Annual Reports in Medicinal Chemistry. Macor JE, editor. Vol. 43. Academic Press; Amsterdam: 2008. pp. 455–497. [Google Scholar]
- 49.Hegde S, Schmidt M. In: Annual Reports in Medicinal Chemistry. Macor JE, editor. Vol. 44. Academic Press; Amsterdam: 2009. pp. 577–632. [Google Scholar]
- 50.Hegde S, Schmidt M. In: Annual Reports in Medicinal Chemistry. Macor JE, editor. Vol. 45. Academic Press; Amsterdam: 2010. pp. 467–537. [Google Scholar]
- 51.Prous JR. Drug News Perspect. 1990;3:19–29. [PubMed] [Google Scholar]
- 52.Prous JR. Drug News Perspect. 1991;4:96–109. [Google Scholar]
- 53.Prous JR. Drug News Perspect. 1992;5:93–101. [Google Scholar]
- 54.Prous JR. Drug News Perspect. 1993;6:95–106. [Google Scholar]
- 55.Prous JR. Drug News Perspect. 1994;7:26–36. [PubMed] [Google Scholar]
- 56.Prous JR. Drug News Perspect. 1995;8:24–37. doi: 10.1358/dnp.2005.18.8.953409. [DOI] [PubMed] [Google Scholar]
- 57.Prous JR. Drug News Perspect. 1996;9:19–32. [PubMed] [Google Scholar]
- 58.Graul AI. Drug News Perspect. 1997;10:5–18. [PubMed] [Google Scholar]
- 59.Graul AI. Drug News Perspect. 1998;11:15–32. [PubMed] [Google Scholar]
- 60.Graul AI. Drug News Perspect. 1999;12:27–43. [Google Scholar]
- 61.Graul AI. Drug News Perspect. 2000;13:37–53. [PubMed] [Google Scholar]
- 62.Graul AI. Drug News Perspect. 2001;14:12–31. [PubMed] [Google Scholar]
- 63.Graul AI. Drug News Perspect. 2002;15:29–43. [PubMed] [Google Scholar]
- 64.Graul AI. Drug News Perspect. 2003;16:22–39. [PubMed] [Google Scholar]
- 65.Graul AI. Drug News Perspect. 2004;17:43–57. [PubMed] [Google Scholar]
- 66.Graul AI, Prous JR. Drug News Perspect. 2005;18:21–36. [PubMed] [Google Scholar]
- 67.Graul AI, Prous JR. Drug News Perspect. 2006;19:33–53. [PubMed] [Google Scholar]
- 68.Graul AI, Sorbera LA, Bozzo J, Serradell N, Revel L, Prous JR. Drug News Perspect. 2007;20:17–44. [PubMed] [Google Scholar]
- 69.Graul AI, Prous JR, Barrionuevo M, Bozzo J, Castañer R, Cruces E, Revel L, Rosa E, Serradell N, Sorbera LA. Drug News Perspect. 2008;21:7–35. [PubMed] [Google Scholar]
- 70.Graul AI, Revel L, Barrionuevo M, Cruces E, Rosa E, Vergés C, Lupone B, Diaz N, Castañer R. Drug News Perspect. 2009;22:7–27. doi: 10.1358/dnp.2009.22.1.1303754. [DOI] [PubMed] [Google Scholar]
- 71.Graul AI, Sorbera LA, Pina P, Tell M, Cruces E, Rosa E, Stringer M, Castañer R, Revel L. Drug News Perspect. 2010;23:7–36. doi: 10.1358/dnp.2010.23.1.1440373. [DOI] [PubMed] [Google Scholar]
- 72.Newman DJ, Cragg GM, O'Keefe BR. In: Modern Biopharmaceuticals, Design, Development and Optimization. Knablein J, editor. Vol. 2. Wiley-VCH; Weinheim: 2005. pp. 451–496. [Google Scholar]
- 73.Boyd MR. In: Current Therapy in Oncology. Neiderhuber J, editor. Decker; Philadelphia: 1993. pp. 11–22. [Google Scholar]
- 74.Sweetman SC. Martindale, The Complete Drug Reference. 33. The Pharmaceutical Press; London: 2002. [Google Scholar]
- 75.Hruby VJ. Nature Rev Drug Disc. 2002;1:847–858. doi: 10.1038/nrd939. [DOI] [PubMed] [Google Scholar]
- 76.Hruby VJ. J Org Chem. 2009;74:9245–9264. doi: 10.1021/jo901767e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Audie J, Boyd C. Curr Pharm Des. 2010;16:567–582. doi: 10.2174/138161210790361425. [DOI] [PubMed] [Google Scholar]
- 78.Vanhee P, van der Sloot AM, Verschueren E, Serrano L, Rousseau F, Schymkowitz J. Trends Biotech. 2011;29:231–239. doi: 10.1016/j.tibtech.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 79.Wan Y, Wallinder C, Plouffe B, Beaudry H, Mahalingam AK, Wu X, Johansson B, Holm M, Botros M, Karlen A, Petterson A, Nyberg F, Fandricks L, Gallo-Payet N, Hallberg A, Alterman M. J Med Chem. 2004;47:5995–6908. doi: 10.1021/jm049715t. [DOI] [PubMed] [Google Scholar]
- 80.Georgsson J, Rosenstrom U, Wallinder C, Beaudry H, Plouffe B, Lindeberg G, Botros M, Nyberg F, Karlen A, Gallo-Payet N, Hallberg A. Bioorg Med Chem. 2006;14:5963–5972. doi: 10.1016/j.bmc.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 81.Georgsson J, Sköld C, Botros M, Lindeberg G, Nyberg F, Karlén A, Hallberg A, Larhed M. J Med Chem. 2007;50:1711–1715. doi: 10.1021/jm0613469. [DOI] [PubMed] [Google Scholar]
- 82.Jones ES, Vinh A, McCarthy CA, Gaspari TA, Widdop RE. Pharmacol Ther. 2008;120:292–316. doi: 10.1016/j.pharmthera.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Newman DJ, Cragg GM, Snader KM. Nat Prod Rep. 2000;17:215–234. doi: 10.1039/a902202c. [DOI] [PubMed] [Google Scholar]
- 84.Breinbauer R, Manger M, Scheck M, Waldmann H. Curr Med Chem. 2002;9:2129–2145. doi: 10.2174/0929867023368773. [DOI] [PubMed] [Google Scholar]
- 85.Breinbauer R, Vetter IR, Waldmann H. Angew Chem Int Ed. 2002;41:2878–2890. doi: 10.1002/1521-3773(20020816)41:16<2878::AID-ANIE2878>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 86.Kingston DGI, Newman DJ. Curr Opin Drug Discov Devel. 2002;5:304–316. [PubMed] [Google Scholar]
- 87.Newman DJ, Cragg GM, Holbeck S, Sausville EA. Curr Cancer Drug Targ. 2002;2:279–308. doi: 10.2174/1568009023333791. [DOI] [PubMed] [Google Scholar]
- 88.Nielsen J. Curr Opin Chem Biol. 2002;6:297–305. doi: 10.1016/s1367-5931(02)00330-7. [DOI] [PubMed] [Google Scholar]
- 89.Perez JJ, Corcho F, Llorens O. Curr Med Chem. 2002;9:2209–2229. doi: 10.2174/0929867023368683. [DOI] [PubMed] [Google Scholar]
- 90.van Huijsduijnen RH, Bombrun A, Swinnen D. Drug Discov Today. 2002;7:1013–1019. doi: 10.1016/s1359-6446(02)02438-8. [DOI] [PubMed] [Google Scholar]
- 91.Barun O, Sommer S, Waldmann H. Angew Chem Int Ed. 2004;43:3195–3199. doi: 10.1002/anie.200353609. [DOI] [PubMed] [Google Scholar]
- 92.Balamurugan R, Dekker FJ, Waldmann H. Mol BioSyst. 2005;1:36–45. doi: 10.1039/b503623b. [DOI] [PubMed] [Google Scholar]
- 93.Ganesan A. Curr Opin Biotech. 2004;15:584–590. doi: 10.1016/j.copbio.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 94.Ganesan A. Curr Opin Chem Biol. 2006;12:306–317. doi: 10.1016/j.cbpa.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 95.Shang S, Tan DS. Curr Opin Chem Biol. 2005;9:248–258. doi: 10.1016/j.cbpa.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 96.Costantino L, Barlocco D. Curr Med Chem. 2006;13:65–85. [PubMed] [Google Scholar]
- 97.Bade R, Chan HF, Reynisson J. Eur J Med Chem. 2010;45:5645–5652. doi: 10.1016/j.ejmech.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 98.Violette A, Fournel S, Frisch B, Briand JP, Monteil H, Guichard G. Chem Biol. 2006;13:531–538. doi: 10.1016/j.chembiol.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 99.Grienke U, Schmidtke M, von Grafenstein S, Kirchmair J, Liedl KR, Rollinger JM. Nat Prod Rep. 2011;29:11–36. doi: 10.1039/c1np00053e. [DOI] [PubMed] [Google Scholar]
- 100.Graul AI, Leeson PA, Castañer J. Drugs Fut. 1999;24:1189–1202. [Google Scholar]
- 101.Urakami K, Zangiacomi V, Yamaguchi K, Kusuhara M. Biomed Res. 2010;31:161–163. doi: 10.2220/biomedres.31.161. [DOI] [PubMed] [Google Scholar]
- 102.Krämer M, Bongaerts J, Bovenberg R, Kremer S, Müller U, Orf S, Wubbolts M, Raeven L. Metab Eng. 2003;5:277–283. doi: 10.1016/j.ymben.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 103.Johansson L, Lindskog A, Silfversparre G, Cimander C, Nielsen KF, Lidén G. Biotechnol Bioeng. 2005;92:541–552. doi: 10.1002/bit.20546. [DOI] [PubMed] [Google Scholar]
- 104.Carter SK, Bakowski MT, Hellmann K. Chemotherapy of Cancer. Wiley; New York: 1977. p. 350. [Google Scholar]
- 105.Cole WH. Chemotherapy of Cancer. Lea and Febiger; Philadelphia: 1970. p. 349. [Google Scholar]
- 106.Scheinfeld N. Drugs Fut. 2008;33:27–30. [Google Scholar]
- 107.Ueda H, Nakajima H, Hori Y, Fujita T, Nishimura M, Goto T, Okuhara M. J Antibiot. 1994;47:301–310. doi: 10.7164/antibiotics.47.301. [DOI] [PubMed] [Google Scholar]
- 108.Ueda H, Nakajima H, Hori Y, Goto T, Okuhara M. Biosci Biotech Biochem. 1994;58:1579–1583. doi: 10.1271/bbb.58.1579. [DOI] [PubMed] [Google Scholar]
- 109.Wang HCR. Drugs Fut. 1999;24:1184–1188. [Google Scholar]
- 110.VanderMolen KM, McCulloch W, Pearce CJ, Oberlies NH. J Antibiot. 2011;64:525–531. doi: 10.1038/ja.2011.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Smith JA, Wilson L, Azarenko O, Zhu X, Lewis BM, Littlefield BA, Jordan MA. Biochemistry. 2010;49:1331–1337. doi: 10.1021/bi901810u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bai R, Nguyen TL, Burnett JC, Atasoylu O, Munro MHG, Pettit GR, Smith AB, III, Gussio R, Hamel E. J Chem Inf Model. 2011;51:1393–1404. doi: 10.1021/ci200077t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Meyers PA, Schwartz CL, Krailo MD, Healey JH, Bernstein ML, Betcher D, Ferguson WS, Gebhardt MC, Goorin AM, Harris M, Kleinerman E, Link MP, Nadel H, Nieder M, Siegal GP, Weiner MA, Wells RJ, Womer RB, Grier HE. J Clin Oncol. 2008;26:633–668. doi: 10.1200/JCO.2008.14.0095. [DOI] [PubMed] [Google Scholar]
- 114.Iwazawa J, Ohue S, Yasumasa K, Mitani T. World J Radiol. 2010;2:468–471. doi: 10.4329/wjr.v2.i12.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gallwitz B. Drugs Fut. 2008;33:13–20. [Google Scholar]
- 116.Marre M, Penfornis A. Diabetes Res Clin Pract. 2011;93:317–327. doi: 10.1016/j.diabres.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 117.Brinkmann V, Billich A, Baumruker T, Heining P, Schmouder R, Francis G, Aradhye S, Burtin P. Nat Rev Drug Discov. 2010;9:883–897. doi: 10.1038/nrd3248. [DOI] [PubMed] [Google Scholar]
- 118.Strader CR, Pearce CJ, Oberlies NH. J Nat Prod. 2011;74:900–907. doi: 10.1021/np2000528. [DOI] [PubMed] [Google Scholar]
- 119.Pchejetski D, Bohler T, Brizuela L, Sauer L, Doumerc N, Golzio M, Salunkhe V, Teissié J, Malavaud B, Waxman J, Cuvillier O. Cancer Res. 2010;70:8651–8661. doi: 10.1158/0008-5472.CAN-10-1388. [DOI] [PubMed] [Google Scholar]
- 120.Wilson RM, Danishefsky SJ. Acc Chem Res. 2006;39:539–549. doi: 10.1021/ar068018n. [DOI] [PubMed] [Google Scholar]
- 121.Newhouse T, Baran PS, Hoffmann RW. Chem Soc Rev. 2009;38:3010–3021. doi: 10.1039/b821200g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shi J, Manolikakes G, Yeh CH, Guerrero CA, Shenvi RA, Shigehisa H, Baran PS. J Amer Chem Soc. 2011;133:8014–8027. doi: 10.1021/ja202103e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Su S, Rodriguez RA, Baran PS. J Amer Chem Soc. 2011;133:13922–13925. doi: 10.1021/ja206191g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Seiple IB, Su S, Young IS, Nakamura A, Yamaguchi J, Jørgensen L, Rodriguez RA, O'Malley DP, Gaich T, Kock M, Baran PS. J Amer Chem Soc. 2011;133:14710–14726. doi: 10.1021/ja2047232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Furst L, Stephenson CRJ. Nat Chem Biol. 2011;7:582–683. doi: 10.1038/nchembio.637. [DOI] [PubMed] [Google Scholar]
- 126.Chen QY, Liu Y, Luesch H. ACS Med Chem Lett. 2011;2:861–865. doi: 10.1021/ml200176m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jackson KL, Henderson JA, Phillips AJ. Chem Rev. 2009;109:3044–3079. doi: 10.1021/cr900016w. [DOI] [PubMed] [Google Scholar]
- 128.Huyck TK, Gradishar W, Manuguid F, Kirkpatrick P. Nat Rev Drug Discov. 2011;10:173–174. doi: 10.1038/nrd3389. [DOI] [PubMed] [Google Scholar]
- 129.Crawford JM, Clardy J. Chem Comm. 2011;47:7559–7566. doi: 10.1039/c1cc11574j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Brakhage AA, Schroeckh V. Fung Genet Biol. 2011;48:15–22. doi: 10.1016/j.fgb.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 131.Onaka H, Mori Y, Igarashi Y, Furumai T. Appl Environ Microbiol. 2011;77:400–406. doi: 10.1128/AEM.01337-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Thomas TRA, Kavlekar DP, LokaBharathi PA. Mar Drugs. 2010;8:1417–1468. doi: 10.3390/md8041417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kumaran RS, Hur BK. Biotechnol Appl Biochem. 2009;54:21–30. doi: 10.1042/BA20080110. [DOI] [PubMed] [Google Scholar]
- 134.Staniek A, Woerdenbag HJ, Kayser O. Planta Med. 2009;75:1561–1566. doi: 10.1055/s-0029-1186181. [DOI] [PubMed] [Google Scholar]
- 135.Staniek A, Woerdenbag HJ, Kayser O. J Plant Interact. 2010;5:189–195. [Google Scholar]
- 136.Flores-Bustamante ZR, Rivera-Ordũa FN, Martínez-Cárdenas A, Flores-Cotera LB. J Antibiot. 2010;63:460–467. doi: 10.1038/ja.2010.83. [DOI] [PubMed] [Google Scholar]
- 137.Soca-Chafre G, Rivera-Orduña FN, Hidalgo-Lara ME, Hernandez-Rodriguez C, Marsch R, Flores-Cotera LB. Fung Biol. 2011;115:143–156. doi: 10.1016/j.funbio.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 138.Bok JW, Hoffmeister D, Maggio-Hall LA, Murillo R, Glasner JD, Keller NP. Chem Biol. 2006;13:31–37. doi: 10.1016/j.chembiol.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 139.Giles SS, Soukup AA, Lauer C, Shaaban M, Lin A, Oakley BR, Wang CCC, Keller NP. Appl Environ Microbiol. 2011;77:3669–3675. doi: 10.1128/AEM.02000-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nützmann HW, Reyes-Dominguez Y, Scherlach K, Schroeckh V, Horn F, Gacek A, Schümann J, Hertweck C, Strauss J, Brakhage AA. Proc Natl Acad Sci USA. 2011;108:14282–14287. doi: 10.1073/pnas.1103523108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gunatilaka AAL. J Nat Prod. 2006;69:509–526. doi: 10.1021/np058128n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Carter GT. Nat Prod Rep. 2011;28:1783–1789. doi: 10.1039/c1np00033k. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


