Abstract
We investigated the role of two key immunoregulatory molecules, indoleamine dioxygenase (IDO) and inducible costimulator ligand (ICOSL), in determining the function of bone marrow (BM)-derived plasmacytoid (p)DC, that offer potential for therapy of allograft rejection. pDC generated from BM of wild-type (WT) or IDO knockout (KO) C57BL/6 mice were used to stimulate T cell proliferation and IFNγ production in response to alloAg via the direct or indirect pathways. In some experiments, pDC were first activated by exposure to CpG ± CTLA4Ig for IDO induction via B7 ligation. Although IDO KO pDC induced enhanced T cell responses compared to WT pDC, use of the IDO inhibitor 1-methyl-tryptophan (1-MT) showed that the inferior stimulatory capacity of WT pDC was not due to production of functional IDO, even under IDO-inducing conditions. DNAX-activating protein of 12kDa (DAP12), that inhibits functional IDO expression, was expressed in BM-pDC. DAP12 silencing increased the T cell stimulatory capacity of WT pDC, but only in the presence of 1-MT. Compared with WT pDC, activated IDO KO DC expressed much lower levels of ICOS-L. Moreover, when ICOSL was blocked on WT pDC, T cell proliferation resembled that induced by IDO KO pDC, and IL-10 secretion in MLR was markedly decreased. These findings implicate ICOSL-induced IL-10, but not IDO in regulation of BM-derived pDC function.
Keywords: Dendritic cells, indoleamine dioxygenase, T cells, DNAX-activating protein of 12kDA, inducible costimulator ligand
1. Introduction
Dendritic cells (DC) are rare, yet ubiquitous, bone marrow (BM)-derived antigen (Ag)-presenting cells (APC), that induce and regulate innate and adaptive immunity [1-3]. Two principal DC subsets have been identified,- ’conventional’ myeloid DC (mDC; CD11c+CD123− in humans; CD11c+ CD8α− CD11b+ in mice) and plasmacytoid DC (pDC; CD11c−CD123+ in humans; CD11cintermediateB220+ in mice [4, 5]). Both subsets exhibit immunostimulatory [3, 4, 6, 7] and tolerogenic [8-10] functions, that reflect their site of origin/isolation, Ag-presenting properties [11], exposure to specific stimuli/inhibitors, and/or their maturation state at the time of T cell interaction.
Following viral stimulation, pDC rapidly produce large amounts of type-1 interferons (IFNs), that activate conventional DC to prime virus-specific T cells [12]. However, pDC can also regulate autoimmune reactivity [13, 14], impede anti-tumor responses [15], mediate oral tolerance [16] and suppress allograft rejection [17-19] and acute graft-versus-host disease [10]. Thus, infusion of donor BM-derived pDC can prolong murine heart allograft survival in an Ag-independent manner [17], while pDC that have acquired alloAg and migrated to host lymphoid tissue induce alloAg-specific Treg and promote transplant tolerance [18]. Understanding the mechanisms that underlie these observations is key to therapeutic targeting of pDC and the potential use of in vitro-propagated BM-derived pDC (BM-pDC) in cell-based therapies.
The inducible costimulator (ICOS)/ICOS ligand (L) (B7RP-1) costimulatory pathway has been implicated in the regulation of T cell responses initiated by pDC [20]. While ICOS-ICOSL interaction stimulates T cells in the context of mDC [21], ICOSL expression on human pDC correlates with diminished T cell responsiveness and increased IL-10 production [20]. ICOS-ICOSL interaction has also recently been shown to expand T helper cell type-2 (Th2) immunity [22] and the size of Foxp3+ regulatory T cell (Treg) and CD62LloCD44hi effector-memory CD4+ T cell populations via modulation of DC maturation [23].
The comparatively poor allostimulatory capacity of BM-pDC may also be regulated by expression of indoleamine dioxygenase (IDO). It is well-recognized, however, that functional expression of IDO by DC subsets (elicited by either cytotoxic T lymphocyte Ag [CTLA4]/CTLA4Ig [24, 25], IFNγ [26, 27] or CD200Ig [28]) results in the breakdown of tryptophan, the rarest essential amino acid, necessary for T cell proliferation. Tryptophan catabolism induces activated T cell apoptosis [26, 29], suppresses T cell proliferation in response to self-peptide in delayed-type hypersensitivity reactions [30, 31] or in mixed leukocyte reactions (MLR) [24], and activates Treg [32]. IDO expression by physiologic DC from mouse spleen is tightly regulated, and is responsive to IFN type I and type II in a rare but distinctive subset of pDCs [24][33].
The role of ICOSL or IDO in the regulation of T cell reactivity initiated by in vitro-propagated BM-pDC has not been evaluated. Our aim was to investigate the roles of these molecules in regulation of alloreactive T cell proliferation and cytokine production by mouse BM-pDC. The findings suggest that IDO expression by BM-pDC is negatively regulated by DNAX-activating protein of 12kDA (DAP12), a transmembrane signaling adapter that inhibits IDO in murine CD8α+ DC [34]. By contrast, the comparatively poor T cell allostimulatory capacity of WT BM-pDC appears to reflect their expression of ICOSL.
2. Materials and methods
2.1. Animals
Six- to eight-week-old C57BL/6 (B6; H2b) and BALB/cByJ (BALB/c; H2d) mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and maintained in the specific pathogen-free Central Animal Facility of the University of Pittsburgh School of Medicine. C57BL/6 IDO−/− (IDO knockout [KO]; H2b) mice, generated at the Medical College of Georgia, Augusta, GA were bred at the University of Pittsburgh. Experiments were conducted under an Institutional Animal Care and Use Committee-approved protocol, and in accordance with National Institutes of Health guidelines. The animals were fed a diet of Purina rodent chow (Ralston Purina, St. Louis, MO) and received tap water ad libitum.
2.2. Media and Reagents
The DC poietin recombinant (r) human fms-like tyrosine kinase 3 ligand (Flt3L) (CHO cell–derived) was a gift from Amgen (Seattle, WA). Mouse recombinant (r) granulocyte-macrophage colong-stimulating factor (GM-CSF) and r human IL-4 were gifts from Schering-Plough (Kenilworth, NJ). Complete medium (CM) comprised RPMI-1640 (BioWhittaker, Walkersville, MD) supplemented with 10% v/v fetal calf serum (Nalgene, Miami, FL), non-essential amino acids, L-glutamine, sodium pyruvate, penicillin-streptomycin and 2-mercaptoethanol (all Life Technologies, Gaithersburg, MD). The Toll-like receptor (TLR) 9 ligand CpG-B, certified endotoxin-free, was obtained from Coley Pharmaceuticals (Wellesley, MA). Monoclonal antibodies (mAbs) used for flow cytometry were anti-CD11c (HL3, CyChrome-conjugated) (eBiosciences, San Diego, CA), anti-CD45R/B220 (RA3-6B2; FITC-conjugated), and PE-conjugated anti-IAb (AF6-120.1), anti-CD19 (1D3), and anti-CD86 (GL1) (BD PharMingen). PE-conjugated anti-B7-H1 (MIH5), anti-B7-DC (TY25), and anti-ICOSL (B7RP-1) (HK5.3), were from eBioscience. Isotype-matched control Igs were from BD PharMingen. CTLA4Ig was a gift from Bristol-Myers Squibb Pharmaceutical Research Institute (Candiac, Quebec, Canada).
2.3. Generation and purification of BM-derived pDC and mDC
DC were generated and purified from freshly-isolated BM as described for pDC and mDC [17, 35, 36], with minor modifications. Briefly, for pDC propagation, BM cells were cultured for 8 days in complete medium in 200 ng/ml Flt3L. On d 4, 50% of the supernatant was replaced with fresh, cytokine-containing medium. On d 8, the cells were enriched for B220+ cells by incubation with anti-mouse B220-coated immunomagnetic beads (Miltenyi Biotec, Auburn, CA), according to the manufacturer's instructions, for 15 min at 4°C, then positively-selected by passage through a paramagnetic column (Miltenyi Biotec), yielding a highly-enriched (>90%) CD11c+B220+ population. In some experiments, DC were then activated by overnight culture (18h) in GM-CSF (20 ng/ml) and CpG (2 μg/ml).
2.4. AlloAg pulsing of DC and MLR cultures
Pulsing of B6 DC with Ag was performed as described [37]. Briefly, bead-purified pDC were incubated with cell-free BALB/c splenocyte lysate at a DC:splenocyte equivalent ratio of 1:10 for 24 h at 37°C. Graded numbers of γ-irradiated (20 Gy) pDC (or control mDC) were then used as stimulators in 72 h MLR, with nylon-wool column-enriched normal syngeneic (B6) T cells as responders (105/ml) in 96-well, round-bottom plates, as described [38]. In allo-MLR, BALB/c T cells were used as responders. Where indicated, neutralizing anti-ICOSL mAb (10 μg/ml) was added to BM-pDC for 30 min prior to the addition of T cells. During the final 18 h, wells were pulsed with 1 μCi [3H]-thymidine (Perkin Elmer Life Sciences/NEN, Woodbridge, Ontario, Canada) and radioisotope incorporation determined using a β-scintillation counter. Results are expressed as mean c.p.m. ± 1 SD of 3-6 replicate wells. In some experiments, T cells were collected and stained for CD4 and intracellular Foxp3, as described [39].
2.5. Cytokine quantitation
IFNγ, IL-4, and IL-10 were quantified by enzyme-linked immunosorbent assay (ELISA) using commercial kits from Biolegend (San Diego, CA) and following the manufacturer's recommended procedures. The detection limits were 4 pg/ml for IFNγ and IL-4, and 30 pg/ml for IL-10.
2.6. 1-methyl-D-tryptophan (1-MT)
1-MT (Aldrich, Milwaukee, WI) was added to cultures at a final concentration of 100 or 200 μM , as described [25, 40].
2.7. Transfection of pDC with DAP12 siRNA and confirmation of gene silencing by RT-PCR
pDC were collected from 7- or 8- d BM cultures and plated at 5×105 cells/500μl CM overnight. They were then transfected using GeneSilencer siRNA Transfection Reagent (Genlantis, San Diego, CA) and following the manufacturer's instructions, with either DAP12 siRNA (400 μM) or Silencer Negative Control #1 (both from Ambion, Foster City, CA). After 6h, the cells were brought to 1.0 ml with CM. In some experiments, pDC were pulsed with alloAg, as described above. Twenty-four h after transfection, the pDC were collected for use in MLR, or to confirm efficient gene silencing by conventional RT-PCR for DAP12 (F: 5′-TGGTGCCTTCTGTTCCTTCC-3′; R: 5′-TTGTTTCCGGGTCCCTTCC-3′) [41] or β-actin (F: ATGGATGACGATATCGCT; R: ATGAGGTAGTCTGTCAGGT).
2.8. Statistical analysis
Statistical analysis was performed using the 2-tailed Student's ‘t’ test. Differences between groups were considered significant at a ‘p’ value of <0.05. Results are expressed as means ± 1 SD.
3. Results
3.1. IDO KO BM-pDC are more potent stimulators of naïve T cell proliferation and IFNγ production in response to alloAg than WT BM-pDC
DC do not express IDO constitutively and an exogenous signal such as IFN type I or type II is required to induce DC to acquire suppressive functions via IDO [25-27, 42]. Thus, in order to establish a baseline level of T cell proliferation and cytokine production in response to alloAg under non-IDO-inducing conditions, we cultured pDC generated from either IDO KO or WT mouse BM with normal T cells in either indirect or direct MLR (Fig. 1).
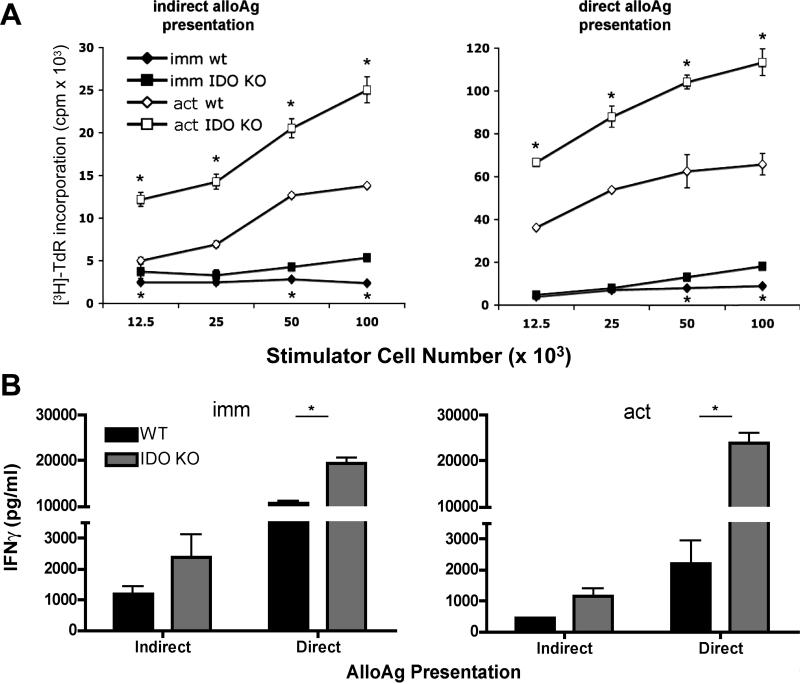
Fig. 1.
IDO KO BM- pDC are more stimulatory than IDO-competent DC via both the direct and indirect pathways of alloAg presentation. Immature and CpG-activated (act) B6 (H2b) IDO KO and WT pDC were pulsed overnight with cell-free BALB/c (H2d) splenocyte lysate, then cultured with syngeneic B6 normal T cells (indirect pathway) for 72h. Alternatively, unpulsed IDO KO and WT B6 DC were co-cultured with allogeneic BALB/c T cells (direct pathway). [3H] TdR was added during the last 18 h for quantification of T cell proliferation (counts per minute; cpm) (A) or supernatants were collected at 72 h for IFNγ quantification by ELISA (B). Results are from a single experiment, representative of at least three performed. Data are means ±1SD of triplicate values. * p < 0.05 between IDO KO and WT DC.
Unstimulated (immature; imm) or CpG-activated (‘act’) WT or IDO KO B6 (H2b) BM-pDC were co-cultured with normal allogeneic BALB/c (H2d) T cells (to assess their ability to induce T cell activation via the direct pathway of allorecognition) or with syngeneic B6 T cells after overnight pulsing of the DC with cell-free BALB/c splenocyte lysate, as described ([37]; indirect pathway). As expected, stimulation via the direct pathway induced higher levels of T cell proliferation than the indirect pathway, for either IDO KO or WT pDC (Fig. 1A). Also, as expected, in MLR with immature pDC, T cell proliferation was much lower than in co-cultures with CpG-activated pDC as stimulators. Interestingly, however, immature IDO KO pDC elicited higher levels of T cell proliferation and IFNγ secretion than WT pDC via the indirect and direct pathways (Fig. 1A & Fig. 1B, left panel). Following their activation, WT pDC remained inferior inducers of T cell proliferation and IFNγ production compared with IDO KO pDC (Fig. 1A and Fig. 1B, right panel).
3.2. Inhibitory effects of CTLA-4Ig on BM-pDC-induced T cell proliferation are independent of IDO
IDO does not exhibit tryptophan-catabolizing properties without prior exposure of DC to specific stimuli. Functional IDO production has been reported in murine CD8α+ DC [26, 30] and human monocyte-derived DC [27] following IFNγ stimulation, and in splenic pDC subsets after B7 ligation by either soluble CTLA4 [24, 43] or CTLA4 expressed on clonally-expanded Treg [24]. However, further analysis of splenic DC revealed that a rare pDC subset expressing the B cell marker CD19 was the only pDC subset capable of expressing IDO in response to CTLA4-Ig and CpG treatment [25, 33]. Because of the marked differences in normal T cell proliferation induced by activated IDO KO and WT pDC (Fig. 1A) in the absence of exogenous stimuli, we hypothesized that, in MLR, functional IDO might be induced in WT BM-pDC in an auto- or paracrine manner via secreted IFNγ (Fig. 1B), resulting in partial suppression of T cell activation. Therefore, we compared T cell proliferation in response to alloAg stimulation in the presence of the IDO inhibitor, 1-MT. However, despite significant differences in IDO-KO versus WT pDC-induced T cell proliferation, addition of 1-MT at the start of cultures did not significantly affect proliferative responses induced by alloAg-pulsed, CpG-activated WT pDC compared to control cultures without IDO (Fig. 2A).
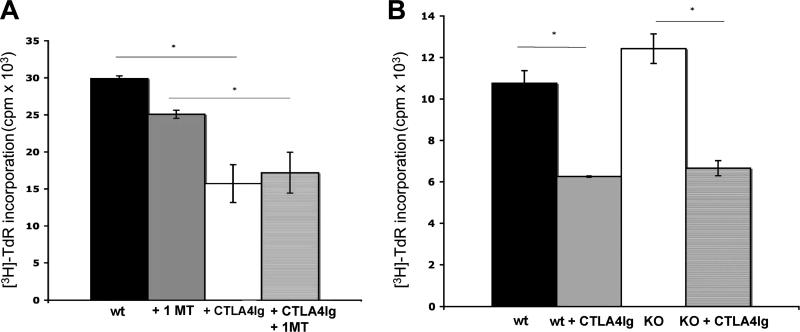
Fig. 2.
Inhibitory effects of CTLA4Ig on BM-pDC are independent of IDO. CpG-activated B6 (H2b) WT (A and B) or IDO KO (B) pDC were pulsed overnight with cell-free BALB/c (H2d) splenocyte lysate, with or without the IDO inhibitor 1-MT (A). After washing, the pDC were incubated with 40 μg/ml CTLA4Ig or control Ig, washed thoroughly, and cultured in 72h MLR with 105 syngeneic B6 normal T cells (1DC: 4 T cells) (indirect pathway). [3H] TdR was added for the last 18 h before quantification of T cell proliferation (cpm). Results are means ±1SD of triplicate cultures. *p<0.05; representative of at least 2 experiments with similar results.
It has been reported that spleen pDC produce functional IDO in response to CTLA4Ig exposure [44]. Therefore we exposed CpG-activated, alloAg-pulsed WT or IDO KO pDC to CTLA4Ig [100 μg/ml; [24]] for 24 h before co-culture with T cells. No differences in CD80/CD86 expression were evident after CTLA4Ig treatment. As expected, in MLR where WT pDC had been exposed to CTLA4Ig, T cell proliferation was reduced significantly (~40%) (Fig. 2B). 1-MT did not rescue T cell proliferation in CTLA4Ig-treated MLR cultures (Fig. 2A). Furthermore, in IDO KO pDC:normal T cell control MLR cultures, where the tryptophan catabolite could not be induced and in which the DC were incubated with CTLA4Ig before the MLR, a quantitatively similar, significant reduction in T cell proliferation was also observed (Fig. 2B).
3.3. BM-pDC express DAP12, a negative regulator of IDO
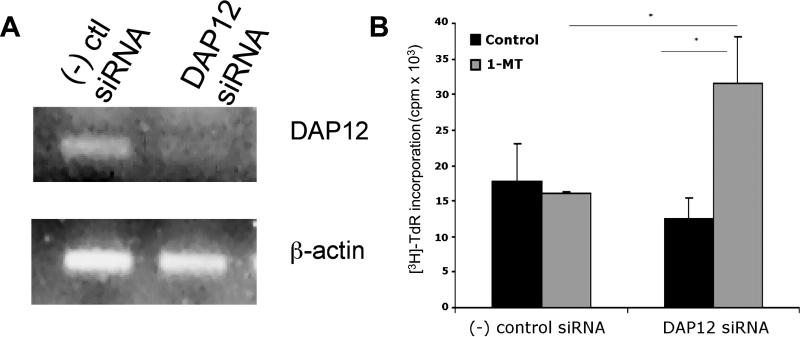
Collectively, the foregoing data suggested that the inferior allostimulatory capacity of WT compared to IDO KO BM-pDC reported in Fig 1 was independent of the induction of functional IDO. We hypothesized that WT BM-pDC might not produce functional IDO. Previous studies have suggested that expression of the immunoreceptor tyrosine-based activation motif-bearing adaptor molecule DAP12 is negatively associated with functional IDO expression [34, 45]. DAP12 RNA was readily detected in purified WT pDC by RT-PCR (Fig. 3A). We next evaluated the role of DAP12 in the regulation of T cell responses by BM-pDC. Silencing DAP12 did not alter surface MHC class II or CD86 expression (data not shown), nor did it affect alloAg-pulsed WT pDC-elicited T cell proliferation when compared to T cell proliferation elicited by WT pDC transfected with non-specific negative control siRNA (Fig. 3B). IFNγ production by T cells stimulated by IDO KO pDC was enhanced (Fig. 1B), suggesting that IFNγ may drive IDO production when DAP12 is silenced in BM-pDC. Consistent with this hypothesis, addition of 1-MT to DAP12-silenced WT pDC MLR resulted in a significant (> 2-fold) increase in T cell allostimulatory ability compared to the T cell allostimulatory ability of WT pDC transected with negative control siRNA (Fig. 3B), confirming that DAP12 silencing induced functional IDO in pDC. Using DAP12-silenced BM-pDC from IDO KO mice as stimulators in MLR also increased T cell proliferation and IFNγ production, though not as robustly as that shown in Fig. 3B (data not shown).
Fig. 3.
DAP12 expression regulates functional IDO production by BM-pDC. (A) Purified B6 (H2b) WT pDC were cultured overnight then transfected with either negative (-) control or DAP12 siRNA. After 6h, cells were washed. To verify DAP12 silencing, cells were collected and assessed by RT-PCR for expression of DAP12 after overnight culture. (B) Alternatively, 6 h after transfection, cells were pulsed overnight with cell-free BALB/c (H2d) splenocyte lysate. MLR were performed with 105 syngeneic normal T cells (1 DC: 4 T cells), either with or without 1-MT (200 mM). [3H] TdR was added during the last 18h. Results are means ± 1SD of triplicate values. *p<0.01; representative of 3 experiments, with similar results.
3.4. IDO-deficient BM-pDC express higher levels of CD86 and lower levels of ICOSL compared to WT BM-pDC
WT BM-pDC induced less IFNγ and lower levels of T cell proliferation compared to IDO KO BM-pDC. However, WT BM-pDC may fail to produce functional IDO due to expression of DAP12 (Fig. 3), suggesting that an alternative, IDO-independent mechanism may account for the differences observed between WT and IDO KO BM-pDC. Therefore, we next evaluated the expression of MHC class II and CD86, as well as other co-stimulatory/co-regulatory B7 family molecules, on WT and IDO KO BM-pDC.
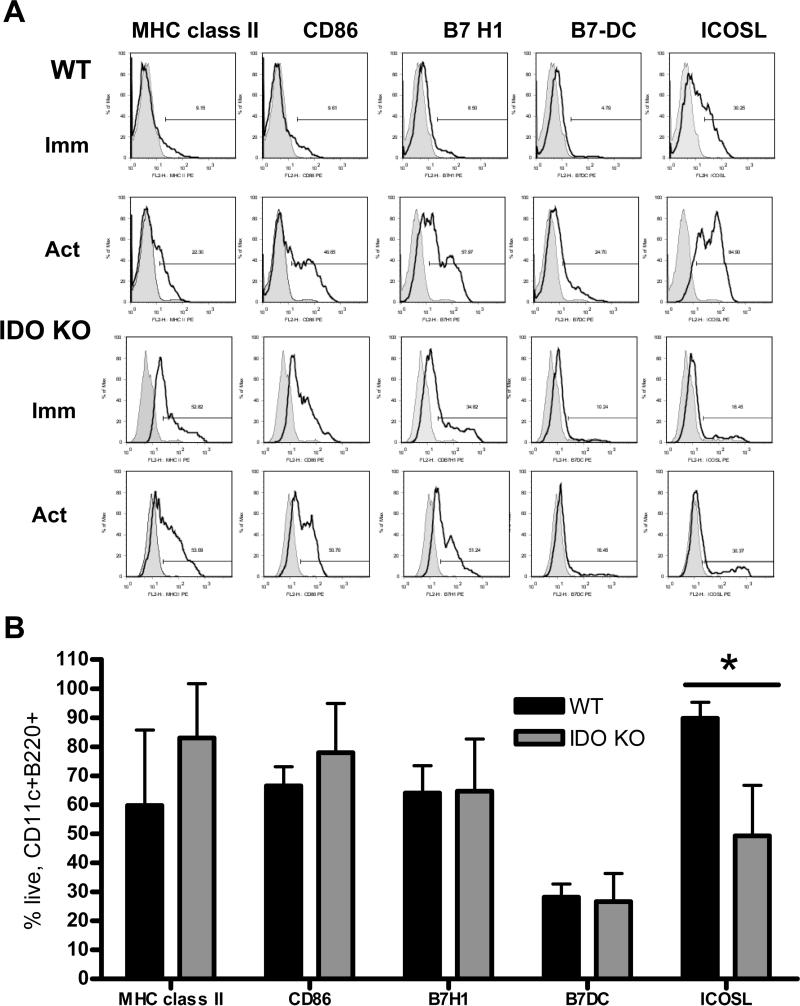
Flow analysis revealed that IDO-deficient pDC consistently expressed higher cell surface MHC class II (IAb) and CD86 (B7-2) (Fig. 4A) compared with WT pDC, especially in the absence of an activating stimulus (CpG). Of the additional B7 family molecules analyzed, including B7-H1 (PD-L1) and B7-DC, only ICOSL/(B7RP-1) expression differed significantly between WT and IDO KO pDC. While immature WT and IDO KO pDC expressed similar low levels of ICOSL, WT but not IDO KO pDC significantly (p<0.001) and markedly upregulated this co-regulatory molecule after CpG-induced activation (Figs. 4A & B).
Fig. 4. WT BM-pDC express elevated ICOSL compared to IDO KO pDC.
(A) BM-pDC were characterized by flow cytometric analysis, before (immature) and after 24 h exposure to CpG (activated; act). Cells were gated on CD11c and B220 (for immunobead-sorted pDC) and then stained for MHC class II, CD86 or B7-H1 (PD-L1), B7-DC (PD-L2) or ICOSL (open histograms). Closed histograms denote appropriate isotype control. Data are from 1 experiment representative of at least 3 performed with similar results. (B) Bars represent the means ± 1SD for live, CD11c+B220+ CpG-stimulated cells expressing the indicated markers from a minimum of 3 experiments. *p<0.01 comparing WT to IDO KO pDC.
3.4. Activated WT BM-pDC regulate T cell function through ICOS-ICOSL interaction
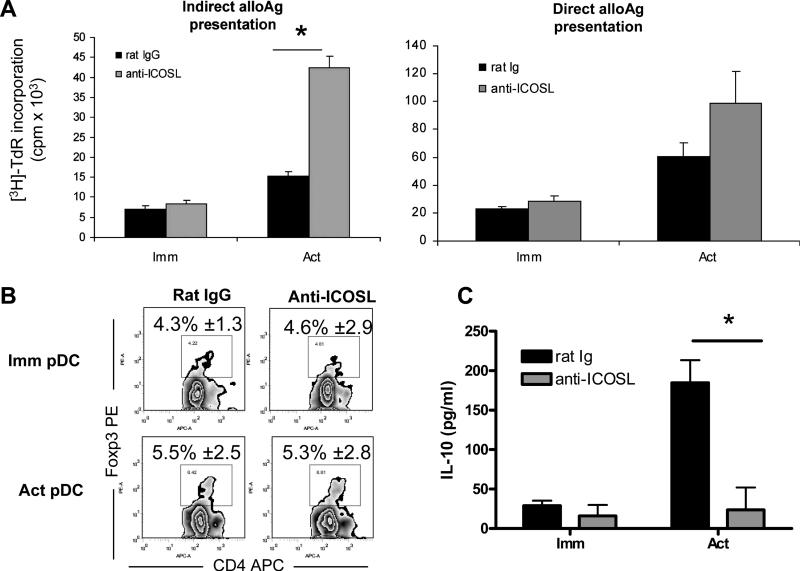
Human pDC can induce the secretion of IL-10 by T cells in an ICOS-ICOSL-dependent fashion [20]. Furthermore, expansion of Foxp3+Treg may rely on functional ICOS expression in vivo [23]. To ascertain the influence of ICOSL expression on CpG-activated WT BM-pDC allostimulatory capacity, neutralizing anti-ICOSL mAb was added to the pDC prior to their addition to T cells in direct or indirect MLR. A marked increase in T cell proliferation was observed when ICOSL was blocked on CpG-activated pDC; no significant effects were observed when ICOSL was blocked on immature pDC (Fig. 5A). To determine if blocking of the ICOS-ICOSL pathway affected Treg in pDC-induced MLR, we stained for intracellular Foxp3, a marker for mouse Treg. In this experiment, only the direct pathway was utilized to amplify any changes that might occur. The size of the CD4+Foxp3+ population was unaffected when ICOSL Ab was added at the start of the MLR, using either immature or mature WT pDC as stimulators (Fig. 5B). Next, the levels of IL-10 in MLR cultures were determined by ELISA. As shown in Fig. 5C, ICOSL neutralization markedly reduced IL-10 levels in MLR supernatants. When parallel experiments were performed using IDO KO BM-pDC, IL-10 was below the limit of detection, regardless of the addition of anti-ICOSL Ab (data not shown; n= 2), suggesting that ICOSL may be required for the induction of IL-10 from T cells when WT BM-pDC are used as stimulators.
Fig. 5. Neutralization of ICOSL enhances T cell proliferation and decreases IL-10 secretion in MLR.
(A) Purified WT pDC were pulsed overnight with cell-free BALB/c (H2d) splenocyte lysate, then stimulated with CpG or left unstimulated before culture with 105 syngeneic normal T cells (indirect pathway; left). Alternatively, immature or CpG-activated (act) B6 pDC were used to stimulate 105 allogeneic BALB/c T cells directly (right). Anti-ICOSL or control (10 μg/ml) was added to pDC prior to the start of MLR cultures (1 pDC:4 T cells). [3H] TdR was added for the last 18 h. In (B), B6 WT BM-pDC were either pretreated with anti-ICOSL mAb or rat IgG (isotype control) (10 μg/ml) for 30 min before their use as stimulators of bulk BALB/c T cells for 72h (1 pDC:4 T cells). Cells were collected and stained for CD4 and intracellular Foxp3. Numbers represent mean percentages ± 1SD of live CD4+ Foxp3+ cells from 3 independent experiments. In (C), supernatants were collected from the MLRs described in (B) and assayed for IL-10 by ELISA. Data shown are representative of 3 independent experiments.
4. Discussion
pDC, like conventional DC, regulate allogeneic T cell responses [9, 17-19, 24, 28, 46]. One mechanism by which secondary lymphoid tissue pDC appear to regulate T cell function is through production of IDO [24, 25, 28]. Thus, we surmised that in vitro-propagated BM-pDC, that exhibit similar characteristics and IFNα production in response to TLR ligation as spleen- and lymph node-isolated pDC [3, 5, 17], would produce functional IDO following B7 ligation, like their secondary lymphoid tissue counterparts. We used IDO KO mice to generate IDO-deficient, BM-pDC to facilitate analysis of the ability of pDC to regulate normal T cell function in a tryptophan-dependent manner.
We found substantial differences in T cell proliferation and IFNγ secretion in MLR using IDO KO vs WT BM-pDC as stimulators that could not be ascribed to CTLA4-induced IDO production. This suggested that the inferior T cell proliferation induced by BM-pDC pre-exposed to CTLA4Ig likely was due to (partial) blockade of the B7-CD28 pathway, rather than to tryptophan-depleting mechanisms, particularly since MLR cultures with CTLA4Ig-treated, IDO KO pDC as stimulators showed similar reductions in T cell stimulation. No differences in B7 molecule expression were evident after CTLA4Ig treatment, but it is conceivable that CTLA4Ig and anti-B7 (CD80/CD86) mAbs bind to different epitopes, and thus blocking of the B7:CD28 pathway is not detected by flow cytometric analysis. Alternatively, changes in B7 co-stimulatory molecule expression may not be evident until after the timing of our phenotypic analyses of the pDC (completed 24-36 h after exposure).
It remains to be determined why in vitro-generated WT BM-pDC, that otherwise share common pDC phenotypic markers and T cell stimulatory function with spleen pDC [17, 47], do not produce functional IDO. They do, however, appear to behave like the majority of ex vivo pDC. Notably, we have been unable to generate the unusual CD19+ DC (that, when isolated from mouse spleen, can express IDO following B7 ligation) from mouse BM under standard conditions. Indeed, these pDC may not share the same lineage as conventional CD19− DC.
It was reported recently that expression of DAP12, an ITAM-bearing transmembrane adaptor protein, typically associated with natural killer and myeloid cells [48], and that may have a role in autoimmune disease [49], is inversely related to functional IDO expression. In functionally mature CD8α− DC, DAP12 blocks IDO activity [34]. In pDC, as well as in macrophages, DAP12 downregulates activation in response to TLR4 [50] and TLR9 ligation [51]. When we silenced DAP12 in BM-pDC in the presence of the IDO inhibitor 1-MT, pDC once again exhibited enhanced ability to induce T cell proliferation. It has been shown that DAP12-silencing enhances constitutive expression of functional IDO in spleen pDC [34]. Further, incubation of spleen pDC with IDO-inducing stimuli has been found to downregulate DAP12 [45]. It will be important to elucidate the function and tolerogenic abilities of BM-pDC to ascertain what effect(s), if any, CTLA4Ig has on the expression of DAP12, and whether B7 ligation will enhance expression of functional IDO in DAP12-silenced BM-pDC.
Interestingly, more extensive phenotypic analyses of WT and IDO KO BM- pDC in our study revealed significant differences in expression of both CD86 and the co-regulatory molecule ICOSL (B7RP-1). Expression of ICOSL by human pDC can prime T cells for IL-10 secretion [20]. While in the present study, CpG-stimulated WT pDC upregulated ICOSL expression markedly, IDO KO pDC ICOSL levels remained similar to those pre-activation. Our finding that the T cell stimulatory function of activated WT pDC increased to that of activated IDO KO pDC after blocking ICOSL expression further emphasizes a relationship between the expression of this TNFR family member and pDC suppressive function.
While original reports suggested that ICOSL may act as a co-stimulatory molecule to induce T cell proliferation [21, 52], in the context of pDC ICOSL appears to act as a co-regulatory molecule [20]. This difference in ICOSL function may reflect the poor allostimulatory ability of pDC compared to conventional mDC, and that high expression of ICOSL, together with low expression of CD86, may allow ICOSL-ICOS interaction with T cells to be “regulatory” rather than stimulatory.
Our data reveal a role for IDO in regulating the maturation status of BM-pDC that has not previously been reported. IDO deficiency results in decreased ICOSL expression in response to TLR9 ligation. The mechanisms driving this phenotype merit further investigation. Although developmental differences between in vitro-propagated and freshly-isolated pDC continue to be characterized [53], the difficulty inherent in recovering tissue-resident pDC (e.g. from blood, spleen or LN) in adequate numbers for evaluation makes them less-than-ideal candidates for adoptive cell therapy [54]. Further, human DC typically must be generated from blood-borne precursors (utilizing various cytokines and/or pharmacologic agents) to derive adequate numbers for potential therapeutic application. Hence, there is a need for more detailed functional study of in vitro-propagated murine BM-pDC. As we have shown, while BM-derived WT pDC can not be manipulated to express functional IDO via CTLA4Ig exposure like their splenic counterparts [24], the fact that, when activated, they express similar high levels of ICOSL to human pDC suggests they may have potential for Treg induction (as reported for human pDC [20]). It will be important to further characterize murine BM-pDC T cell stimulatory and regulatory function to more fully assess the potential of these cells for regulation of immune-mediated disorders.
Acknowledgments
The work was supported by National Institutes of Health (NIH) Grant R01 AI60994 (to AWT), HD41187 (to ALM), an American Society of Transplantation (AST) Basic Science Fellowship (to BLC) and the American Liver Foundation Roger L. Jenkins Postdoctoral Research Fellowship (to TLS). TLS and JS were supported by NIH training grants T32 CA82084 and T35 DK65521, respectively, and by a non-concurrent AST basic science fellowship (TLS). We thank Amgen for providing Flt3L and Ms. Miriam Freeman for excellent administrative support.
Abbreviations
- BM-pDC
bone marrow-derived plasmacytoid DC
- CTLA4 (Ig)
cytotoxic T lymphocyte antigen 4 (immunoglobulin)
- DAP12
DNAX-activating protein of 12kDa
- Flt3L
fms-like tyrosine kinase 3 ligand
- ICOS (L)
inducible costimulator (ligand)
- 1-MT
1-methyl-tryptophan
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. Conflict of Interest
ALM is a member of the Scientific Advisory Board of NewLink Genetics Inc. and receives consulting income from this source. Other authors declare no financial or commercial conflict of interest.
References
- 1.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–96. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 3.Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5(12):1219–26. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- 4.Shortman K, Naik SH. Steady-state and inflammatory dendritic-cell development. Nat Rev Immunol. 2007;7(1):19–30. doi: 10.1038/nri1996. [DOI] [PubMed] [Google Scholar]
- 5.Wu L, Liu YJ. Development of dendritic-cell lineages. Immunity. 2007;26(6):741–50. doi: 10.1016/j.immuni.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Fonteneau JF, Gilliet M, Larsson M, Dasilva I, Munz C, Liu YJ, Bhardwaj N. Activation of influenza virus-specific CD4+ and CD8+ T cells: a new role for plasmacytoid dendritic cells in adaptive immunity. Blood. 2003;101(9):3520–6. doi: 10.1182/blood-2002-10-3063. [DOI] [PubMed] [Google Scholar]
- 7.Lou Y, Liu C, Kim GJ, Liu YJ, Hwu P, Wang G. Plasmacytoid dendritic cells synergize with myeloid dendritic cells in the induction of antigen-specific antitumor immune responses. J Immunol. 2007;178(3):1534–41. doi: 10.4049/jimmunol.178.3.1534. [DOI] [PubMed] [Google Scholar]
- 8.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 9.Morelli AE, Thomson AW. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat Rev Immunol. 2007;7(8):610–21. doi: 10.1038/nri2132. [DOI] [PubMed] [Google Scholar]
- 10.Hadeiba H, Sato T, Habtezion A, Oderup C, Pan J. Butcher EC: CCR9 expression defines tolerogenic plasmacytoid dendritic cells able to suppress acute graft-versus-host disease. Nat Immunol. 2008 doi: 10.1038/ni.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Young LJ, Wilson NS, Schnorrer P, Proietto A, ten Broeke T, Matsuki Y, Mount AM, Belz GT, O'Keeffe M, Ohmura-Hoshino M, Ishido S, Stoorvogel W, Heath WR, Shortman K, Villadangos JA. Differential MHC class II synthesis and ubiquitination confers distinct antigen-presenting properties on conventional and plasmacytoid dendritic cells. Nat Immunol. 2008;9(11):1244–52. doi: 10.1038/ni.1665. [DOI] [PubMed] [Google Scholar]
- 12.Barchet W, Cella M, Colonna M. Plasmacytoid dendritic cells--virus experts of innate immunity. Semin Immunol. 2005;17(4):253–61. doi: 10.1016/j.smim.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 13.Bailey-Bucktrout SL, Caulkins SC, Goings G, Fischer JA, Dzionek A, Miller SD. Cutting edge: central nervous system plasmacytoid dendritic cells regulate the severity of relapsing experimental autoimmune encephalomyelitis. J Immunol. 2008;180(10):6457–61. doi: 10.4049/jimmunol.180.10.6457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilliet M, Cao W, Liu YJ. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat Rev Immunol. 2008;8(8):594–606. doi: 10.1038/nri2358. [DOI] [PubMed] [Google Scholar]
- 15.Munn DH, Sharma MD, Hou D, Baban B, Lee JR, Antonia SJ, Messina JL, Chandler P, Koni PA, Mellor AL. Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. J Clin Invest. 2004;114(2):280–90. doi: 10.1172/JCI21583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goubier A, Dubois B, Gheit H, Joubert G, Villard-Truc F, Asselin-Paturel C, Trinchieri G, Kaiserlian D. Plasmacytoid dendritic cells mediate oral tolerance. Immunity. 2008;29(3):464–75. doi: 10.1016/j.immuni.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abe M, Wang Z, de Creus A, Thomson AW. Plasmacytoid dendritic cell precursors induce allogeneic T-cell hyporesponsiveness and prolong heart graft survival. Am J Transplant. 2005;5(8):1808–19. doi: 10.1111/j.1600-6143.2005.00954.x. [DOI] [PubMed] [Google Scholar]
- 18.Ochando JC, Homma C, Yang Y, Hidalgo A, Garin A, Tacke F, Angeli V, Li Y, Boros P, Ding Y, Jessberger R, Trinchieri G, Lira SA, Randolph GJ, Bromberg JS. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat Immunol. 2006;7(6):652–62. doi: 10.1038/ni1333. [DOI] [PubMed] [Google Scholar]
- 19.Fugier-Vivier IJ, Rezzoug F, Huang Y, Graul-Layman AJ, Schanie CL, Xu H, Chilton PM, Ildstad ST. Plasmacytoid precursor dendritic cells facilitate allogeneic hematopoietic stem cell engraftment. J Exp Med. 2005;201(3):373–83. doi: 10.1084/jem.20041399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ito T, Yang M, Wang YH, Lande R, Gregorio J, Perng OA, Qin XF, Liu YJ. Gilliet M: Plasmacytoid dendritic cells prime IL-10-producing T regulatory cells by inducible costimulator ligand. J Exp Med. 2007;204(1):105–15. doi: 10.1084/jem.20061660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nurieva R, Thomas S, Nguyen T, Martin-Orozco N, Wang Y, Kaja MK, Yu XZ. Dong C: T-cell tolerance or function is determined by combinatorial costimulatory signals. EMBO J. 2006;25(11):2623–33. doi: 10.1038/sj.emboj.7601146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tesciuba AG, Shilling RA, Agarwal MD, Bandukwala HS, Clay BS, Moore TV, Weinstock JV, Welcher AA. Sperling AI: ICOS costimulation expands Th2 immunity by augmenting migration of lymphocytes to draining lymph nodes. J Immunol. 2008;181(2):1019–24. doi: 10.4049/jimmunol.181.2.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burmeister Y, Lischke T, Dahler AC, Mages HW, Lam KP, Coyle AJ, Kroczek RA, Hutloff A. ICOS controls the pool size of effector-memory and regulatory T cells. J Immunol. 2008;180(2):774–82. doi: 10.4049/jimmunol.180.2.774. [DOI] [PubMed] [Google Scholar]
- 24.Mellor AL, Chandler P, Baban B, Hansen AM, Marshall B, Pihkala J, Waldmann H, Cobbold S, Adams E, Munn DH. Specific subsets of murine dendritic cells acquire potent T cell regulatory functions following CTLA4-mediated induction of indoleamine 2,3 dioxygenase. Int Immunol. 2004;16(10):1391–401. doi: 10.1093/intimm/dxh140. [DOI] [PubMed] [Google Scholar]
- 25.Mellor AL, Baban B, Chandler P, Marshall B, Jhaver K, Hansen A, Koni PA, Iwashima M, Munn DH. Cutting edge: induced indoleamine 2,3 dioxygenase expression in dendritic cell subsets suppresses T cell clonal expansion. J Immunol. 2003;171(4):1652–5. doi: 10.4049/jimmunol.171.4.1652. [DOI] [PubMed] [Google Scholar]
- 26.Fallarino F, Vacca C, Orabona C, Belladonna ML, Bianchi R, Marshall B, Keskin DB, Mellor AL, Fioretti MC, Grohmann U, Puccetti P. Functional expression of indoleamine 2,3-dioxygenase by murine CD8 alpha(+) dendritic cells. Int Immunol. 2002;14(1):65–8. doi: 10.1093/intimm/14.1.65. [DOI] [PubMed] [Google Scholar]
- 27.Munn DH, Sharma MD, Lee JR, Jhaver KG, Johnson TS, Keskin DB, Marshall B, Chandler P, Antonia SJ, Burgess R, Slingluff CL, Jr., Mellor AL. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science. 2002;297(5588):1867–70. doi: 10.1126/science.1073514. [DOI] [PubMed] [Google Scholar]
- 28.Fallarino F, Asselin-Paturel C, Vacca C, Bianchi R, Gizzi S, Fioretti MC, Trinchieri G, Grohmann U, Puccetti P. Murine plasmacytoid dendritic cells initiate the immunosuppressive pathway of tryptophan catabolism in response to CD200 receptor engagement. J Immunol. 2004;173(6):3748–54. doi: 10.4049/jimmunol.173.6.3748. [DOI] [PubMed] [Google Scholar]
- 29.Fallarino F, Grohmann U, Vacca C, Bianchi R, Orabona C, Spreca A, Fioretti MC, Puccetti P. T cell apoptosis by tryptophan catabolism. Cell Death Differ. 2002;9(10):1069–77. doi: 10.1038/sj.cdd.4401073. [DOI] [PubMed] [Google Scholar]
- 30.Grohmann U, Fallarino F, Bianchi R, Belladonna ML, Vacca C, Orabona C, Uyttenhove C, Fioretti MC, Puccetti P. IL-6 inhibits the tolerogenic function of CD8 alpha+ dendritic cells expressing indoleamine 2,3-dioxygenase. J Immunol. 2001;167(2):708–14. doi: 10.4049/jimmunol.167.2.708. [DOI] [PubMed] [Google Scholar]
- 31.Grohmann U, Fallarino F, Silla S, Bianchi R, Belladonna ML, Vacca C, Micheletti A, Fioretti MC, Puccetti P. CD40 ligation ablates the tolerogenic potential of lymphoid dendritic cells. J Immunol. 2001;166(1):277–83. doi: 10.4049/jimmunol.166.1.277. [DOI] [PubMed] [Google Scholar]
- 32.Sharma MD, Baban B, Chandler P, Hou DY, Singh N, Yagita H, Azuma M, Blazar BR, Mellor AL, Munn DH. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J Clin Invest. 2007;117(9):2570–82. doi: 10.1172/JCI31911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baban B, Hansen AM, Chandler PR, Manlapat A, Bingaman A, Kahler DJ, Munn DH, Mellor AL. A minor population of splenic dendritic cells expressing CD19 mediates IDO-dependent T cell suppression via type I IFN signaling following B7 ligation. Int Immunol. 2005;17(7):909–19. doi: 10.1093/intimm/dxh271. [DOI] [PubMed] [Google Scholar]
- 34.Orabona C, Tomasello E, Fallarino F, Bianchi R, Volpi C, Bellocchio S, Romani L, Fioretti MC, Vivier E, Puccetti P, Grohmann U. Enhanced tryptophan catabolism in the absence of the molecular adapter DAP12. Eur J Immunol. 2005;35(11):3111–8. doi: 10.1002/eji.200535289. [DOI] [PubMed] [Google Scholar]
- 35.Morelli AE, Larregina AT, Shufesky WJ, Zahorchak AF, Logar AJ, Papworth GD, Wang Z, Watkins SC, Falo LD, Jr., Thomson AW. Internalization of circulating apoptotic cells by splenic marginal zone dendritic cells: dependence on complement receptors and effect on cytokine production. Blood. 2003;101(2):611–20. doi: 10.1182/blood-2002-06-1769. [DOI] [PubMed] [Google Scholar]
- 36.Brawand P, Fitzpatrick DR, Greenfield BW, Brasel K, Maliszewski CR, De Smedt T. Murine plasmacytoid pre-dendritic cells generated from Flt3 ligand-supplemented bone marrow cultures are immature APCs. J Immunol. 2002;169(12):6711–9. doi: 10.4049/jimmunol.169.12.6711. [DOI] [PubMed] [Google Scholar]
- 37.Taner T, Hackstein H, Wang Z, Morelli AE, Thomson AW. Rapamycin-treated, alloantigen-pulsed host dendritic cells induce ag-specific T cell regulation and prolong graft survival. Am J Transplant. 2005;5(2):228–36. doi: 10.1046/j.1600-6143.2004.00673.x. [DOI] [PubMed] [Google Scholar]
- 38.Hackstein H, Taner T, Zahorchak AF, Morelli AE, Logar AJ, Gessner A, Thomson AW. Rapamycin inhibits IL-4--induced dendritic cell maturation in vitro and dendritic cell mobilization and function in vivo. Blood. 2003;101(11):4457–63. doi: 10.1182/blood-2002-11-3370. [DOI] [PubMed] [Google Scholar]
- 39.Raimondi G, Shufesky WJ, Tokita D, Morelli AE, Thomson AW. Regulated compartmentalization of programmed cell death-1 discriminates CD4+CD25+ resting regulatory T cells from activated T cells. J Immunol. 2006;176(5):2808–16. doi: 10.4049/jimmunol.176.5.2808. [DOI] [PubMed] [Google Scholar]
- 40.Manlapat AK, Kahler DJ, Chandler PR, Munn DH, Mellor AL. Cell-autonomous control of interferon type I expression by indoleamine 2,3-dioxygenase in regulatory CD19+ dendritic cells. Eur J Immunol. 2007;37(4):1064–71. doi: 10.1002/eji.200636690. [DOI] [PubMed] [Google Scholar]
- 41.Orabona C, Belladonna ML, Vacca C, Bianchi R, Fallarino F, Volpi C, Gizzi S, Fioretti MC, Grohmann U, Puccetti P. Cutting edge: silencing supprssor of cytokine signaling 3 expression in dendritic cells turns CD28-Ig from immune adjuvant to suppressant. J Immunol. 2005;174(11):6582–6. doi: 10.4049/jimmunol.174.11.6582. [DOI] [PubMed] [Google Scholar]
- 42.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4(10):762–74. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 43.Grohmann U, Orabona C, Fallarino F, Vacca C, Calcinaro F, Falorni A, Candeloro P, Belladonna ML, Bianchi R, Fioretti MC, Puccetti P. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat Immunol. 2002;3(11):1097–101. doi: 10.1038/ni846. [DOI] [PubMed] [Google Scholar]
- 44.Fallarino F, Orabona C, Vacca C, Bianchi R, Gizzi S, Asselin-Paturel C, Fioretti MC, Trinchieri G, Grohmann U, Puccetti P. Ligand and cytokine dependence of the immunosuppressive pathway of tryptophan catabolism in plasmacytoid dendritic cells. Int Immunol. 2005;17(11):1429–38. doi: 10.1093/intimm/dxh321. [DOI] [PubMed] [Google Scholar]
- 45.Orabona C, Puccetti P, Vacca C, Bicciato S, Luchini A, Fallarino F, Bianchi R, Velardi E, Perruccio K, Velardi A, Bronte V, Fioretti MC, Grohmann U. Toward the identification of a tolerogenic signature in IDO-competent dendritic cells. Blood. 2006;107(7):2846–54. doi: 10.1182/blood-2005-10-4077. [DOI] [PubMed] [Google Scholar]
- 46.Abe M, Colvin BL, Thomson AW. Plasmacytoid dendritic cells: in vivo regulators of alloimmune reactivity? Transplant Proc. 2005;37(9):4119–21. doi: 10.1016/j.transproceed.2005.09.111. [DOI] [PubMed] [Google Scholar]
- 47.Lau AH, Abe M, Thomson AW. Ethanol affects the generation, cosignaling molecule expression, and function of plasmacytoid and myeloid dendritic cell subsets in vitro and in vivo. J Leukoc Biol. 2006;79(5):941–53. doi: 10.1189/jlb.0905517. [DOI] [PubMed] [Google Scholar]
- 48.Lanier LL, Bakker AB. The ITAM-bearing transmembrane adaptor DAP12 in lymphoid and myeloid cell function. Immunol Today. 2000;21(12):611–4. doi: 10.1016/s0167-5699(00)01745-x. [DOI] [PubMed] [Google Scholar]
- 49.Bakker AB, Hoek RM, Cerwenka A, Blom B, Lucian L, McNeil T, Murray R, Phillips LH, Sedgwick JD, Lanier LL. DAP12-deficient mice fail to develop autoimmunity due to impaired antigen priming. Immunity. 2000;13(3):345–53. doi: 10.1016/s1074-7613(00)00034-0. [DOI] [PubMed] [Google Scholar]
- 50.Hamerman JA, Tchao NK, Lowell CA, Lanier LL. Enhanced Toll-like receptor responses in the absence of signaling adaptor DAP12. Nat Immunol. 2005;6(6):579–86. doi: 10.1038/ni1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sjolin H, Robbins SH, Bessou G, Hidmark A, Tomasello E, Johansson M, Hall H, Charifi F, Hedestam GBK, Biron CA, Karre K, Hoglund P, Vivier E, Dalod M. DAP12 signaling regulates plasmacytoid dendritic cell homeostasis and down-modulated their function during viral infection. J Immunol. 2006;177(5):2908–16. doi: 10.4049/jimmunol.177.5.2908. [DOI] [PubMed] [Google Scholar]
- 52.Yoshinaga SK, Whoriskey JS, Khare SD, Sarmiento U, Guo J, Horan T, Shih G, Zhang M, Coccia MA, Kohno T, Tafuri-Bladt A, Brankow D, Campbell P, Chang D, Chiu L, Dai T, Duncan G, Elliott GS, Hui A, McCabe SM, Scully S, Shahinian A, Shaklee CL, Van G, Mak TW, Senaldi G. T-cell co-stimulation through B7RP-1 and ICOS. Nature. 1999;402(6763):827–32. doi: 10.1038/45582. [DOI] [PubMed] [Google Scholar]
- 53.Xu Y, Zhan Y, Lew AM, Naik SH, Kershaw MH. Differential development of murine dendritic cells by GM-CSF versus Flt3 ligand has implications for inflammation and trafficking. J Immunol. 2007;179(11):7577–84. doi: 10.4049/jimmunol.179.11.7577. [DOI] [PubMed] [Google Scholar]
- 54.Bluestone JA, Thomson AW, Shevach EM, Weiner HL. What does the future hold for cell-based tolerogenic therapy? Nat Rev Immunol. 2007;7(8):650–4. doi: 10.1038/nri2137. [DOI] [PubMed] [Google Scholar]