Abstract
The cystic larvae of Taenia solium commonly infect the human nervous system, resulting in neurocysticercosis, a major contributor to seizure disorders in most of the world. Inflammation around the parasites is a hallmark of neurocysticercosis pathophysiology. Although mechanisms regulating this inflammation are poorly understood, anti-inflammatory drugs, particularly corticosteroids, have been long used alone or with anthelmintics to manage disease and limit neurological complications and perhaps damage to neural tissues. Only scarce controlled data exist to determine when and what type of corticosteroids and the treatment regime to use. This article revisits the mechanisms of action, rationale, evidence of benefit, safety and problems of corticosteroids in the context of neurocysticercosis, as well as alternative anti-inflammatory strategies to limit the damage caused by inflammation in the CNS.
Keywords: central nervous system, corticosteroids, cysticercosis, neurocysticercosis, seizures, Taenia solium
Taenia solium cysticercosis affects the human brain and other tissues where the larval stages of the parasite locate, establish and eventually degenerate. When present in the CNS, it causes seizures, intracranial hypertension or other neurological symptoms. inflammation accompanies this process and plays an important role in causing morbidity and occasional mortality. Corticosteroids are commonly used to modulate inflammation in neurocysticercosis (NCC). However, our knowledge of the specific mechanisms of action, doses and timing of steroid treatment in NCC are surprisingly incomplete. This article attempts to summarize the existing information on the use of corticosteroids and other immunomodulatory agents in NCC.
Taenia solium infection, cysticercosis & NCC
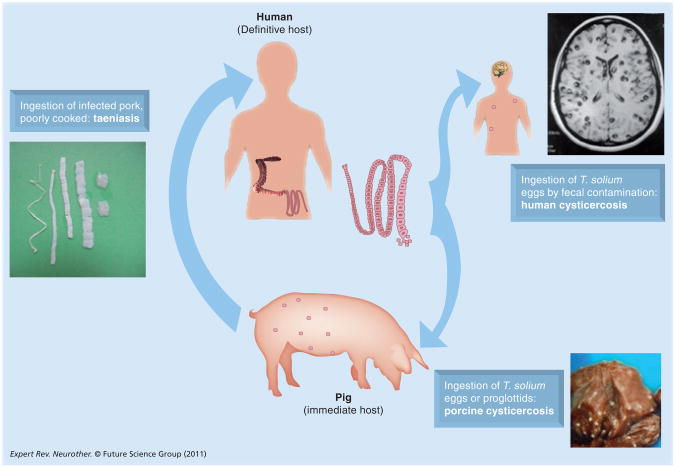
Neurocysticercosis is an infection with the larval form of the cestode tapeworm T. solium. The adult tapeworm resides in the human small intestine and produces proglottids containing infectious ova. When proglottids or liberated eggs excreted in feces are ingested by freeranging pigs, they develop into cysts, primarily in the muscles and brain. Humans acquire tapeworms after the ingestion of undercooked infected pork. Like the pig, humans are infected after the accidental ingestion of ova, which can originate from themselves or another tapeworm carrier (Figure 1). In humans, cysts can be found in virtually any perfused organ, but tend to develop most commonly in muscles, the brain and subcutaneous tissue [1,2].
Figure 1.
Life-cycle of Taenia solium.
Disease pathogenesis
Most patients present with brain manifestations, whose nature and severity depend on the number, location, size and manner of growth of cysts, as well as their stage of degeneration and accompanying inflammation [3]. The disease caused by NCC results from three basic pathophysiological mechanisms: mass effects, mechanical obstruction and inflammation [1,4]. Cysts lodged in the parenchyma of the brain (Figure 2) commonly cause seizures, while cysts lodged in the ventricles frequently lead to mechanical obstruction and hydrocephalus. Although a majority of subarachnoid cysts have a usual morphology, and are often closely adherent to the outer surface of the brain and act similar to parenchymal cysts, others grow abnormally, mostly in the subarachnoid spaces as proliferating vesicle-forming membranes, commonly referred to as racemose cysts [5]. These may become large, resulting in mass effect and/or infltrate among the spaces of the brain and spinal cord. Clinically, these cause severe chronic meningitis, resulting in hydrocephalus, mass effect, infarcts, arachnoid cysts, nerve entrapment and, uncommonly, hemorrhage [5,6]. This form is frequently among the most difficult to treat and is the most morbid.
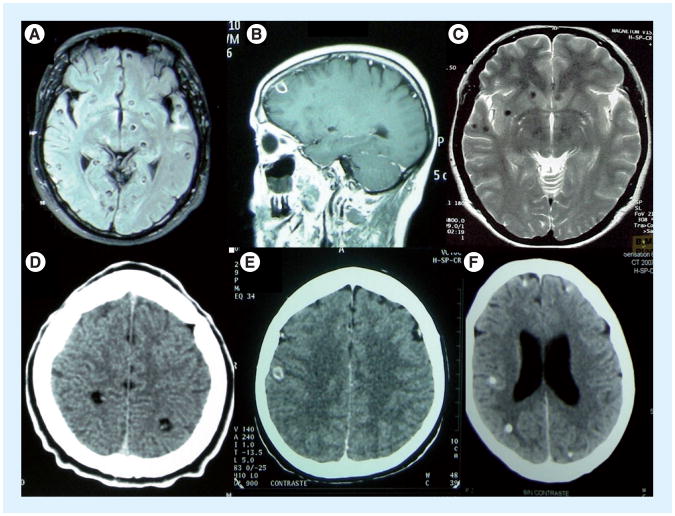
Figure 2.
Parenchymal cysticercosis. Viable (A & D), degenerating (B & E) and calcified (C & F) cysts on MRI (A–C) and CT (D–F).
Role of CNS inflammation in disease
Of the three mechanisms listed previously, inflammation is responsible for the majority of the clinical manifestations of disease. In most aspects, NCC can best be thought of as a chronic inflammatory infection of the brain, and this is the principal rationale for corticosteroid use in this infection.
In order to remain viable and infectious, cysts circumvent harmful host inflammatory responses that commonly give rise to symptoms. However, eventually, the host immune response does target the parasite and the cysts begin to degenerate. Clinically, when a patient with a single or multiple viable parenchymal cysts presents with a seizure, the single cyst or one or more of the multiple cysts enhance and/or show perilesional edema on MRI imaging. Histological examination reveals lesions with accompanying inflammation [7]. Parenchymal cysts degenerate through predictable stages over time, characterized by increased cyst destruction and decreased granulomatous response, resulting in either lesion resolution or calcification. Pathological changes in lesions are reflected in MRI and, to a lesser extent, in computed tomography examinations [8]. Both imaging modalities not only serve as the best way to establish a diagnosis, but are also an excellent way to gauge presence and degree of inflammation and the effectiveness of anthelmintic treatment [9]. Changes in the size and state or stage of the lesions, and presence or change in the degree of enhancement and edema are measures of effectiveness of anthelmintic treatment and suppression of inflammation by corticosteroids [8,9].
Degenerating cysts either calcify in 3 months to 2 years or resolve completely. The timing and degree of accompanying inflammation are extremely variable. Some lesions are in degenerative, inflammatory stages for prolonged periods, but even though the tendency is for decreased seizure activity as the accompanying inflammation abates and the lesion resolves or calcifes, seizures may occur at any degenerative stage. Calcified lesions are associated with seizure activity, but less frequently than degenerating viable parenchymal cysts. However, because they persist and are the most prevalent radiological finding in endemic population-based and clinical series, they are associated with more seizures and epilepsy due to NCC than other forms of the disease [10]. Calcified lesions are commonly described as inert noninflammatory granulomas. However, it is likely that some calcified lesions maintain some degree of inflammation, since many calcified lesions enhance on MRI [11] and there is preliminary histological confrmation that some calcified lesions contain significant inflammatory responses [12]. The episodic occurrence of seizures or focal neurological findings in the presence of perilesional edema around calcified T. solium granulomas is a relatively common phenomenon [10,13,14] and may be a consequence of an intermittent host response to sequestered antigen.
Basal subarachnoid cysticercosis is the most severe form of the infection owing to frequent and extensive involvement of the base of the brain, unrelenting growth, extensive mass and large antigenic load, all resulting in extensive arachnoiditis and hydrocephalus. In addition, the host may lack mechanisms to adequately process macroscopic membranes in the subarachnoid space. In contrast to parenchymal brain cysts, which for the most part give rise to inflammatory responses over a finite time period, racemose cysts, large cysts and occasionally ventricular cysts tend to invoke long-lasting, continuous, unremitting inflammatory responses, often involving critical brain structures [5,6,15,16].
Nature of inflammation in NCC
The inflammatory responses in humans are not well studied, but probably reflect the stage of degeneration. The responses usually consist of varying degrees of mononuclear infiltrates, mostly made up of lymphocytes, macrophages and plasma cells. Eosinophils, depending on the stage, are present to varying degrees and may make up a significant part of the inflammation. Giant cells are occasionally present in granulomatous inflammation. In infected pigs, which are the only available animal models, there are varying degrees of granulomatous infltration surrounding the parasite, with more developed granulomas showing increased cellularity and the presence of epithelioid cells adjacent to the parasite. Eosinophils are commonly noted and occasionally predominate, sometimes forming microabscesses.
Although the few calcified granulomas pictured in the literature show lesions devoid of inflammation, there is a recent example of a calcified lesion surgically removed from a patient experiencing episodes of perilesional edema in which the histopathology showed mononuclear inflammation coursing through the capsule, as well as adjacent to the brain. Eosinophils were present sparingly [12].
Cellular responses in the cerebrospinal fluid of patients with subarachnoid basilar disease show a moderate lymphocytic pleocytosis. Eosinophils are usually not present to a significant degree, but a minority of patients develop eosinophilic meningitis.
Local & systemic immune responses in humans
Studies in human NCC have generally been limited to descriptions of the profiles of inflammatory mediators or phenotypes of T cells in subsets of patients manifesting varying degrees of inflammatory pathology. Most of these studies have reported proinflammatory states during symptomatic periods (defined by the mitogen- or antigen-driven secreted cytokine patterns by peripheral blood mononuclear cells or cells from the cerebrospinal fluid) and asymptomatic chronic disease states associated with regulatory (or an ‘anti-inflammatory’) milieu [17–20]. Based on these observations, investigators have suggested that acute symptomatic individuals with NCC have a Th1-type profile in the peripheral blood, whereas asymptomatic individuals (with live parasites or calcified lesions) have predominantly Th2 responses to parasite antigens [21]. However, this is too simplistic an explanation and does not account for very different forms of clinical disease (live cysts, parenchymal vs subarachnoid disease, or calcific disease). Detailed analyses of local (pericystic) immune responses and the effects of chronic infections on neural tissues with regulatory functions (microglia, astrocytes and neurons) are also lacking.
Studies in murine intracerebral cysticercosis, modeled with Mesocestoides corti, have reported several mediators of inflammation that act locally to recruit cells and, perhaps, disrupt the BBB [20,22–24]. These studies implicated γδ T cells as important early actors in the inflammatory responses generated by the invading parasite [25]. However, although there is undoubtedly induction of severe inflammatory responses around the degenerating cysts, as discussed earlier, in human infections with T. solium, the specific roles of these cells and mediators in the inflammation that accompanies degenerating parasites remain to be elucidated.
Murine studies with Taenia crassiceps and M. corti have also shed light on regulatory immunological mechanisms that promote parasite survival and a paucity of host inflammatory reactions to the living parasite. This state of ‘tolerance’ of the parasite appears to be mediated by regulatory T cells and a subset of functionally distinct macrophages termed ‘alternatively activated’ macrophages, characterized by high levels of expression of the mannose receptor (MR) and arginase-1 (Arg1), among other markers [21]. These macrophages secrete regulatory cytokines, such as IL-10 and related cytokines (IL-19, IL-20 and so on), and TGF-β, and are dependent on IL-4 secreted by unknown cell populations early in infection, which also promotes a Th2 phenotype in the T-cell response to the parasite. In murine models of infection, Th2 responses favor susceptibility to infection, but also appear to protect from neurological symptoms. Limited studies in humans have yielded insufficient data for definitive conclusions in this regard. The interdependent, yet contradictory, relationship between inflammation, immunopathology and parasite killing is particularly important in the CNS, which is an immunologically privileged organ and tolerates inflammation poorly [1,7]. Thus, there is a strong imperative to use anti-inflammatory agents to strongly suppress inflammation in NCC.
Effects of corticosteroids on immune responses
Glucocorticoids (GC), as a class of steroid hormones, exhibit potent immunomodulatory activities, almost all of which occur following binding of the glucocorticoid receptor (GR) in the cytoplasm of immune cells [26]. GR, a ligand-activated transcription factor, mediates most, if not all, the effects of GC in cells of the immune system [27,28]. GR is a cytoplasmic receptor for GC, and is normally bound to and kept inactive by chaperone proteins, such as heat-shock proteins (e.g., Hsp90, Hsp70 and Hsp23) and immunophilins (e.g., FKBP51, FKBP52, Cyp44 and PP5) [29]. However, there appears to be continuous shuttling of the receptor between the two cellular compartments.
Activation of the GR results in numerous direct and indirect immunomodulatory effects on the innate and adaptive immune responses [26]. Inhibitory effects on a variety of immune cell populations (including the ones implicated in NCC in animal models; discussed earlier in the article) and their function have been reported. In addition, a number of mechanisms interfering with inflammatory responses have been described, such as: inhibition of lymphocyte binding to endothelial cells resulting in decreased egress of circulating immune cells to the site of inflammation [30,31]; increased apoptosis of T cells and B cells in the thymus and the periphery, via both antibody-induced cell death and caspase activation [32,33]; inhibition of Toll-like receptor (TLR) pathways in antigen-presenting cells (macrophages and dendritic cells) via direct inhibitory effects on p38/MAPK phosphorylation, resulting in decreased NFκ-B [34] and downregulation of dendritic cell migration and maturation [35–38]
Glucocorticoid receptor-mediated negative gene regulation targets a large number of proinflammatory genes via the transrepression mechanism or so-called tethering mechanism without binding DNA itself [39]. This concept refers to the negative interference of GR with the activity of other DNA-bound transcription factors, such as NFκ-B, cAMP response element-binding protein (CREB), interferon regulatory factor 3 (IRF3), nuclear factor of activated T cells (NFAT), signal transducer and activator of transcription (STAT), T-box expressed in T cells (T-Bet), GATA-3 and activating protein (AP)-1 [3,40]. The downstream effects of the action is seen on a vast number of inflammatory proteins, including IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-12, IL-18, COX-2, E-selectin, inducible NO synthase (iNOS), IFN-γ, TNF-α, ICAM, monocyte chemoattractant protein 1 (MCP-1) and VCAM [28].
Owing to interference with multiple proinflammatory immune mechanisms by GCs, they have served as potent anti-inflammatory agents in the treatment of neurological inflammation of diverse etiologies, and NCC is one of many infectious and inflammatory diseases in which use of GC has become standard practice, as discussed later. However, as more information emerges about the mediators that regulate inflammation in NCC-targeted anti-inflammatory therapy, such as the blockade of inflammatory mediators, including as TNF-α, IL-1β or IL-6, rational alternatives with potentially less severe long-term adverse outcomes than GC may be discovered [3,16,41,42].
Management of CNS inflammation: role of corticosteroids
As summarized previously, inflammatory responses play a dominant role in the pathophysiology of disease, and corticosteroids are frequently used to control inflammation and associated disease in NCC. Corticosteroids have been used in NCC for decades, long before any specific therapeutic approach other than surgery was available. However, with the exception of single enhancing lesions, which are solitary degenerating T. solium granulomas, there are no randomized studies of the benefit of corticosteroids or how to use them in other forms of the disease.
Corticosteroids are also commonly employed to prevent or modulate brain inflammation that follows anthelmintic treatment of parasitic cysts with the cysticidal drugs albendazole or praziquantel. When praziquantel was first used, investigators noted the development of acute clinical symptoms/signs such as seizures, mass effect and death, which were observed within the first week of initiating therapy [43]. Through unclear mechanisms, treatment disrupts the usual stable, noninflammatory host–parasite relationship, initiating an acute immune response to the parasite. These side effects were rapidly identified as being secondary to inflammation and effectively managed with corticosteroids. Side effects were also noted with albendazole, which was introduced a few years later as the second effective cysticidal agent. In a previously published double-blind trial, the number of seizures with generalization were increased in the albendazole treatment group in the first month after treatment and then decreased thereafter compared with controls [44]. Paradoxically, the inflammatory state that one is trying to otherwise suppress is induced by therapy. As all parasites are affected at the same time, clinical deterioration is frequent and, in the absence of potent corticosteroid treatment, may be lethal. These side effects are a major impediment to antiparasitic therapy and, until recently, how best to treat and/or prevent them was never studied. While almost any conceivable regimen, duration, dose or specific corticosteroid agent have been used when treating viable brain cysticercosis cysts, there are no controlled studies of coticosteroid use in the literature at all.
The only published controlled studies of corticosteroid use in NCC relate to the management of single enhancing granulomatous lesions (SEL). In the Indian subcontinent, SELs (a single T. solium cyst undergoing degeneration with associated edema and inflammation) are the most common clinical presentation of NCC and a frequent cause of seizures. Treatment of these patients is well studied in India and includes the only studies of the usefulness of corticosteroids in comparison with no treatment. Four studies compared corticosteroid treatment in patients with SEL who presented with seizures within the previous 14 days (TABLE 1). Two studies by Mall et al.[45] and Garg et al.[46] compared 1 mg/kg prednisolone for 10 days per os and a 4-day taper plus antiepileptic drugs (AED) to AED alone, while a third study by Kishore et al. compared the same drug regimen without a taper to AED alone [47]. The fourth study compared 1 g/1.72 m2 intravenous methyl prednisolone for 5 days with AED to AED alone [48]. All four studies found a decrease in seizures (statistically significant in three out of four), mostly at 6–9 months, but three also found an accompanying significant disappearance of cysts on CT scan [45,47,48]. By contrast, the only blinded randomized trial found no difference in disappearance at 6 months [46]. These studies suggest a benefit of cortico steroid treatment in the treatment of SELs with a recent onset of seizures. The inference is that other types of inflammation-induced damage may also be amendable to corticosteroid treatment.
Table 1.
Controlled studies on steroid therapy of neurocysticercosis.
| Study (year) | Details | Entry criteria | Intervention | Imaging results (resolution) | Seizures | Ref. |
|---|---|---|---|---|---|---|
| Mall et al. (2003) | Randomized, not blinded. All ages | Seizure <10 days and SEL | 1 mg/kg prednisolone × 10 days with 4-day taper | Significantly improved in steroid group (88 vs 52%) at 6 months | Significantly fewer in steroid group (2 vs 13%) at 6 months | [45] |
| Garg et al. (2006) | Randomized, blinded. All ages. | Seizure <14 days and SEL | 1 mg/kg prednisolone × 10 days with 4-day taper | No difference at 6 months | Significantly fewer in steroid group (12 vs 48%) at 9 months | [46] |
| Prakash et al. (2006) | Randomized. All ages | Seizure <14 days and SEL | 1 g/1.72 m2 iv. methyl prednisolone × 5 days | Significantly improved in steroid group (60 vs 18.5%) at 2 months | No significant decrease in seizures in steroid group (16 vs 33%) | [48] |
| Kishore et al. (2007) | Randomized. All ages | Seizure <7 days and SEL | 1 mg/kg prednisolone × 7 days + 3-day taper | Significantly improved in steroid group (68 vs 53%) at 8–12 weeks | Significantly fewer seizures in steroid group (10.6 vs 26.7%) | [47] |
iv.: Intravenous; SEL: Single enhancing granulomatous lesion.
A trial by Singhi et al. compared groups with SEL and an onset of seizures of less than 3 months duration receiving albendazole at 15 mg/kg for 4 weeks, albendazole as above plus prednisolone for 1 week at 2 mg/kg, and prednisolone for 3 weeks and a 1-week taper [49]. Although the authors found significantly more seizures in the corticosteroid group on follow-up compared with the other groups, the efficacy of corticosteroids alone compared with no corticosteroid treatment was not studied. In addition, the two corticosteroid groups could not be directly compared since they received different drug dosing. They found no benefit of corticosteroids for 1 week combined with a 4-week course of albendazole compared with albendazole alone. There was no difference in the disappearance of lesions among the three groups [49].
A few studies examined the usefulness of anthelmintic treatment combined with corticosteroids and AEDs [50–52] to corticosteroids and AEDs alone. No inference about the utility of corticosteroids compared with controls could be discerned.
Despite the absence of controlled trials, corticosteroid treatment is normally used to control inflammation in almost any clinical setting where there is potential for or there is active inflammation. They are also used in conjunction with anthelmintic treatment in multicystic parenchymal, ventricular and subarachnoid disease, and have been used alone in control groups in studies of the effectiveness of anthelmintics drugs. In some cases, corticosteroid use is immediately and dramatically beneficial. Examples include control of symptoms in intramedullary brain or spinal involvement, spinal compression, orbital cysticercosis with compression of the orbit, acute encephalitis, disseminated NCC and meningitis, just to mention a few. In some situations, such as disseminated disease and encephalitis, corticosteroids are used alone as the use of anthelmintics in these conditions is controversial. Its broad use is testament to a general view that there is clinical benefit, but their effectiveness has never been studied in randomized trials. Since there are no controlled studies, there is no regimen in standard use, and in addition these regimens vary greatly depending on the perceived need and practice. The duration, dose and type of corticosteroids also vary widely. To employ corticosteroids together with anthelmintics in the treatment of parenchymal disease is a common approach. Even in this setting, extensive variability exists. Some use corticosteroids only as the clinical need arises, while others also use them prophylactically, as well as during and after therapy. In particular, extensive subarachnoid disease requires high-dose prolonged treatment in conjunction with extended anthelmintic therapy.
There are no data concerning the usefulness of corticosteroids in promoting healing in multicystic parenchcymal disease compared with untreated control. There is an inference from some studies of SELs that corticosteroids are helpful in the healing of degenerating lesions.
The best time to administer corticosteroids has not yet been studied in a randomized trial. However, a retrospective comparison in the treatment of subarachnoid disease suggested a clinical benefit of pretreatment with corticosteroids before administration of antihelminthics [53].
Corticosteroids are commonly employed to suppress ongoing inflammation in chronic subarachnoid and ventricular NCC, either associated with treatment or as a consequence of the natural course of disease. Long-term use frequently leads to bothersome and sometimes severe side effects, such as diabetes, weight gain, aseptic necrosis of the hips and numerous other complications. Attempts to control side effects include the use of every-other-day corticosteroid dosing [54] to prevent shunt malfunction, use of the lowest dose of corticosteroids that are effective, and administration of cortico steroid-sparing agents such as methotrexate. There is brief mention of the use of cyclohexamide [53] or azathioprine in severe disease [55,56].
The practice of the authors is to commonly use 10–16 mg dexamethasone in critical and severe NCC associated with inflammation. Subsequent dosing depends upon the effectiveness of treatment, degree of clinical improvement, loss of edema and enhancement as assessed by MRI. We fnd that short courses of high-dose corticosteroids and abrupt termination frequently leads to rebound effects so we commonly taper over a period of 6–8 weeks or longer. Shorter tapers may be effective, but have not been studied. There is no direct information favoring one type of corticosteroid over another. Because dexamethasone has little mineral cortical activity, we favor its use.
Corticosteroids in perilesional edema around calcified cysticerci
Although corticosteroids are intuitively and commonly employed to treat brain edema due to a variety of causes, there are no studies suggesting that corticosteroids are of benefit for perilesional brain edema seen around already calcified brain cysts at the time of a symptomatic episode. There is a report and anecdotal experience suggesting worsening of edema and/or symptoms when lowering or stopping corticosteroids [57].
Blood levels of albendazole sulfoxide and praziquantel are particularly variable from person to person and are affected by a number of drugs [55]. Dexamethasone decreases the serum levels of praziquantel [58] and affects the metabolism of albendazole sulfoxide, the active metabolite of albendazole. In one study dexamethasone increased the concentration of albendazole sulfoxide [59] and in another it decreased the metabolism of albendazole sulfoxide, leading to an increase in the area under the curve [60].
Perspectives, other potential therapies & research agenda
Although short-term use of corticosteroids causes relatively few side effects, longer use, which is a common requirement in the treatment of complicated subarachnoid disease, leads to a long list of morbid complications that can be life threatening and can be difficult to manage medically. The authors have used methotrexate as a corticosteroid-sparing agent in patients who are likely to require more than 3 months of moderate-to-high-dose corticosteroids. In doses of 20 mg/week or less with folic acid daily (except the day methotrexate is used), it appears to be safe and effective in controlling chronic disease. However, it cannot be used to control acute inflammation, as it acts too slowly [61].
Expert commentary
Although little studied, corticosteroids are currently important, if not essential, in the control of inflammation that occurs during the natural course of disease, or as a result of treatment-induced inflammation. Better understanding of their actions and effects should lead to sound therapeutic schemes. Long-term steroid use is accompanied by multiple side effects, and alternative therapies are needed. Despite increasing interest in biological modulators of brain and/or systemic inflammation that may be able to replace or reduce the use of corticosteroids, their application is and will be limited by the lack of animal models to test efficacy and the safety and costs both to perform trials and to the patient. Nevertheless, proof-of-principle studies are important to provide treatment for severe, life-threatening disease.
Five-year view
Determination of how best to use corticosteroids based on mechanisms of inflammation and controlled trials should be obtained. One ongoing trial is currently studying whether enhanced corticosteroid dosing is more effective at reducing seizure rates in the treatment of parenchymal NCC. Although corticosteroids will remain the most common drug used due to its effectiveness, availability and affordability, in more complicated disease, corticosteroid side effects associated with long-term therapy are often severe. Other immunomodulatory agents (probably including anti-TNF agents) need to be found as corticosteroid-sparing or replacement agents. It is also likely that more efficient anthelmintic treatments arising from higher doses or combinations of antiparasitic drugs will change the need for and dosing of anti-inflammatory agents.
Key issues.
Disease in neurocysticercosis is primarily caused by inflammation.
Inflammation occurs naturally and is also induced by anthelmintic treatment.
Corticosteroids are commonly used to control inflammation that occurs during the natural evolution of disease and as a consequence of anthelmintic treatment.
How best to use corticosteroids has only been evaluated in randomized trials in single enhancing granulomatous lesions, where it uniformly decreases seizures rates.
The immune response in human disease is only partially known.
Corticosteroids have broad effects on the immune system.
Extraparenchymal neurocysticercosis commonly requires high-dose and long-term steroid use that is associated with severe side effects, limiting their use.
Corticosteroid-sparing agents are needed to limit severe side effects.
Acknowledgments
Financial & competing interests disclosure: Funding for this study was in part provided by the US National Institute of Allergy and Infectious Diseases (NIAID), US NIH.
Footnotes
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Garcia HH, Del Brutto OH. Neurocysticercosis: updated concepts about an old disease. Lancet Neurol. 2005;4(10):653–661. doi: 10.1016/S1474-4422(05)70194-0. [DOI] [PubMed] [Google Scholar]
- 2.Flisser A. Taeniasis and cysticercosis due to Taenia solium. Prog Clin Parasitol. 1994;4:77–116. [PubMed] [Google Scholar]
- 3.Mahanty S, Garcia HH. Cysticercosis and neurocysticercosis as pathogens affecting the nervous system. Prog Neurobiol. 2010;91(2):172–184. doi: 10.1016/j.pneurobio.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 4.Lobato RD, Lamas E, Portillo JM, et al. Hydrocephalus in cerebral cysticercosis Pathogenic and therapeutic considerations. J Neurosurg. 1981;55(5):786–793. doi: 10.3171/jns.1981.55.5.0786. [DOI] [PubMed] [Google Scholar]
- 5.Bickerstaff ER, Cloake PCP, Hughes B, Smith WT. The racemose form of cerebral cysticercosis. Brain. 1952;75:1–16. doi: 10.1093/brain/75.1.1. [DOI] [PubMed] [Google Scholar]
- 6.Estanol B, Corona T, Abad P. A prognostic classifcation of cerebral cysticercosis: therapeutic implications. J Neurol Neurosurg Psychiatry. 1986;49(10):1131–1134. doi: 10.1136/jnnp.49.10.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nash TE, Singh G, White AC, et al. Treatment of neurocysticercosis: current status and future research needs. Neurology. 2006;67(7):1120–1127. doi: 10.1212/01.wnl.0000238514.51747.3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dumas JL, Vusy JM, Belin C. Parenchymal neurocysticercosis: follow up and staging by MRI. Neuroradiology. 1997;39:12–16. doi: 10.1007/s002340050358. [DOI] [PubMed] [Google Scholar]
- 9.Garcia HH, Del Brutto OH. Imaging findings in neurocysticercosis. Acta Tropica. 2003;87:71–78. doi: 10.1016/s0001-706x(03)00057-3. [DOI] [PubMed] [Google Scholar]
- 10.Nash TE, Del Brutto OH, Butman JA, et al. Calcific neurocysticercosis and epileptogenesis. Neurology. 2004;62(11):1934–1938. doi: 10.1212/01.wnl.0000129481.12067.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sheth TN, Pillon L, Keystone J, Kucharczyk W. Persistent MR contrast enhancement of calcified neurocysticercosis lesions. AJNR Am J Neuroradiol. 1998;19(1):79–82. [PMC free article] [PubMed] [Google Scholar]
- 12.Ooi WW, Wijemanne S, Thomas CB, Quezado CR, Brown CR, Nash TE. A calcified Taenia solium granuloma associated with recurrent perilesiona edema causing refractory seizures: histopathological features. Am J Trop Med Hyg. 2011 doi: 10.4269/ajtmh.2011.11-0221. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13•.Nash TE, Pretell EJ, Lescano AG, et al. Perilesional brain oedema and seizure activity in patients with calcified neurocysticercosis: a prospective cohort and nested case-control study. Lancet Neurol. 2008;7(12):1099–1105. doi: 10.1016/S1474-4422(08)70243-6. Prospective study demonstrating the association between perilesional brain edema and seizures in individuals with calcified neurocysticercosis (NCC) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14••.Nash TE, Pretell J, Garcia HH. Calcified cysticerci provoke perilesional edema and seizures. Clin Infect Dis. 2001;33(10):1649–1653. doi: 10.1086/323670. Very good description of perilesional edema and the clinical consequences of this phenomenon. [DOI] [PubMed] [Google Scholar]
- 15.Henneberg R. Parasites of the central nervous system. In: Lewandowsky M, editor. Handbuch Der Neurologie. Verlag Von Julius Springer; Berlin, Germany: 1912. pp. 643–712. [Google Scholar]
- 16.Fleury A, Escobar A, Fragoso G, Sciutto E, Larralde C. Clinical heterogeneity of human neurocysticercosis results from complex interactions among parasite, host and environmental factors. Trans R Soc Trop Med Hygiene. 2010;104(4):243–250. doi: 10.1016/j.trstmh.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 17.De Melo CS, Vaz AJ, Nakamura PM, Da Silva MV, Machado Ade B. Human neurocysticercosis. IgE in cerebrospinal fluid. Arq Neuropsiquiatr. 1997;55(1):8–11. doi: 10.1590/s0004-282x1997000100002. [DOI] [PubMed] [Google Scholar]
- 18.Bueno EC, dos Ramos Machado L, Livramento JA, Vaz AJ. Cellular immune response of patients with neurocysticercosis (inflammatory and non-inflammatory phases) Acta Trop. 2004;91(2):205–213. doi: 10.1016/j.actatropica.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 19.Chavarria A, Alcocer-Varela J. Is damage in central nervous system due to inflammation? Autoimmun Rev. 2004;3(4):251–260. doi: 10.1016/j.autrev.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Alvarez JI, Rivera J, Teale JM. Differential release and phagocytosis of tegument glycoconjugates in neurocysticercosis: implications for immune evasion strategies PLoS Negl Trop Dis. 2008;2(4):e218. doi: 10.1371/journal.pntd.0000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21•.Terrazas LI. The complex role of pro- and anti-inflammatory cytokines in cysticercosis: immunological lessons from experimental and natural hosts. Curr Top Med Chem. 2008;8(5):383–392. doi: 10.2174/156802608783790848. Good review of NCC i m munopatho genes is, summarizing a broad range of animal model studies and discussing human immune responses. [DOI] [PubMed] [Google Scholar]
- 22.Cardona AE, Restrepo BI, Jaramillo JM, Teale JM. Development of an animal model for neurocysticercosis: immune response in the central nervous system is characterized by a predominance of γ δ T cells. J Immunol. 1999;162(2):995–1002. [PubMed] [Google Scholar]
- 23.Cardona AE, Teale JM. γ/δ T cell-defcient mice exhibit reduced disease severity and decreased inflammatory response in the brain in murine neurocysticercosis. J Immunol. 2002;169(6):3163–3171. doi: 10.4049/jimmunol.169.6.3163. [DOI] [PubMed] [Google Scholar]
- 24••.Alvarez JI, Teale JM. Breakdown of the blood brain barrier and blood–cerebrospinal fluid barrier is associated with differential leukocyte migration in distinct compartments of the CNS during the course of murine NCC. J Neuroimmunol. 2006;173:1–2. 45–55. doi: 10.1016/j.jneuroim.2005.11.020. Detailed study of BBB in an Mesocestoides corti mouse NCC model; clear demonstration of vascular leakage and inflammatory infltration. [DOI] [PubMed] [Google Scholar]
- 25.Alvarez JI, Colegial CH, Castano CA, Trujillo J, Teale JM, Restrepo BI. The human nervous tissue in proximity to granulomatous lesions induced by Taenia solium metacestodes displays an active response. J Neuroimmunol. 2002;127(1–2):139–144. doi: 10.1016/s0165-5728(02)00101-7. [DOI] [PubMed] [Google Scholar]
- 26.Tuckermann JP, Kleiman A, McPherson KG, Reichardt HM. Molecular mechanisms of glucocorticoids in the control of inflammation and lymphocyte apoptosis. Crit Rev Clin Lab Sci. 2005;42(1):71–104. doi: 10.1080/10408360590888983. [DOI] [PubMed] [Google Scholar]
- 27.Lowenberg M, Stahn C, Hommes DW, Buttgereit F. Novel insights into mechanisms of glucocorticoid action and the development of new glucocorticoid receptor ligands. Steroids. 2008;73(9–10):1025–1029. doi: 10.1016/j.steroids.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 28•.Baschant U, Tuckermann J. The role of the glucocorticoid receptor in inflammation and immunity. J Steroid Biochem Mol Biol. 2010;120:2–3. 69–75. doi: 10.1016/j.jsbmb.2010.03.058. Comprehensive review of glucocorticoid (GC) signaling pathways and the role of GC receptors in the actions of GCs. [DOI] [PubMed] [Google Scholar]
- 29.Pratt WB, Toft DO. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp Biol Med. 2003;228(2):111–133. doi: 10.1177/153537020322800201. [DOI] [PubMed] [Google Scholar]
- 30.Cronstein BN, Kimmel SC, Levin RI, Martiniuk F, Weissmann G. A mechanism for the antiinflammatory effects of corticosteroids: the glucocorticoid receptor regulates leukocyte adhesion to endothelial cells and expression of endothelial-leukocyte adhesion molecule 1 and intercellular adhesion molecule 1. Proc Natl Acad Sci USA. 1992;89(21):9991–9995. doi: 10.1073/pnas.89.21.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pitzalis C, Pipitone N, Bajocchi G, et al. Corticosteroids inhibit lymphocyte binding to endothelium and intercellular adhesion: an additional mechanism for their anti-inflammatory and immunosuppressive effect. J Immunology. 1997;158(10):5007–5016. [PubMed] [Google Scholar]
- 32.Brewer JA, Kanagawa O, Sleckman BP, Muglia LJ. Thymocyte apoptosis induced by T cell activation is mediated by glucocorticoids in vivo. J Immunol. 2002;169(4):1837–1843. doi: 10.4049/jimmunol.169.4.1837. [DOI] [PubMed] [Google Scholar]
- 33.Herold MJ, McPherson KG, Reichardt HM. Glucocorticoids in T cell apoptosis and function. Cell Mol Life Sci. 2006;63(1):60–72. doi: 10.1007/s00018-005-5390-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhattacharyya S, Brown DE, Brewer JA, Vogt SK, Muglia LJ. Macrophage glucocorticoid receptors regulate Toll-like receptor 4-mediated inflammatory responses by selective inhibition of p38 MAP kinase. Blood. 2007;109(10):4313–4319. doi: 10.1182/blood-2006-10-048215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kitajima T, Ariizumi K, Bergstresser PR, Takashima A. A novel mechanism of glucocorticoid-induced immune suppression: the inhibiton of T cell-mediated terminal maturation of a murine dendritic cell line. J Clin Invest. 1996;98(1):142–147. doi: 10.1172/JCI118759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cumberbatch M, Dearman RJ, Kimber I. Inhibition by dexamethasone of Langerhans cell migration: infuence of epidermal cytokine signals. Immunopharmacology. 1999;41(3):235–243. doi: 10.1016/s0162-3109(99)00037-5. [DOI] [PubMed] [Google Scholar]
- 37.Matyszak MK, Citterio S, Rescigno M, Ricciardi-Castagnoli P. Differential effects of corticosteroids during different stages of dendritic cell maturation. Eur J Immunol. 2000;30(4):1233–1242. doi: 10.1002/(SICI)1521-4141(200004)30:4<1233::AID-IMMU1233>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 38.Kim KD, Choe YK, Choe IS, Lim JS. Inhibition of glucocorticoid-mediated, caspase-independent dendritic cell death by CD40 activation. J Leukoc Biol. 2001;69(3):426–434. [PubMed] [Google Scholar]
- 39.Reichardt HM, Tuckermann JP, Gottlicher M, et al. Repression of inflammatory responses in the absence of DNA binding by the glucocorticoid receptor. EMBO J. 2001;20(24):7168–7173. doi: 10.1093/emboj/20.24.7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40•.De Bosscher K, Haegeman G. Minireview: latest perspectives on antiinflammatory actions of glucocorticoids. Mol Endocrinol. 2009;23(3):281–291. doi: 10.1210/me.2008-0283. Review of recent advances in the understanding of mechanisms by which GC inhibit inflammatory pathways. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.White AC., Jr New developments in the management of neurocysticercosis. J Infect Dis. 2009;199(9):1261–1262. doi: 10.1086/597758. [DOI] [PubMed] [Google Scholar]
- 42.Alvarez JI, Mishra BB, Gundra UM, Mishra PK, Teale JM. Mesocestoides corti intracranial infection as a murine model for neurocysticercosis. Parasitology. 2010;137(3):359–372. doi: 10.1017/S0031182009991971. [DOI] [PubMed] [Google Scholar]
- 43.Spina-Franca A, Nobrega JP, Livramento JA, Machado LR. Administration of praziquantel in neurocysticercosis. Tropenmed Parasitol. 1982;33(1):1–4. [PubMed] [Google Scholar]
- 44.Garcia HH, Pretell EJ, Gilman RH, et al. A trial of antiparasitic treatment to reduce the rate of seizures due to cerebral cysticercosis. N Engl J Med. 2004;350(3):249–258. doi: 10.1056/NEJMoa031294. [DOI] [PubMed] [Google Scholar]
- 45••.Mall RK, Agarwal A, Garg RK, Kar AM, Shukla R. Short course of prednisolone in Indian patients with solitary cysticercus granuloma and new-onset seizures. Epilepsia. 2003;44(11):1397–1401. doi: 10.1046/j.1528-1157.2003.08003.x. One of a few controlled studies on steroid therapy in NCC. [DOI] [PubMed] [Google Scholar]
- 46••.Garg RK, Potluri N, Kar AM, et al. Short course of prednisolone in patients with solitary cysticercus granuloma: a double blind placebo controlled study. J Infect. 2006;53(1):65–69. doi: 10.1016/j.jinf.2005.09.002. One of a few controlled studies on steroid therapy in NCC. [DOI] [PubMed] [Google Scholar]
- 47••.Kishore D, Misra S. Short course of oral prednisolone on disappearance of lesion and seizure recurrence in patients of solitary cysticercal granuloma with single small enhancing CT lesion: an open label randomized prospective study. J Assoc Physicians India. 2007:55, 419–424. One of a few controlled studies on steroid therapy in NCC. [PubMed] [Google Scholar]
- 48.Prakash S, Garg RK, Kar AM, et al. Intravenous methyl prednisolone in patients with solitary cysticercus granuloma: a random evaluation. Seizure. 2006;15(5):328–332. doi: 10.1016/j.seizure.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 49.Singhi P, Singhi S. Neurocysticercosis in children. Indian J Pediatr. 2009;76(5):537–545. doi: 10.1007/s12098-009-0139-5. [DOI] [PubMed] [Google Scholar]
- 50.Carpio A, Kelvin EA, Bagiella E, et al. Effects of albendazole treatment on neurocysticercosis: a randomised controlled trial. J Neurol Neurosurg Psychiatry. 2008;79(9):1050–1055. doi: 10.1136/jnnp.2008.144899. [DOI] [PubMed] [Google Scholar]
- 51.Carpio A, Santillan F, Leon P, Flores C, Hauser WA. Is the course of neurocysticercosis modifed by treatment with antihelminthic agents? Arch Intern Med. 1995;155(18):1982–1988. [PubMed] [Google Scholar]
- 52.Takayanagui OM, Odashima NS. Clinical aspects of neurocysticercosis. Parasitol Int. 2006;55(Suppl):S111–S115. doi: 10.1016/j.parint.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 53.Marquez-Caraveo C, Gongora-Rivera F, Santos Zambrano J, Hernandez R, Soto-Hernandez JL. Pre-treatment with corticosteroids and a single cycle of high dose albendazole for subarachnoidal cysticercosis. J Neurol Neurosurg Psychiatry. 2004;75(6):938–939. doi: 10.1136/jnnp.2003.023572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sotelo J. Treatment of brain cysticercosis. Surg Neurol. 1997;48(2):110–112. doi: 10.1016/s0090-3019(97)00179-1. [DOI] [PubMed] [Google Scholar]
- 55.Sotelo J, Jung H. Pharmacokinetic optimisation of the treatment of neurocysticercosis. Clin Pharmacokinet. 1998;34(6):503–515. doi: 10.2165/00003088-199834060-00006. [DOI] [PubMed] [Google Scholar]
- 56.Agapejev S, Da Silva MD, Ueda AK. Severe forms of neurocysticercosis: treatment with albendazole. Arq Neuropsiquiatr. 1996;54(1):82–93. doi: 10.1590/s0004-282x1996000100014. [DOI] [PubMed] [Google Scholar]
- 57•.Poeschl P, Janzen A, Schuierer G, Winkler J, Bogdahn U, Steinbrecher A. Calcified neurocysticercosis lesions trigger symptomatic inflammation during antiparasitic therapy. AJNR Am J Neuroradiol. 2006;27(3):653–655. Case report highlighting edema exacerbation around nonviable brain lesions at the time of antiparasitic treatment. [PMC free article] [PubMed] [Google Scholar]
- 58.Vazquez ML, Jung H, Sotelo J. Plasma levels of praziquantel decrease when dexamethasone is given simultaneously. Neurology. 1987;37(9):1561–1562. doi: 10.1212/wnl.37.9.1561. [DOI] [PubMed] [Google Scholar]
- 59.Jung H, Hurtado M, Medina MT, Sanchez M, Sotelo J. Dexamethasone increases plasma levels of albendazole. J Neurol. 1990;237(5):279–280. doi: 10.1007/BF00314741. [DOI] [PubMed] [Google Scholar]
- 60.Takayanagui OM, Lanchote VL, Marques MP, Bonato PS. Therapy for neurocysticercosis: pharmacokinetic interaction of albendazole sulfoxide with dexamethasone. Ther Drug Monit. 1997;19(1):51–55. doi: 10.1097/00007691-199702000-00009. [DOI] [PubMed] [Google Scholar]
- 61•.Mitre E, Talaat KR, Sperling MR, Nash TE. Methotrexate as a corticosteroid-sparing agent in complicated neurocysticercosis. Clin Infect Dis. 2007;44(4):549–553. doi: 10.1086/511040. Initial report of the use of methotrexate to reduce or replace corticosteroid treatment in NCC. [DOI] [PubMed] [Google Scholar]