Abstract
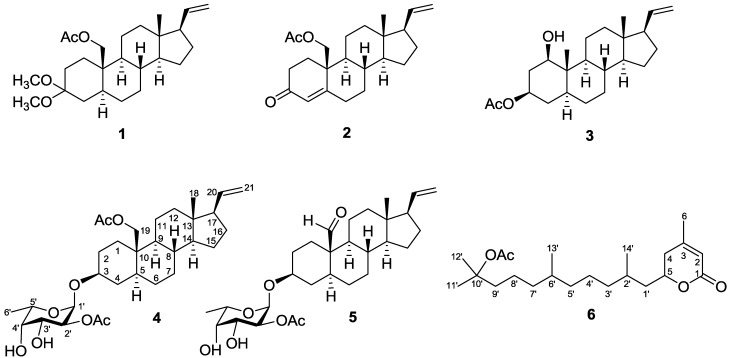
Five new pregnane-type steroids, sclerosteroids J–N (1–5), and a diterpenoid with a new chemotype 3-methyl-5-(10′-acetoxy-2′,6′,10′-trimethylundecyl)-2-penten-5-olide (6), have been isolated from a soft coral Scleronephthya gracillimum. The structures of the metabolites were determined by extensive spectroscopic analysis. Compound 4 exhibited cytotoxicity against HepG2, A549, and MDA-MB-231 cancer cell lines. Furthermore, steroids 2 and 4 were found to significantly inhibit the accumulation of the pro-inflammatory iNOS protein, and 1, 2, 4 and 5 could effectively reduce the accumulation of COX-2 protein in LPS-stimulated RAW264.7 macrophage cells.
Keywords: soft coral, Scleronephthya gracillimum, cytotoxicity activity, anti-inflammatory activity
1. Introduction
Soft corals have proven to be rich sources of terpenoids [1]. In previous studies, a series of novel secondary metabolites, including two alkyl glycerol ethers, one cembrane-based diterpenoid, one indole alkaoid and pregnane-type steroids have been isolated from the soft corals of the genus Scleronephthya [2,3,4,5,6,7]. During the course of search for bioactive metabolites from marine invertebrates, several pregnane-type compounds also have been isolated from soft corals, such as Carijoa sp. [8], Dendronephthya griffini [9], Dendronephthya sp. [10], Eunicella cavolini [11], Eunicella verrucosa [12], Muricea austera [13], Stereonephthya crystallina [14], and Subergorgia suberosa [15]. Our recent study of the chemical constituents of the Green Island soft coral Scleronephthya gracillimum [7] has yielded sclerosteroids A–I, of which some were found to exhibit significant cytotoxicity and anti-inflammatory activities. Our continuing chemical investigation on the same collection of this organism, with the aim of discovering other biologically active natural products, again led to the isolation of five new sclerosteroids J–N (1–5), and a new type of diterpenoidal δ-lactone, 3-methyl-5-(10′-acetoxy-2′,6′,10′-trimethylundecyl)-2-penten-5-olide (6). (Chart 1). The structures of 1–6 have been established by extensive spectroscopic analysis, including 2D NMR (1H–1H COSY, HMQC, HMBC, and NOESY) correlations. The cytotoxicity of compounds 1–6 against human liver carcinoma (HepG2), human lung carcinoma (A-549), and human breast carcinoma (MDA-MB-231) cell lines was measured. The ability of 1, 2, and 4–6 to inhibit the up-regulation of pro-inflammatory iNOS (inducible nitric oxide synthase) and COX-2 (cyclooxygenase-2) proteins in LPS (lipopolysaccharide)-stimulated RAW264.7 macrophage cells also was evaluated.
Chart 1.
Structures of metabolites 1–6.
2. Results and Discussion
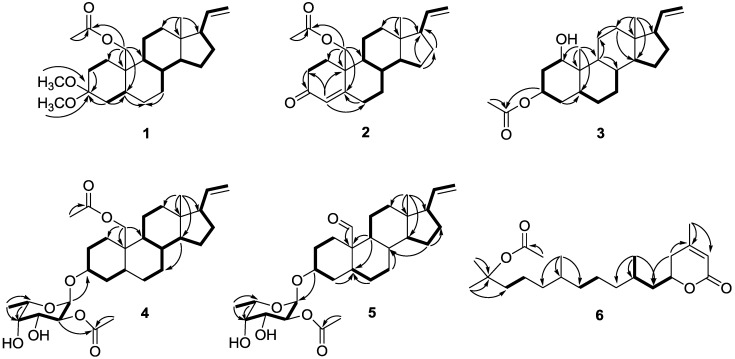
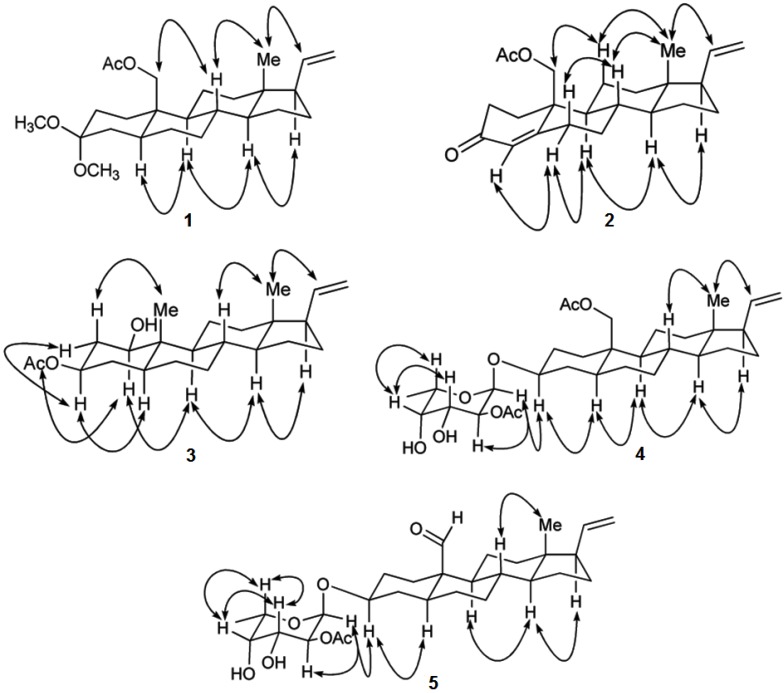
Sclerosteroid J (1) was obtained as colorless oil. The HRESIMS of 1 showed a pseudomolecular ion peak at m/z 427.2827 [M + Na]+, consistent with a molecular formula of C25H40O4 and requiring six degrees of unsaturation. The IR spectrum revealed the presence of ester-carbonyl group (1739 cm−1) in 1. The 13C NMR and DEPT spectroscopic data (Table 1) showed the presence of 25 carbon signals, including 4 methyls, 10 sp3 methylenes, 5 sp3 methines, 1 sp2 methine, 1 sp2 methylene, 1 sp2 quaternary and 3 sp3 quaternary carbons. The 1H NMR showed the presence of a tertiary methyl at δH 0.57 (3H, s), two methoxyls at δH 3.15 (3H, s), and 3.19 (3H, s), an acetoxymethyl at δH 4.16 (1H, d, J = 12.0 Hz), 4.35 (1H, d, J = 12.0 Hz), and 2.05 (3H, s), and a vinyl group at δH 4.96 (1H, br d, J = 17.0 Hz), 4.97 (1H, br d, J = 10.5 Hz), and 5.74 (1H, ddd, J = 17.0, 10.5, 7.5 Hz). These spectroscopic data showed that 1 might be a pregnane with an acetoxymethyl substituent at C-10 on the basis of the disappearance of an H3-19 singlet around δH 0.80–1.10 and the presence of an AB doublet at δH 4.16 (J = 12.0 Hz), and 4.35 (J = 12.0 Hz). The molecular skeleton of 1 was determined by 1H–1H COSY and HMBC correlations as shown in Figure 1, in which C-3 (δC 100.0) was HMBC correlated by protons of two methoxy groups and CH2-2. Thus, similar to known compound 11α-acetoxy-3,3-dimethoxy-5α-pregn-20-ene [11], 1 also has two methoxyl substituents at C-3. The presence of an sp3 methylene substituent at C-19 was further confirmed by the HMBC correlations from H2-19 to C-1, C-5, C-9 and C-10. The relative stereochemistry of 1 was determined by correlations of a 2D NOE experiment (Figure 2). The observed NOE correlations between H-20 and H3-18, H3-18 and H-8, H-8 and H2-19, H-17 and H-14, H-14 and H-9, and H-9 and H-5 revealed the β-orientation of H-8, H3-18, H2-19 and H-20, and α-orientation of H-5, H-9, H-14 and H-17. On the basis of the above spectroscopic data, the structure of sclerosteroid J (1) was established as 19-acetoxy-3,3-dimethoxy-5α-pregn-20-ene.
Table 1.
1H and 13C NMR spectroscopicdata of1–3.
| Position | 1 | 2 | 3 | ||||
|---|---|---|---|---|---|---|---|
| δH (J in Hz) a | δC (mult.) b | δH (J in Hz) a | δC (mult.) b | δH (J in Hz) a | δC (mult.) b | ||
| 1 | α | 1.01 m | 29.7, CH2 c | 1.84 m | 33.7, CH2 | 3.51 td (11.5, 5.0) d | 76.8, CH |
| β | 2.05 m | 2.34 m | |||||
| 2 | α | 1.36 m | 28.4, CH2 | 2.35 m | 34.7, CH2 | 2.04 m | 38.1, CH2 |
| β | 1.92 m | 2.61 m | 1.53 m | ||||
| 3 | 100.0, C | 199.5, C | 4.70 tt (11.5, 5.0) | 70.2, CH | |||
| 4 | α | 1.42 m | 35.8, CH2 | 5.91d (1.5) | 126.7, CH | 1.33 m | 34.0, CH2 |
| β | 1.70 m | 1.56 m | |||||
| 5 | 1.46 m | 42.7, CH | 166.0, C | 1.08 m | 42.2, CH | ||
| 6 | α | 1.26 m | 28.0, CH2 | 2.34 m | 33.2, CH2 | 1.37 m | 28.3, CH2 |
| β | 2.41 m | ||||||
| 7 | α | 0.97 m | 31.9, CH2 | 1.08 m | 32.3, CH2 | 1.67 m | 32.0, CH2 |
| β | 1.73 m | 1.91 m | 0.87 m | ||||
| 8 | 1.49 m | 35.9, CH | 1.62 m | 36.3, CH | 1.34 m | 36.0, CH | |
| 9 | 0.84 m | 54.3, CH | 1.10 m | 54.4, CH | 0.88 m | 54.9, CH | |
| 10 | 38.3, C | 41.9, C | 41.6, C | ||||
| 11 | α | 1.63 m | 21.8, CH2 | 1.67 m | 21.2, CH2 | 1.42 m | 24.1, CH2 |
| β | 1.30 m | 1.41 m | 2.07 m | ||||
| 12 | α | 0.97 m | 37.9, CH2 | 1.03 m | 37.5, CH2 | 1.06 m | 37.8, CH2 |
| β | 1.65 m | 1.73 m | 1.68 m | ||||
| 13 | 43.6, C | 43.4, C | 43.0, C | ||||
| 14 | 1.00 m | 55.9, CH | 1.00 m | 55.4, CH | 0.99 m | 55.5, CH | |
| 15 | α | 1.67 m | 24.7, CH2 | 1.70 m | 24.6, CH2 | 1.66 m | 25.1, CH2 |
| β | 1.17 m | 1.23 m | 1.14 m | ||||
| 16 | α | 1.77 m | 27.2, CH2 | 1.81 m | 27.1, CH2 | 1.74 m | 27.0, CH2 |
| β | 1.54 m | 1.58 m | 1.53 m | ||||
| 17 | 1.93 m | 55.3, CH | 1.96 m | 55.1, CH | 1.93 m | 55.5, CH | |
| 18 | 0.57 s | 13.0, CH3 | 0.64 s | 12.9, CH3 | 0.58 s | 12.8, CH3 | |
| 19 | 4.16 d (12.0) d | 62.5, CH2 | 4.17 d (11.0) | 67.0, CH2 | 0.87 s | 6.7, CH3 | |
| 4.35 d (12.0) | 4.67 d (11.0) | ||||||
| 20 | 5.74 ddd (17.0, 10.5, 7.5) | 140.0, CH | 5.74 ddd (17.0, 11.0, 6.5) | 139.2, CH | 5.75 ddd (17.0, 11.0, 7.5) | 139.8, CH | |
| 21 | 4.96 br d (17.0) | 114.5, CH2 | 4.98 br d (17.0) | 114.9, CH2 | 4.95 br d (17.0) | 114.4, CH2 | |
| 4.97 br d (10.5) | 4.99 br d (11.0) | 4.96 br d (11.0) | |||||
| OH | 1.25 d (11.5) | ||||||
| OAc | 2.05s | 21.2, CH3 | 2.00 s | 21.0, CH3 | 2.02 s | 21.3, CH3 | |
| 171.2, C | 170.7, C | 170.6, C | |||||
| OMe | 3.15 s | 47.5, CH3 | |||||
| 3.19 s | 47.6, CH3 | ||||||
a Spectra recorded at 500 MHz in CDCl3; b Spectra recorded at 125 MHz in CDCl3; c Deduced from DEPT; d J values (Hz) in parentheses.
Figure 1.
1H–1H COSY (▬) and HMBC (→)correlations for 1–6.
Figure 2.
Key NOESY Correlations for 1–5.
Sclerosteroid K (2) has a molecular formula of C23H32O3 as determined by HRESIMS, appropriate for eight degrees of unsaturation. The 1H and 13C NMR, including DEPT spectrum, exhibited the presence of a tertiary methyl (δH 0.64, 3H, s; δC 12.9), a primary acetoxymethyl group (δH 4.17, 1H, d, J = 11.0 Hz; 4.67, 1H, d, J = 11.0 Hz; δC 67.0), a vinyl group (δH 4.98, 1H, br d, J = 17.0 Hz; 4.99, 1H, br d, J = 11.0 Hz; 5.74, 1H, ddd, J = 17.0, 11.0, 6.5 Hz; δC 114.9, 139.2), and an enone group (δH 5.91, 1H, d, J = 1.5 Hz; δC 126.7, 166.0, 199.5) (Table 1). These spectroscopic data showed that 2 might have a 4,20-dien-3-one-pregnane skeleton [16,17] with an acetoxymethyl substituent at C-10 on the basis of the disappearance of an H3-19 singlet around δH 0.80–1.10 and the presence of an AB doublet at δH 4.17 (J = 11.0 Hz) and 4.67 (J = 11.0 Hz) and a doublet vinyl proton at δH 5.91 (J = 1.5 Hz). From COSY spectrum measured in CDCl3, it was possible to establish thirteen proton sequences from H2-1 to H2-2, H2-6 to H2-7, H2-7 to H-8, H-8 to H-9 and H-14, H-9 to H2-11, H2-11 to H2-12, H-14 to H2-15, H-17 to H-20, and H-20 to H2-21 (Figure 1). The HMBC correlations of H2-19 to C-1, C-5, C-9 and C-10; H3-18 to C-12, C-13 and C-17; H2-2 to C-3; H-4 to C-2, C-6 and C-10; H2-15 to C-16 and C-17, permitted the connection of the carbon skeleton. The observed NOESY correlations between H3-18 and both H-20 and H-8, H-8 and H-6β, H-11β and H2-19, H-14 and both H-17 and H-9, H-6α and both H-4 and H-9 revealed the β-orientation of H-6β, H-8, H-11β, H3-18, H2-19, and H-20 and α-orientation of H-4, H-6α, H-9, H-14, and H-17 (Figure 2). On the basis of the above spectroscopic data, the structure of 2 was established as 19-acetoxypregna-4,20-dien-3-one.
Sclerosteroid L (3) was shown by HRESIMS to possess the molecular formula C23H36O3 (m/z 383.2559 [M + Na]+). The IR absorptions at 3568 and 1712 cm−1 suggested the presence of hydroxy and carbonyl groups. The 13C NMR and DEPT spectrum of 3 were similar to those of 5α-pregn-20-en-1α,3α-diol 3-acetate [18], with small differences observed in the A-ring. This was confirmed by the COSY correlations between H-1/H2-2, H2-2/H-3, and H-3/H2-4, revealing that 3 is an epimer of 5α-pregn-20-en-1α,3α-diol 3-acetate (Figure 1). The planar structure of 3 was further confirmed by analysis of 1H–1H COSY and HMBC correlations (Figure 1). The 1H–1H COSY correlations allowed the establishment of two additional spin systems from H-5 to H-8 and H-14 to H-17 through H-20 to H2-21. Key HMBC correlations of H3-19 to C-1, C-5, C-9 and C-10; H3-18 to C-12, C-13, C-14 and C-17; H-4 to and C-5; H-9 to C-8 and C-11; H2-12 to C-11 and C-14, permitted establishment of the planar structure of 3. The observed NOESY correlations between H-20 and H3-18, H3-19 and H-2β, H3-18 and H-8, H-17 and H-14, H-14 and H-9, H-9 and H-1α, H-5 and H-3α, H-3α and H-2α, and H-2α and H-1α revealed the β-orientation of H-2β, H-8, H3-18, H3-19, and H-20 and α-orientation of H-1α, H-2α, H-3α, H-5, H-9, H-14, and H-17 (Figure 2). Analysis of the coupling constants of H-1 (δH 3.51, 1H, td, J = 11.5, 5.0 Hz) and H-3 (δH 4.70, 1H, tt, J = 11.5, 5.0 Hz) confirming the β-orientations of 1-OH and 3-OH. Therefore, the structure of 3 could be established unambiguously as 5α-pregn-20-en-1β,3β-diol 3-acetate.
The molecular formula of sclerosteroid M (4) was found to be C31H48O8, as established HRESIMS, 13C NMR, and DEPT data, indicating eight degrees of unsaturation. The 1H and 13C NMR spectra of 4 displayed the signals of a vinyl group (δH 4.95, 1H, br d, J = 18.0 Hz; 4.96, 1H, br d, J = 10.0 Hz; 5.74, 1H, ddd, J = 18.0, 10.0, 8.0 Hz; δC 114.5, 139.8), two ester carbonyl (δC 171.3, 171.5), and two acetate methyl groups (δH 2.06, 3H, s; 2.13, 3H, s; δC 21.1, 21.2). Therefore, 4 possesses five rings. The 1H and 13C NMR spectroscopic data of 4 were similar to those of sclerosteroid A [7], except for the appearing of six additional carbon signals at δC 94.6 (CH), 72.0 (CH), 68.6 (CH), 72.3 (CH), 65.3 (CH), and 16.1 (CH3), an anomeric proton signal at δH 5.13 (1H, d, J = 4.0 Hz), as well as a methyl doublet at δH 1.28, suggesting the presence of a 6′-deoxyhexose unit. This hexose appeared to be the C-2′ monoacetate derivative of fucopyranose by comparison of 1H and 13C NMR data with those reported previously [7] and on the basis of the results of 1H–1H COSY, HMBC, and NOESY experiments, in particular the HMBC correlation from H-2′ (δH 4.86) to the acetate carbonyl carbon (δC 171.5) (Figure 1). The sugar moiety was found to be connected with C-3 of the aglycon by HMBC correlation of H-1′ and C-3. The anomeric proton H-1′ (δH 5.13) has a small coupling constant, indicating the equatorial orientation of this proton. The relative configuration of the aglycon of 4 was further determined by NOESY experiment (Figure 2). On the basis of the above analysis, the structure of 4 was established as 3β-(2′-O-acetyl-α-l-fucopyranosyloxy)-5α-pregn-20-en-19-ol 19-acetate.
Sclerosteroid N (5) had a molecular formula of C29H44O7 as established by HRESIMS. The 1H and 13C NMR spectroscopic data of 5 were similar to those of 4, except for the replacement of the C-10 acetoxymethyl group in 4 by an aldehyde (δH 10.0, 1H, s; δC 208.5) in 5 (Table 2). The relative configuration and connection of the aglycon and sugar residue of 5 were further determined by 1H–1H COSY, HMBC, and NOESY experiments (Figure 1, Figure 2). Thus, the structure of 5 was assigned as 3β-(2′-O-acetyl-α-l-fucopyranosyloxy)-5α-pregna-20-en-19-al.
Table 2.
1H and 13C NMR spectroscopic data of 4–6.
| Position | 4 | 5 | 6 | ||||
|---|---|---|---|---|---|---|---|
| δH (J in Hz) a | δC (mult.) b | δH (J in Hz) c | δC (mult.) d | δH (J in Hz) c | δC (mult.) d | ||
| 1 | α | 0.91 m | 31.9, CH2e | 0.93 m | 31.0, CH2 | 165.4, C | |
| β | 2.23 m | 2.40 dt (14.0, 4.0) | |||||
| 2 | α | 1.83 m | 29.3, CH2 | 1.40 m | 30.3, CH2 | 5.81 s | 116.6, CH |
| β | 1.44 m | 1.87 m | |||||
| 3 | 3.53 m | 76.7, CH | 3.50 m | 76.7, CH | 157.0, C | ||
| 4 | α | 1.62 m | 34.4, CH2 | 1.71 m | 35.9, CH2 | 2.22 d (4.4) f | 35.0, CH2 |
| β | 1.33 m | 1.23 m | 2.27 m | ||||
| 5 | 1.25 m | 44.8, CH | 1.36 m | 43.3, CH | 4.46 m | 75.7, CH | |
| 6 | α | 1.27 m | 28.3, CH2 | 1.53 m | 28.3, CH2 | 1.99 s | 23.0, CH3 |
| β | 1.91 m | ||||||
| 7 | α | 0.92 m | 32.0, CH2 | 1.07 m | 32.0, CH2 | ||
| β | 1.75 m | 1.90 m | |||||
| 8 | 1.50 m | 35.9, CH | 1.43 m | 37.0, CH | |||
| 9 | 0.75 m | 54.5, CH | 0.95 m | 52.8, CH | |||
| 10 | 38.1, C | 51.8, C | |||||
| 11 | α | 1.65 m | 21.8, CH2 | 1.26 m | 21.4, CH2 | ||
| β | 1.35 m | 1.69 m | |||||
| 12 | α | 0.97 m | 37.8, CH2 | 0.98 m | 37.3, CH2 | ||
| β | 1.68 m | 1.66 m | |||||
| 13 | 43.6, C | 43.4, C | |||||
| 14 | 1.02 m | 55.9, CH | 0.93 m | 55.7, CH | |||
| 15 | α | 1.68 m | 24.7, CH2 | 1.14 m | 24.6, CH2 | ||
| β | 1.18 m | 1.66 m | |||||
| 16 | α | 1.79 m | 27.2, CH2 | 1.50 m | 27.1, CH2 | ||
| β | 1.56 m | 1.76 m | |||||
| 17 | 1.95 dt (18.0,8.0) f | 55.3, CH | 1.93 m | 55.3, CH | |||
| 18 | 0.57 s | 13.0, CH3 | 0.52 s | 12.8, CH3 | |||
| 19 | 4.20 d (12.0) | 62.8, CH2 | 10.0 s | 208.5, CH | |||
| 4.34 d (12.0) | |||||||
| 20 | 5.74 ddd (18.0, 10.0, 8.0) | 139.8, CH | 5.72 ddd (18.0, 10.0, 8.0) | 139.5, CH | |||
| 21 | 4.95 br d (18.0) | 114.5, CH2 | 4.96 br d (18.0) | 114.7, CH2 | |||
| 4.96 br d (10.0) | 4.97 br d (10.0) | ||||||
| 1′ | 5.13 d (4.0) | 94.6, CH | 5.12 d (3.6) | 94.8, CH | 1.52 m | 42.3, CH2 | |
| 1.68 m | |||||||
| 2′ | 4.86 dd (10.5, 4.0) | 72.0, CH | 4.84 dd (10.0, 3.6) | 72.0, CH | 2.66 m | 28.9, CH | |
| 3′ | 4.00 dd (10.5, 3.5) | 68.6, CH | 3.98 dd (10.0, 3.2) | 68.5, CH | 1.34 m | 36.9, CH2 | |
| 4′ | 3.81 d (3.5) | 72.3, CH | 3.80 d (3.2) | 72.3, CH | 1.20 m | 24.2, CH2 | |
| 1.34 m | |||||||
| 5′ | 4.09 q (6.5) | 65.3, CH | 4.06 q (6.8) | 65.4, CH | 1.08 m | 37.2, CH2 | |
| 6′ | 1.28 d (6.5) | 16.1, CH3 | 1.27 d (6.8) | 16.1, CH3 | 1.38 m | 32.7, CH | |
| 7′ | 1.08 m | 37.3, CH2 | |||||
| 1.26 m | |||||||
| 8′ | 1.24 m | 21.3, CH2 | |||||
| 9′ | 1.70 m | 41.0, CH2 | |||||
| 10′ | 82.5, C | ||||||
| 11′ | 1.42 s | 26.1, CH3 | |||||
| 12′ | 1.42 s | 26.1, CH3 | |||||
| 13′ | 0.85 d (6.4) | 19.6, CH3 | |||||
| 14′ | 0.93 d (6.0) | 19.9, CH3 | |||||
| OAc | 2.06 s | 21.1, CH3 | 2.12 s | 21.1, CH3 | 1.97 s | 22.5, CH3 | |
| 2.13 s | 21.2, CH3 | 171.5, C | 170.5, C | ||||
| 171.3, C | |||||||
| 171.5, C | |||||||
a Spectra recorded at 500 MHz in CDCl3; b Spectra recorded at 125 MHz in CDCl3; c Spectra recorded at 400 MHz in CDCl3; d Spectra recorded at 100 MHz in CDCl3; e Deduced from DEPT; f J values (Hz) in parentheses.
3-Methyl-5-(10′-acetoxy-2′,6′,10′-trimethylundecyl)-2-penten-5-olide (6) was obtained as colorless oil with [α]D25 −22 (c 0.17, CHCl3). The HRESIMS of 6 exhibited a [M + Na]+ peak at m/z 389.2666, and established a molecular formula of C22H38O4, implying four degrees of unsaturation. Above information and the UV absorption at 211 nm and the IR absorption at 1729 cm−1 suggested the presence of an α,β-unsaturated δ-lactone [19]. The 13C NMR and DEPT spectroscopic data (Table 2) showed 22 carbon signals, including six methyls, eight sp3 methylenes, three sp3 methines, one sp2 methine, three sp2 quaternary and one sp3 quaternary carbons. The signals in the 1H NMR spectrum at δH (5.81, 1H, s), 2.22 (1H, d, J = 4.4 Hz), 2.27 (1H, m), 4.46 (1H, m), and 1.99 (3H, s) verified the presence of a subunit of 3-methyl-2-penten-5-olide [20,21]. The planar structure and all of the 1H and 13C chemical shifts of 6 were elucidated by 2D NMR experiments, in particular the 1H–1H COSY and HMBC experiments (Figure 1). The HMBC correlations from H2-5 to C-4 and C-1′; H3-11′ to C-9′ and C-10′; H3-12′ to C-10′; H3-13′ to C-5′ and C-6′; H3-14′ to C-1′, C-2′ and C-3′; and H3-6 to C-3 and C-4. The structure of 6 thus was established and was found to be a α,β-unsaturated δ-lactone, 3-methyl-5-(10′-acetoxy-2′,6′,10′-trimethylundecyl)-2-penten-5-olide, which is a new chemotype of diterpene.
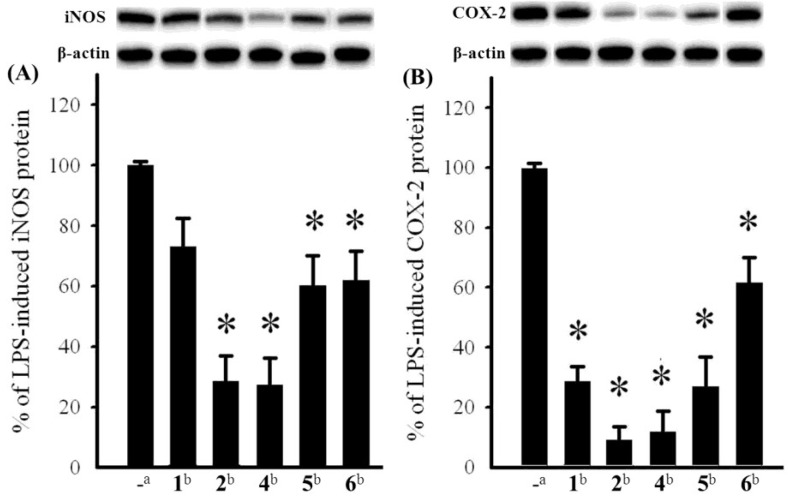
The cytotoxicity of compounds 3–5 against the proliferation of a limited panel of cancer cell lines, including HepG2, A549, and MDA-MB-231 carcinoma cell lines was evaluated. The results showed that compound 4 exhibited moderate cytotoxicity against HepG2, A549, and MDA-MB-231 with IC50 values of 23.3, 21.9, and 24.3 μM, respectively. The anti-inflammatory activities of 1, 2, and 4–6 against the accumulation of pro-inflammatory iNOS and COX-2 proteins in RAW264.7 macrophage cells stimulated with LPS, were also evaluated using immunoblot analysis (Figure 3). At a concentration of 10 μM, compounds 2, and 4 significantly reduced the levels of iNOS protein to 28.4 ± 8.4%, and 27.2 ± 9.0%, respectively, relative to control cells stimulated with LPS only. Meanwhile, compounds 1, 5, and 6 moderately reduced iNOS level to 72.8 ± 9.5%, 60.3 ± 9.7%, and 61.8 ± 9.8%, respectively. At the same concentration, compounds 1, 2, and 4–6 also could reduce COX-2 expression to 28.4 ± 4.9%, 9.0 ± 4.4%, 11.8 ± 6.8%, 26.6 ± 10.0%, and 61.7 ± 8.3%, respectively. These results indicated that compounds 2 and 4 might become the effective anti-inflammatory agents as they can potently inhibit the expression of both iNOS and COX-2 proteins in LPS-induced macrophage cells. Compounds 1 and 5 might also be useful anti-inflammatory compounds as they could effectively reduce COX-2 expression, too. Thus, soft coral S. gracillimum is an important source of anti-inflammatory agents, as except 1, 2, 4 and 5, effective anti-inflammatory compounds sclerosteroids A (7), B (8), and E (9) (Chart 2) were also discovered from this organism by our previous investigation [7]. The exhibited anti-inflammatory activity for sclerosteroids A, B, E, J, K, M, and N revealed that the oxidation at C-19 in pregnanes could enhance anti-inflammatory activity.
Figure 3.
Effect of isolates (10 μM) from S. gracillimum on the lipopolysaccharide (LPS)-induced pro-inflammatory iNOS and on COX-2 protein expression of RAW264.7 macrophage cells by immunoblot analysis. (A) Immunoblots iNOS and β-actin; (B) Immunoblots COX-2 and β-actin. The values are means ± SEM (n = 6). The relative intensity of the LPS alone stimulated group was taken as 100%. * Significantly different from LPS alone stimulated group (* p < 0.05). a stimulated with LPS; b stimulated with LPS in the presence of 1, 2, and 4–6 (10 μM).
Chart 2.
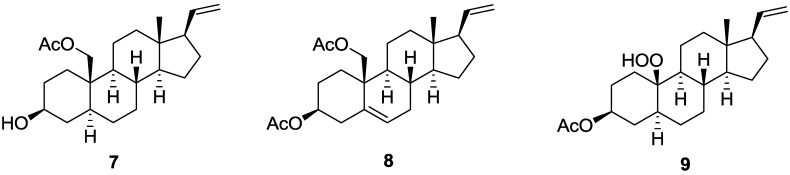
Structures of known anti-inflammatory compounds 7–9.
3. Experimental Section
3.1. General Experimental Procedures
Melting point was determined using a Fisher-Johns melting point apparatus. Optical rotations were measured with a JASCO P-1020 polarimeter. Ultraviolet spectrum was recorded on a JASCO V-650 spectrophotometer. IR spectrum was recorded on a JASCO FT/IR-4100 spectrophotometer. The NMR spectra were recorded on a Varian MR-400 FT-NMR or Varian Unity INOVA 500 FT-NMR instrument at 400 MHz or 500 MHz for 1H (referenced to TMS, δH7.27 ppm for CDCl3) and 100 or 125 MHz for 13C (referenced to δC 77.0 for CDCl3). ESIMS and HRESIMS were recorded by ESI FT-MS on a Bruker APEX II mass spectrometer. Silica gel 60 (Merck, 230–400 mesh) was used for column chromatography. Precoated silica gel plates (Merck, Kieselgel 60 F254, 0.25 mm) and precoated RP-18 F254S plates (Merck, 1.05560) were used for TLC analyses. High-performance liquid chromatography (HPLC) was performed on a Hitachi L-7100 pump equipped with a Hitachi L-7400 UV detector and a semi-preparative reversed-phase column (YMC-Pack Pro C18, 5 μm, 250 × 10 mm).
3.2. Animal Material
The soft coral Scleronephthya gracillimum was collected at Green Island, Taiwan, in January, 2008, at a depth of 10 m, and was stored in a freezer until extraction. This soft coral was identified by one of the authors (C.-F.D.). A voucher specimen (NSYSU-SG001) was deposited in the Department of Marine Biotechnology and Resources, National Sun Yat-sen University.
3.3. Extraction and Isolation
The frozen bodies of S. gracillimum (1.3 kg, fresh wet) were minced and extracted with EtOH (3 L × 2). The organic extract was concentrated to an aqueous suspension which was further partitioned between EtOAc and H2O. The EtOAc extract (9.6 g) was fractionated by open column chromatography on silica gel using n-hexane–EtOAc and EtOAc–MeOH mixtures of increasing polarity to yield 40 fractions. Fraction 13, eluting with n-hexane–EtOAc (4:1), was further separated by silica gel column chromatography (n-hexane–EtOAc, 10:1) and followed by reversed-phase HPLC (MeOH–H2O, 85:15) to afford 1 (1.0 mg), 2 (1.0 mg), and 6 (1.7 mg). Fraction 14, eluting with n-hexane–EtOAc (2:1), was further separated by silica gel column chromatography (n-hexane–EtOAc, 10:1) and followed by reversed-phase HPLC (MeOH–H2O, 85:15) to afford 3 (0.9 mg). Fraction 18, eluting with n-hexane–EtOAc (1:6), was rechromatographed over a Sephadex LH-20 column using acetone as the mobile phase to afford six subfractions (A1–A6). Subfractions A2 was separated by reversed-phase HPLC (CH3CN–H2O, 75:25) to afford compounds 4 (2.0 mg), and 5 (2.5 mg).
Sclerosteroid J (1): colorless oil; [α]D25 −60 (c 0.1, CHCl3); IR (neat) νmax 2926, 2865, 1739, 1576, 1452, 1236, and 1051 cm−1; 13C and 1H NMR data, see Table 1; ESIMS m/z 427 [M + Na]+; HRESIMS m/z 427.2827 [M + Na]+ (calcd for C25H40O4Na, 427.2824).
Sclerosteroid K (2): colorless oil; [α]D25 −41 (c 0.1, CHCl3); IR (neat) νmax 2926, 2870, 1744, 1677, 1233, and 1036 cm−1; UV (MeOH) λmax 239 (log ε = 4.2); 13C and 1H NMR data, see Table 1; ESIMS m/z 379 [M + Na]+; HRESIMS m/z 379.2251 [M + Na]+ (calcd for C23H32O3Na, 379.2249).
Sclerosteroid L (3): white powder; mp 150–152 °C; [α]D25 −18 (c 0.07, CHCl3); IR (neat) νmax 3568, 2922, 2870, 1712, 1244, and 1025 cm−1; 13C and 1H NMR data, see Table 1; ESIMS m/z 383 [M + Na]+; HRESIMS m/z 383.2559 [M + Na]+ (calcd for C23H36O3Na, 383.2562).
Sclerosteroid M (4): yellow oil; [α]D25 −60 (c 0.1, CHCl3); IR (neat) νmax 3448, 2926, 2868, 1739, 1369, 1241, and 1034 cm−1; 13C and 1H NMR data, see Table 2; ESIMS m/z 571 [M + Na]+; HRESIMS m/z 571.3249 [M + Na]+ (calcd for C31H48O8Na, 571.3247).
Sclerosteroid N (5): colorless oil; [α]D25 −24 (c 0.25, CHCl3); IR (neat) νmax 3390, 2926, 2870, 1739, 1374, 1250, and 1036 cm−1; 13C and 1H NMR data, see Table 2; ESIMS m/z 527 [M + Na]+ ; HRESIMS m/z 527.2989 [M + Na]+ (calcd for C29H44O7Na, 527.2985).
3-Methyl-5-(10′-acetoxy-2′,6′,10′-trimethylundecyl)-2-penten-5-olide (6): colorless oil; [α]D25 −22 (c 0.17, CHCl3); IR (neat) νmax 2926, 2865, 1729, 1363, 1250, and 1014 cm−1; UV (MeOH) λmax 211 (log ε = 4.0); 13C and 1H NMR data, see Table 2; ESIMS m/z 389 [M + Na]+ ; HRESIMS m/z 389.2666 [M + Na]+ (calcd for C22H38O4Na, 389.2668).
3.4. Cytotoxicity Testing
Cell lines were purchased from the American Type Culture Collection (ATCC). Cytotoxicity assays of compounds 1–6 were performed using the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide] colorimetric method [22,23].
3.5. In Vitro Anti-Inflammatory Assay
Macrophage (RAW264.7) cell line was purchased from ATCC. In vitro anti-inflammatory activities of tested compounds were measured by examining the inhibition of lipopolysaccharide (LPS) induced upregulation of iNOS (inducible nitric oxide synthase) and COX-2 (cyclooxygenase-2) proteins in macrophages cells using western blotting analysis [24,25].
4. Conclusions
Our present investigation again demonstrated that the Formosan soft coral Scleronephthya gracillimum could be a source of anti-inflammatory natural products. Anti-inflammatory activity assay revealed that compounds 1, 2 and 4–6, in particular 2 and 4, and the previously discovered pregnanes sclerosteroids A, B, and E, deserve further study for therapeutic potential against inflammation-related diseases.
Acknowledgements
This work was supported by a grant from National Science Council of Taiwan NSC-100-2320-B-110-001-MY2 to J.-H.S.
Supplementary Files
Supplementary Information (PDF, 1469 KB)
Footnotes
Samples Availability: Not available.
References
- 1.Blunt J.W., Copp B.R., Keyzers R.A., Munro M.H.G., Prinsep M.R. Marine natural products. Nat. Prod. Rep. 2012;29:144–222. doi: 10.1039/c2np00090c. [DOI] [PubMed] [Google Scholar]
- 2.Huo J., Tang H., Li L., Liu B.-S., Sun P., Yi Y.-H., Zhang W. Study on bioactive constituents of the South China Sea soft coral Scleronephthya sp. Acad. J. Sec. Mil. Med. Univ. 2011;32:21–24. [Google Scholar]
- 3.Han L., Wang C.-Y., Huang H., Shao C.-L., Liu Q.-A., Qi J., Sun X.-P., Zhai P., Gu Y.-C. A new pregnane analogue from Hainan soft coral Scleronephthya gracillimum Kükenthal. Biochem. Syst. Ecol. 2010;38:243–246. doi: 10.1016/j.bse.2009.12.030. [DOI] [Google Scholar]
- 4.Yan X.-H., Liu H.-L., Guo Y.-W. Ximaosteroids A–D, new steroids from the Hainan soft coral Scleronephthya sp. Steroids. 2009;74:1061–1065. doi: 10.1016/j.steroids.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Yan X.-H., Guo Y.-W., Zhu X.-Z., Mollo E., Cimino G. Studies on Chemical Constituents of the Soft Coral Scleronephthya sp. from the South China Sea. Chin. J. Org. Chem. 2004;24:1233–1238. [Google Scholar]
- 6.Kittakoop P., Suttisri R., Chaichantipyuth C., Vethchagarun S., Suwanborirux K. Norpregnane glycosides from a Thai soft coral, Scleronephthya pallida. J. Nat. Prod. 1999;62:318–320. doi: 10.1021/np980273w. [DOI] [PubMed] [Google Scholar]
- 7.Fang H.-Y., Liaw C.-C., Chao C.-H., Wen Z.-H., Wu Y.-C., Hsu C.-H., Dai C.-F., Sheu J.-H. Bioactive pregnane-type steroids from the soft coral Scleronephthya gracillimum. Tetrahedron. 2012;68:9694–9700. doi: 10.1016/j.tet.2012.09.060. [DOI] [Google Scholar]
- 8.Zhao H.Y., Shao C.L., Li Z.Y., Han L., Cao F., Wang C.Y. Bioactive pregnane steroids from a South China sea gorgonian Carijoa sp. Molecules. 2013;18:3458–3466. doi: 10.3390/molecules18033458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chao C.-H., Wen Z.-H., Su J.-H., Chen I.-M., Huang H.-C., Dai C.-F., Sheu J.-H. Further study on anti-inflammatory oxygenated steroids from the octocoral Dendronephthya griffini. Steroids. 2008;73:1353–1358. doi: 10.1016/j.steroids.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 10.Tomono Y., Hirota H., Imahara Y., Fusetani N. Four new steroids from two octocorals. J. Nat. Prod. 1999;62:1538–1541. doi: 10.1021/np990246l. [DOI] [PubMed] [Google Scholar]
- 11.Ioannou E., Abdel-Razik A.F., Alexi X., Vagias C., Alexis M.N., Roussis V. Pregnanes with antiproliferative activity from the gorgonian Eunicella cavolini. Tetrahedron. 2008;64:11797–11801. doi: 10.1016/j.tet.2008.09.078. [DOI] [PubMed] [Google Scholar]
- 12.Kashman Y., Green D., Garcia C., Garcia Arevalos D. Verrucoside, a new cytotoxic pregnane glycoside from a gorgonian Eunicella verrucosa. J. Nat. Prod. 1991;54:1651–1655. doi: 10.1021/np50078a026. [DOI] [PubMed] [Google Scholar]
- 13.Gutiérrez M., Capson T.L., Guzmán H.M., González J., Ortega-Barría E., Quiñoá E., Riguera R. Antiplasmodial metabolites isolated from the marine octocoral Muricea austera. J. Nat. Prod. 2006;69:1379–1383. doi: 10.1021/np060007f. [DOI] [PubMed] [Google Scholar]
- 14.Wang S.-K., Dai C.-F., Duh C.-Y. Cytotoxic pregnane steroids from the Formosan soft coral Stereonephthya crystallina. J. Nat. Prod. 2006;69:103–106. doi: 10.1021/np050384c. [DOI] [PubMed] [Google Scholar]
- 15.Qi S.H., Zhang S., Yang L.H., Qian P.Y. Antifouling and antibacterial compounds from the gorgonians Subergorgia suberosa and Scripearia gracillis. Nat. Prod. Res. 2008;22:154–166. doi: 10.1080/14786410701642441. [DOI] [PubMed] [Google Scholar]
- 16.Krubiner A.M., Gottfried N., Oliveto E.P. The synthesis of 17-deoxy-17-α and -17β 20-pregnynes and -20-pregnenes. J. Org. Chem. 1969;34:3502–3505. doi: 10.1021/jo01263a061. [DOI] [PubMed] [Google Scholar]
- 17.Wu S.-L., Wang G.-H., Dai C.-F., Sheu J.-H. Pregnane-based steroids from a Formosan gorgonian Subergorgia mollis. J. Chin. Chem. Soc. 2004;51:205–208. [Google Scholar]
- 18.Higgs M.D., Faulkner D.J. 5α-Pregna-1,20-dien-3-one and related compounds from a soft coral. Steroids. 1977;30:379–388. doi: 10.1016/0039-128X(77)90028-9. [DOI] [PubMed] [Google Scholar]
- 19.Gueldner R.C., Thompson A.C., Hedin P.A. Stereoselective synthesis of racemic grandisol. J. Org. Chem. 1972;37:1854–1856. doi: 10.1021/jo00976a053. [DOI] [Google Scholar]
- 20.Shimomura H., Sashida Y., Mimaki Y., Adachi T., Yoshinari K. A new mevalonolactone glucoside derivative from the bark of Prunus buergeriana. Chem. Pharm. Bull. 1989;37:829–830. doi: 10.1248/cpb.37.829. [DOI] [Google Scholar]
- 21.Henrick C.A., Willy W.E., Mckean D.R., Baggiolini E., Siddall J.B. Approaches to the synthesis of the insect juvenile hormone analog ethyl 3,7,11-trimethyl-2,4-dodecadienoate and its photochemistry. J. Org. Chem. 1975;40:8–14. doi: 10.1021/jo00889a002. [DOI] [PubMed] [Google Scholar]
- 22.Alley M.C., Scudiero D.A., Monks A., Hursey M.L., Czerwinski M.J., Fine D.L., Abbott B.J., Mayo J.G., Shoemaker R.H., Boyd M.R. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988;48:589–601. [PubMed] [Google Scholar]
- 23.Scudiero D.A., Shoemaker R.H., Paull K.D., Monks A., Tierney S., Nofziger T.H., Currens M.J., Seniff D., Boyd M.R. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988;48:4827–4833. [PubMed] [Google Scholar]
- 24.Lu Y., Huang C.-Y., Lin Y.-F., Wen Z.-H., Su J.-H., Kao Y.-H., Chiang M.-Y., Sheu J.-H. Anti-inflammatory cembranoids from the soft corals Sinularia querciformis and Sinularia granosa. J. Nat. Prod. 2008;71:1754–1759. doi: 10.1021/np8003563. [DOI] [PubMed] [Google Scholar]
- 25.Jean Y.-H., Chen W.-F., Sung C.-S., Duh C.-Y., Huang S.-Y., Lin C.-S., Tai M.-H., Tzeng S.-F., Wen Z.-H. Capnellene, a natural marine compound derived from soft coral, attenuates chronic constriction injury-induced neuropathic pain in rats. Br. J. Pharmacol. 2009;158:713–725. doi: 10.1111/j.1476-5381.2009.00323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information (PDF, 1469 KB)