Abstract
Cancer/Testis (CT) antigens are normally only expressed in germ cells and yet are aberrantly activated in a wide variety of human cancers. Most chromosome X-encoded CT antigens (CT-X) show restricted expression in pre-meiotic germ cells in adult testis, except for the expression of SPANX in post-meiotic germ cells. In the present study, the expression of eight CT-X antigens (MAGE-A, NY-ESO-1, GAGE, MAGE-C1/CT7, MAGE-C2/CT10, CT45, SAGE1, and SPANX) in non-seminomatous germ cell tumors was evaluated immunohistochemically, including 24 embryonal carcinomas, 20 yolk sac tumors, 9 teratomas, and 3 choriocarcinomas, and the results were compared to our previous study of 77 classic seminomas and 2 spermatocytic seminomas. SPANX was not detected in any germ cell tumors tested. Spermatocytic seminoma showed strong expression of all CT-X antigens tested (except SPANX), reflecting their origin from adult CT-Xpositive pre-meiotic germ cells. Classic seminomas, originating from prenatal gonocytes, showed widely variable frequency of CT-X antigen expression, ranging from > 80% (CT7, CT10, CT45, and GAGE), 63% (MAGE-A), 18% (NY-ESO-1) to only 4% (SAGE1). In comparison, non-seminomatous germ cell tumors expressed CT-X antigens much less frequently and usually only in small subsets of tumor cells. Intratubular germ cell neoplasia (ITGCN) were mostly CT-X-negative, even in CT-X positive classic seminomas. These findings indicate that CT-X antigens are not expressed in the fetal precursor cells for germ cell tumors, and their expression likely reflects germ cell differentiation of the neoplastic cells (in seminomas) or aberrant gene activation as cancer antigens (in non-seminomatous tumors).
Keywords: CT-X antigen, tumor antigens, germ cell tumors
Introduction
Cancer/Testis (CT) antigens were initially identified during the search for immunogenic tumor antigens capable of eliciting spontaneous immune responses in cancer patients. MAGE, BAGE, and GAGE antigens (1), the first group of tumor antigens shown to elicit cell-mediated immune responses in melanoma patients, were found to have their mRNA expression limited to testis, and no expression in any other normal adult tissue was detected. Subsequent serological cloning of antigens that elicited antibody responses in cancer patients identified SSX, NY-ESO-1, and CT7, all of which also share this distinctive characteristic of testis-restricted expression and aberrant activation in various types of human cancer. This unique feature led us to designate this group of antigens as CT antigens (2, 3), and clinical trials using CT antigens as cancer vaccine targets are ongoing (4-6).
The distinctive CT mRNA expression pattern also provided in silico analytic tools and led to subsequent identification of many novel genes with similar characteristics, and the number of CT and CT-like genes in the literature expanded from 20 in 2002 (7), 44 in 2004 (8), to more than 110 in the most recent version of CTpedia (9, 10), a CT-database established by the Ludwig Institute for Cancer Research (11). These genes can be separated into two main groups. The first group consists of genes on non-X chromosomes, many of them encoding proteins with known biological functions either in spermatids or in the process of spermatogenesis, including ACRBP (acrosinbinding protein), ADAM2, and meiotic proteins SCP1, SYCE, and HORMAD1 (12-14). Most of these genes are expressed during or after meiosis, but not in the pre-meiotic spermatogonia or spermatocytes. In contrast, the second group comprises approximately 30 genes (or gene families) on X chromosome, most of them encoding proteins with unknown functions. These genes have been referred to as CT-X genes (3, 10). By immunohistochemical studies, we recently showed that most of these genes, including MAGE-A, NY-ESO-1, GAGE, CT7, CT10, CT45, and SAGE1, are expressed in spermatogonia and/or primary spermatocytes in adult testis, but not in post-meiotic germ cells. The only exception to this rule is the SPANX gene family that encodes proteins that are involved in the morphogenesis of mature sperm cells and are expressed in spermatids and sperm cells (15, 16).
In fetal testis, all germ cells are derived from OCT3/4-positive primordial germ cells (PGCs). As these OCT3/4-positive pluripotent cells migrate to the gonads from the dorsal yolk sac and develop into germ cells, the expression of OCT3/4 is lost (17-19). By immunohistochemical analysis, we recently showed that the expression of OCT3/4 and CT-X antigens are almost always mutually exclusive, and CT-X expression in fetal testis only appears when fetal testicular gonocytes have lost OCT3/4 expression and committed to spermatogenesis (20). This finding of CT-X antigen expression in fetal testicular germ cells, possibly including primordial germ cells, raises the possibility that CT-X antigens might be frequently expressed in germ cell tumors (GCTs). Although several studies have investigated this issue, the analyses were limited to one or two CT antigens, e.g., MAGE-A (21-24), NY-ESO-1(25), SSX (26), GAGE (27), and CT45 (28), and the results were discrepant, particularly in terms of their expression in non-seminomatous germ cell tumors and in intratubular germ cell neoplasia (ITGCN). We recently evaluated the expression of multiple CT antigens in fetal gonads and in seminoma (20), and we have now expanded this study to non-seminomatous germ cell tumors (NSGCTs) to explore the possible differential expression of CT antigens in this heterogeneous group of tumors that might reflect different mechanisms of gene activation and evaluate their potential values as diagnostic markers or therapeutic targets.
Results
Of the eight CT-X antigens (MAGE-A, NY-ESO-1, GAGE, CT7, CT10, CT45, SAGE1, and SPANX) examined, no SPANX expression was identified in any germ cell tumors. In comparison, all seven other CT-X antigens were expressed at variable frequencies in different types of germ cell tumors, more abundantly in spermatocytic seminomas and classic seminomas, as previously reported (20). Non-seminomatous germ cell tumors expressed CT-X antigens at variable but in general much lower frequencies, and these results are summarized in Table 1 in conjunction with the previous results on seminomas.
Table 1. Expression of CT-X antigens in germ cell tumors.
Embryonal carcinoma
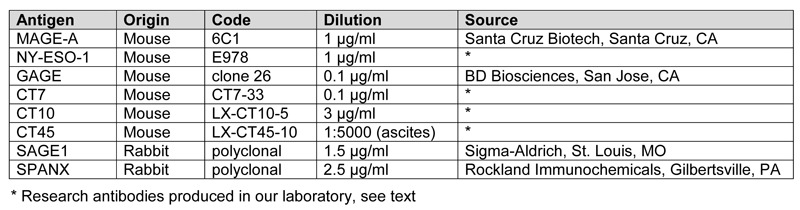
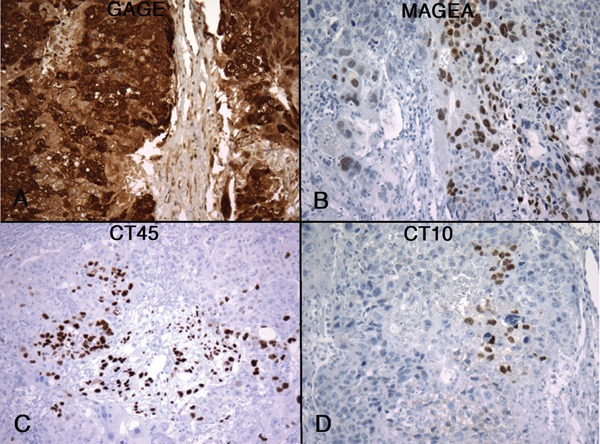
In comparison to seminomas, embryonal carcinoma showed no or minimal expression of CT-X antigens. Of 24 embryonal carcinomas, 10 were totally negative for all CT-X antigens and 11 showed positivity in a very small subset of tumor cells, comprising < 1% of the tumor cells in most cases. The only exception to this very focal pattern of CT-X expression was seen in a case of mixed germ cell tumors that contained yolk sac tumor, choriocarcinoma, and embryonal carcinoma components. In this particular case, GAGE expression was observed in all three components, including in > 90% of the embryonal carcinoma component (Figure 1). The other 7 CT antigens tested were all negative in the embryonal carcinoma component.
Figure 1. Expression of CT-X antigens in embryonal carcinoma. Examples of MAGE-A (A), NY-ESO-1 (B), GAGE (C), and CT7 (D) expression are shown, illustrating the highly focal nature of CT-X expression in embryonal carcinoma, the only exception being the expression of GAGE in a case of mixed germ cell tumor with embryonal carcinoma component (C). (Magnification, 200X).
Among the eight CT-X antigens analyzed, GAGE was most frequently expressed in embryonal carcinomas, observed in 10 of 24 (42%) cases. Other CT-X antigens were only expressed in 4% to 21% of cases, all in extremely focal patterns.
Yolk sac tumor
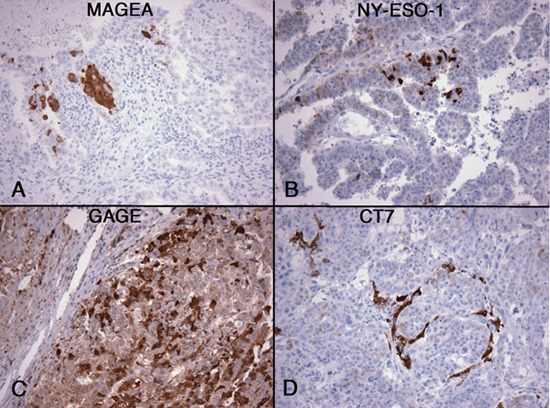
Among non-seminomatous GCTs, yolk sac tumors showed most frequent expression of CT-X antigens. Twenty yolk sac tumors were examined, including 3 pediatric yolk sac tumors, and CT-X expression was detected in 12 (60%) cases, 9 of them expressing 3 or more CT-X antigens. Two of the three pediatric yolk sac tumors were CT-X-positive but expressed only CT45 and not any other CT-X antigens. The extent of positivity is highly variable, from < 1% of tumor cells being positive to diffuse positivity in 80-100% of the tumor cells (Figure 2).
Figure 2. Expression of CT-X antigens in yolk sac tumor. Examples of MAGE-A (A), CT45 (B), CT7 (C), and SAGE1 (D) expression are shown. The expression pattern is patchy in most cases, but often positive in a larger proportion of tumor cells when compared to CT-X antigen-positive embryonal carcinomas. (Magnification, 200X).
Among the CT-X antigens, CT45, CT7, and MAGE-A were most frequently expressed in adult yolk sac tumors, detected in 59%, 43%, and 47% of cases, respectively. In comparison, CT10, GAGE, SAGE1, and NY-ESO-1 were expressed in 24-35% of the cases.
Teratoma
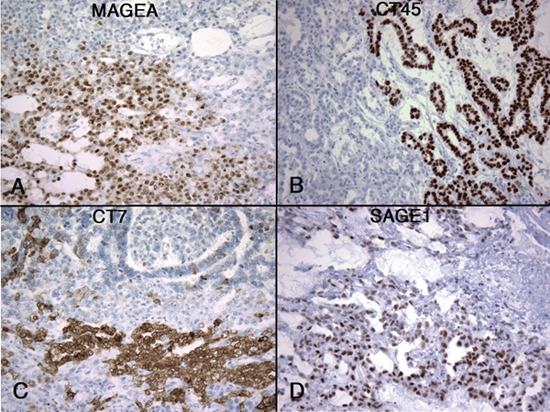
A component of mature or immature teratoma was identified in nine cases as part of mixed non-seminomatous germ cell tumor. These teratoma components showed either no CT-X expression (5/9) or minimal (4/9) expression in < 1% of the tumor cells. In one of the 4 positive cases, the same small cluster of cells were found to co-express MAGE-A, CT7, GAGE, and NY-ESO-1 (Figure 3). The other three cases were positive for one to three CT-X antigens.
Figure 3. Expression of CT-X antigens in a case of teratoma. Teratomasarerarelypositive,but one case was positive for MAGE-A (A), CT7 (B), GAGE (C), and NY-ESO-1 (D). The same small clusters of positive cells (< 1% of the tumor) were noted to simultaneously express these CT-X antigens in a CT-X-negative background. (Magnification, 200X).
Choriocarcinoma
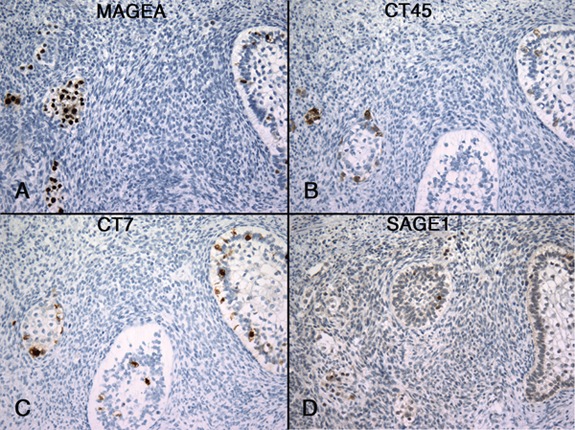
Only three choriocarcinomas were in this series and two of three were CT-X antigen-positive. One case showed expression of GAGE, MAGE-A, CT10, and CT45, but in highly variable percentages of cells, estimated to be 90%, 30%, < 5%, and < 2%, respectively (Figure 4). Positive staining was observed in both cytotrophoblasts and syncytiotrophoblasts. The other case was only positive for CT45, detected in about 40% of the tumor cells.
Figure 4. Expression of CT-X antigens in a case of choriocarcinoma. Heterogeneous staining patterns were observed, more diffusely positive for GAGE (A) and MAGE-A (B) than for CT45 (C) and CT10 (D). Both syncytiotrophoblasts and cytotrophoblasts were MAGE-A-positive. (Magnification, 200X).
Intratubular germ cell neoplasia (ITGCN)
CT-X-positive germ cells were often identified in atrophic seminiferous tubules adjacent to the germ cell tumors regardless of the CT-X expression status of the main tumor. Whether these represent ITGCN or residual spermatogonia or both could be difficult to distinguish histologically (Figure 5A vs. 5C). To separate residual non-neoplastic spermatogonia from ITGCN, OCT3/4 was used to define ITGCN and possible co-expression of CT-X was evaluated by double-staining of OCT3/4 and CT7, or OCT3/4 and MAGE-A. CT7 shows cytoplasmic staining in spermatogonia and MAGE-A is present as both nuclear and cytoplasmic staining, allowing their detection in ITGCN cells with nuclear OCT3/4 positivity (Figure 5B and 5D).
Figure 5. Expression of CT-X antigens in ITGCN. Seminiferous tubules in two different cases of seminoma showed possible ITGCN (A and C). Using OCT3/4 (nuclear, brown) and CT7 (cytoplasmic, red) dual staining, one case (B) showed ITGCN with mutually exclusive expression between CT7-negative; OCT3/4-positive ITGCN cells; and CT7-positive, OCT3/4-negative residual spermatogonia in the same tubules, whereas the other case (D) showed only spermatogonia (i.e., CT7-positive, OCT3/4negative), negative for ITGCN. The invasive component of this second tumor (D, on the right) was OCT3/4-positive and CT7-negative. In most CT-X positive cases (E, a case of CT7-positive mixed embryonal carcinoma and yolk sac tumor), the ITGCN component (F, OCT3/4-positive cells in brown) was negative for CT-X expression (CT7, red). However, CT-X-positive ITGCN cells could be identified in some cases, evidenced by co-expression of OCT3/4 and CT7 (E) [or OCT3/4 and MAGE-A (F)], co-existing with CT-X-negative ITGCN cells. (Magnification, 400X).
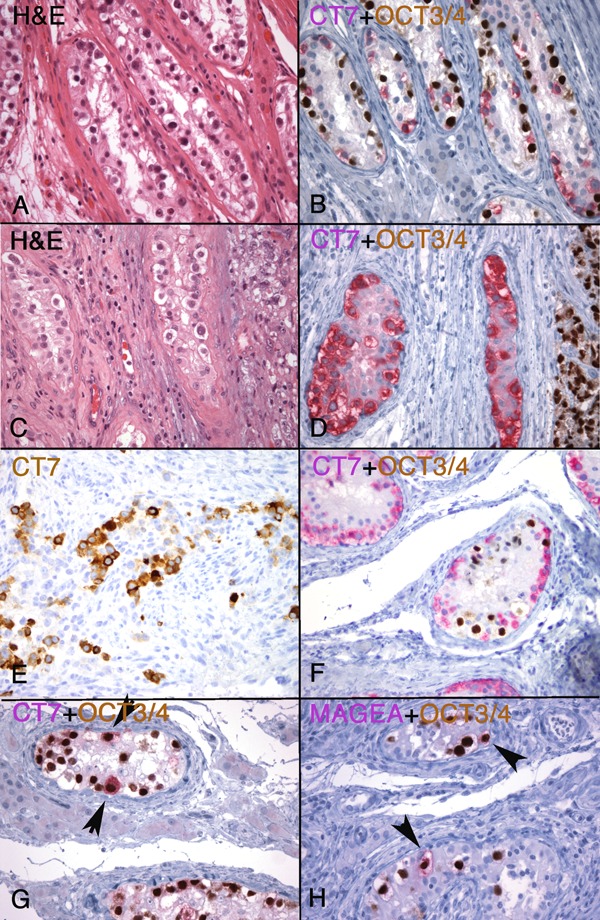
These double-staining experiments showed that the majority (14/18, 78%) of ITGCN were negative for CT7 (or MAGE-A) expression, including 5 cases in which the invasive component was CT7 (or MAGE-A)-positive. An example of this is shown in Figure 5E and 5F. As illustrated, ITGCN often co-existed with non-neoplastic spermatogonia in the same tubules, evidenced by their positive CT7 (Figure 5B, 5F, and 5G) or MAGE-A (Figure 5H) staining. Exceptions to this negative expression of CT-X in ITGCN cells, however, were observed in two cases each for CT7 and MAGE-A in which occasional OCT3/4 and CT7 (or MAGE-A) dual-positive cells were identified (Figure 5G and 5H), co-existing with OCT3/4-positive and CT7 (or MAGE-A)negative ITGCN cells.
Intratubular spematocytic seminoma
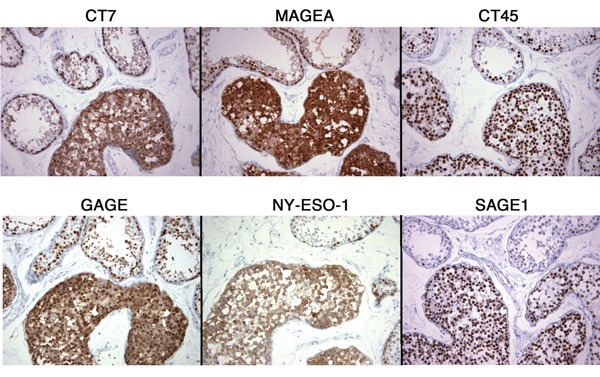
In contrast to the rare expression of CT-X antigens in ITGCN, the intratubular component of the spermatocytic seminoma that is present in one of the two cases expressed all seven CT-X antigens in a homogeneous manner, similar to its invasive component (Figure 6).
Figure 6. Expression of CT-X antigens in the intratubular component of spermatocytic seminoma and adjacent non-neoplastic seminiferous tubules. All intratubular tumor cells showed diffuse expression of CT-X antigens, as pure nuclear antigens (CT45 and SAGE1), predominantly cytoplasmic antigens (CT7 and NY-ESO-1), or mixed nuclear and cytoplasmic antigens (MAGE-A and GAGE). Adjacent non-neoplastic tubules showed strong staining of the spermatogonia, with variable staining of the more mature forms. (Magnification, 400X).
Discussion
Germ cell tumors originate from germ cells at different stages of maturation and most of the phenotypic characteristics found in this heterogeneous group of tumors are believed to reflect the gene expression profile at that specific stage of germ cell development. Oosterhius and Looijenga (29) proposed a classification scheme that separated GCTs into 5 types based on this notion. Type I GCTs, the teratomas and yolk sac tumors seen in neonates and childhood, presumably derived from early primordial germ cell (PGC) or gonocytes. Type II GCTs, adult seminomatous and non-seminomatous GCTs, including most testicular GCTs, are believed to originate from PGC/gonocytes in utero, but at a maturation stage slightly later than the type I precursors, evidenced by the erased pattern of genomic imprinting in these tumors. The only adult testicular GCT that does not belong to type II is spermatocytic seminoma, which originates from adult pre-meiotic germ cells rather than prenatal PGC/ gonocytes and is classified as type III. Dermoid cyst of the ovary and hydatiform mole of pregnancy represent special categories of GCTs from oogonia/oocyte and empty ovum/spermatozoa, respectively, and are classified as types IV and V.
This concept that different GCTs originate from germ cells at different stages of maturation is best supported by the different gene expression profiles of classic seminoma and spermatocytic seminoma. Seminomas express markers typically seen in embryonic stem cells and PGCs, including OCT3/4, c-KIT, PLAP, and NANOG (18, 30). In contrast, spermatocytic seminomas have completely lost these markers of pluripotency and express markers characteristic of spermatogenesis, including CT antigens. This difference was convincingly shown in the mRNA expression microarray study by Looijenga et al. (31) in which CT antigen genes GAGE, SSX, MAGE, SAGE, and BORIS all showed 20-to 100-fold higher expression in spermatocytic seminoma than in seminomas, and OCT3/4 and NANOG were 100-fold higher in seminomas. Previous protein expression studies by immunohistochemistry confirmed the lack of OCT3/4 and NANOG in spermatocytic seminoma (18, 19, 32) and the universal expression of SSX [in 13 of 13 cases by Stoop et al. (26)] and MAGE-A [in 25 of 25 cases by Rajpert-De Meyts et al. (24)] in spermatocytic seminoma. Supporting these prior findings and expanding on our recent study (20), we now found that all pre-meiotically expressed CT-X proteins, including GAGE, SAGE, NY-ESO-1, CT7, CT10, and CT45, are diffusely expressed in spermatocytic seminomas, including in the intratubular in situ component. This finding supports the notion that spermatocytic seminomas are derived from CT-positive pre-meiotic adult germ cells. With the exception of CT10 and CT45 that show strongest expression in primary spermatocytes (27, 30), all antigens above have strongest expression in spermatogonia, indicating that spermatocytic seminomas likely originate from spermatogonia, as was previously proposed (24). However, since all CT-X antigens examined in the study are expressed at both spermatogonia and primary spermatocytes stages, albeit at different levels, it remains possible that spermatocytic seminomas can originate from primary spermatocytes (31). Different from these finding of 100% CT-X expression in spermatocytic seminoma, however, Satie et al. (25) detected NY-ESO-1 expression in only 8 of 16 spermatocytic seminomas. This less frequent expression of NY-ESO-1 might reflect the fact that all spermatogonia, while positive for MAGE-A, are not NY-ESO-1-positive (20, 33). Alternatively, the absence of NY-ESO-1 expression in some spermatocytic seminomas in their study might be due to a lower sensitivity in their immunohistochemical detection of NY-ESO-1, as different antibodies were used in their and our studies.
In contrast to the expression of almost all CT-X antigens in the intratubular spermatocytic seminomas, the in situ precursors of all testicular seminomatous and non-seminomatous germ cell tumors, ITGCN cells, were mostly CT-negative in our study, even when the corresponding invasive tumors were CT-positive. This finding was also observed by Stoop et al. (26) who noted that ITGCN cells in all three SSX-positive seminomas in their series were SSX-negative. In contrast to this finding, however, were studies that found ITGCN cells to be at least partially positive for MAGE-A in 13 of 15 cases (21), for NY-ESO-1 in 7 of 15 cases (25), and for CT45 expression in most, if not all, cases (28). This reason for this discrepancy in the prevalence of CT-X expression in ITGCN is not entirely clear. One main difference between the current and the earlier studies, however, was that we defined our ITGCN population by positive OCT3/4-positive, rather than relying on histological criteria alone. We found this to be necessary as atrophic tubules adjacent to the GCTs often contain residual spermatogonia that are histologically almost indistinguishable from ITGCN cells (Figure 5A vs. 5C). Based on this observation, we suspect that at least some of the CT-positive ITGCN cases described in the earlier literature might represent residual benign spermatogonia in atrophic tubules, and not ITGCN cells. Combining our data with those of earlier studies, we believe that ITGCN cells could be either CT-X-positive or -negative, and these two populations can co-exist in the same case. Since the precursors of type II GCTs are OCT3/4-positive pluripotent PGC/gonocytes that normally would be CT-X antigen-negative, it is most likely that early ITGCN cells are CT-X-negative, which subsequently become CT-X antigen-positive in some, but not all, cases. One likely explanation for this gain of CT expression would be the partial differentiation of tumor cells along the pathway of spermatogenesis. Since seminoma represents a differentiation of ITGCN cells toward germ cell lineage (29, 34), it is fully conceivable that CT antigen expression can be switched on during this differentiation process, either during the in situ stage or in the subsequent invasive phase. This concept is corroborated by two findings in our current study. One is the much more frequent expression of CT antigens in seminoma than in GCTs that are either undifferentiated, i.e., embryonal carcinomas, or differentiated towards somatic lineages, i.e., teratomas. The other is the highly variable expression frequency for individual CT antigen in seminomas, ranging from > 80% (CT7, CT10, CT45, and GAGE) to < 5% (SAGE) or negative (SPANX). This cascade of CT expression frequency in seminoma, high in CT7 and GAGE and low in SAGE, roughly correlates with the relative abundance of these CT-X antigens in developing fetal germ cells (20) and suggests a parallel germ cell differentiation process in both benign and malignant germ cells. The high frequency of CT45 expression in classic seminoma (92%) that we observed confirmed the findings of Rudolph et al. (100% in 55 cases), and the lower frequency of MAGE-A expression (63%) in our data also falls within the previously described frequency of 42%-71% (21-23). However, NY-ESO-1, observed in 18% (14/76) of our cases, was not detected in any of the 13 cases previously analyzed by Satie et al. (25). The reason for this discrepancy is unclear but it is likely attributable to our larger sample size and/or to the different sensitivity of the anti-NY-ESO-1 antibodies used in these two studies.
In contrast to seminomas, expression of CT-X antigens in non-seminomatous GCTs cannot be explained by this notion of germ cell differentiation, and two other mechanisms could be postulated. One is the aberrant epigenetic activation of CT-X genes as cancer antigens similar to their activation in non-germ cell malignancy and the other is that some non-seminomatous GCTs might have foci of tumor cells that have retained or acquired partial germ cell features, resulting in their CT-X positivity. Which one of the two mechanisms (or both) is at play is unknown to us at present. Although additional studies, e.g., methylation studies of the promoter regions of these CT genes, might help to clarify this, the highly focal nature of CT expression in these tumors would mean that laser microdissection is necessary to separate CT-negative from CT-positive areas for such experiments. Given this technical challenge and the quantitative nature of the methylation assays, whether this approach could unequivocally distinguish these two possible mechanisms is uncertain. It is clear, however, that either mechanism only occurs rather infrequently, as indicated by the much lower frequency of CT-X expression in non-seminomatous GCTs, as well as the focal and often patchy expression pattern in individual tumor. In fact, two previous studies showed no MAGE-A4 expression in 10 cases of non-seminomatous GCTs (21) and no NY-ESO-1 expression in 13 cases (25), leading to the notion that CT-X genes are always silent in these tumors. With a larger series of samples, we now know that CT-X antigens can be expressed in non-seminomatous GCTs, albeit at significantly lower frequencies than in seminomas. Our observation of CT-X expression in non-seminomatous GCTs is also supported by the previously described expression of CT45 in about 50% of yolk sac tumor (28) and GAGE expression in a teratoma (27). Similarly, mRNA studies (35) have demonstrated CT expression in various types of NSGCTs, including yolk sac tumor, embryonal carcinoma, and mixed NSGCTs. Of interest, yolk sac tumors, either from pediatric or adult patients, appear to express CT-X antigens more frequently than other types of non-seminomatous GCTs [(28) and the present study], suggesting that the second mechanism (of retained gonocyte phenotype) described above might be involved in this specific tumor type.
In summary, CT antigens, normally expressed in pre-spermatogonia in fetal testis and in spermatogonia and pre-meiotic spermatocytes in adult, are expressed most frequently in spermatocytic seminomas, less in classic seminomas, and least in NSGCTs. Given their overlapping expression patterns in these tumors, CT antigens are not useful diagnostic markers for GCTs. However, their expression at a significant proportion of GCTs does suggest CT antigens as candidates for targeted therapy for GCTs. Biologically, our findings indicate three distinctive mechanisms of CT gene activation. In spermatocytic seminoma, CT expression reflects the intrinsic characteristics of the precursor adult pre-meiotic germ cells and the expression is most homogeneous in pattern. In classic seminoma, CT expression is acquired as a result of germ cell differentiation, likely from CT-negative ITGCN cells. In NSGCTs, CT expression is likely due to epigenetic aberrant activation or retained gonocyte phenotype, and this would account for the heterogeneous and often focal CT-X antigen expression pattern in these tumors.
Abbreviations
CT, Cancer/Testis; ITGCN, intratubular germ cell neoplasia; NSGCT, non-seminomatous germ cell tumor
Materials and methods
Tissues
Formalin-fixed paraffin-embedded tissue blocks of normal and tumor tissues were obtained from the Department of Pathology and Laboratory Medicine at Weill Cornell Medical College and Washington University School of Medicine following protocols approved by the two Institutional Review Boards (IRB). All hematoxylin and eosin stained tissue sections were evaluated and the diagnoses of seminoma and various components of NSGCTs were confirmed.
Monoclonal and polyclonal antibodies
The antibodies used are summarized in Table 2. Antibodies against GAGE, SAGE1, MAGE-A, and SPANX were purchased commercially. GAGE antibody produced against GAGE-7 is expected to react with all GAGE gene products due to the extreme high sequence homology among the GAGE proteins. MAGE-A monoclonal antibody 6C1 produced against MAGE-A1 has been shown to be broad-reactive for gene products of MAGE-A multigene family, including MAGE-A1, A2, A3, A4, A6, A10, and A12 protein (36). SPANX antibody, obtained commercially, was produced against SPANX-C. However, due to the polyclonal nature of this antibody, it is highly likely that this antibody would also recognize SPANX-A and SPANX-B proteins. Antibodies against the other CT-X antigens NY-ESO-1, CT7, CT10, and CT45 were produced and characterized in our laboratory and have been previously described (37-40).
Table 2. Anti-CT-X antibodies used in the present study.
Immunohistochemical analysis
Immunohistochemical (IHC) analysis was performed using formalin-fixed paraffin-embedded tissues. Whole sections were used for non-seminomatous germ cell tumors, and expression in seminoma was evaluated using a tissue microarray (TMA), each case represented by three 0.6 mm tissue cores. Selective cases of seminoma were also evaluated on whole sections for the evaluation of ITGCN. Five μM tissue sections on coated slides were deparaffinized, rehydrated, and treated in H2O2 to block the endogenous peroxidase activity. The sections were then subjected to antigen retrieval by autoclaving for 15 minutes in 10 mM citrate buffer, pH 6.0. The sections were incubated with the primary antibody for one hour at room temperature, followed by detection using Dako EnVision+ horseradish peroxidase mouse (or rabbit) detection system (DakoCytomation) and DAB as the chromogen. The slides were counterstained with hematoxylin and evaluated. Any staining of the tumor cells is regarded as positive, and positive immunoreactivity was recorded as + to +++ based on the staining intensity. The staining pattern, either focal (< 50%) or diffuse (> 50%), was also recorded. Adult testis was used as positive control and immunoglobulin isotype controls were also included in all experiments.
For double immunostaining, the first antibody staining was performed as above, using DAB as chromogen. After DAB chromogen reaction, the slides were washed with Tris-buffered saline pH 7.0, blocked by Dual Endogenous Enzyme Block (DakoCytomation), and incubated with the second antibody for 30 minutes at room temperature, followed by detection using LSAB 2 System-Alkaline Phosphatase (DakoCytomation) and developed by Permanent Red chromogen. The slides were then counterstained and scored as described above.
References
- 1.Boon T, Coulie PG, Van den Eynde B. Tumor antigens recognized by T cells. Immunol Today. 1997;18:267–268. doi: 10.1016/s0167-5699(97)80020-5. [DOI] [PubMed] [Google Scholar]
- 2.Caballero OL, Chen YT. Cancer/testis (CT) antigens: potential targets for immunotherapy. Cancer Sci. 2009;100:2014–2021. doi: 10.1111/j.1349-7006.2009.01303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simpson AJ, Caballero OL, Jungbluth A, Chen YT, Old LJ. Cancer/testis antigens gametogenesis and cancer. Nat Rev Cancer. 2005;5:615–625. doi: 10.1038/nrc1669. [DOI] [PubMed] [Google Scholar]
- 4.Brichard VG, Lejeune D. GSK’s antigen-specific cancer immunotherapy programme: pilot results leading to Phase III clinical development. Vaccine. 2007;(25 Suppl 2):B61–B71. doi: 10.1016/j.vaccine.2007.06.038. [DOI] [PubMed] [Google Scholar]
- 5.Davis ID, Chen W, Jackson H, Parente P, Shackleton M, Hopkins W, Chen Q, Dimopoulos N, Luke T, Murphy R, Scott AM, Maraskovsky E, McArthur G, MacGregor D, Sturrock S, Tai TY, Green S, Cuthbertson A, Maher D, Miloradovic L, Mitchell SV, Ritter G, Jungbluth AA, Chen YT, Gnjatic S, Hoffman EW, Old LJ, Cebon JS. Recombinant NY-ESO-1 protein with ISCOMATRIX adjuvant induces broad integrated antibody and CD4(+) and CD8(+) T cell responses in humans. Proc Natl Acad Sci U S A. 2004;101:10697–10702. doi: 10.1073/pnas.0403572101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marchand M, van Baren N, Weynants P, Brichard V, Dreno B, Tessier MH, Rankin E, Parmiani G, Arienti F, Humblet Y, Bourlond A, Vanwijck R, Lienard D, Beauduin M, Dietrich PY, Russo V, Kerger J, Masucci G, Jager E, De Greve J, Atzpodien J, Brasseur F, Coulie PG, van der Bruggen P, Boon T. Tumor regressions observed in patients with metastatic melanoma treated with an antigenic peptide encoded by gene MAGE-3 and presented by HLA-A1. Int J Cancer. 1999;80:219–230. doi: 10.1002/(sici)1097-0215(19990118)80:2<219::aid-ijc10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 7.Scanlan MJ, Güre AO, Jungbluth AA, Old LJ, Chen YT. Cancer/testis antigens: an expanding family of targets for cancer immunotherapy. Immunol Rev. 2002;188:22–32. doi: 10.1034/j.1600-065x.2002.18803.x. [DOI] [PubMed] [Google Scholar]
- 8.Scanlan MJ, Simpson AJ, Old LJ. The cancer/testis genes: review, standardization, and commentary. Cancer Immun. 2004;4(1) [PubMed] [Google Scholar]
- 9.Almeida LG, Sakabe NJ, deOliveira AR, Silva MC, Mundstein AS, Cohen T, Chen YT, Chua R, Gurung S, Gnjatic S, Jungbluth AA, Caballero OL, Bairoch A, Kiesler E, White SL, Simpson AJ, Old LJ, Camargo AA, Vasconcelos AT. CTdatabase: a knowledge-base of high-throughput and curated data on cancer-testis antigens. Nucleic Acids Res. 2009;37:D816–D819. doi: 10.1093/nar/gkn673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hofmann O, Caballero OL, Stevenson BJ, Chen YT, Cohen T, Chua R, Maher CA, Panji S, Schaefer U, Kruger A, Lehvaslaiho M, Carninci P, Hayashizaki Y, Jongeneel CV, Simpson AJ, Old LJ, Hide W. Genome-wide analysis of cancer/testis gene expression. Proc Natl Acad Sci U S A. 2008;105:20422–20427. doi: 10.1073/pnas.0810777105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.CTpedia. Accessed from: http://www.cta.lncc.br/ [Google Scholar]
- 12.Chen YT, Venditti CA, Theiler G, Stevenson BJ, Iseli C, Güre AO, Jongeneel CV, Old LJ, Simpson AJ. Identification ofCT46/HORMAD1, an immunogenic cancer/testis antigen encoding a putative meiosis-related protein. Cancer Immun. 2005;5(9) [PubMed] [Google Scholar]
- 13.Ono T, Kurashige T, Harada N, Noguchi Y, Saika T, Niikawa N, Aoe M, Nakamura S, Higashi T, Hiraki A, Wada H, Kumon H, Old LJ, Nakayama E. Identification of proacrosin binding protein sp32 precursor as a human cancer/testis antigen. Proc Natl Acad Sci U S A. 2001;98:3282–3287. doi: 10.1073/pnas.041625098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Türeci O, Sahin U, Zwick C, Koslowski M, Seitz G, Pfreundschuh M. Identification of a meiosis-specific protein as a member of the class of cancer/testis antigens. Proc Natl Acad Sci U S A. 1998;95:5211–5216. doi: 10.1073/pnas.95.9.5211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Westbrook VA, Diekman AB, Klotz KL, Khole VV, von Kap-Herr C, Golden WL, Eddy RL, Shows TB, Stoler MH, Lee CY, Flickinger CJ, Herr JC. Spermatid-specific expression of the novel X-linked gene product SPAN-X localized to the nucleus of human spermatozoa. Biol Reprod. 2000;63:469–481. doi: 10.1093/biolreprod/63.2.469. [DOI] [PubMed] [Google Scholar]
- 16.Kouprina N, Noskov VN, Pavlicek A, Collins NK, Schoppee Bortz PD, Ottolenghi C, Loukinov D, Goldsmith P, Risinger JI, Kim JH, Westbrook VA, Solomon G, Sounders H, Herr JC, Jurka J, Lobanenkov V, Schlessinger D, Larionov V. Evolutionary diversification of SPANX-N sperm protein gene structure and expression. PLoS One. 2007;2:e359. doi: 10.1371/journal.pone.0000359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaskell TL, Esnal A, Robinson LL, Anderson RA, Saunders PT. Immunohistochemical profiling of germ cells within the human fetal testis: identification of three subpopulations. Biol Reprod. 2004;71:2012–2021. doi: 10.1095/biolreprod.104.028381. [DOI] [PubMed] [Google Scholar]
- 18.Looijenga LH, Stoop H, de Leeuw HP, de Gouveia Brazao CA, Gillis AJ, van Roozendaal KE, van Zoelen EJ, Weber RF, Wolffenbuttel KP, van Dekken H, Honecker F, Bokemeyer C, Perlman EJ, Schneider DT, Kononen J, Sauter G, Oosterhuis JW. POU5F1 (OCT3/4) identifies cells with pluripotent potential in human germ cell tumors. Cancer Res. 2003;63:2244–2250. [PubMed] [Google Scholar]
- 19.Rajpert-De Meyts E, Hanstein R, Jorgensen N, Graem N, Vogt PH, Skakkebaek NE. Developmental expression of POU5F1 (OCT-3/4) in normal and dysgenetic human gonads. Hum Reprod. 2004;19:1338–1344. doi: 10.1093/humrep/deh265. [DOI] [PubMed] [Google Scholar]
- 20.Chen YT, Chiu R, Lee P, Beneck D, Jin B, Old LJ. Chromosome X-encoded cancer/testis antigens show distinctive expression patterns in developing gonads and in testicular seminoma. Hum Reprod. 2011;26:3232–3243. doi: 10.1093/humrep/der330. [DOI] [PubMed] [Google Scholar]
- 21.Aubry F, Satie AP, Rioux-Leclercq N, Rajpert-De Meyts E, Spagnoli GC, Chomez P, De Backer O, Jegou B, Samson M. MAGE-A4, a germ cell specific marker, is expressed differentially in testicular tumors. Cancer. 2001;92:2778–2785. doi: 10.1002/1097-0142(20011201)92:11<2778::aid-cncr10125>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 22.Cheville JC, Roche PC. MAGE-1 and MAGE-3 tumor rejection antigens in human germ cell tumors. Modern Pathol. 1999;12:974–978. [PubMed] [Google Scholar]
- 23.Grobholz R, Verbeke CS, Schleger C, Kohrmann KU, Hein B, Wolf G, Bleyl U, Spagnoli GC, Coplan K, Kolb D, Iversen K, Jungbluth AA. Expression of MAGE antigens and analysis of the inflammatory T-cell infiltrate in human seminoma. Urol Res. 2000;28:398–403. doi: 10.1007/s002400000143. [DOI] [PubMed] [Google Scholar]
- 24.Rajpert-De Meyts E, Jacobsen GK, Bartkova J, Aubry F, Samson M, Bartek J, Skakkebaek NE. The immunohistochemical expression pattern of Chk2, p53, p19INK4d, MAGE-A4 and other selected antigens provides new evidence for the premeiotic origin of spermatocytic seminoma. Histopathology. 2003;42:217–226. doi: 10.1046/j.1365-2559.2003.01587.x. [DOI] [PubMed] [Google Scholar]
- 25.Satie AP, Rajpert-De Meyts E, Spagnoli GC, Henno S, Olivo L, Jacobsen GK, Rioux-Leclercq N, Jegou B, Samson M. The cancer-testis gene, NY-ESO-1, is expressed in normal fetal and adult testes and in spermatocytic seminomas and testicular carcinoma in situ. Lab Invest. 2002;82:775–780. doi: 10.1097/01.lab.0000017169.26718.5f. [DOI] [PubMed] [Google Scholar]
- 26.Stoop H, van Gurp R, de Krijger R, van Kessel AG, Koberle B, Oosterhuis W, Looijenga L. Reactivity of germ cell maturation stage-specific markers in spermatocytic seminoma: diagnostic and etiological implications. Lab Invest. 2001;81:919–928. doi: 10.1038/labinvest.3780302. [DOI] [PubMed] [Google Scholar]
- 27.Gjerstorff MF, Harkness L, Kassem M, Frandsen U, Nielsen O, Lutterodt M, Mollgard K, Ditzel HJ. Distinct GAGE and MAGE-A expression during early human development indicate specific roles in lineage differentiation. Hum Reprod. 2008;23:2194–2201. doi: 10.1093/humrep/den262. [DOI] [PubMed] [Google Scholar]
- 28.Rudolph P, Kellner U, Schmidt D, Kirchner V, Talerman A, Harms D, Parwaresch R. Ki-A10, a germ cell nuclear antigen retained in a subset of germ cell-derived tumors. Am J Pathol. 1999;154:795–803. doi: 10.1016/S0002-9440(10)65326-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oosterhuis JW, Looijenga LH. Testicular germ-cell tumours in a broader perspective. Nat Rev Cancer. 2005;5:210–222. doi: 10.1038/nrc1568. [DOI] [PubMed] [Google Scholar]
- 30.Stoop H, Honecker F, van de Geijn GJ, Gillis AJ, Cools MC, de Boer M, Bokemeyer C, Wolffenbuttel KP, Drop SL, de Krijger RR, Dennis N, Summersgill B, McIntyre A, Shipley J, Oosterhuis JW, Looijenga LH. Stem cell factor as a novel diagnostic marker for early malignant germ cells. J Pathol. 2008;216:43–54. doi: 10.1002/path.2378. [DOI] [PubMed] [Google Scholar]
- 31.Looijenga LH, Hersmus R, Gillis AJ, Pfundt R, Stoop HJ, van Gurp RJ, Veltman J, Beverloo HB, van Drunen E, van Kessel AG, Pera RR, Schneider DT, Summersgill B, Shipley J, McIntyre A, van der Spek P, Schoenmakers E, Oosterhuis JW. Genomic and expression profiling of human spermatocytic seminomas: primary spermatocyte as tumorigenic precursor and DMRT1 as candidate chromosome 9 gene. Cancer Res. 2006;66:290–302. doi: 10.1158/0008-5472.CAN-05-2936. [DOI] [PubMed] [Google Scholar]
- 32.Jones TD, Ulbright TM, Eble JN, Baldridge LA, Cheng L. OCT4 staining in testicular tumors: a sensitive and specific marker for seminoma and embryonal carcinoma. Am J Surg Pathol. 2004;28:935–940. doi: 10.1097/00000478-200407000-00014. [DOI] [PubMed] [Google Scholar]
- 33.Gjerstorff MF, Kock K, Nielsen O, Ditzel HJ. MAGE-A1, GAGE and NY-ESO-1 cancer/testis antigen expression during human gonadal development. Hum Reprod. 2007;22:953–960. doi: 10.1093/humrep/del494. [DOI] [PubMed] [Google Scholar]
- 34.van de Geijn GJ, Hersmus R, Looijenga LH. Recent developments in testicular germ cell tumor research. Birth Defects Res C Embryo Today. 2009;87:96–113. doi: 10.1002/bdrc.20140. [DOI] [PubMed] [Google Scholar]
- 35.Yuasa T, Okamoto K, Kawakami T, Mishina M, Ogawa O, Okada Y. Expression patterns of cancer testis antigens in testicular germ cell tumors and adjacent testicular tissue. J Urol. 2001;165:1790–1794. [PubMed] [Google Scholar]
- 36.Rimoldi D, Salvi S, Schultz-Thater E, Spagnoli GC, Cerottini JC. Anti-MAGE-3 antibody 57B and anti-MAGE-1 antibody 6C1 can be used to study different proteins of the MAGE-A family. Int J Cancer. 2000;86:749–751. doi: 10.1002/(sici)1097-0215(20000601)86:5<749::aid-ijc24>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 37.Chen YT, Hsu M, Lee P, Shin SJ, Mhawech-Fauceglia P, Odunsi K, Altorki NK, Song CJ, Jin BQ, Simpson AJ, Old LJ. Cancer/testis antigen CT45: Analysis of mRNA and protein expression in human cancer. Int J Cancer. 2009;124:2893–2898. doi: 10.1002/ijc.24296. [DOI] [PubMed] [Google Scholar]
- 38.Jungbluth AA, Chen YT, Busam KJ, Coplan K, Kolb D, Iversen K, Williamson B, Van Landeghem FK, Stockert E, Old LJ. CT7 (MAGE-C1) antigen expression in normal and neoplastic tissues. Int J Cancer. 2002;99:839–845. doi: 10.1002/ijc.10416. [DOI] [PubMed] [Google Scholar]
- 39.Jungbluth AA, Chen YT, Stockert E, Busam KJ, Kolb D, Iversen K, Coplan K, Williamson B, Altorki N, Old LJ. Immunohistochemical analysis of NY-ESO-1 antigen expression in normal and malignant human tissues. Int J Cancer. 2001;92:856–860. doi: 10.1002/ijc.1282. [DOI] [PubMed] [Google Scholar]
- 40.Zhuang R, Zhu Y, Fang L, Liu XS, Tian Y, Chen LH, Ouyang WM, Xu XG, Jian JL, Güre AO, Fortunato S, Ritter G, Old LJ, Simpson AJ, Chen YT, Jin B, Jungbluth AA. Generation of monoclonal antibodies to cancer/testis (CT) antigen CT10/MAGE-C2. Cancer Immun. 2006;6(7) [PubMed] [Google Scholar]


