Abstract
Background
The cyclin B1/CDC2 complex governs entry into mitosis by regulating the G2/M checkpoint, and it can be repressed by the tumor suppressor p53. We aimed to determine cyclin B1 expression in squamous cell carcinomas of the head and neck (SCCHN) and correlate it with p53 status and clinicopathological parameters.
Patients and Methods
Cyclin B1 and p53 protein expression was analyzed by immunohistochemistry, and p53 mutation analyses were performed.
Results
Cytoplasmic expression of cyclin B1 was found in all 26 SCCHN studied. In contrast, nuclear staining was seen in the basal layers of normal mucosa. A total of 46% of tumors showed high cyclin B1 expression. p53 was overexpressed in 53.8% of cases, and of these 79% carried a p53 gene mutation. High cyclin B1 expression significantly correlated with the high tumor grade, but not with gender, tumor size, nodal status, local tumor recurrence or p53 expression.
Conclusion
Cyclin B1 is frequently overexpressed in SCCHN, and its high expression is significantly associated with a high tumor grade. These data suggest that cyclin B1 may serve as a potential prognostic biomarker in SCCHN.
Keywords: Cyclin B1, cell cycle, p53, squamous cell carcinoma, head and neck cancer
Uncontrolled proliferation is a hallmark of malignant cells. In normal cells, progression through the cell cycle is controlled by cyclins. They bind to corresponding cyclin-dependent kinases and initiate a complex signaling cascade that regulates the timing of cell cycle phase transitions. Cyclin levels are tightly regulated to coordinate peak expression with kinase activation. Cyclins A, D and E regulate the passage from G1 phase to S phase, whereas cyclins A and B regulate the transition from G2 phase to M phase. Specifically, the onset of mitosis is regulated by the activation of a complex of cyclin B1 and CDC2 (1, 2). Since CDC2 is generally present abundantly throughout the cell cycle, synthesis and deactivation of cyclin B1 is the main mechanism for controlling the activity of the cyclin B1/CDC2 complex. Hence, inappropriate expression of cyclin B1 may result in premature entry into mitosis, uncontrolled cell proliferation and neoplastic transformation. Accordingly, it has been shown that cancer cells can express cyclin B1 as early as in the G1 phase (3), and overexpression of cyclin B1 has been reported in several cancer types including tumors of the breast, prostate, colon and the oral cavity (4–8).
Squamous cell carcinoma of the head and neck (SCCHN) is the most common histological subtype of head and neck cancer. Despite the fact that radio- and chemotherapy have made progress in recent years, the prognosis of SCCHN is still not yet satisfactory (9, 10). Therefore, detailed tumor characterization is of importance to improve outcome in SCCHN (11).
In SCCHN, the p53 tumor suppressor gene is often mutated leading to dysfunctional protein (12–15). p53 is an important regulator of the G2/M transition, and it blocks entry into mitosis by inhibition of CDC2 and by repression of the cyclin B1 gene (16). Recent data indicate that cyclin B1 is overexpressed in laryngeal and tongue SCCHN and may be associated with aggressive biological behavior (17, 18). However to date, the expression of cyclin B1 and its possible correlation with p53 status in SCCHN has not yet been investigated in detail and little is known about its clinical significance.
Here we investigated the expression of cyclin B1 in tumor specimens from 26 SCCHN patients and assessed its correlation with clinicopathological factors and p53 expression.
Patients and Methods
Patients
Tumor tissue was obtained from 26 patients (22 males and 4 females) with histo-morphologically confirmed SCCHN. Normal epithelium adjacent to the tumor provided a negative internal control for immunoreaction and was additionally obtained from each patient. All patients were treated at the University of Pittsburgh with surgery and/or radiotherapy and/or chemotherapy according to their tumor stage. The study was approved by the IRB at the University of Pittsburgh and written informed consent was obtained from each individual. Hospital records and pathology slides were reviewed and TNM staging was carried out according to the UICC classification (10). No distant metastases were detected in any of the patients at time of first diagnosis. The follow-up time for recurrence was 4.2 years. The clinicopathological characteristics of the patients with SCCHN are shown in Table I.
Table I.
Clinicopathological parameters of the patients with SCCHN.
| Characteristic | Patients |
|---|---|
| SCCHN | 26 |
| Gender | |
| Female | 4 |
| Male | 22 |
| Age (years) | |
| Range | 54–94 |
| Median | 73.77 |
| Tumor site | |
| Oral cavity | 4 |
| Oropharynx | 5 |
| Hypopharynx | 2 |
| Larynx | 13 |
| Lip | 1 |
| CUP | 1 |
| Tumor status | |
| T1 | 7 |
| T2 | 9 |
| T3 | 1 |
| T4 | 8 |
| Nodal status | |
| N0 | 18 |
| N1 | 4 |
| N2 | 4 |
| N3 | 0 |
| Distant metastasis | |
| M0 | 26 |
| M1 | 0 |
| Differentiation | |
| Well (G1) | 3 |
| Moderate (G2) | 16 |
| Poor (G3) | 7 |
| Recurrence | |
| Yes | 7 |
| No | 19 |
Immunohistochemistry
For immunohistochemical staining, formalin-fixed, paraffin-embedded tumor tissues were sectioned (3–5 µm), air-dried overnight at 37°C, deparaffinized and dehydrated. For p53 immunohistochemistry, a mAb against p53, D0–7 (Dako, Carpinteria, CA, USA), was used, which recognizes an epitope in the n-terminus between amino acids 35–45 and reacts with wild-type and most mutant forms of p53 protein. The avidin-biotin-peroxidase method was used to visualize the p53, according to the instructions supplied by the manufacturer (Dako). The immunostained slides were evaluated by light microscopy for p53 accumulation. The tumor was considered p53-positive when more than 25% of the tumor cells showed staining intensity of 2+ and higher on scale of 0–4+. IgG isotype mAb used at the same concentration as the primary mAb served as a negative control.
Immunohistochemical detection of cyclin B1 was performed with the anti-cyclin B1 antibody, GNS-1 (BD PharMingen, Heidelberg, Germany), as described previously (19). The avidin-biotin-peroxidase method was applied as above according to the instructions supplied by the manufacturer (Dako).
For semiquantitative scoring of the cyclin B1 expression, the number of stained cells and the staining intensity were taken into consideration. For further analyses, cyclin B1 expression was categorized into two groups: no or low (=low, score 1) cyclin B1 expression and moderate or high (=high, score 2) cyclin B1 expression (Table II). All slides were scored by two independent investigators (TKH, ES).
Table II.
Cyclin B1 expression in 26 SCCHN correlated to the clinicopathological parameters and the p53 status.
| Cyclin B1 | |||
|---|---|---|---|
| Feature | Total no. | Score 1 (low) | Score 2 (high) |
| Gender | |||
| Male | 22 | 12 (55%) | 10 (45%) |
| Female | 4 | 2 (50%) | 2 (50%) |
| Nodal status | |||
| N0 | 18 | 9 (50%) | 9 (50%) |
| N1–N2 | 8 | 5 (63%) | 3 (37%) |
| Tumor status | |||
| T1–T2 | 16 | 8 (50%) | 8 (50%) |
| T3–T4 | 9 | 6 (67%) | 3 (33%) |
| Tumor grade | |||
| G1 | 3 | 3 (100%) | 0 (0%) |
| G2 | 16 | 9 (57%) | 7 (43%) |
| G3 | 7 | 2 (29%) | 5 (71%) |
| Recurrence | |||
| N | 19 | 11 (58%) | 8 (42%) |
| Y | 7 | 3 (43%) | 4 (57%) |
| p53 overexpression | |||
| N | 12 | 7 (58%) | 5 (42%) |
| Y | 14 | 7 (50%) | 7 (50%) |
p53 mutation analysis
Twenty-five cases of SCCHN included in this study were available as paraffin blocks archived at the University of Pittsburgh Medical Center (#10 not available). The histology of each case was reviewed by a pathologist, and representative tissue sections containing areas of invasive SCCHN were selected for microdissection. Normal-appearing salivary gland tissue or skeletal muscle was microdissected separately to serve as an internal non-tumor control. Using 4 µm-thick recut unstained histologic sections, normal and malignant tissue samples were removed under stereomicroscopic observation. Sufficient material was collected from a single histologic section to afford replicate analysis. Samples were treated with Proteinase K at a final concentration of 100 µg/ml for 2 h and then boiled for 5 min to remove protease activity. Polymerase chain reaction (PCR) utilized sets of amplification primers flanking exons 5 through 8 of the p53 gene in four separate PCRs. Amplified DNA from microdissected tissues also included splice sites. PCR products were electrophoresed in 4% agarose and the ethidium bromide-stained bands were excised and then isolated with glassmilk. DNA sequencing utilized antisense PCR primers for each exon with 33P-dATP as the reporter molecule, and sequence analysis was read from autoradiograms of 6% polyacylamide gels exposed overnight.
Statistical analysis
The association of cyclin B1 and p53 expression with clinicopathological parameters was analyzed by means of the Chi-square test or the nonparametric Mann-Whitney U-test. A p-value of less than 0.05 (two-sided) was considered significant.
Results
Patients characteristics
The clinicopathological characteristics of patients with SCCHN are summarized in Table I. The cohort of 26 SCCHN patients included 22 men and 4 women, with a median age of 73.8 years (range 54–94 years). The 26 histologically verified squamous cell carcinomas originated from the oral cavity, oropharynx, hypopharynx, larynx, and lips and included one carcinoma of unknown primary (CUP). Tumor cell differentiation was high (G1) in 3 cases, moderate (G2) in 16 cases and poor (G3) in 7 cases (Table I).
Patients were classified according to the TNM system, describing size and range of the tumor, the lymph nodes and the metastasis status. All patients were without evidence of distant metastases (M0) at the time of first diagnosis. Seven (27%) patients developed a local recurrence of the tumor within a median follow-up time of 4.2 years.
Cyclin B1 expression in SCCHN
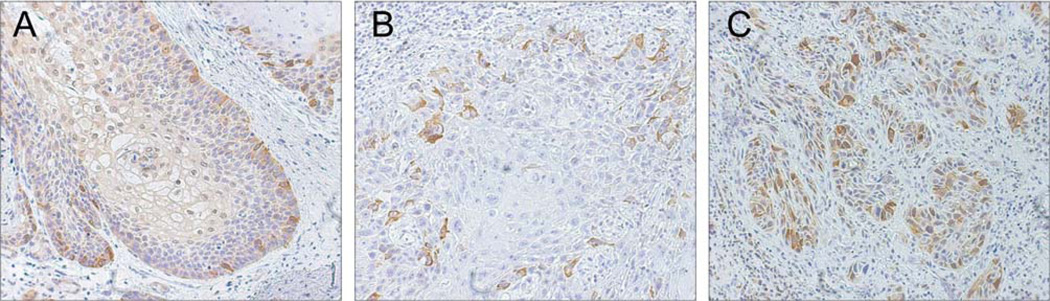
Immunohistochemical analysis of cyclin B1 expression in 26 cases of SCCHN showed that cyclin B1 was expressed in all tumor samples. Cyclin B1 expression was also detected in the surrounding normal mucosa, however, with a different localization pattern. In normal mucosa, cells with nuclear staining were located in the basal and parabasal layers of the stratified squamous epithelium, marking areas with regular mitotic activity (Figure 1A), and only few cells exhibited a cytoplasmatic staining pattern (Figure 1A). By contrast, cyclin B1 protein was expressed predominantly in the cytoplasm of the tumor cells. Cells with cyclin B1 expression in the cytoplasm were distributed throughout the tumor islands (Figure 1 B and C). Out of 26 tumors, 14 (53.8%) had low (score 1) and 12 (46.2%) high (score 2) cyclin B1 expression (Table II and Figure 1).
Figure 1.
Immunohistochemical staining of cyclin B1 in SCCHN. A: Nuclear staining in the basal and parabasal layers of normal epithelium, marking areas with high cell cycle activity and a normal proliferation rate. B and C: SCCHN showing mainly cytoplasmic staining of cyclin B1 in tumor cells. Original magnification: ×250.
Association of cyclin B1 expression with clinipathological parameters
Next, we investigated the potential correlation of cyclin B1 expression with clinicopathological parameters.
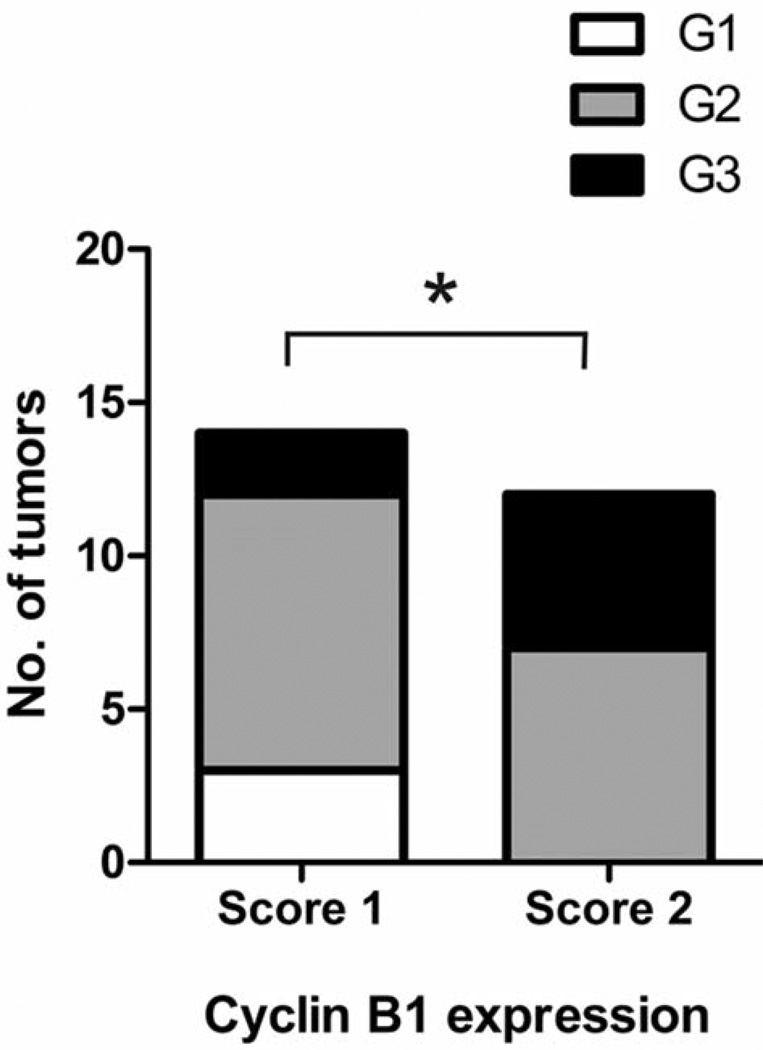
High cyclin B1 expression was more common among poorly differentiated tumors in comparison to moderately or well differentiated tumors. While 5/7 of G3 tumors had high cyclin B1 expression, only 7/16 of G2 tumors and none (0/3) of the G1 tumors exhibited high expression of cyclin B1 (Table II). Accordingly, the histopathological grade was significantly higher in tumors with high cyclin B1 expression compared with tumors with low cyclin B1 expression (Figure 2) (Mann-Whitney U-test, p<0.05).
Figure 2.
Association of cyclin B1 expression and the grade of histopathological differentiation in SCCHN. Cyclin B1 expression was assessed by immunohistochemistry in 26 SCCHN (score 1, low expression; score 2, high expression). Well-differentiated tumors, G1; moderately differentiated tumors, G2; poorly differentiated tumors, G3. *p<0.05, Mann-Whitney U-test.
Cyclin B1 expression showed no significant correlation with gender, tumor size (T), nodal status (N) or local recurrence (p>0.05 for all, Chi-square test).
p53 expression and mutation analysis
In SCCHN, the p53 tumor suppressor gene is often mutated or dysfunctional. Immunhistochemistry of p53 protein and sequencing of genomic PCR product of p53 exons 5–8 were performed and related to cyclin B1 expression. Immunohistochemical analysis indicated that 53.8% of tumors (14 of 26) showed accumulation of p53 protein (Table II and Table III).
Table III.
p53 immunohistochemistry and mutation analysis compared to cyclin B1 expression.
| Patient | p53 overexpression | p53 Genotype | Cyclin B1 score |
|---|---|---|---|
| #1 | 1 | E8 E271K | 2 |
| #2 | 1 | E7 R248W | 1 |
| #3 | 1 | wt | 2 |
| #4 | 0 | wt | 2 |
| #5 | 0 | wt | 1 |
| #6 | 0 | wt | 2 |
| #7 | 1 | wt | 1 |
| #8 | 0 | wt | 2 |
| #9 | 1 | Y220C | 2 |
| #10 | 1 | N/A | 1 |
| #11 | 0 | E6 213 Stop | 1 |
| #12 | 0 | wt | 1 |
| #13 | 1 | E8 R273H | 2 |
| #14 | 0 | Exon 6 deletion | 1 |
| #15 | 1 | E5 S149C | 1 |
| #16 | 1 | E5 H168Y | 1 |
| #17 | 1 | wt | 1 |
| #18 | 0 | wt | 1 |
| #19 | 0 | wt | 1 |
| #20 | 0 | wt | 2 |
| #21 | 1 | E5 V157F | 2 |
| #22 | 0 | wt | 1 |
| #23 | 1 | E8 E286K | 2 |
| #24 | 1 | E6 G226R | 1 |
| #25 | 0 | wt | 2 |
| #26 | 1 | E5 T150R | 2 |
Twenty-five tumors underwent sequencing of genomic PCR products of exons 5–8 (#10 not available). Out of 14 tumors showing p53 overexpression, 10 were found to express missense mutations within p53 exons 5–8 (Table III). Three tumors (#3, #7, #17) had a wild-type p53 genotype but overexpressed p53, which could possibly be a consequence of a mutation outside the exons 5–8 or in genes impacting on the stability of p53 (12).
Association between p53 status and clinicopathological parameters
A possible association of p53 overexpression with clinicopathological parameters in the 26 SCCHN patients was tested. p53 expression showed no association with gender, tumor size (T status), nodal status or local recurrence (p>0.05 for all, Chi-square test).
Moreover, p53 expression showed no correlation with the differentiation grade of the tumors. Among G1 tumors, all (3 out of 3) accumulated p53; among G2 tumors, 6 out of 16 accumulated p53; of the 7 poorly differentiated tumors included in the study, 5 had p53 overexpression.
Association between p53 status and cyclin B1 expression
Since p53 plays an important role in the regulation of the cell cycle, including the G2/M transition, we investigated the possible association of p53 status and cyclin B1 level.
p53 was overexpressed in 14 out of 26 samples. Among the p53-overexpressing tumors, 7 tumors exhibited low cyclin B1 levels (score 1), and 7 tumors (50%) had high cyclin B1 levels (score 2) (Table III). Among 12 tumor samples without p53 overexpression, 7 had low levels (score 1) and 5 had high levels (score 2) of cyclin B1. There was no significant association between cyclin B1 expression and p53 immunoreactivity.
Among 12 tumors with high (score 2) cyclin B1 expression, there were 5 with wt p53 and no accumulation of the p53 protein (Table III). The other 7 tumors had either p53 mutations or accumulation of p53 protein: 6 tumors had p53 mutations and accumulation (# 1, 9, 13, 21, 23 and 26). One tumor (# 3) did not have a p53 mutation but a p53 accumulation.
In the group of 13 patients with low (score 1) cyclin B1 expression there were 5 with wt p53 and normal p53 protein (#5, 12, 18, 19, 22); 4 (#2, 15, 16, 24) had p53 mutation and accumulation, 2 tumors (#11 and 14) had p53 mutations but no overexpression, and 2 tumors (#7 and 17) were genomically wt p53 with p53 protein overexpression.
Altogether, the frequency of tumors with dysfunctional p53 (either mutated p53 or p53 overexpression) did not differ significantly between those with high and those with low cyclin B1 expression.
Discussion
Cyclins play an essential role in orchestrating normal cell cycle progression. While normal cells turn on transient expression of small amounts of cyclin proteins at specific points of the cell cycle, many tumors have constitutively high levels of one or more cyclins (2, 20, 21). Most studies investigating cell cycle regulation in SCCHN have focused on the G1/S transition phase, and showed that overexpression of cyclin D1 is common in SCCHN (22–25). However, deregulation of proteins in subsequent stages of cell proliferation, such as the G2/M transition phase, can also lead to uncontrolled cell growth. In this study, we demonstrated the increased expression and altered subcellular localization of cyclin B1 in SCCHN, and showed that high cyclin B1 expression is associated with higher differentiation grade.
Cyclin B1 was highly expressed in 46% of tumors. While normal epithelial controls had low cyclin B1 expression with exclusively nuclear staining in the basal and parabasal layers, corresponding to cells in the G2/M transition, staining of SCCHN by immunohistochemistry showed areas with high cyclin B1 expression predominantly in the cytoplasm. Altered subcellular localization and overexpression of cyclin B1 in tumor cells indicate that control over the cell cycle has been lost in these cells. In line with our data, cytoplasmic cyclin B1 expression has been shown in breast cancer cells (26). Moreover, a study investigating the association of cyclin B1 expression with radiotherapy response in SCCHN also showed predominantly cytoplasmic cyclin B1 expression and the frequency of tumors with high cyclin B1 expression comparable to that shown here (27).
Our results showed an association of higher cyclin B1 expression with higher tumor grade. Notably, none of the well-differentiated tumors had high cyclin B1 expression, while 7/16 of the moderately differentiated tumors and 5/7 of the poorly differentiated tumors expressed cyclin B1 at a high level. Regarding the clinical features of tumor grade and differentiation, this finding suggests that cyclin B1 expression is associated with a more aggressive phenotype and worse prognosis (28, 29). In our analysis, cyclin B1 expression was not associated with other clinicopathological parameters such as gender, tumor size and nodal status. Local recurrence was slightly more common for tumors with high cyclin B1 expression compared with those having low levels of cyclin B1. In line with this observation, in a previous study investigating the association of cyclin B1 expression with resistance to radiotherapy in SCCHN, a higher risk for recurrence was observed for tumors with high cyclin B1 expression (27). Altogether these results suggest that cyclin B1 may be a predictor of prognosis in SCCHN. Notably, a recent study including 1348 breast tumors demonstrated that high cyclin B1 levels were associated with a worse prognosis (30). Moreover, a prognostic value for cyclin B1 has been suggested in patients with squamous cell carcinoma of the esophagus and the lung, as well as laryngeal cancer, with a tendency for poor prognosis for those with high cyclin B1 expression (7, 17, 31). Future studies with larger patient numbers will be needed to better evaluate the prognostic value of cyclin B1 in SCCHN.
The tumor suppressor p53 is an important regulator of the cell cycle, and dysfunction of p53 due to missense mutations or deletions is a common finding in SCCHN. Missense mutations can result in the accumulation of dysfunctional p53 and, therefore, p53 overexpression is an indicator of p53 dysfunction. Among the 26 tumors studied, 14 exhibited p53 protein overexpression and an additional 2 tumors had mutations in p53 without protein overexpression. p53 was not significantly associated with any of the clinicopathological parameters.
p53 controls mitotic initiation and has been shown to regulate cyclin B1 in tumor cells (32–34). In our study, no significant association between p53 overexpression or p53 mutation and cyclin B1 expression was found, suggesting that in addition to p53, other factors are important in regulating cyclin B1 in tumor cells in vivo. In line with this finding, another study did not find any correlation between p53 reactivity and cyclin B1 expression in breast cancer (26). By contrast, a recent study using a larger cohort of breast cancer patients showed that high cyclin B1 expression was significantly associated with p53 positivity (30). Extensive studies with large sample numbers will be needed to determine whether such cell cycle regulators are also correlated in SCCHN and whether cyclin B1 and p53 can be used as biomarkers in combination to predict prognosis and/or therapy response.
In conclusion, this study has demonstrated overexpression of cyclin B1 in the cytoplasm of SCCHN cells. High expression of cyclin B1 was associated with poor tumor cell differentiation. These results suggest that cyclin B1 may be a potential biomarker in SCCHN and its prognostic value, possibly in combination with other markers, should be assessed in future studies.
Acknowledgements
This work was supported in part by the NIH grant PO1-DE 12321 to TLW and a postdoctoral training fellowship from the Dr. Mildred Scheel Stiftung fur Krebsforschung to TKH (grant D/99/08916).
Footnotes
Conflict of Interest Statement
None declared.
References
- 1.Nurse P. Universal control mechanism regulating onset of M-phase. Nature. 1990;344:503–508. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- 2.Hunt T. Cyclins and their partners: from a simple idea to complicated reality. Seminars in cell biology. 1991;2:213–222. [PubMed] [Google Scholar]
- 3.Shen M, Feng Y, Gao C, Tao D, Hu J, Reed E, Li QQ, Gong J. Detection of cyclin B1 expression in G(1)-phase cancer cell lines and cancer tissues by postsorting Western blot analysis. Cancer Res. 2004;64:1607–1610. doi: 10.1158/0008-5472.can-03-3321. [DOI] [PubMed] [Google Scholar]
- 4.Kawamoto H, Koizumi H, Uchikoshi T. Expression of the G2-M checkpoint regulators cyclin B1 and cdc2 in nonmalignant and malignant human breast lesions: immunocytochemical and quantitative image analyses. Am J Pathol. 1997;150:15–23. [PMC free article] [PubMed] [Google Scholar]
- 5.Kushner J, Bradley G, Young B, Jordan RC. Aberrant expression of cyclin A and cyclin B1 proteins in oral carcinoma. J Oral Pathol Med. 1999;28:77–81. doi: 10.1111/j.1600-0714.1999.tb02000.x. [DOI] [PubMed] [Google Scholar]
- 6.Mashal RD, Lester S, Corless C, Richie JP, Chandra R, Propert KJ, Dutta A. Expression of cell cycle-regulated proteins in prostate cancer. Cancer Res. 1996;56:4159–4163. [PubMed] [Google Scholar]
- 7.Soria JC, Jang SJ, Khuri FR, Hassan K, Liu D, Hong WK, Mao L. Overexpression of cyclin B1 in early-stage non-small cell lung cancer and its clinical implication. Cancer Res. 2000;60:4000–4004. [PubMed] [Google Scholar]
- 8.Wang A, Yoshimi N, Ino N, Tanaka T, Mori H. Overexpression of cyclin B1 in human colorectal cancers. J Cancer Res Clin Oncol. 1997;123:124–127. doi: 10.1007/BF01269891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Argiris A, Karamouzis MV, Raben D, Ferris RL. Head and neck cancer. Lancet. 2008;371:1695–1709. doi: 10.1016/S0140-6736(08)60728-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marur S, Forastiere AA. Head and neck cancer: changing epidemiology, diagnosis, and treatment. Mayo Clinic Proc. 2008;83:489–501. doi: 10.4065/83.4.489. [DOI] [PubMed] [Google Scholar]
- 11.Molinolo AA, Amornphimoltham P, Squarize CH, Castilho RM, Patel V, Gutkind JS. Dysregulated molecular networks in head and neck carcinogenesis. Oral Oncol. 2009;45:324–334. doi: 10.1016/j.oraloncology.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balz V, Scheckenbach K, Gotte K, Bockmuhl U, Petersen I, Bier H. Is the p53 inactivation frequency in squamous cell carcinomas of the head and neck underestimated? Analysis of p53 exons 2–11 and human papillomavirus 16/18 E6 transcripts in 123 unselected tumor specimens. Cancer Res. 2003;63:1188–1191. [PubMed] [Google Scholar]
- 13.Hoffmann TK, Sonkoly E, Hauser U, van Lierop A, Whiteside TL, Klussmann JP, Hafner D, Schuler P, Friebe-Hoffmann U, Scheckenbach K, Erjala K, Grenman R, Schipper J, Bier H, Balz V. Alterations in the p53 pathway and their association with radio- and chemosensitivity in head and neck squamous cell carcinoma. Oral Oncol. 2008;44:1100–1109. doi: 10.1016/j.oraloncology.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Koch WM, Brennan JA, Zahurak M, Goodman SN, Westra WH, Schwab D, Yoo GH, Lee DJ, Forastiere AA, Sidransky D. p53 mutation and locoregional treatment failure in head and neck squamous cell carcinoma. J Natl Cancer Inst. 1996;88:1580–1586. doi: 10.1093/jnci/88.21.1580. [DOI] [PubMed] [Google Scholar]
- 15.Somers KD, Merrick MA, Lopez ME, Incognito LS, Schechter GL, Casey G. Frequent p53 mutations in head and neck cancer. Cancer Res. 1992;52:5997–6000. [PubMed] [Google Scholar]
- 16.Taylor WR, Stark GR. Regulation of the G2/M transition by p53. Oncogene. 2001;20:1803–1815. doi: 10.1038/sj.onc.1204252. [DOI] [PubMed] [Google Scholar]
- 17.Dong Y, Sui L, Watanabe Y, Sugimoto K, Tokuda M. Clinical relevance of cyclin B1 overexpression in laryngeal squamous cell carcinoma. Cancer Lett. 2002;177:13–19. doi: 10.1016/s0304-3835(01)00770-4. [DOI] [PubMed] [Google Scholar]
- 18.Hassan KA, El-Naggar AK, Soria JC, Liu D, Hong WK, Mao L. Clinical significance of cyclin B1 protein expression in squamous cell carcinoma of the tongue. Clin Cancer Res. 2001;7:2458–2462. [PubMed] [Google Scholar]
- 19.Kao H, Marto JA, Hoffmann TK, Shabanowitz J, Finkelstein SD, Whiteside TL, Hunt DF, Finn OJ. Identification of cyclin B1 as a shared human epithelial tumor-associated antigen recognized by T-cells. J Exp Med. 2001;194:1313–1323. doi: 10.1084/jem.194.9.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collecchi P, Santoni T, Gnesi E, Giuseppe Naccarato A, Passoni A, Rocchetta M, Danesi R, Bevilacqua G. Cyclins of phases G1, S and G2/M are overexpressed in aneuploid mammary carcinomas. Cytometry. 2000;42:254–260. doi: 10.1002/1097-0320(20000815)42:4<254::aid-cyto6>3.3.co;2-j. [DOI] [PubMed] [Google Scholar]
- 21.Kim JK, Diehl JA. Nuclear cyclin D1: an oncogenic driver in human cancer. J Cell Physiol. 2009;220:292–296. doi: 10.1002/jcp.21791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bartkova J, Lukas J, Muller H, Strauss M, Gusterson B, Bartek J. Abnormal patterns of D-type cyclin expression and G1 regulation in human head and neck cancer. Cancer Res. 1995;55:949–956. [PubMed] [Google Scholar]
- 23.Bellacosa A, Almadori G, Cavallo S, Cadoni G, Galli J, Ferrandina G, Scambia G, Neri G. Cyclin D1 gene amplification in human laryngeal squamous cell carcinomas: prognostic significance and clinical implications. Clin Cancer Res. 1996;2:175–180. [PubMed] [Google Scholar]
- 24.Kotelnikov VM, Coon JSt, Mundle S, Kelanic S, LaFollette S, Taylor SI, Hutchinson J, Panje W, Caldarelli DD, Preisler HD. Cyclin D1 expression in squamous cell carcinomas of the head and neck and in oral mucosa in relation to proliferation and apoptosis. Clin Cancer Res. 1997;3:95–101. [PubMed] [Google Scholar]
- 25.Michalides R, van Veelen N, Hart A, Loftus B, Wientjens E, Balm A. Overexpression of cyclin D1 correlates with recurrence in a group of forty-seven operable squamous cell carcinomas of the head and neck. Cancer Res. 1995;55:975–978. [PubMed] [Google Scholar]
- 26.Winters ZE, Hunt NC, Bradburn MJ, Royds JA, Turley H, Harris AL, Norbury CJ. Subcellular localisation of cyclin B, CDC2 and p21(WAF1/CIP1) in breast cancer. association with prognosis. Eur J Cancer. 2001;37:2405–2412. doi: 10.1016/s0959-8049(01)00327-6. [DOI] [PubMed] [Google Scholar]
- 27.Hassan KA, Ang KK, El-Naggar AK, Story MD, Lee JI, Liu D, Hong WK, Mao L. Cyclin B1 overexpression and resistance to radiotherapy in head and neck squamous cell carcinoma. Cancer Res. 2002;62:6414–6417. [PubMed] [Google Scholar]
- 28.Arduino PG, Carrozzo M, Chiecchio A, Broccoletti R, Tirone F, Borra E, Bertolusso G, Gandolfo S. Clinical and histopathologic independent prognostic factors in oral squamous cell carcinoma: a retrospective study of 334 cases. J Oral Maxillofac Surg. 2008;66:1570–1579. doi: 10.1016/j.joms.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 29.Cojocariu OM, Huguet F, Lefevre M, Perie S. Prognosis and predictive factors in head-and-neck cancers. Bull Cancer. 2009;96:369–378. doi: 10.1684/bdc.2009.0777. (in French). [DOI] [PubMed] [Google Scholar]
- 30.Aaltonen K, Amini RM, Heikkila P, Aittomaki K, Tamminen A, Nevanlinna H, Blomqvist C. High cyclin B1 expression is associated with poor survival in breast cancer. Br J Cancer. 2009;100:1055–1060. doi: 10.1038/sj.bjc.6604874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murakami H, Furihata M, Ohtsuki Y, Ogoshi S. Determination of the prognostic significance of cyclin B1 overexpression in patients with esophageal squamous cell carcinoma. Virchows Arch. 1999;434:153–158. doi: 10.1007/s004280050319. [DOI] [PubMed] [Google Scholar]
- 32.Innocente SA, Abrahamson JL, Cogswell JP, Lee JM. p53 regulates a G2 checkpoint through cyclin B1. Proc Natl Acad Sci USA. 1999;96:2147–2152. doi: 10.1073/pnas.96.5.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Innocente SA, Lee JM. p53 is a NF-Y- and p21-independent, Sp1-dependent repressor of cyclin B1 transcription. FEBS Lett. 2005;579:1001–1007. doi: 10.1016/j.febslet.2004.12.073. [DOI] [PubMed] [Google Scholar]
- 34.Yu M, Zhan Q, Finn OJ. Immune recognition of cyclin B1 as a tumor antigen is a result of its overexpression in human tumors that is caused by non-functional p53. Mol Immunol. 2002;38:981–987. doi: 10.1016/s0161-5890(02)00026-3. [DOI] [PubMed] [Google Scholar]