Abstract
Deep brain stimulation (DBS) has shown promise in the treatment of many neurological and psychiatric disorders as well as a disorder of consciousness, the minimally conscious state (MCS). In the clinic, DBS is always monotonic standard pulses; however, we have hypothesized that temporally patterned pulses might be more efficient in achieving desired behavioral responses. Here we present two experiments on DBS of the central thalamus to increase arousal, as measured by motor activity, and to affect the electroencephalogram (EEG). In the first, we optimized amplitude and frequency in standard stimulation of the central thalamus in intact mice. In the second, the optimized fixed frequency was compared to two alternative temporal patterns, chaotic and random, which were physically identical to each other and fixed frequency in all ways except temporal pattern. In both experiments and with all types of stimulation, DBS of the central thalamus increased arousal as measured by motor activity. These data also revealed that temporal patterning of pulses can modulate response to stimulation. That temporal patterns in DBS of the central thalamus were found to alter motor activity response implies possible usefulness of temporal patterns in DBS of other contexts. More investigation into exactly how temporally patterned stimulation may affect neuronal circuit dynamics is necessary.
Keywords: Deep brain stimulation, temporal patterns, generalized arousal, logistic equation
1. Introduction
Deep brain stimulation (DBS) is growing in popularity as a neurosurgical technique because of its wide applicability, adjustability, and reversibility. It is used in a large number of brain targets to treat widespread neurological and psychiatric illnesses and has been successfully applied for the treatment of movement disorders [1–3], affective disorders [4–6], and chronic pain [7–9]. Another promising application of DBS is the treatment of patients with disorders of consciousness; in particular, a diagnosis of minimally conscious state (MCS) is amenable to treatments such as DBS. Central thalamus DBS (CT/DBS) has been shown to facilitate functional recovery in MCS patients. In a double-blinded crossover study of one MCS patient, Schiff and colleagues show great improvement of patient awareness and ability to communicate with CT/DBS [10]. Six years after the initial brain injury, the patient presented with behavior fluctuations consistent with MCS, and with no improvement since two years post-injury. Almost immediately after stimulation started, the patient began showing improvement, and by the end of the six months of the study, he had much greater functional ability including being able to feed himself and communicate with words.
For the particular application of DBS for MCS, the chosen target is the central thalamus. The central thalamus is uniquely poised for modulation in the severely injured brain because of its neuroanatomical placement and role in regulating generalized arousal. Anatomically located between the major ascending and basal forebrain arousal systems and the cortex, the central thalamus is recruited to support overall cerebral activation and maintain that activation during high arousal states [11]. This level of cerebral activation is obviously absent in the severely injured brain; Schiff theorizes that while MCS patients retain thalamo-cortical connections needed to support cerebral activation, they lack sufficient innervation from the arousal systems. It is suggested that CT/DBS in a severely injured brain approximates this missing arousal input, allowing the central thalamus to function normally in supporting cerebral activity and cognition [12].
Assuming DBS for increasing arousal does mimic a missing arousal input, what is the nature of that input? There are various theories of how information is coded in neuronal action potentials; one such hypothesis is that information is contained in temporal patterns of neuronal spike trains [13–15]. If information is carried by temporal patterns, responses to DBS of different patterns might be larger than standard stimulation. Temporally patterned DBS might be more effective for increasing arousal than conventional fixed frequency stimulation. Assuming this is true, it would be possible to minimize electric current directed into brain by optimizing temporal pattern to maximize response to DBS. In our previously published work [16], we showed that temporal patterning of pulses altered motor activity response to DBS.
There is a multitude of patterns or ways to pattern a series of pulses. To narrow down the possibilities, we first chose to use deterministic chaos to generate temporal patterns. In our previously published work [16], the logistic equation was chosen for our first temporal pattern because it is a simple discrete chaotic equation and because it coincides with our previous hypothesis that nonlinear dynamics may be important in the control of CNS arousal systems [17]. Chaotic systems have the advantage of being able to quickly amplify the smallest of perturbations. In that original study, we compared two different temporal patterns generated by the logistic equation to the standard fixed frequency. Here in addition to our chaotic temporal pattern, we wanted an alternate temporal pattern that would also serve as a control. Since our chaotic temporal pattern is deterministic, we wanted our second temporal pattern to be internally independent; therefore, a true random number generator was chosen. True random number generators use physical measurements (such as measurements of atmospheric noise) instead of deterministic algorithms to create series of numbers.
In this paper we present two experiments; in the first, we optimize amplitude and frequency CT/DBS, and in the second we compare our optimized fixed frequency with chaotic and random temporal patterns. While the highest amplitude tested (125 μA) significantly increased behavioral response to DBS, we were conservative in our choice of 100 μA for subsequent studies. There were no significant differences between the four frequencies we tried, but 125 hz increased behavioral activity more than the other three. We also saw a significant effect of light phase, and this difference seems to be due to the low baselines of behavior in the light which result in larger relative changes with stimulation than in the dark. In the second experiment, we found a general effect of temporal pattern. In both studies, CT/DBS increased generalized arousal as measured by motor activity and EEG waves. We also confirmed that temporal patterning makes a difference in behavioral response, but using conservative statistical methods, these differences were not always significant.
2. Methods
2.1 Animals and Materials
A total of 26 six to nine week old C57BL/6 mice were singly housed on a reversed 12 h light/dark cycle with food and water available ad libitum for the duration of the study. Monopolar stainless steel electrodes (Plastics One) were implanted bilaterally into the central thalamus under ketamine/xylazine anesthesia (80/12 mg/kg) using a Kopf stereotaxic apparatus. Coordinates used were as follows: anterior-posterior, −1.70 mm from bregma; lateral, +/− 0.75 mm from midline; and depth, −3.00 mm from the surface of the brain. Only mice histologically confirmed with at least unilateral electrode placement in the central thalamus are reported here. Mice were also subcutaneously implanted with a small animal transmitter (Data Sciences International) capable of transmitting electroencephalogram (EEG). EEG electrodes were positioned to touch the intact dura and secured to the skull with super glue; the drilled holes for the electrodes were located on the left posterior and right anterior portions of the parietal bone of the skull. Post-operative care included recovery from anesthesia under a heat lamp and flunazine analgesia (50 mg/kg) for 2 days. Before any handling or manipulation, mice were allowed to recover from surgery for 5–7 days. At the end of the study, mice were euthanized by isoflurane anesthesia and decapitation. Fresh frozen brains were collected and then cut at 40 μm on a cryostat, and electrode placements were confirmed by acetylcholinesterase staining [18] (see Figure S1 for diagram of electrode placements). All animal procedures were in compliance with National Institutes of Health guidelines and approved by the Rockefeller University Institutional Animal Care and Use Committee.
2.2 Parametric Experiment
In this experiment, mice (n=10) were stimulated for 10 minutes for a maximum of 24 stimulations over the course of three days. To space stimulations evenly over the course of the study, stimulation epochs occurred every three hours. Three amplitudes (75, 100, and 125 μA) and four frequencies (50, 125, 175, and 225 hz) were tested. While amplitude was increased systematically over the three days in all animals, frequency was counterbalanced for order.
2.3 Temporal Pattern Experiment
Based on the motor activity data from the parametric experiment, we chose 125 hz and 100 μA as the parameters for the temporal pattern experiment. Two temporal patterns were chosen in addition to our optimized fixed frequency pattern. One pattern, based on the logistic equation, was chosen subsequent to our previous study using nonlinear patterns [16] as well as our hypothesis that nonlinear dynamics may be important in the control of CNS arousal systems [17]. The second pattern, based on a true random number generator, was chosen as a patterned control; based on independent true random numbers, the random temporal pattern was used to give perspective on the internally structured chaotic temporal pattern. Mice (n=16) were stimulated for 10 minutes for a total of six stimulations over the course of one day. To space stimulations evenly over the course of that day, stimulation epochs occurred every four hours. Each mouse was challenged with all three temporal patterns, counterbalanced for order.
The other two temporal patterns were generated using a true random number generator (based on atmospheric noise) and the logistic equation. The logistic equation, Xn = R Xn−1(1−Xn−1), where X is the output at time n, has a constant modifier, R, that creates chaotic output at certain values. The true random number generator used here takes atmospheric noise measurements to generate three thousand uniformly distributed numbers [19]. Output to the logistic equation was calculated to two or three thousand iterations with initial conditions to ensure chaotic behavior of the equation (R = 3.90 and X0 = 0.2). To ensure that depolarization block did not occur, a minimum interpulse interval (IPI) was defined as 0.3 ms, and chaotic and random sequences were scaled to meet that minimum. For both sequences of numbers, a consecutive set of numbers was found such that the number of elements in the set divided by the sum of the scaled output equaled the desired average frequency. In this manner, we defined a set of 50 pulses from the logistic equation output and named it Chaotic and a set of 50 pulses from the true random number generator output and named it Random. To ensure the only difference between the three patterns was the temporal patterning of pulses, the three temporal patterns were identical with respect to pulse shape, pulse duration, amplitude, average frequency, and stimulation duration. All stimulation, in both experiments, was constant current, monopolar, symmetric biphasic stimulation with a total pulse duration of 0.2 ms.
2.4 Data Analysis
During the course of each study, one channel EEG and three behavioral data measures were collected. Two data collection systems were utilized; a 3D infrared beam home cage activity monitor (Accuscan Instruments) was used to collect motor activity data, and an implantable transmitter telemetry system (Data Sciences International or DSI) was used to collect EEG as well as motor activity data. The three behavioral measures observed included: 1) activity counts (whole body activity, designated “Counts” below), collected by the DSI transmitter and representing changes in field strength between the transmitter and receiver as the mouse moves; 2) horizontal activity (fidgeting movements, designated “Hactv” below), collected by the home cage Accuscan system and representing the number of infrared beams broken in the horizontal plane; and 3) total distance (ambulation, designated “Totdist” below), collected by the home cage Accuscan system and representing non-repeating infrared beam breaks in the horizontal plane. EEG, collected by the DSI system, was divided into minute long epochs and its power spectra were estimated using the multitaper method. EEG waves were integrated over the following frequency bins: delta (0.5–4 hz), theta (4.5–8 hz), alpha (8.5–12 hz), beta (12.5–20 hz), and gamma (35–45 hz). EEG data are reported as the relative power of each frequency bin to the summed power in all five frequency bins. Data were reported for 10 minutes before, 10 minutes during (only for behavioral measures), and 10 minutes after each stimulation. Data collected during and after stimulation were normalized to data collected directly before stimulation. Because motor activity and EEG inherently change over the course of the light dark cycle, we restricted our analyses to the half hour surrounding stimulation to avoid the intrinsic fluctuations of our outcome measures.
For statistical evaluation, each data measure was analyzed by multiple factor ANOVA and post-hoc two-tailed t-tests with Bonferroni corrections for multiple comparisons. Multiple factor ANOVA is a common method for determining the relative effects of multiple independent variables as well as their interactions on a single outcome measure [20,21]. The factors included in our analyses were stimulation, stimulation parameters (amplitude and frequency in the Parametric experiment, temporal pattern in the Temporal pattern experiment), phase of the light dark cycle, as well as any interactions between these factors. Since no significant interactions were found, these analyses were not included in the following for discussion. Despite obvious strong differences where the standard error of the means did not overlap, several post-hoc comparisons were not significant with Bonferroni-corrected t-tests. Even though these Bonferroni corrections were very conservative, we included them during our interpretation of the results. In subsequent evaluation of the behavioral data, we utilized multiple factor MANOVA with Wilks lambda to determine if our findings held true when considering the behavioral data as a whole [21]. MANOVA analysis of both experiments confirmed all of our major findings except for the main effect of light phase in the Parametric experiment.
Extensive analyses of data from both experiments were done to determine if any sex differences in response to stimulation exist. All differences found were small and inconsistent across data measures and between the two data sets; therefore, data presented here are pooled from males and females.
3. Results
3.1 Parametric Experiment
In addition to looking for differences caused by the stimulation itself, we investigated differences caused by amplitude of stimulation, frequency of stimulation, light phase during which stimulation occurred, and any correlation between data measures (scattergrams and correlation coefficients for EEG data can be found in Figure S2). First and foremost, we found an effect of central thalamus DBS (CT/DBS) in all three behavioral measures (Counts: F2,100= 14.23, p<0.001; Hactv: F2,902 = 32.33, p<0.001; Totdist: F2,914 = 5.66, p<0.01; see Figure 1) and four of five EEG waves (Theta: F1,780 = 69.78, p<0.001; Alpha: F1,772= 14,58, p<0.001; Beta: F1,760 = 21.89, p<0.001; Gamma: F1,771 = 42.44, p<0.001; see Figure 2). CT/DBS increased motor activity, decreased theta waves in the EEG, and increased alpha, beta, and gamma waves in the EEG. These data replicate our finding that CT/DBS increases generalized arousal.
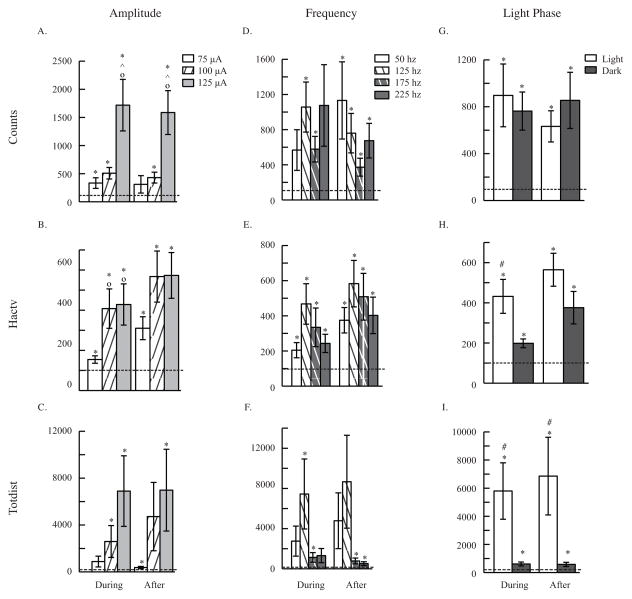
Figure 1. Parametric Experiment: Behavior.
Behavioral response to amplitude (A. B. and C.), frequency (D. E. and F.), and light phase (G. H. and I.) of CT/DBS. Three behavioral measures are shown: activity counts (whole body movement), horizontal activity (fidgeting), and total distance (ambulation). ANOVA analyses revealed an overall effect of CT/DBS in activity counts (p<0.001), horizontal activity (p<0.001), and total distance (p<0.01). ANOVA also showed an effect of amplitude in activity counts (A. p<0.001), horizontal activity (B. p<0.001), and total distance (C. p<0.01). An overall effect of frequency was found in horizontal activity (E. p<0.05) and total distance (F. p<0.001). Light phase of CT/DBS was found to be significant in horizontal activity (H. p<0.001) and total distance (I. p<0.001). Panels include 10 minutes of data during and 10 minutes of data after CT/DBS, all normalized to before stimulation. Data are presented as mean ± s.e.m. * p<0.05 compared to before, ° p<0.05 compared to 75 μA, ^ p<0.05 compared to 100 μA, # p<0.05 compared to dark phase.
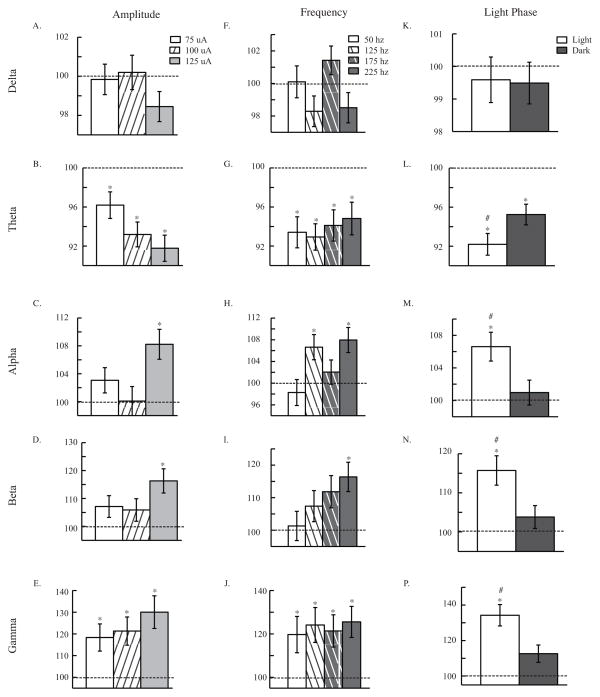
Figure 2. Parametric Experiment: EEG.
EEG response to amplitude (A. B. C. D. and E.), frequency (F. G. H. I. and J.), and light phase (K. L. M. N. and P.) of CT/DBS. EEG waves are defined by the following frequency bins: delta (0.5–4 hz), theta (4.5–8 hz), alpha (8.5–12 hz), beta (12.5–20 hz), and gamma (35–45 hz). ANOVA analyses revealed an overall effect of CT/DBS in theta (p<0.001), alpha (p<0.001), beta (p<0.001), and gamma (p<0.001) waves. ANOVA also showed an effect of amplitude in theta (B. p<0.05) and alpha (C. p<0.01) waves. An effect of frequency was found in alpha (H. p<0.05) waves. Light phase of CT/DBS was found to be significant in theta (L. p<0.05), alpha (M. p<0.01), beta (N. p<0.01), and gamma (P. p<0.001) waves. Panels include 10 minutes of data after CT/DBS, normalized to before stimulation. Data are presented as mean ± s.e.m. * p<0.05 compared to before, # p<0.05 compared to dark phase.
3.1.1 Amplitude effects
ANOVA analyses also revealed an effect of amplitude, when all other factors are held constant, in all three behavioral measures (Counts: F2,1000 = 15.68, p<0.001; Hactv: F2,902 = 7.83, p<0.001; Totdist: F2,914 = 5.13, p<0.01; see Figure 1). Comparing individual amplitudes, 125 μA stimulation increased activity counts greater than either 75 μA (during: t197 = −3.06, p<0.01; after: t200= −3.15, p<0.01) or 100 μA (during: t202 = −2.72, p<0.05; after: t202= −3.03, p<0.01) during and after stimulation (see Figure 1A). Looking at horizontal activity, 75 μA stimulation increased motor activity less than 100 μA (t178= −2.76, p<0.05) or 125 μA (t175= −2.90, p<0.05) during stimulation (see Figure 1B). Confirming an effect of stimulation, all amplitudes increased all three behavioral measures during and after stimulation compared to before.
In the ANOVA analyses of EEG waves, an effect of amplitude, holding all other factors constant, was found in two of five EEG waves (Theta: F2,780 = 3.06, p<0.05; Alpha: F2,772 = 5.08, p<0.01; see Figure 2). Despite the overall effect of amplitude in theta and alpha waves, there were no statistically significant differences between individual amplitudes in those two EEG waves. Comparing response after stimulation to before however, significant differences were found. Theta waves decreased with all three amplitudes of stimulation (75 μA: t278 = 2.92, p<0.01; 100 μA: t279= 5.56 p<0.001; 125 μA: t 250 = 6.32, p<0.001; see Figure 2B). 125 μA increased alpha (t248= −3.98, p<0.001; see Figure 2C) and beta (t235= −4.14, p<0.001; see Figure 2D) waves after stimulation. Gamma waves were increased after all three amplitudes (75 μA:t283= −3.00, p<0.01; 100 μA: t279= −3.41, p<0.01; 125 μA: t236= −4.38, p<0.001; see Figure 2E) of stimulation. These data also support the idea that more current in CT/DBS increases generalized arousal to a higher degree. To avoid negative side effects of injecting too much current into the brain, we decided on the conservative choice of 100 μA for future studies.
3.1.2 Frequency effects
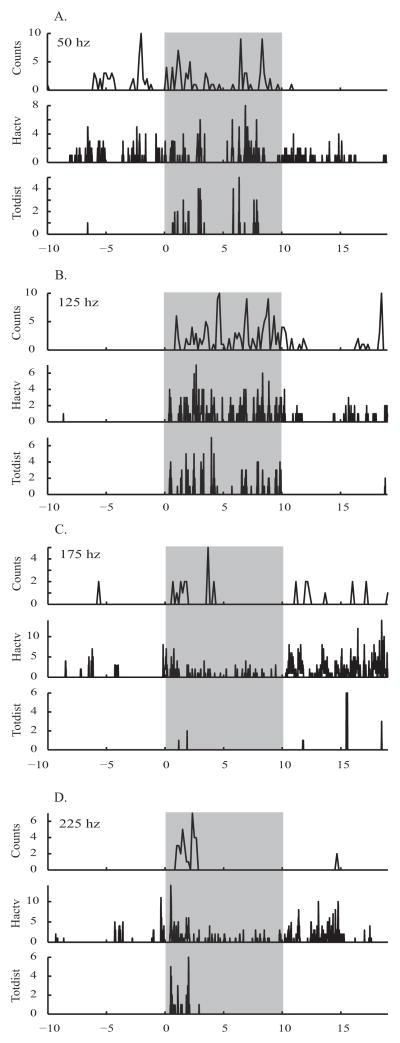
Investigation of the frequency of stimulation led to the determination there was a significant effect of frequency in two of behavioral measures (Hactv: F3,902 = 3.70, p<0.05; Totdist: F3,914 = 5.98, p<0.001; see Figures 1E and 1F). Despite this overall effect of frequency, none of the Bonferroni-corrected t-tests showed significant differences between individual frequencies. Comparing response during and after stimulation to before, 125 hz stimulation increased motor activity in activity counts during (t196= −4.00, p<0.001) and after (t196= −3.50, p<0.01; see Figure 1D) stimulation, horizontal activity during (t184= −4.12, p<0.001) and after (t184= −4.72, p<0.001; see Figure 1E) stimulation, and total distance during (t187 = −2.66, p<0.05; see Figure 1F) stimulation. An example of high temporal resolution raw behavioral data for all four frequencies in a single mouse can be found in Figure 3.
Figure 3. Parametric Experiment - example of behavior at high temporal resolution.
Motor activity behavior of one mouse at 4 frequencies of stimulation, 100 μA, in the light phase. A. 50 hz, B. 125 hz, C. 175 hz, D. 225 hz. Activity counts ('counts') shown at 1 sample every 10 seconds, Horizontal activity ('Hactv') and Total distance ('Totdist') shown at 1 sample every 1 second. Grey boxes mark epochs of stimulation.
Only one of the EEG waves displayed an effect of frequency when all other factors are held constant (Alpha: F3,772 = 3.57, p<0.05; see Figure 2H), and again, despite this ANOVA result, none of the Bonferroni-corrected t-tests showed significant differences between individual frequencies. EEG response after stimulation was also compared to before stimulation. Theta waves decreased with all four frequencies (50 hz: t198 = 4.30, p<0.001; 125 hz: t223 = 5.40, p<0.001; 175 hz: t197 = 3.86, p<0.001; 225 hz: t187 = 3.22, p<0.01; see Figure 2g) after stimulation. Alpha waves increased with 125 hz (t215= −3.06, p<0.01) and 225 hz (t184= −3.64, p<0.01; see Figure 2H) while Beta waves only increased significantly with 225 hz (t186= −3.80, p<0.001; see Figure 2I) after stimulation. Finally, gamma waves increased with all four frequencies (50 hz: t192= −2.53, p<0.05; 125 hz: t213= −3.25, p<0.01; 175 hz: t203= −2.92, p<0.05; 225 hz: t188= −3.69, p<0.001; see Figure 2J) after stimulation. While we do see an overall effect of frequency in some data measures, these effects of CT/DBS are inconsistent across data measures. Since 125 hz tended to increase arousal more than the other frequencies, we chose 125 hz for future studies.
Secondary analysis uncovered a multivariate interaction effect of amplitude and frequency (F18,2048 = 2.59, p<0.001). This effect was primarily exhibited in the behavioral measure horizontal activity (F6,902 = 3.82, p<0.01). The highest increases in horizontal activity during and after stimulation occurred with 125 hz stimulation at 100 μA. This result further supported our choice of 125 hz and 100 μA as stimulation parameters for future studies.
3.1.3 Daily light phase effects
In addition to the various parameters of stimulation manipulated above, we found that the light phase during which stimulation occurred had an effect, holding all other factors constant, on response to DBS in two behavioral measures (Hactv: F1,902 = 14.03, p<0.001; Totdist: F1,914= 16.07, p<0.001; see Figures 1H and 1I). Motor activity increased with light phase stimulation more than dark phase stimulation in horizontal activity during stimulation (t257 = 2.82, p<0.01; see Figure 1H) and total distance during (t263 = 2.49, p<0.01) and after stimulation (t264 = 2.20, p<0.05; see Figure 1I).
In ANOVA analyses of EEG waves, light phase of stimulation had a significant effect on response to stimulation in four EEG waves (Theta: F1,780 = 5.80, p<0.05; Alpha: F1,772 = 8.10, p<0.01; Beta: F1,760 = 9.05, p<0.01; Gamma: F1,771 = 12.40, p<0.001; see Figures 2L-2P). Theta waves decreased more with stimulation in the light phase than dark phase (t389= −1.99, p<0.05; see Figure 2L). Additionally, alpha (t381 = 2.42, p<0.05; see Figure 2M), beta (t369 = 2.54, p<0.05; see Figure 2N), and gamma (t380 = 2.82, p<0.01; see Figure 2P) waves all increased more with DBS during the light phase than the dark. These data coincided with previous observations that increases to arousal due to stimulation are larger in the light than the dark.
3.2 Temporal Pattern Experiment
In the analysis of the temporal pattern experiment, we looked for differences caused by the stimulation itself and investigated differences caused by pattern of stimulation, light phase during which stimulation occurred, and any correlation between data measures (scattergrams and correlation coefficients for EEG data can be found in Figure S3). As with the Parametric data set, we found a significant effect of stimulation in all three behavioral measures (Counts: F2,453= 21.47, p<0.001; Hactv: F2,513 = 15.19, p<0.001; Totdist: F2,504 = 12.56, p<0.001; see Figure 4) and four of the EEG waves (Delta: F1,271 = 8.76, p<0.01; Theta: F1,258 = 13.29, p<0.001; Beta: F1,258 = 5.57, p<0.05; Gamma: F1,261 = 8.25, p<0.01; see Figure 5). Again, we replicate findings here and elsewhere that CT/DBS increases generalized arousal as measured by motor activity and EEG response.
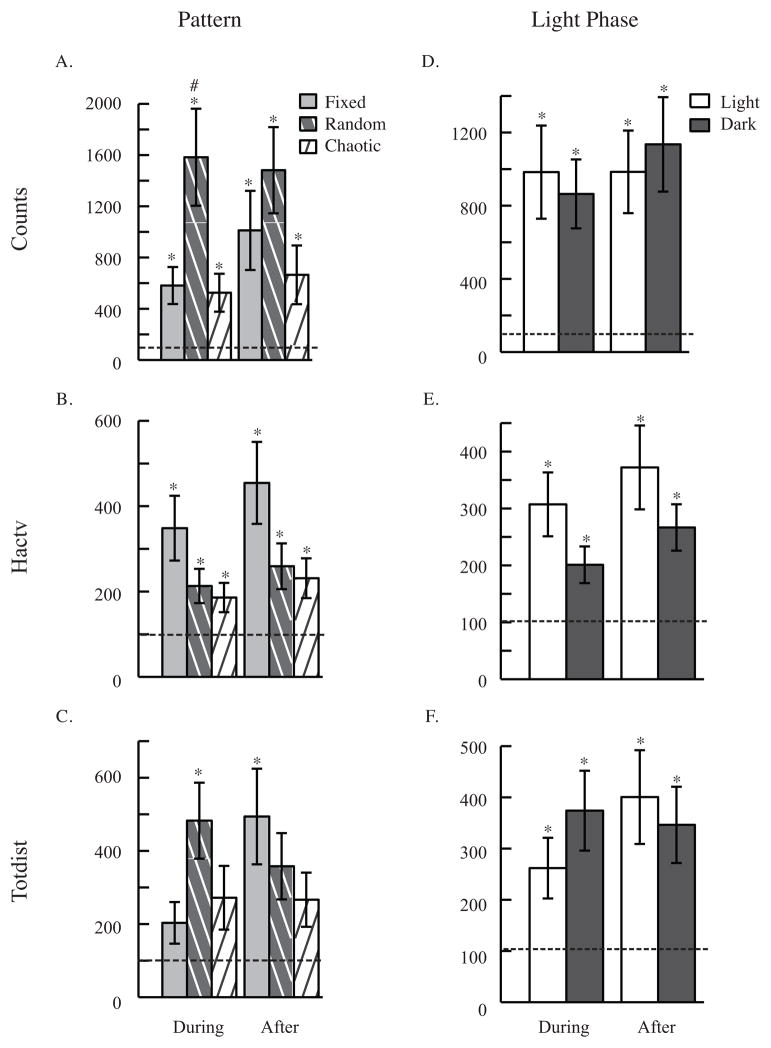
Figure 4. Temporal Pattern Experiment: Behavior.
Behavioral response to temporal pattern (A. B. and C.) and light phase (D. E. and F.) of CT/DBS. Three behavioral measures are shown: activity counts (whole body movement), horizontal activity (fidgeting), and total distance (ambulation). ANOVA analyses revealed an overall effect of temporal pattern in activity counts (A. p<0.001) and horizontal activity (B. p<0.01). Panels include 10 minutes of data during and 10 minutes of data after CT/DBS, all normalized to before stimulation. Data are presented as mean ± s.e.m. * p<0.05 compared to before, # p<0.05 compared to chaotic.
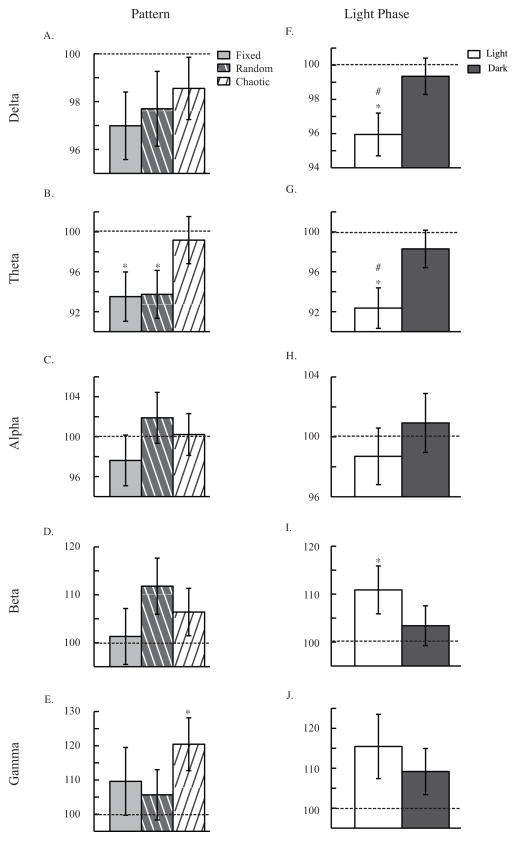
Figure 5. Temporal Pattern Experiment: EEG.
EEG response to temporal pattern (A. B. C. D. and E.) and light phase (F. G. H. I. and J.) of CT/DBS. EEG waves are defined by the following frequency bins: delta (0.5–4 hz), theta (4.5–8 hz), alpha (8.5–12 hz), beta (12.5–20 hz), and gamma (35–45 hz). ANOVA analyses revealed an effect of CT/DBS in activity counts (p<0.001), horizontal activity (p<0.001), and total distance (p<0.001) Light phase of CT/DBS also had a significant effect in delta (F. p<0.05) and theta (G. p<0.05) waves. Panels include 10 minutes of data after CT/DBS, normalized to before stimulation. Data are presented as mean ± s.e.m. * p<0.05 compared to before, # p<0.05 compared to dark phase.
3.2.1 Temporal pattern effects
ANOVA analyses also revealed an effect of temporal patterning, while all other factors remain constant, in two behavioral measures (Counts: F2,453 = 8.56, p<0.001; Hactv: F2,513= 6.44, p<0.01; see Figures 4A and 4B). While an overall effect of pattern was found, only one post-hoc comparison between patterns was significant. Random stimulation increased activity counts significantly more than Chaotic during stimulation (t96 = 2.52, p<0.05; see Figure 3A). Comparing response during and after stimulation to before, all patterns increased motor activity compared to before in activity counts during (Fixed: t103= −3.83, p<0.001; Random: t113= −4.39, p<0.001; Chaotic: t105= −2.35, p<0.01) and after stimulation (Fixed: t104= −3.38, p<001; Random: t114= −4.56, p<0.001; Chaotic: t106= −2.76, p<0.05; see Figure 4A), horizontal activity during (Fixed: t116= −3.33, p<0.01; Random: t121 = −2.93, p<0.05; Chaotic: t116= −2.55, p<0.05) and after stimulation (Fixed: t115= −3.79, p<0.01; Random: t121= − 3.10, p<0.01; Chaotic: t116= −2.86, p<0.05; see Figure 4B), and total distance during (Random: t120= −3.87, p<0.001) and after stimulation (Fixed: t114= −3.12, p<0.01; Random: t119= −3.02, p<0.01; see Figure 4C). An example of high temporal resolution raw behavioral data for all three patterns in a single mouse can be found in Figure 6.
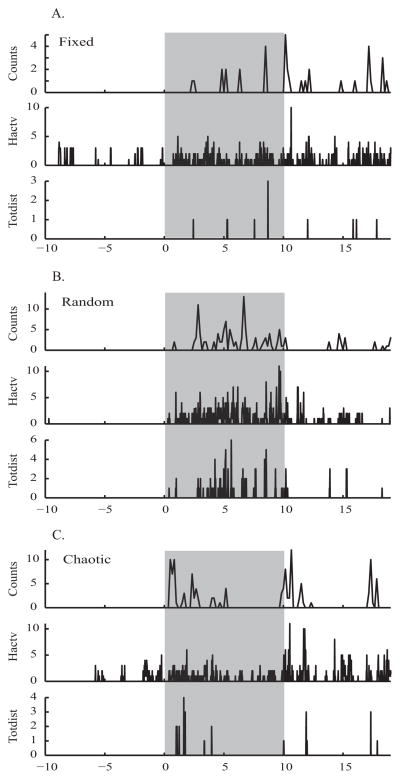
Figure 6. Temporal Pattern Experiment - example of behavior at high temporal resolution.
Motor activity behavior of one mouse at 3 temporal patterns of stimulation, 100 μA, in the light phase. A. Fixed, B. Random, and C. Chaotic. Activity counts ('counts') shown at 1 sample every 10 seconds, Horizontal activity ('Hactv') and Total distance ('Totdist') shown at 1 sample every 1 second. Grey boxes mark epochs of stimulation.
In the EEG analysis, there was no overall effect of pattern in any of the EEG waves, but in comparing responses after DBS to before, some significant differences emerged. Fixed and Random DBS decreased theta waves after stimulation (Fixed: t84 = 2.96, p<0.05; Random: t95= 2.81, p<0.05; see Figure 5B), and chaotic DBS increased gamma waves after stimulation (t90= −2.77, p<0.05; see Figure 5E). Despite an overall effect of temporal pattern and large obvious differences, conservative Bonferroni-corrected post hoc analyses reveal few significant differences between specific temporal patterns.
3.2.2 Daily light phase effects
An effect of daily light phase was found in two of the EEG waves (Delta: F1,271 = 4.24, p<0.05; Theta: F1,258 = 6.49, p<0.05; see Figures 5F and 5G). This general effect was confirmed by post-hoc t tests comparing light to dark (Delta: t136= −2.09, p<0.05; Theta: t123= −2.14, p<0.05) after stimulation (see Figures 5F and 5G). In three EEG waves, response after light phase DBS was significantly different from before. Delta (t132= 3.38, p<0.001) and theta (t127= 4.10, p<0.001) waves decreased with stimulation in the light (see Figures 5F and 5G), and beta (t124= −2.44, p<0.05) waves increased with stimulation in the light (see Figure 5I). This recapitulates our result that higher relative responses to stimulation occur during the light phase.
4. Discussion
4.1 Major Findings
4.1.1 Behavior
Considering the behavioral results of both experiments presented above, we conclude that generalized arousal as measured by spontaneous motor activity can be increased by CT/DBS. This increase can be modulated by various parameters of stimulation. Amplitude of DBS increases behavioral response in an expected way; more current yields a concomitant increase in motor activity. Behavioral responses to various frequencies of DBS also differ, but in this particular circumstance the differences between the specific frequencies chosen were not significant. Light phase during which DBS occurs can also affect degree of increase in behavior; larger increases during and after stimulation occur during the light phase and are most likely due to the low baseline activity during the light. Most importantly we have found that temporal pattern of stimulation affects behavioral response to CT/DBS. This effect of temporal pattern in behavioral response to DBS replicates our previous findings [16] that temporal patterning makes a difference.
Our behavioral data revealed an overall effect of temporal pattern, but only one specific significant difference in the Bonferroni-corrected post-hoc analysis and only in the activity counts behavioral measure. In that instance, Random CT/DBS increased motor activity during stimulation more than Chaotic. This result is unexpected. Since we theorized [17] that nonlinear dynamics play a role in controlling arousal systems, we hypothesized that Chaotic CT/DBS would increase arousal more than either Fixed or Random; instead we see that Random does better. At the moment, it is unclear why one temporal pattern did better than another in one behavioral measure. Given that our results ran counter to our expectations, it might be that some singular undefined characteristic of the Random temporal pattern was responsible for the temporal pattern s success instead of theoretical method by which it was generated.
4.1.2 EEG
EEG responses to DBS were as expected. In both experiments, EEG waves changed significantly with CT/DBS and in a direction consistent with an increase in arousal. Delta and theta waves, associated with sleep and quiet wakefulness [22], decreased with CT/DBS, while alpha, beta, and gamma waves, associated with wakefulness and higher cognitive functions such as attention [23], increased with CT/DBS. Degree of response to DBS was modulated by amplitude of stimulation, but these changes were small and often not significant. Differences between response to various frequencies of stimulation were also subtle and only significant in alpha waves. EEG response to DBS during the two phases of the light cycle were also different; changes after DBS were larger in the light phase than in the dark. We assume these larger responses during the light phase are due to the nocturnal nature of mice; since mice are often quiescent during the light phase, they respond more dramatically to an arousing stimulus then. We saw no differences in response to our three patterns of stimulation. The EEG data presented here do not coincide with our previously published results [16]. It was discovered that a typographical error in the analysis script for these previously collected EEG data altered the results and their interpretation. Upon reanalyzing these data, we found that delta waves did not increase significantly with either hippocampal or central thalamus stimulation and that gamma waves did increase significantly with central thalamus stimulation. These corrected results coincide with our expectations as well as the results presented here. Other differences can be explained by different brain regions stimulated as well as different stimulation parameters used.
4.2 Literature
Due to the novelty of this work, there is only a handful of articles, reporting work done in varying contexts, to compare our results to. CT/DBS has been investigated in intact rats [24], brain injured rats [25], macaque monkeys [26], mice [16], and one human case study [10]. Concurrent with what we observe here, CT/DBS increased motor activity or enhanced performance on a cognitive task in all of these studies; however, none of the work mentioned above explored the importance of temporal pattern of stimulation. Shirvalkar et al. showed increased early action gene expression in cortical and basal ganglia regions as well as enhanced performance on a novel object recognition task with CT/DBS in intact rats [24]. In a DMTP rat model, Mair et al showed enhanced working memory with CT/DBS [25]. Smith et al. used bayesian statistical methods to analyze the effect of CT/DBS on a sustained attention task in macaque monkeys [26]. This method was also used to further analyze data collected from the human case study of CT/DBS in a MCS patient. It was confirmed that CT/DBS helped facilitate functional recovery in this patient [10, 26]. These studies represent the body of evidence that CT/DBS can increase arousal and enhance cognition, but there are still questions to explore such as what stimulation parameters, including pattern, are best and how do these parameters need to be adjusted, patient to patient.
4.3 Potential Caveats
Like many behavioral studies with multiple subjects, especially mice, these experiments show a large variability. Three potential sources of variance are considered: baseline activity level, exact electrode placement, and sex differences. First, it is clear that baseline activity level does influence response to stimulation. Mice respond differentially to stimulation in the light and dark; responses during the light phase, when mice are more often quiescent, are relatively larger than those during the dark phase. Fluctuations of baseline activity on a smaller scale are likely causes of intra-subject variability. Second, small variations in electrode placement might affect inter-subject variability. These small position differences change the electrical field of stimulation and which specific neurons are influenced by this field. These changes might alter the efficiency of stimulation as well as the magnitude of response during and directly after stimulation. Finally, we observed small, inconsistent sex differences as mentioned in section 2.1. While these differences were not statistically significant, it is likely they affected inter-subject variability once the data from males and females were pooled.
4.4 Conclusions
Here we have presented more evidence that temporal patterning of DBS can affect the magnitude of desired responses. While a comprehensive exploration of temporal patterns in DBS is outside the scope of this paper, possible avenues to continue this work would be to investigate 1) longer sequences of pulses that would presumably permit still more entropy, 2) specific local characteristics of the temporal patterns presented here to determine why Random was better than Chaotic, 3) other deterministic chaotic equations [27], and 4) playbacks or temporal patterns recorded from central thalamus or from arousal systems that project to the central thalamus. Additionally, it stands to reason that because we found that temporal patterns of CT/DBS are important in modulating motor activity and EEG response, temporal patterns of stimulation might modulate desired responses to DBS in other, medically important contexts. More research is needed into how neuronal circuits of interest function in normal and diseased states as well as how DBS can be used to effect desired changes in these circuits. This is essential to the future of DBS therapy because the more we know about diseased neuronal circuits and how DBS affects them, the better able we will be to logically choose stimulation parameters and design DBS regimes. Choosing a stimulation regime for a specific disorder or set of symptoms will be much more efficient than the current method for finding stimulation parameters, by trial and error.
Supplementary Material
A. Coronal figure at bregma −1.70 mm of whole mouse brain and close up of the thalamus. Electrode placements indicated by black dots. To be included in analysis, mice must have at least one hit within the central thalamus. Parametric experiment mice: close up on just the thalamus at B. bregma −1.70 mm, C. bregma −1.94 mm, and D. bregma −2.06 mm. Temporal Pattern experiment mice: close up on just the thalamus at E. bregma −1.70 mm, F. bregma −1.94 mm, and G. bregma −2.06 mm. Abbreviations for thalamic nuclei include CL (central lateral thalamic nucleus), PC (paracentral thalamic nucleus), CM (central medial thalamic nucleus), MD (mediodorsal thalamic nucleus), PF (parafascicular thalamic nucleus).
Data are correlated in expected ways. Delta is negatively correlated with alpha (r = −0.7943) and beta waves (r = −0.6077). Alpha and beta waves are positively correlated (r = 0.7116). Gamma is positively correlated with alpha (r = 0.6575) and beta waves (r = 0.9106). Data plotted are only after stimulation and are normalized to before stimulation. * p<0.0001.
Data are correlated in expected ways. Delta is negatively correlated with alpha (r = −0.7717) and beta waves (r = −.7278). Alpha and beta waves are positively correlated (r = 0.6905). Gamma is positively correlated with beta waves (r = 0.8332). Data plotted are only after stimulation and are normalized to before stimulation. * p<0.0001.
Research Highlights.
We optimized amplitude and frequency of DBS of the central thalamus for increasing arousal
We chose 100 μA and 125 hz for rest of our experiments
Temporal pattern of DBS modulates increases in motor activity seen with stimulation
Random temporal pattern did better than Chaotic temporal pattern, against our expectations
Future: learn how temporal pattern of DBS can modulate/disrupt brain region or circuit of interest
Acknowledgments
We appreciate the intellectual support of Nicolas D Schiff as well as the funding from his NIH grant NS067249. This work was also funded in part by Intelect Medical, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Benabid AL, Chabardes S, Mitrofanis J, Pollak P. Deep brain stimulation of the subthalamic nucleus for the treatment of Parkinson's disease. Lancet Neurology. 2009;8:67–81. doi: 10.1016/S1474-4422(08)70291-6. [DOI] [PubMed] [Google Scholar]
- 2.Castrioto A, Lozano AM, Poon YY, Lang AE, Fallis M, Moro E. Ten-Year Outcome of Subthalamic Stimulation in Parkinson Disease: A Blinded Evaluation. Arch Neurol. 2011 doi: 10.1001/archneurol.2011.182. [DOI] [PubMed] [Google Scholar]
- 3.Obeso JA, Guridi J, Rodriguez-Oroz MC, Agid Y, Bejjani P, Bonnet AM, et al. Deep-brain stimulation of the subthalamic nucleus or the pars interna of the globus pallidus in Parkinson's disease. New England Journal of Medicine. 2001;345:956–63. doi: 10.1056/NEJMoa000827. [DOI] [PubMed] [Google Scholar]
- 4.Greenberg BD, Suzanne SLR, Haber SN. Invasive Circuitry-Based Neurotherapeutics: Stereotactic Ablation and Deep Brain Stimulation for OCD. Neuropsychopharmacology. 2010;35:317–36. doi: 10.1038/npp.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirschfeld RMA. Deep Brain Stimulation for Treatment-Resistant Depression. American Journal of Psychiatry. 2011;168:455–6. doi: 10.1176/appi.ajp.2011.11020231. [DOI] [PubMed] [Google Scholar]
- 6.Wichmann T, DeLong MR. Deep brain stimulation for neurologic and neuropsychiatric disorders. Neuron. 2006;52:197–204. doi: 10.1016/j.neuron.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 7.Coffey RJ. Deep brain stimulation for chronic pain: results of two multicenter trials and a structured review. Pain Med. 2001;2:183–92. doi: 10.1046/j.1526-4637.2001.01029.x. [DOI] [PubMed] [Google Scholar]
- 8.Levy R, Deer TR, Henderson J. Intracranial Neurostimulation for Pain Control: A Review. Pain Physician. 2010;13:157–65. [PubMed] [Google Scholar]
- 9.Rasche D, Rinaldi PC, Young RF, Tronnier VM. Deep brain stimulation for the treatment of various chronic pain syndromes. Neurosurg Focus. 2006;21:E8. doi: 10.3171/foc.2006.21.6.10. [DOI] [PubMed] [Google Scholar]
- 10.Schiff ND, Giacino JT, Kalmar K, Victor JD, Baker K, Gerber M, et al. Behavioural improvements with thalamic stimulation after severe traumatic brain injury. Nature. 2007;448:600–3. doi: 10.1038/nature06041. [DOI] [PubMed] [Google Scholar]
- 11.Schiff ND. Central thalamic contributions to arousal regulation and neurological disorders of consciousness. Molecular and Biophysical Mechanisms of Arousal, Alertness, and Attention. 2008;1129:105–18. doi: 10.1196/annals.1417.029. [DOI] [PubMed] [Google Scholar]
- 12.Schiff ND. Central thalamic deep-brain stimulation in the severely injured brain: rationale and proposed mechanisms of action. Ann N Y Acad Sci. 2009;1157:101–16. doi: 10.1111/j.1749-6632.2008.04123.x. [DOI] [PubMed] [Google Scholar]
- 13.Kumar A, Rotter S, Aertsen A. Spiking activity propagation in neuronal networks: reconciling different perspectives on neural coding. Nature Reviews Neuroscience. 2010;11:615–27. doi: 10.1038/nrn2886. [DOI] [PubMed] [Google Scholar]
- 14.Panzeri S, Brunel N, Logothetis NK, Kayser C. Sensory neural codes using multiplexed temporal scales. Trends in Neurosciences. 2010;33:111–20. doi: 10.1016/j.tins.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Wasserman GS. Isomorphism, task dependence, and the multiple meaning theory of neural coding. Biol Signals. 1992;1:117–42. doi: 10.1159/000109318. [DOI] [PubMed] [Google Scholar]
- 16.Quinkert AW, Schiff ND, Pfaff DW. Temporal patterning of pulses during deep brain stimulation affects central nervous system arousal. Behavioural Brain Research. 2010;214:377–85. doi: 10.1016/j.bbr.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 17.Pfaff D, Banavar JR. A theoretical framework for CNS arousal. Bioessays. 2007;29:803–10. doi: 10.1002/bies.20611. [DOI] [PubMed] [Google Scholar]
- 18.Paul CA, Beltz BS, Berger-Sweeney J. Discovering Neurons: The Experimental Basis of Neuroscience. Cold Spring Harbor Laboratory Press; 1997. [Google Scholar]
- 19.Haahr M. [Accessed 16 June 2010.];Random Integer Generator. Random.org. 1998 Available at http://www.random.org/integers.
- 20.Brown MB, Forsythe AB. 372: The Anova and Multiple Comparisons for Data with Heterogeneous Variances. Biometrics. 1974:719–724. [Google Scholar]
- 21.Tabachnick BG, Fidell LS. Using multivariate statistics. 4. Boston, MA: Allyn and Bacon; 2001. [Google Scholar]
- 22.Niedermeyer E. Sleep and EEG. In: Niedermeyer E, Lopes da Silva F, editors. Electroencephalography: Basic Principles, Clinical Applications, and Related Fields. 5. Philadelphia: Lippincott Williams and Wilkins; 2005. [Google Scholar]
- 23.Niedermeyer E. The Normal EEG of the Waking Adult. In: Niedermeyer E, Lopes da Silva F, editors. Electroencephalography: Basic Principles, Clinical Applications, and Related Fields. 5. Philadelphia: Lippincott Williams and Wilkins; 2005. [Google Scholar]
- 24.Shirvalkar P, Seth M, Schiff ND, Herrera DG. Cognitive enhancement with central thalamic electrical stimulation. Proc Natl Acad Sci U S A. 2006;103:17007–12. doi: 10.1073/pnas.0604811103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mair RG, Hembrook JR. Memory Enhancement with Event-Related Stimulation of the Rostral Intralaminar Thalamic Nuclei. Journal of Neuroscience. 2008;28:14293–300. doi: 10.1523/JNEUROSCI.3301-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith AC, Shah SA, Hudson AE, Purpura KP, Victor JD, Brown EN, et al. A Bayesian statistical analysis of behavioral facilitation associated with deep brain stimulation. Journal of Neuroscience Methods. 2009;183:267–76. doi: 10.1016/j.jneumeth.2009.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen JE. Unexpected dominance of high frequencies in chaotic nonlinear population models. Nature. 1995;378:610–6. doi: 10.1038/378610a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A. Coronal figure at bregma −1.70 mm of whole mouse brain and close up of the thalamus. Electrode placements indicated by black dots. To be included in analysis, mice must have at least one hit within the central thalamus. Parametric experiment mice: close up on just the thalamus at B. bregma −1.70 mm, C. bregma −1.94 mm, and D. bregma −2.06 mm. Temporal Pattern experiment mice: close up on just the thalamus at E. bregma −1.70 mm, F. bregma −1.94 mm, and G. bregma −2.06 mm. Abbreviations for thalamic nuclei include CL (central lateral thalamic nucleus), PC (paracentral thalamic nucleus), CM (central medial thalamic nucleus), MD (mediodorsal thalamic nucleus), PF (parafascicular thalamic nucleus).
Data are correlated in expected ways. Delta is negatively correlated with alpha (r = −0.7943) and beta waves (r = −0.6077). Alpha and beta waves are positively correlated (r = 0.7116). Gamma is positively correlated with alpha (r = 0.6575) and beta waves (r = 0.9106). Data plotted are only after stimulation and are normalized to before stimulation. * p<0.0001.
Data are correlated in expected ways. Delta is negatively correlated with alpha (r = −0.7717) and beta waves (r = −.7278). Alpha and beta waves are positively correlated (r = 0.6905). Gamma is positively correlated with beta waves (r = 0.8332). Data plotted are only after stimulation and are normalized to before stimulation. * p<0.0001.