Abstract
Previously, we reported that peripheral vaccination of mice with modified autologous tumor cells secreting granulocyte-macrophage colony-stimulating factor (GM-CSF) combined with ionizing radiation to the whole brain cured 50% of mice using a syngeneic, intracranial model of murine high-grade glioma. Here, we tested the combination of radiotherapy (4 Gy × 2) with an immunotherapeutic approach using an anti-CD137 antibody directed to the co-stimulatory molecule CD137. The CD137 antibody has shown promise in generating effective antitumor responses in several animal models and has demonstrated a favorable toxicity profile in the clinic. The combination of radiation and anti-CD137 therapy resulted in complete tumor eradication and prolonged survival in six of nine (67%) mice with established brain tumors (P = 0.0009). Five of six (83%) long-term survivors in the combination group demonstrated antitumor immunity by rejecting challenge tumors. Antitumor immunity was associated with an increased number of tumor-infiltrating lymphocytes (TILs) in brain tumors and increased tumor-specific production of γ IFN. In view of the finding that radiation enhanced the antitumor effect of anti-CD137 therapy, this approach should be studied further for clinical translation.
Introduction
The current standard of care for glioma uses adjuvant chemoradiotherapy with the alkylating agent temozolomide (1). Most recently, the anti-angiogenic monoclonal antibody bevacizumab has been used in patients with recurrent glioma in combination with radiotherapy and irinotecan or carboplatin (2). Despite all these approaches, only a small increase in overall survival has been achieved.
To improve these disappointing results, immunotherapy for gliomas has been explored, including passive and active immunotherapy strategies (3). Antibodies targeting the epidermal growth factor receptor such as cetuximab (Bristol-Myers Squibb) have been shown to increase the effects of radiotherapy and chemotherapy. Adoptive T-cell therapy uses autologous CD8+ T cells specific for a given antigen, such as the glioma-associated antigen gp100, are expanded ex vivo and reinfused into the patient. Another immunotherapeutic approach for gliomas has been a form of active immunotherapy that uses tumor-derived vaccines. In this case a lysate derived from the tumor is used to expand autologous CD8+ T cells specific for a given antigen, such as the glioma-associated antigen gp100, ex vivo for reinfusion into the patient. To date these trials have demonstrated safety and some preliminary efficacy (4–6).
Our group has explored strategies to merge standard radiotherapy with immunotherapy. We have used for preclinical testing an experimental mouse glioma model that mimics the aggressive and invasive growth observed in human brain tumors (7). In this model, we have shown that peripheral vaccination of mice with modified autologous tumor cells secreting granulocyte-macrophage colony-stimulating factor (GM-CSF) combined with a modest dose of ionizing radiation to the whole brain can cure well-established brain tumors in about half of the animals (8). In the present study we tested an alternative immunotherapeutic approach using an antibody directed to the co-stimulatory molecule CD137 (4-1BB), which has shown promise in generating effective antitumor responses in various animal models of cancer (9, 10).
CD137 is a membrane protein, a member of the tumor necrosis factor receptor (TNFR) family, that has been shown to augment CD4 and CD8 T-cell responses (11–14). It is expressed on activated CD4+ and CD8+ T cells, NK cells and monocytes (15–17). Binding of 4-1BB to its ligand (4-1BBL) induces a signaling cascade in T cells that promotes their activation, survival and growth (18, 19). Anti-CD137 antibody treatment of tumor-bearing animals has been shown to enhance antitumor immunity in several preclinical models of cancer including P815 mastocytoma, AG104A sarcoma, GL261 glioma, 10.2 fibrosarcoma, CT26 colon carcinoma, EL4 lymphoma and B16F10 melanoma (20–25).
The growing awareness that radiotherapy-mediated effects can make tumors more amenable to immune recognition has encouraged testing its combination with novel immunotherapy approaches (26, 27). We hypothesized that a low therapeutic dose of ionizing radiation would induce local tumor cell death, providing signals to enhance presentation of tumor-derived antigens to antitumor T cells (28, 29).
Administration of whole-brain radiation treatment first was based on the rationale that T-cell activation could occur prior to anti-CD137 treatment that then would support the expansion and survival of antitumor T cells. Since the human version of the CD137 antibody is currently in clinical trials with promising results, it appears to be a good candidate to test with radiotherapy in preclinical models.
Materials and Methods
Mice
Female C57BL/6 mice were obtained from Taconic (Germantown, NY) and maintained under aseptic conditions in microisolator cages. All animal studies were performed under a protocol approved by the Institutional Animal Care and Use Committee at New York University School of Medicine. The mice used for the experiments weighed 20 g and were 10 to 12 weeks old, as described previously (8).
Tumor
The GL261 is a poorly immunogenic glioma line that was induced through intracranial implantation of 20-methylcholanthrene pellets into brains of C57BL/6 CRL mice (30). Cells were cultured in 5% CO2 and 95% humidified air atmosphere at 37°C in Dulbecco's modified Eagle's medium (DMEM) (Gibco BRL, Grand Island, NY) supplemented with 10% fetal bovine serum (Atlanta Biologicals, Norcross, GA), 0.25% gentamicin (Gibco BRL) and 1% l-glutamine (Gibco BRL) as described previously (7). GL261 cells were cultured to subconfluence, trypsinized, washed twice in DMEM without serum, and resuspended in DMEM for inoculation into the brains of mice.
Anti-CD137 Antibody
A rat IgG2a mAb against mouse CD137 (BMS-469492, clone 1D8) was produced and purified by Bristol-Myers Squibb (Princeton, NJ). Anti-CD137 mAb was certified to have <0.5 EU/mg endotoxin level, >95% purity and <5% high-molecular-weight species. Bristol-Myers Squibb provided us with the anti-CD137 mAb for these experiments. Stock solutions of anti-CD137 mAb were kept at −80°C and were thawed on ice prior to use.
Ionizing Radiation and Anti-CD137 Therapy
Mice were anesthetized to ensure immobilization to allow radiation to be delivered directly to the tumor-bearing hemisphere. Radiation was delivered to the head of the mouse centered in a 5-cm radiation field using a 60Co radiation source (Theratron 780-C, AECL Medical) on days 15 and 17 after implantation. The total radiation dose administered was 8 Gy (4 Gy × 2) given at 48-h intervals as described (8). We have shown that GL261 cells express low levels of major histocompatibility complex (MHC) molecules that become up-regulated in response to 4 Gy ionizing radiation in vitro and in vivo (8). In our experience, use of fractionated radiotherapy given at 48-h intervals compared to a single dose of radiation prolonged animal survival (31). Anti-CD137 antibody was given by intraperitoneal injection at a dose of 200 mg 3 days apart on days 18, 21 and 24 (32). Control mice received isotype control rat IgG2a (clone 2A3, Bio X Cell, West Lebanon, NH).
Treatment Protocol
To establish intracerebral (i.c.) tumors, GL261 glioma cells (1 × 105) were implanted in the brains of 10- to 12-week-old female C57BL/6 mice (20 g) as described previously (8). Briefly, animals were anesthetized with xylazine/ketamine (10 mg/kg xylazine/90 mg/kg ketamine) and a burr hole was drilled into the skull 0.1 mm posterior to the bregma and 2.32 mm lateral to the midline. GL261 cells (5 × 107/ml) in 2 ml of medium were inoculated stereotactically using a head frame (David Kopf Instruments, Tujunga, CA) in the defined location of the caudate/putamen (0.1 mm posterior to the bregma, 2.32 mm lateral to the midline) using a 10-μl Hamilton syringe (no. 80301, Reno, NV) with a 1-in. 30-gauge needle attached and inserted into a Kopf microinjection unit (Model 5000 with Model 5001 Hamilton syringe holder). The needle was advanced to a depth of 2.35 mm from the cortical surface and the cell suspension was delivered slowly over the course of 3–4 min. After injection, the needle was left in place for 2 min, after which time it was raised to a depth of 1.5 mm below the dura and left in place for an additional minute. Upon withdrawal of the needle, the burr hole was immediately sealed with bone wax and the incision was sutured.
On day 15 after implantation, mice were randomly assigned to four treatment groups: (1) sham irradiation and non-specific rat IgG; (2) whole-brain irradiation given in two fractions of 4 Gy, 48 h apart on days 15 and 17 and rat IgG; (3) anti-CD137 mAb alone; and (4) whole-brain irradiation and anti-CD137 mAb. The experiment was repeated twice, with similar results. The combined results represent nine mice per group.
Survival was followed and recorded as the percentage of surviving mice over time (in days) after tumor inoculation. Mice were observed twice weekly, and when they showed signs of neurological deficit (lethargy, failure to ambulate or lack of feeding resulting in loss of >20% body weight) they were euthanized. Early signs of motor deficit in the animals were demonstrated by allowing them to grab the top of the cage with their front paws. Failure to grasp the cage top with their paws is an indication of weak paresis.
Animals were anesthetized and then perfused transcardially with PBS followed by 4% paraformaldehyde. Brains were removed and placed in cold 4% paraformaldehyde overnight, then sliced into 2-mm coronal sections prior to processing and embedding in paraffin. Tumors were measured grossly in three orthogonal axes to determine tumor volumes. Hematoxylin and eosin (H&E)-stained coronal sections were used as a check of tumor volumes and for overall tumor histopathology.
Assessment of Antitumor Immune Response
Antitumor immunity in the long-term survivors was assessed using subcutaneous (s.c.) tumor rechallenge to assess their systemic state of immunity. Control naïve 6- to 8-week-old female C57BL/6 mice or long-term surviving animals were challenged by s.c. injection in the hind limb of GL261 cells (2 × 106) in 0.05 ml of serum-free medium. Long-term survivors were challenged at 8 or 16 weeks after i.c. implantation in the two independent experiments, respectively. Tumor growth was measured twice weekly using calipers and tumor volumes were calculated using the formula (length × width2)/2, where the length represents the longest axis and the width was measured at right angles to the length. Animals were observed for an additional 60 days and/or killed when tumor volumes reached approximately 2000 mm3. Brains from long-term survivors were harvested and H&E-stained coronal sections were examined for overall brain histopathology.
Assessment of Tumor-Infiltrating Lymphocytes (TILs)
On day 22 after implantation, mice were euthanized for the collection of brains and spleens (see γIFN Assay). Brains were harvested and tumor-bearing hemispheres from two mice per treatment group were processed into single cell suspensions and the lymphocyte-enriched fraction was obtained using a Percoll gradient as described previously (8). Each brain was removed and the tumor was finely minced with a razor blade and then homogenized in 5 ml of PBS in a Dounce Homogenizer (7 ml, Pyrex no. 7727-07). The homogenate was drawn up into a 12-ml syringe fitted with a 21-gauge needle and passaged 10 times through the needle. The final cell suspension was filtered through a 40-μm nylon mesh strainer into a 50-ml tube. The cell suspension was centrifuged at 400g for 10 min at room temperature. The pellet was resuspended in 4 ml of 30% isotonic Percoll and overlaid on a Percoll gradient and centrifuged at 500g for 20 min. Lymphocytes were collected from the 37% to 70% interface, washed once in PBS, and counted in a hemacytometer. Aliquots of cells (1 × 105) were blocked in 10% normal mouse serum in PBS for 15 min at room temperature followed by staining with FITC-CD3, PE-CD4 and PE-Cy5-CD8 monoclonal antibodies (BD Pharmingen, San Jose, CA) for 30 min on ice. Cells were washed, resuspended in 1% paraformaldehyde, and analyzed using a FACScan flow cytometer (BD Biosciences, Bedford, MA) and FlowJo software version 6.4.4 (Tree Star, Ashland, OR). The total numbers of CD3+CD4+ and CD3+CD8+ T cells were calculated by multiplying the percentages of cells positive for each marker by the total number of viable cells obtained from the two pooled brains by the percentage of cells in the lymphocyte gate, as described previously (8). Two independent experiments were performed with similar results.
γIFN Assay
On day 22 after implantation, mice were euthanized for the collection of spleens and brains (see TIL Assay). Splenocytes (5 × 106) were cultured for 5 days in a six-well plate in the absence or presence of irradiated GL261 cells (1 × 105) in RPMI 1640 medium supplemented with 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 50 μM 2-mercapthoethanol, 10% FBS (T-cell medium) and 30 U/ml human rIL-2 (provided by the National Cancer Institute BRB Preclinical Repository), as described previously (33). γIFN was measured in cell-free supernatants by ELISA (Diaclone Tepnel, Lifecodes Corp., Stamford, CT). Tumor-specific γIFN production was calculated by subtracting the background values measured in supernatants of spleen cells cultured with medium alone. Two independent experiments were performed with similar results.
Statistical Analysis
Comparisons of survival curves of different groups were made by the log-rank test. P values <0.05 were considered significant.
Results
Radiation Enhances Antitumor Effect of Anti-CD137 Therapy
In agreement with our previous results, the median survival time was 31 days for control animals treated with rat IgG and 37 days for animals treated with whole-brain radiation and rat IgG (Fig. 1B) (8). Administration of anti-CD137 in the absence of radiation resulted in a median survival time of 42 days. Mice treated with the combination of radiation and anti-CD137 had a median survival of 114 days. This result was significantly superior when compared to the rat IgG group (P = 0.0009), the group treated with anti-CD137 alone (P = 0.0038), and the radiation plus rat IgG group (P = 0.036).
FIG. 1.

Radiation enhances antitumor effect of anti-CD137 therapy. Panel A: Schematic of the treatment protocol. Panel B: Survival curves of animals in the different treatment groups (n = 9/group). The experiment was repeated twice with similar results; the combined results are shown. Panel C: Long-term survivors and naïve mice were challenged in the hind limb with a tumorigenic inoculum of GL261 cells and observed for 60 days. The percentages of tumor-free mice are shown. WBRT: whole-brain irradiation.
To test whether the long-term survivors had developed a protective antitumor memory response, the eight surviving animals (2/9 from radiation alone group and 6/9 from the combination radiation and anti-CD137 group) were challenged with a tumorigenic inoculum of GL261 cells in the hind limb together with a control group of naïve animals who received the same tumorigenic inoculum (n = 9). Tumor development was monitored for an additional 60 days (Fig. 1C). All nine naïve animals had palpable tumors by 21 days. In contrast, only two of eight (25%) long-term survivors developed tumors at the site of challenge: one animal from the radiation group and one animal from the combination radiation and anti-CD137 group (Fig. 1C). Brains from all eight long-term survivors lacked histopathological evidence of residual tumor.
Antitumor Response Correlates with Increased TILs in the Tumor
The evidence of a protective antitumor response in mice that survived long-term (Fig. 1C) supports the interpretation that treatment elicited an adaptive immune response. To monitor the immune response associated with the inhibition of the i.c. gliomas, brains from animals in each of the treatment group were harvested on day 22 to determine the numbers of CD8 and CD4 T cells infiltrating the tumors. As predicted, at this early time, tumors were smaller in all mice from the three treatment arms compared to the control group (Fig. 2A).
FIG. 2.
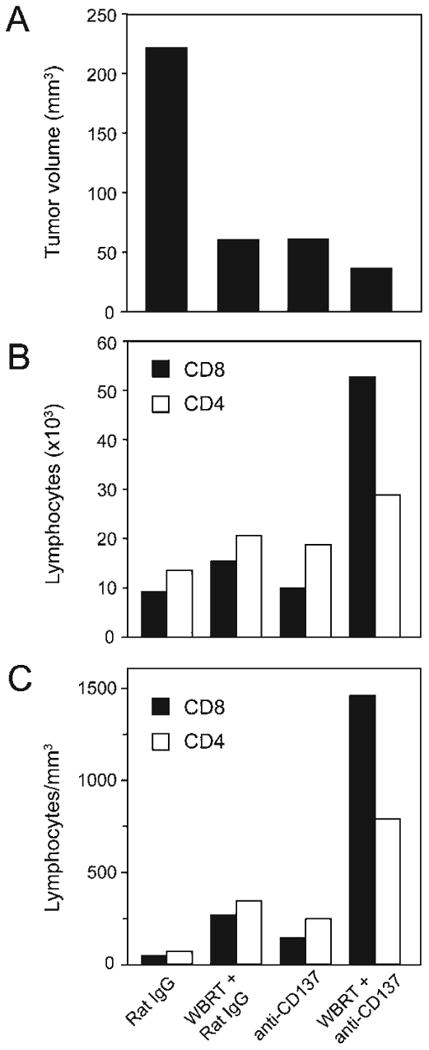
Antitumor response correlates with increased TILs in the tumor. Animals were treated as shown in Fig. 1A. Brains were harvested on day 22 for TIL analysis. Panel A: Tumor volumes. Panel B: Numbers of CD4 (white bars) and CD8 (black bars) cells. Panel C: Density of lymphocytes per tumor volume. Results are the average of two pooled brain tumors in each group. One representative experiment from two independent experiments performed is shown. WBRT: whole-brain irradiation.
The density of TILs was highest in the brains of the radiation + anti-CD137-treated animals (Fig. 2B and C). Specifically, TILs in this group showed a 36-fold increase of CD8 and a 13-fold increase of CD4 T cells compared to control, rat IgG2a-treated mice. Radiation treatment alone increased CD8 and CD4 cells by sixfold while anti-CD137-treated animals showed a fourfold increase of CD8 and CD4 cells compared with the control group (Fig. 2C).
Antitumor Response Correlates with Increased γIFN Production
Splenocytes from mice in each treatment arm from the same experiment were also isolated at day 22 after i.c. inoculation of GL261 glioma cells and assessed for γIFN production. In mice treated with radiation + anti-CD137 tumor-specific γIFN production was increased 13-fold compared with the rat IgG2a-treated mice (Fig. 3). Anti-CD137-treated animals showed a threefold increase in γIFN production compared with the control.
FIG. 3.

Antitumor response correlates with increased γIFN production. Splenocytes were harvested on day 22 for γIFN production and co-cultured in the absence or presence of irradiated GL261 cells for 5 days. γIFN secretion was measured in cell-free supernatants by ELISA in duplicate. Tumor-specific γIFN production was calculated by subtracting the background value obtained with medium alone. Results are averages of two pooled spleens in each group. One representative experiment from two independent experiments performed is shown. WBRT: whole-brain irradiation.
Discussion
We showed previously in the GL261 mouse model that whole-brain irradiation can be combined successfully with vaccination with modified autologous tumor cells for the treatment of i.c. gliomas. Here we tested the combination of radiation with a different immunotherapeutic approach based on the administration of antibodies against the co-stimulatory molecule CD137. Overall, the results support the interpretation that the combination of radiation + CD137 co-stimulation results in activation of an antitumor immune response that is effective at rejecting the i.c. tumors. The antitumor immune response can be monitored by analysis of peripheral T cells.
Ionizing radiation has been shown to induce an immunogenic cancer cell death and promote cross-priming, i.e., the presentation of tumor-derived antigens to antitumor T cells (34–36). Consistent with the concept that radiation can promote antitumor immunity, a small percentage of mice treated with radiation alone survived long-term and showed a memory response capable of rejecting a peripheral challenge with GL261 cells (Fig. 1B and C). However, radiation by itself was unable to induce tumor-specific γIFN production (Fig. 3), suggesting that T cells primed in the absence of CD137 co-stimulation tend to either proliferate less or survive less. In contrast, anti-CD137 by itself was able to enhance tumor-specific γIFN production, suggesting that it can expand T cells that were spontaneously primed by tumor antigens, possibly derived from cells dying during tumor growth. However, the therapeutic effect of anti-CD137 was limited, likely due to the inefficient infiltration of the tumor by effector T cells (Fig. 2C). Overall, our data suggest that CD137 co-stimulation and some of the effects of radiation on the immune system are independent and that they can potentially complement each other. Whereas CD137 co-stimulation mostly supports the development and survival/expansion of tumor-specific T cells (18, 19, 22), whole-brain irradiation, even at these moderate doses, has a cytocidal effect on the tumor, enhances MHC class I expression on invading glioma cells (8), and improves the recruitment and/or infiltration of T cells into the tumors (26, 37). While the combination of radiation and CD137 co-stimulation achieves a markedly improved tumor control as reflected by a significant therapeutic effect, the precise mechanism of this cooperation remains elusive.
Only two antibody-based therapeutics targeting co-inhibitory or co-stimulatory pathways have been tested in preclinical mouse models, monkeys and humans. Two monoclonal antibodies to CD152 [also known as cytotoxic T-lymphocyte antigen-4 (CTLA4)], have been generated ipilimumab (MDX-010; by Medarex and Bristol-Myers Squibb) and tremelimuab (CP-675,206; by Pfizer). Antibody-mediated CTLA-4 blockade enhances the ability of T cells to become activated in conditions of suboptimal co-stimulation (38). After extensive preclinical testing in animal models, the antibodies entered clinical trials for the treatment of melanoma patients due to acceptable toxicity profiles and demonstration of modest antitumor activity (39).
CD137, also known as 4-1BB, has been shown to augment CD4 and CD8 T-cell responses (40). CD137 is expressed on CD4 and CD8 T cells only after immune system stimulation. CD137 is also expressed on activated NK cells and monocytes. Binding of 4-1BB to its ligand (4-1BBL) induces a signaling cascade in T cells that promotes their activation, survival and growth. After preclinical testing in several animal models, the fully human agonist antibody specific to CD137 receptor (BMS-663513, Bristol-Myers Squibb) was entered into clinical trials for patients with metastatic or locally advanced solid tumors. Overall, the treatment was very well tolerated (41).
In summary, the antibody-based targeting of the immune molecules CD152 and CD137 were first tested in preclinical mouse models and then successfully translated to the clinic for treatment of patients with advanced solid malignancies with acceptable toxicity levels. In view of these promising preliminary results, this combination approach should be studied further for clinical translation. The findings are particularly relevant since re-treatment of initially irradiated recurrent glioma patients is often feasible only with low doses of radiation. CD137 and re-irradiation could present a viable alternative.
Acknowledgments
This work was supported by NIH grant NS057829-03 (EWN), the Long Island League to Abolish Cancer (EWN), NIH CA113851 and Research Scholar award RSG-05-145-01-LIB from the American Cancer Society (SD), NIH CA016087 (SCF), Department of Defense Center of Excellence Grant BC030282 (SCF), Breast Cancer Research Foundation (SCF), Radiological Society of North America Research and Education Foundation (MA-B), and Bristol-Myers Squibb (SD and SCF). Financial disclosure: all authors have declared that they have no financial conflicts of interest in regard to this work.
References
- 1.Stupp R, Hegi ME, Gilbert MR, Chakravarti A. Chemoradiotherapy in malignant glioma; Standard of care and future directions. J Clin Oncol. 2007;25:4127–4136. doi: 10.1200/JCO.2007.11.8554. [DOI] [PubMed] [Google Scholar]
- 2.Narayana A, Kelly P, Golfinos J, Parker E, Johnson G, Knopp E, Zagzag D, Fischer I, Raza S, Gruber ML. Antiangiogenic therapy using bevacizumab in recurrent high-grade glioma: impact on local control and patient survival. J Neurosurg. 2009;110:173–180. doi: 10.3171/2008.4.17492. [DOI] [PubMed] [Google Scholar]
- 3.Okada H, Kohanbash G, Zhu X, Kastenhuber ER, Hoji A, Ueda R, Fujita M. Immunotherapeutic approaches for glioma. Crit Rev Immunol. 2009;29:1–42. doi: 10.1615/critrevimmunol.v29.i1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rutowski S, De Vleeschouwer S, Kaempgen E, Wolff JEA, Kuhl J, Demaerel P, Warmuth-Metz M, Flamen P, Van Calenbergh F, Van Gool SW. Surgery and adjuvant dendritic cell-based tumour vaccination for patients with relapsed malignant glioma, a feasibility study. Br J Cancer. 2004;91:1656–1662. doi: 10.1038/sj.bjc.6602195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steiner HH, Bonsanto MM, Beckhove P, Brysch M, Geletneky K, Ahmadi R, Schuele-Freyer R, Kremer P, Ranaie G, Herold-Mende C. Antitumor vaccination of patients with glioblastoma multiforme: A pilot study to assess feasibility, safety, and clinical benefit. J Clin Oncol. 2004;22:4272–4281. doi: 10.1200/JCO.2004.09.038. [DOI] [PubMed] [Google Scholar]
- 6.Wheeler CJ, Black KL, Liu G, Mazer M, Zhang XX, Pepkowitz S, Goldfinger D, Ng H, Irvin D, Yu JS. Vaccination elicts correlated immune and clinical responses in glioblastoma multiforme patients. Cancer Res. 2008;68:5955–5964. doi: 10.1158/0008-5472.CAN-07-5973. [DOI] [PubMed] [Google Scholar]
- 7.Zagzag D, Miller DC, Chiriboga L, Yee H, Newcomb EW. Green fluorescent protein immunohistochemistry as a novel experimental tool for the detection of glioma cell invasion in vivo. Brain Pathol. 2003;13:34–37. doi: 10.1111/j.1750-3639.2003.tb00004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newcomb EW, Demaria S, Lukyanov Y, Shao Y, Schnee T, Kawashima N, Lan L, Dewyngaert JK, Zagzag D, Formenti SC. The combination of ionizing radiation and peripheral vaccination produces long-term survival of mice bearing established invasive GL261 gliomas. Clin Cancer Res. 2006;12:4730–4737. doi: 10.1158/1078-0432.CCR-06-0593. [DOI] [PubMed] [Google Scholar]
- 9.Lynch DH. The promise of 4-1BB (CD137)-mediated immunomodulation and the immunotherapy of cancer. Immunol Rev. 2008;222:277–286. doi: 10.1111/j.1600-065X.2008.00621.x. [DOI] [PubMed] [Google Scholar]
- 10.Croft M. The role of TNF superfamily members in T-cell function and diseases. Nat Rev Immunol. 2009;9:271–285. doi: 10.1038/nri2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodwin RG, Din WS, Davis-Smith T, Anderson DM, Gimpel SD, Sato TA, Maliszewski CR, Brannan CI, Copeland NG, Beckmann MP. Molecular cloning of a ligand for the inducible T-cell gene 4-1BB: A member of an emerging family of cytokines with homology to tumor necrosis factor. Eur J Immunol. 1993;23:2631–2641. doi: 10.1002/eji.1830231037. [DOI] [PubMed] [Google Scholar]
- 12.Alderson MR, Smith CA, Tough TW, Davis-Smith T, Armitage RJ, Falk B, Roux E, Baker E, Sutherland GR, Din WS. Molecular and biological characterization of human 4-1BB and its ligand. Eur J Immunol. 1994;24:2219–2227. doi: 10.1002/eji.1830240943. [DOI] [PubMed] [Google Scholar]
- 13.Shuford WW, Klussman K, Tritchler DD, Loo DT, Chalupny J, Siadak AW, Brown TJ, Emswiler J, Raecho H, Mittler RS. 4-1BB costimulatory signals preferentially induce CD8+ T cell proliferation and lead to the amplification in vivo of cytotoxic T cell responses. J Exp Med. 1997;186:47–55. doi: 10.1084/jem.186.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gramaglia I, Cooper D, Miner KT, Kwon BS, Croft M. Co-stimulation of antigen-specific CD4 T cells by 4-1BB ligand. Eur J Immunol. 2000;30:392–402. doi: 10.1002/1521-4141(200002)30:2<392::AID-IMMU392>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 15.Pollok KE, Kim YJ, Zhou Z, Hurtado J, Kim KK, Pickard RT, Kwon BS. Inducible T cell antigen 4-1BB. An analysis of expression and function. J Immunol. 1993;150:771–781. [PubMed] [Google Scholar]
- 16.Hurtado JC, Kim SH, Pollock KE, Lee ZH, Kwon BS. Potential role of 4-1BB in T cell activation. Comparison with the costimulatory molecule CD28. J Immunol. 1995;155:3360–3367. [PubMed] [Google Scholar]
- 17.Melero I, Johnston JV, Shuford WW, Mittler RS, Chen L. NK1.1 cells express 4-1BB (CDw137) costimulatory molecule and are required for tumor immunity eleicited by anti-4-1-BB monoclonal antibodies. Cell Immunol. 1998;190:167–172. doi: 10.1006/cimm.1998.1396. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi C, Mittler RS, Vella AT. Cutting edge: 4-1BB is a bona fide CD8 T cell survival signal. J Immunol. 1999;162:5037–5040. [PubMed] [Google Scholar]
- 19.Cannons JL, Lau P, Ghumann B, DeBenedette MA, Yagita H, Okumura K, Watts TH. 4-1BB ligand induces cell division, sustains survival, and enhances effector function of CD4 and CD8 cells with similar efficacy. J Immunol. 2001;167:1313–1324. doi: 10.4049/jimmunol.167.3.1313. [DOI] [PubMed] [Google Scholar]
- 20.Melero I, Shuford WW, Newby SA, Aruffo A, Ledbetter JA, Hellstrom KE, Mittler RS, Chen L. Monoclonal antibodies against the 4-1BB T-cell activation molecule can eradicate established tumors. Nat Med. 1997;3:682–685. doi: 10.1038/nm0697-682. [DOI] [PubMed] [Google Scholar]
- 21.Kim JA, Averbook BJ, Chambers K, Rothchild K, Kjaergaard J, Papay R, Shu S. Divergent effects of 4-1BB antibodies on antitumor immunity and on tumor-reactive T-cell generation. Cancer Res. 2001;61:2031–2037. [PubMed] [Google Scholar]
- 22.Miller RE, Jones J, Le T, Whitmore J, Boiani N, Gliniak B, Lynch DH. 4-1BB-specific monoclonal antibody promotes the generation of tumor-specific immune responses by direct activation of CD8 T cells in a CD40-dependent manner. J Immunol. 2002;169:1792–1800. doi: 10.4049/jimmunol.169.4.1792. [DOI] [PubMed] [Google Scholar]
- 23.Taraban VY, Rowley TF, O′Brien L, Chan HT, Haswell LE, Green MH, Tutt AL, Glennie MJ, Al-Shamkhani A. Expression and costimulatory effects of the TNF receptor superfamily member CD134 (OX40) and CD137 (4-1BB), and their role in the generation of antitumor immune responses. Eur J Immunol. 2002;32:3617–3627. doi: 10.1002/1521-4141(200212)32:12<3617::AID-IMMU3617>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 24.Wilcox RA, Flies DB, Zhu G, Johnson AJ, Tamada K, Chapoval AI, Strome SE, Pease LR, Chen L. Provision of antigen and CD137 signaling breaks immunological ignorance, promoting regression of poorly immunogenic tumors. J Clin Invest. 2002;109:651–659. doi: 10.1172/JCI14184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ju S, Lee SC, Kwon TH, Heo SK, Park SM, Paek HN, Suh JH, Cho HR, Kwon B, Kim BS. Immunity to melanoma mediated by 4-1BB is associated with enhanced activity of tumour-infiltrating lymphocytes. Immunol Cell Biol. 2005;83:344–351. doi: 10.1111/j.1440-1711.2005.01330.x. [DOI] [PubMed] [Google Scholar]
- 26.Demaria S, Formenti SC. Sensors of ionizing radiation effects on the immunological microenvironment of cancer. Int J Radiat Biol. 2007;83:819–825. doi: 10.1080/09553000701481816. [DOI] [PubMed] [Google Scholar]
- 27.Teitz-Tennenbaum S, Li Q, Okuyama R, Davis MA, Sun R, Whitfield J, Knibbs RN, Stoolman LM, Chang AE. Mechanisms involved in radiation enhancement of intratumoral dendritic cell therapy. J Immunother. 2008;31:345–358. doi: 10.1097/CJI.0b013e318163628c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Formenti SC, Demaria S. Local control by radiotherapy: is that all there is? Breast Cancer Res. 2008;10:215. doi: 10.1186/bcr2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Formenti SC, Demaria S. Systemic effects of local radiotherapy. Lancet Oncol. 2009;10:718–726. doi: 10.1016/S1470-2045(09)70082-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seligman AM, Shear MJ. Studies in carcinogenesis. VIII. Experimental production of brain tumors in mice with methylcholanthrene. Am J Cancer. 1939;37:364–395. [Google Scholar]
- 31.Demaria S, Kawashima N, Yang AM, Devitt ML, Babb JS, Allison JP, Formenti SC. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res. 2005;15:727–734. [PubMed] [Google Scholar]
- 32.Ito F, Li Q, Shreiner AB, Okuyama R, Jure-Kunkel MN, Teitz-Tennenbaum S, Chang AE. Anti-Cd137 monoclonal antibody administration augments the antitumor efficacy of dendritic cell-based vaccines. Cancer Res. 2004;64:8411–8419. doi: 10.1158/0008-5472.CAN-04-0590. [DOI] [PubMed] [Google Scholar]
- 33.Prins RM, Odesa SK, Liau LM. Immunotherapeutic targeting of shared melanoma-associated antigens in a murine glioma model. Cancer Res. 2003;63:8487–8491. [PubMed] [Google Scholar]
- 34.Obeid M, Panaretakis T, Joza N, Tufi R, Tesniere A, van Endert P, Zitvogel L, Kroemer G. Calreticulin exposure is required for the immunogenicity of gamma-irradiation and UVC light-induced apoptosis. Cell Death Differ. 2007;14:1848–1850. doi: 10.1038/sj.cdd.4402201. [DOI] [PubMed] [Google Scholar]
- 35.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Mauri MC, Ullrich E, Kroemer G. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 36.Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 2005;174:7516–7523. doi: 10.4049/jimmunol.174.12.7516. [DOI] [PubMed] [Google Scholar]
- 37.Matsumura S, Wang B, Kawashima N, Braunstein S, Badura M, Cameron TO, Babb JS, Schneider RJ, Formenti SC, Demaria S. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J Immunol. 2008;181:3099–3107. doi: 10.4049/jimmunol.181.5.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Egen JG, Kuhns MS, Allison JP. CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nat Immunol. 2002;3:611–618. doi: 10.1038/ni0702-611. [DOI] [PubMed] [Google Scholar]
- 39.Peggs KS, Quezada SA, Korman AJ, Allison JP. Principles and use of anti-CTLA4 antibody in human cancer immunotherapy. Curr Opin Immunol. 2006;18:206–213. doi: 10.1016/j.coi.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 40.Myers LM, Vella AT. Interfacing T-cell effector and regulatory function through CD137 (4-1BB) co-stimulation. Trends Immunol. 2005;26:440–446. doi: 10.1016/j.it.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 41.Sznol M, Hodi FS, Margolin DF, McDermott DF, Ernstoff MS, Kirkwood JM, Wojtaszedk C, Feltquate D, Logan T. Phase I study of BMS-663513, a fully human anti-CD137 agonist monoclonal antibody, in patients (pts) with advanced cancer (CA) J Clin Oncol. 2008;26(Suppl.) abstract 3007. [Google Scholar]


