Abstract
Interleukin-15 (IL-15) has a major role in NK-cell homeostasis. Modulation of the relative frequency and expression intensity of the NK-cell receptors by IL-15 may increase NK cell-mediated cytotoxicity in cancer patients. We investigated the receptor repertoire and measured NK-cell activity in newly diagnosed AML patients and evaluated the ex vivo effects of IL-15. The expression of the activating NK cell receptors was significantly decreased in the AML patients compared to that in NK cells of healthy donors. When NK cells obtained from AML patients were cultured with IL-15, expression of the activating receptors was significantly upregulated compared to pre-culture levels. Concomitantly, cytotoxic activity of NK cells against autologous leukemic blasts increased following IL-15 stimulation. This IL-15 induced increase in activity was blocked by neutralizing antibodies specific for the NK cell activating receptors. These pre-clinical data support the future use of IL-15 for NK cell- based therapies for AML patients.
Keywords: Acute myeloid leukemia, Natural killer cells, Activating receptors, NCRs, NKG2D, Interleukin -15
Introduction
Natural killer (NK) cells play a critical role in innate immunity through their capacity to lyse malignant cells without prior antigen-specific priming. Human peripheral blood NK cells can be divided into two functional subsets: (a) CD56dimCD16bright which represent ~90% of all NK cells and mediate cytotoxicity and (b) CD56brightCD16−, the remaining 10% of NK cells, which regulate immune responses via cytokine production [1].
Natural killer cell-mediated cytotoxicity is controlled through the balance of signals mediated by activating and inhibitory receptors found on each NK cell, which act as a fail-safe system. Thus, the engagement of activating receptors identifies potential targets, while signaling via inhibitory receptors blocks killing of normal autologous cells [2]. Multiple structurally distinct activating NK cell receptors have been characterized [2]. Those linked to NK cell-mediated killing of tumor cells are NKG2D and natural cytotoxicity receptors (NCR). NKG2D, a C-type lectin-like receptor, recognizes two distinct families of ligands, the MHC class I chain-related molecules (MICA and MICB) and the UL-16 binding proteins (ULBP) [3, 4]. These NKG2D ligands are overexpressed in malignant cells, and NK cytotoxicity correlates with expression levels of MICA, MICB and ULBP on tumor cell targets [5–7]. The NCR such as NKp30 and NKp46, which bind to as yet unidentified tumor antigens, are constitutively expressed on both resting and activated NK cells and are involved in NK cell-mediated killing of a variety of cancer cell targets. Inhibitory NK cell receptors include the killer immunoglobulin-like receptors (KIRs) that bind to classical MHC Class Ia ligands (HLA–A, –B or –C) and the CD94–NKG2A C-type lectin receptor heterodimers that recognize leader peptides derived from HLA–A, –B or –C presented by the non-classical class I molecule, HLA–E [1, 8]. Normal autologous cells which express self-MHC class I ligands are not susceptible to NK cell-mediated killing, but cells that have reduced ligand expression due to malignant transformation are a subject to NK-cell attack [9].
Acute myeloid leukemia (AML) is a disease with considerable phenotypic and genotypic heterogeneity, which is characterized by acquisition of somatic mutations in hematopoietic progenitors. These progenitors acquire a proliferative and/or survival advantage and impair hematopoietic differentiation. NK cells are derived from hematopoietic progenitors in the bone marrow. Patients with AML have depressed NK cell function and cytokine production [10]. Further, NK cell activity correlates positively with relapse-free survival [11]. The inability of NK cells to kill autologous leukemic blasts has also been linked to low expression of NCR, and the NCRdull phenotype has been associated with decreased survival in AML patients [12].
As previously demonstrated, IL-2 and IL-12 play an important role in NK cell biology [13–15]. However, in the last few years IL-15 has been shown to be the major NK cell homeostatic cytokine. NK cell differentiation is cytokine dependent, and interleukin-15 (IL-15) plays a major role in NK-cell differentiation, their expansion in the periphery and survival [16, 17]. Mice lacking expression of either IL-15 or the IL-15Rα chain fail to develop NK cells, and NK cells transferred into IL-15−/− hosts fail to survive [18]. In man, IL-15 induces NK cell differentiation in stroma-free cultures from CD34+ progenitor cells and expands mature NK cells in culture. We have demonstrated that mature human circulating NK cells treated with IL-15 up-regulate the expression of their receptors, and this up-regulation is associated with a concomitant increase of the NK cell activity [19].
Based on the significant role of IL-15 in NK cell regulation, we hypothesize that IL-15 may enhance anti-leukemic effects of autologous NK cells in patients with AML either through increasing the NK cell frequency or by alternating the intensity of NK-cell receptor expression. Here, we evaluate the ex vivo effects of IL-15 on the NK-cell subsets, their receptor repertoire and NK cell activity in the peripheral blood of patients with newly diagnosed AML prior to any treatment and in normal donors.
Materials and methods
AML patients and healthy volunteers
Blood samples were obtained from newly diagnosed AML patients prior to any treatment (n = 17) and from age-matched healthy volunteers (n = 10). All subjects signed an informed consent approved by the Institutional Review Board at the University of Pittsburgh. The collected blood (20–50 mL) was drawn into heparinized tubes. The samples were hand-carried to the laboratory and immediately processed using Ficoll–Hypaque gradients. PBMC were recovered, washed in AIM-V medium (Invitrogen, Carlsbad, CA, USA) counted in a trypan blue dye and immediately used for experiments.
Immunophenotyping
The two NK subsets (CD56dimCD16+ and CD56brightCD16−) and their receptor expression were evaluated in PBMC obtained from AML patients and normal controls (NC). The following anti-human monoclonal antibodies (mAb) were used for flow cytometry: CD16-FITC, NKG2D-PE, CD25-PE all from BD Pharmingen, San Diego, CA; CD3-ECD, CD56-PC5, CD94-PE, NKp30-PE, NKp44-PE, NKp46-PE, CD158a-PE, CD158b-PE, CD158e1/e2-PE, CD158i-PE, CD122-PE all from Beckman Coulter, Miami, FL; and NKG2A-PE and NKG2C-PE both from R&D System, Minneapolis, MN. Prior to use, all mAbs were titrated using normal resting or activated PBMC to establish optimal staining dilutions. Isotype controls were included in all experiments. Flow cytometry was performed using a Beckman Coulter flow cytometer equipped with the Expo32 software.
Cell culture and IL-15 stimulation
Natural killer cells were isolated from PBMC of healthy donors and from patients with AML using magnetic beads (AutoMACS, Miltenyi Biotec, Auburn, CA, USA) or flow cytometry sorting. NK cells were cultured in the CM—RPMI-1640 complete medium (InVitrogen-Lifetech) supplemented with 10% fetal calf serum (FCS), l-glutamine, sodium pyruvate, non-essential amino acids and penicillin/streptomycin). Cells cultured in the presence of rhIL-15 (Peprotech, Rocky Hill, NJ, USA) at concentrations ranging from 10 to 200 ng/mL were harvested after 24 and 72 h, and the NK receptor repertoire were assessed by multiparameter flow cytometry.
NK cell cytotoxicity
Natural killer cell activity was measured in 4 h 51Cr release assays using K562 cells as well as autologous myeloid blasts as targets as previously described [20]. Briefly, target cells were labeled with 100 μCi of 51Cr for 1 h at 37°C at 5% CO2, washed twice in a complete medium (CM), resuspended in CM, and counted. Co-cultures of PBMC and target cells established in triplicate at the effector to target (E:T) cell ratios of 6, 3, 1.5, and 0.75–1 were co-incubated for 4 h at 37°C. Controls included targets incubated in medium alone for spontaneous release and targets in 5% (v/v) Triton X-100 in PBS for maximum release. Radioactivity was measured by Wallac Wizard 1470 Automatic Gamma Counter. The percentage of cytotoxic activity was calculated using the following formula: % specific lysis = (sample cpm − spontaneous cpm)/(maximal cpm − spontaneous cpm) × 100%. Lytic units (LU) were calculated as the number of effector cells needed to lyse 20% of 5 × 103 target cells, and expressed as LU/107 cells [17]. For the blocking assays neutralizing Abs to NKG2D, NKG2C, NKp46, NKp30 were pre-titered at (0.01–100 μg/mL) to determine the optimal concentration and added to NK cell cultures for 1 h prior to cytotoxicity assays.
Interleukin-15 levels
The IL-15 QuantiGlo ELISA (R&D Systems, Minneapolis, MN, USA) was used to measure the patient’s levels of plasma IL-15 prior to any treatment, 14 days after the initiation of induction chemotherapy and in the plasma of patients who achieved complete remission (CR). The sensitivity of the assay was 0.03 pg/mL of IL-15. The ELISA assay was performed in triplicate wells for each patient sample.
Statistical analysis
Data were summarized by descriptive statistics (mean and standard error (SE) for continued variables; frequency and percentage for categorical variables). Statistical analyses were performed using the paired and unpaired two-tailed Student t tests. The value of P < 0.05 was considered significant.
Results
NK subsets in AML patients
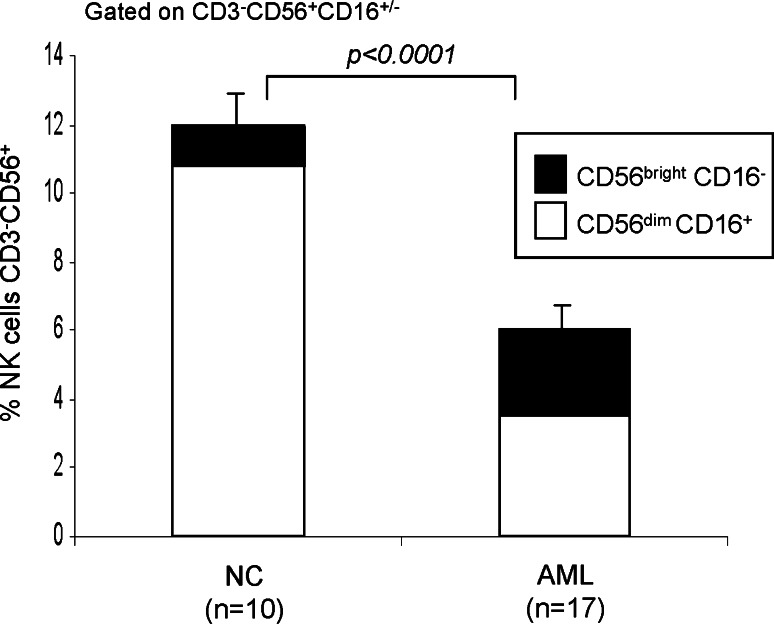
Natural killer cells were evaluated in AML patients at the time of diagnosis and prior to any treatment. The results were compared to those obtained for NK cells of normal controls (NC). The percentage of circulating NK cells was lower (P < 0.0001) in the AML patients (6% ± 0.7, range 1–17%) compared to the NK cells of NC (12% ± 1, range 9–17%). Relative to NC, the proportion of the CD56brightCD16− NK cells in the AML patients was significantly higher, and the frequency of CD56dimCD16+ cells was significantly lower (Fig. 1).
Fig. 1.
Percentages of CD56brightCD16− and CD56dimCD16+ in the peripheral blood of AML patients and NC. The data are mean percentages ± SE
Activating receptor expression in NK cells of AML patients
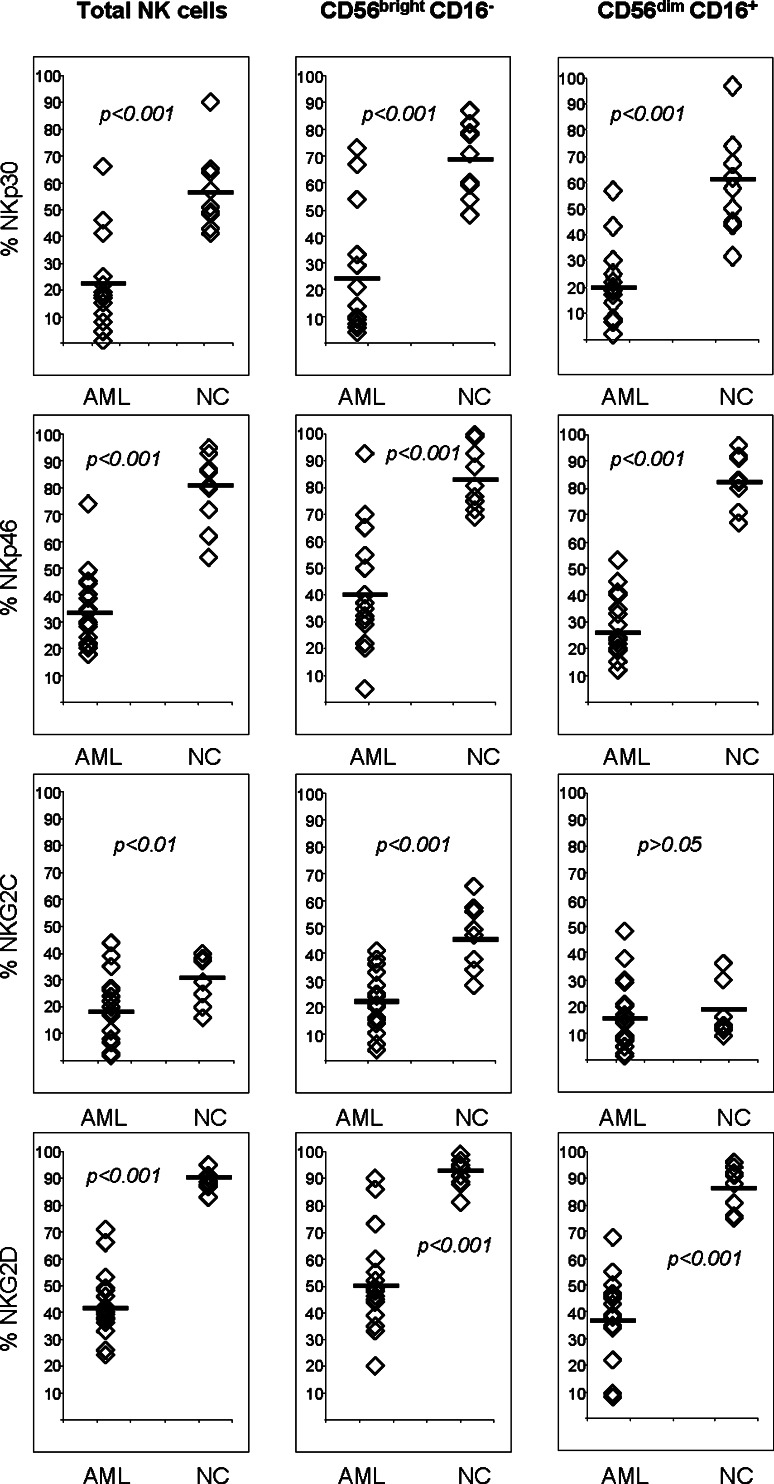
The frequency of NK cells expressing the activating NCR, NKp30, NKp44 and NKp46, and the C-type lectin receptors, NKG2D and NKG2C, was significantly decreased in the AML patients compared to the NK cells of NC (Table 1). In addition, the mean fluorescence intensity (MFI) of the activating receptors was significantly lower in AML patients compared to NC (data not shown). We then determined percentages of cells expressing activating NK receptors within the CD56brightCD16− and CD56dimCD16+ subsets of NK cells in the peripheral circulation of AML patients. Both NK cell subsets in these patients contained significantly fewer cells expressing activating receptors compared to the same NK cell subsets in NC (Fig. 2). No significant differences in the frequency and MFI of NK cell inhibitory receptors were observed in these subsets between AML patients and NC.
Table 1.
Frequency of NK cells expressing activating NK cell receptors in patients with acute myelogenous leukemia and in normal controls
| NK cell activating receptors | % Positive | P value | |
|---|---|---|---|
| NK cells in NC (n = 10) | NK cells in AML pts at diagnosis (n = 17) | ||
| NKp30 | 51 | 24 | 0.0001 |
| NKp44 | 10 | 4 | 0.0001 |
| NKp46 | 73 | 32 | 0.0001 |
| NKG2C | 28 | 17 | 0.03 |
| NKG2D | 83 | 43 | 0.0001 |
Percentages of NK cells gated as CD3−CD56+ cells expressing activating receptors were determined by flow cytometry as described in “Materials and methods”. NC Normal controls
Fig. 2.
Percentages of NK cells and NK cell subsets expressing activating receptors in AML patients and NC. Horizontal bars represent the mean values
IL-15 increases the expression of activating NK cell receptors in AML patients
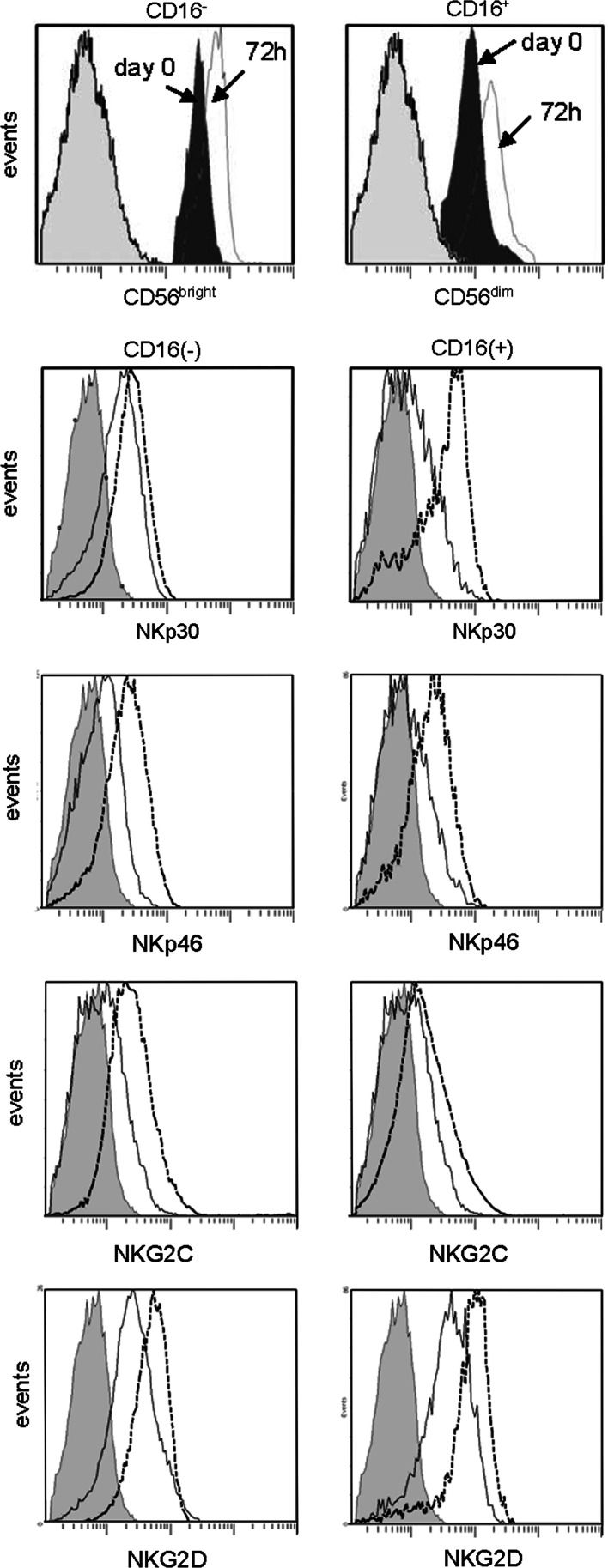
To assess the effects of IL-15 on NK cell receptor expression, sorted NK cells were cultured in the presence of IL-15. We observed an up-regulation of the NK cell activating receptors, including an increase in the percentage of cells expressing the receptors (Table 2) and the MFI for each receptor (Fig. 3). These changes were evident as early as 24 h after the start of culture, reaching a maximum level of expression within 72 h.
Table 2.
Effects of IL-15 on the expression of activating receptors in patients with acute myelogenous leukemia
| NK cell activating receptors | % Positive | P value | |
|---|---|---|---|
| NK cells in AML pts at diagnosis | NK cells in AML pts after IL-15 stimulation | ||
| NKp30 | 24 | 84 | 0.001 |
| NKp46 | 32 | 83 | 0.001 |
| NKG2C | 17 | 58 | 0.005 |
| NKG2D | 43 | 87 | 0.01 |
Percentages of NK cells gated as CD3−CD56+ cells expressing activating receptors were determined by flow cytometry as described in “Materials and methods”
Fig. 3.
Up-regulation of expression of NK cell activating receptors by IL-15 in the NK cell subsets in AML patients. Flow cytometry histograms illustrate the levels of expression of NCRs, NKG2D and NKG2C in both NK subsets after culture with IL-15. Gray-filled histograms indicate isotype control, black lines indicate day 0 values and dotted lines indicate expression levels after 72 h culture with IL-15. The representative data were obtained with NK cells of one AML patient
IL-15 increases cytotoxicity in cultured NK cells obtained from AML patients
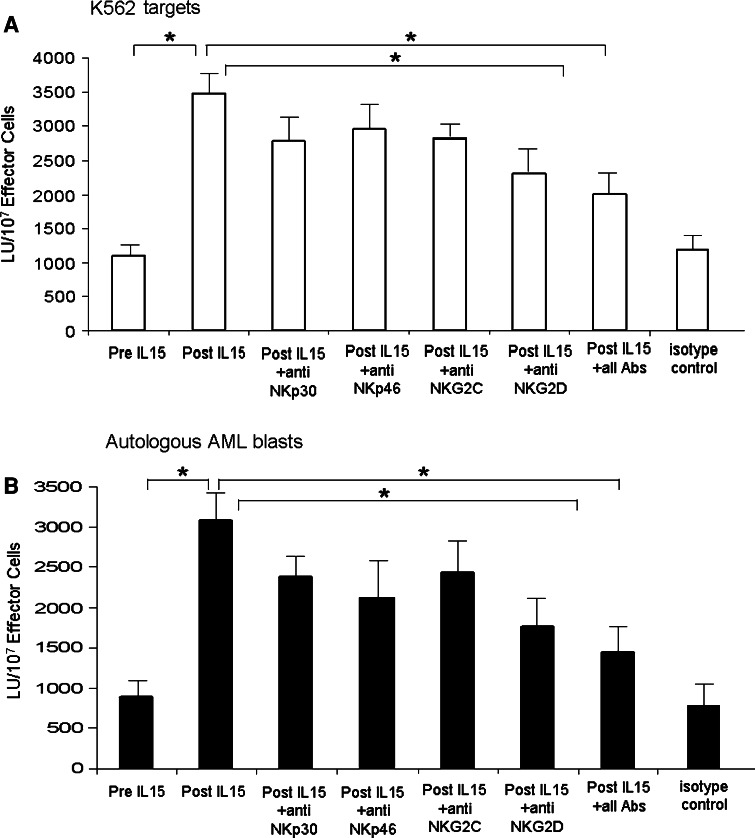
Natural killer cell cytotoxicity was significantly lower in AML patients than in NC (1,102 vs. 1,646 LU, P < 0.001). When NK cells sorted from AML patients were cultured with IL-15, NK cell cytotoxicity against K562 targets was increased (P < 0.05) (Fig. 4a). When sorted autologous AML blasts were used as targets in cytotoxicity assays, a significant increase in NK cell cytotoxicity against autologous leukemic blasts (P < 0.05) was observed (Fig. 4b), which was concomitant with the upregulation of the activating receptors following 3 days of IL-15 stimulation. To assess the contribution of activating receptors on NK cells to the observed increases in NK cell cytotoxicity in response to culture in IL-15, we used neutralizing antibodies specific for the activating receptors in cytotoxicity assays. The addition of the neutralizing antibodies specific for any of the receptors or the simultaneous addition of all four antibodies significantly reduced the level of cytotoxicity (Fig. 4a, b).
Fig. 4.
Increased NK cell cytotoxicity after IL-15 stimulation of NK cells sorted from the peripheral blood of AML patients. a NK cytotoxicity was tested using K562 targets after NK cell culture with IL-15 ± neutralizing Abs to activating receptors. b NK cytotoxicity was tested using autologous AML blasts after NK cell culture with IL-15 ± neutralizing Abs to activating receptors at concentrations ranging from 0.1 to 10 μg/mL. The data are mean LU ± SE from 7 experiments (asterisks indicate significant differences)
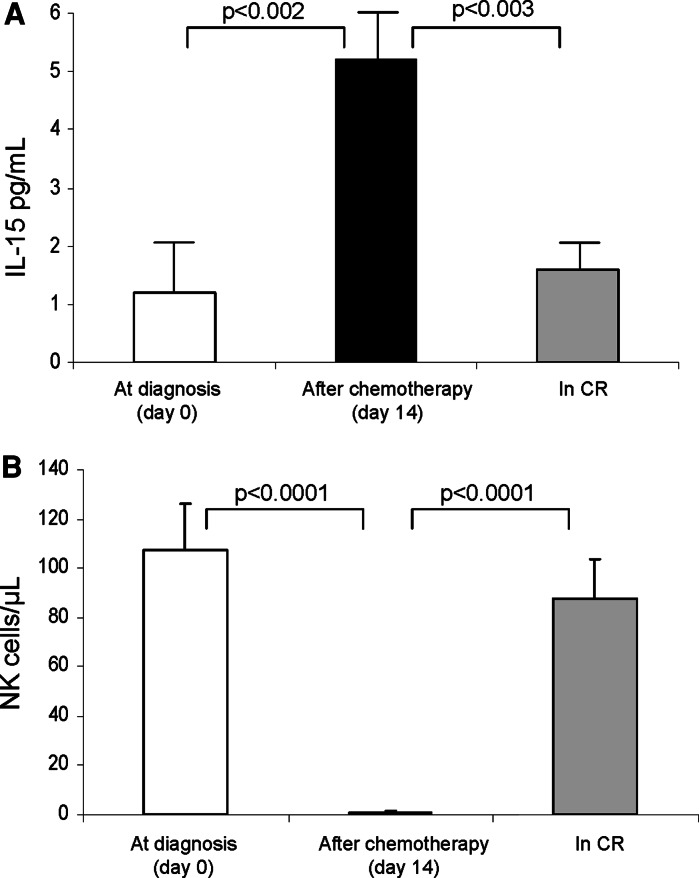
NK recovery following induction chemotherapy inversely correlates with plasma IL-15 levels
To further assess the role of IL-15 regulating NK cell behavior in AML patients, we measured plasma IL-15 levels at diagnosis, after induction chemotherapy and when patients achieved a complete remission (CR). At diagnosis, the mean IL-15 level in the patient’s plasma was 1.2 pg/mL (range 0.03–2.29 pg/mL), and it increased to 5.2 pg/mL (range 0.06–13.4 pg/mL; P < 0.002) after induction chemotherapy was completed when the NK cell numbers were reduced to 0 cells/microliter. Subsequently, the level of IL-15 in the plasma decreased to pre-treatment levels when NK cells reconstituted (mean 1.6 pg/mL, range 0.4–2.3 pg/mL) in the AML patients who achieved CR (Fig. 5).
Fig. 5.
IL-15 levels in the plasma and NK cell absolute numbers in the AML patients. The levels of IL-15 increased following induction chemotherapy and subsequently declined when NK cells recovered in patients who achieved CR. The data are mean values ± SE
Discussion
In this study, we examined NK cells and their receptor repertoire in patients with AML prior to any treatment. We observed that the CD56bright NK subset was disproportionately increased in the circulation of newly diagnosed AML patients relative to the CD56dim subset. It is believed that the progression of CD56bright to CD56dim represents the continuum of their normal development and differentiation [1, 21]. The higher proportion of CD56bright observed in AML patients relative to NC could be due to the increased production of NK cells in response to a rapid replacement of the bone marrow by leukemic blasts or to changes in the cytokine milieu which accompany tumor progression and drive the generation of CD56bright NK cells.
Activated NK cells have long been known to be effective in killing a broad range of tumor targets. However, NK cell activity in the peripheral blood of untreated acute leukemia patients has been shown to be significantly decreased in this and previous studies [10, 12]. This decrease in NK cell-activity appears to be related to: (a) a lower frequency of NK cells in the peripheral blood of AML patients; (b) up-regulation in expression of MHC class I molecules in leukemia blasts; and (c) decreased expression levels of the ULBP and NCR ligands on the AML blasts [22], possibly as a consequence of the maturation arrest during differentiation of the myelomonocytic cell lineages. Such an arrest could result in an increased resistance of AML targets to NK cell-mediated lysis, and it could also impair the clearance of AML blasts by NK cells bearing the activating NKG2D and NCR receptors. The decreased expression of NK cell activating receptors in AML patients reported here provides yet another explanation for reduced NK cell activity in AML patients. Although the molecular mechanisms responsible for the reduced receptor expression remains elusive, elevated serum levels of soluble NK cell receptor ligands shed by tumor cells have been associated with down-regulation of the NK cell receptors and might contribute to the decreased levels of NK cell activity [23].
Alloreactive NK cells have beneficial anti-tumor effects in haploidentical hematopoietic stem cell transplantation of patients with AML. The enhanced anti-tumor effect of NK cells is mainly due to the incompatibility between the recipient’s HLA class I antigens and the KIR repertoire expressed by the donor NK cells [24]. Another consideration for exploiting NK cell anti-tumor effects could be the preferential up-regulation of the NK cell activating receptors. We demonstrated that NK cells from AML patients up-regulate the receptor expression in the presence of exogenous IL-15, with a concomitant increase in receptor-dependent cytotoxicity against NK cell-sensitive tumor targets (K562) and, more importantly, against autologous AML blasts, suggesting that the use of IL-15 can overcome net negative effects of the inhibitory NK cell receptors. These observations are consistent with the concept that IL-15 plays an important role in NK cell anti-tumor activity.
Plasma IL-15 levels have been demonstrated to increase significantly following cytoreductive therapy [25]. We have recently demonstrated that the NK levels in the peri-transplant period are inversely proportional to the dramatic rise and fall in plasma levels of IL-15 [19]. Similarly, in the current study, we found elevated IL-15 levels after induction chemotherapy that decreased following NK cell recovery in those patients who achieved CR. The elevated plasma IL-15 levels could be related to the induction regimen-induced depletion of lymphoid populations that normally consume circulating IL-15 or could be secondary to inflammatory stimulation induced by chemotherapy.
The results of this study demonstrate that NK cells of AML patients acquire high levels of cytotoxic activity against autologous AML blasts upon ex vivo stimulation with IL-15. These data support the use of IL-15 in the design of future immunotherapeutic strategies for these patients and for the generation of therapeutic products for adoptive NK cell transfers [26].
Ethical standards
All human studies have been approved by the appropriate ethics committee. Please see “Materials and methods”, first paragraph.
Acknowledgments
This article was supported in part by Production Assistance for Cellular Therapies (PACT) under contract N01-HB-37165 from the NHLBI (TLW, MS), The Mario Lemieux Foundation (MB) and PO1-CA109688 from the NCI (TLW).
Conflict of interest statement
All authors agree with the contents of the manuscript and all authors declare that they have no conflict of interest.
References
- 1.Caligiuri MA. Human natural killer cells. Blood. 2008;112:461–469. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farag SS, Caligiuri MA. Human natural killer cell development and biology. Blood Rev. 2006;20:123–137. doi: 10.1016/j.blre.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, Spies T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–729. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 4.Cosman D, Mullberg J, Sutherland CL, Chin W, Armitage R, Fanslow W, Kubin M, Chalupny NJ. ULBPs, novel MHC class I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity. 2001;14:123–133. doi: 10.1016/S1074-7613(01)00095-4. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez S, Groh V, Spies T. Immunobiology of human NKG2D and its ligands. Curr Topics Microbiol Immunol. 2006;298:121–138. doi: 10.1007/3-540-27743-9_6. [DOI] [PubMed] [Google Scholar]
- 6.Carbone E, Neri P, Mesuraca M, Fulciniti MT, Otsuki T, Pende D, Groh V, Spies T, Pollio G, Cosman D, Catalano L, Tassone P, Rotoli B, Venuta S. HLA class I, NKG2D, and natural cytotoxicity receptors regulate multiple myeloma cell recognition by natural killer cells. Blood. 2005;105:251–258. doi: 10.1182/blood-2004-04-1422. [DOI] [PubMed] [Google Scholar]
- 7.Pende D, Rivera P, Marcenaro S, Chang CC, Biassoni R, Conte R, Kubin M, Cosman D, Ferrone S, Moretta L, Moretta A. Major histocompatibility complex class I-related chain A and UL16-binding protein expression on tumor cell lines of different histotypes: analysis of tumor susceptibility to NKG2D-dependent natural killer cell cytotoxicity. Cancer Res. 2002;62:6178–6186. [PubMed] [Google Scholar]
- 8.Vivier E, Nunes JA, Vely F. Natural killer cell signaling pathways. Science. 2004;306:1517–1519. doi: 10.1126/science.1103478. [DOI] [PubMed] [Google Scholar]
- 9.Bryceson YT, March ME, Ljunggren HG, Long EO. Activation, coactivation, and costimulation of resting human natural killer cells. Immuno Rev. 2006;214:73–91. doi: 10.1111/j.1600-065X.2006.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Costello RT, Sivori S, Marcenaro E, Lafage-Pochitaloff M, Mozziconacci MJ, Reviron D, Gastaut JA, Pende D, Olive D, Moretta A. Defective expression and function of natural killer cell-triggering receptors in patients with acute myeloid leukemia. Blood. 2002;99:3661–3667. doi: 10.1182/blood.V99.10.3661. [DOI] [PubMed] [Google Scholar]
- 11.Tajima F, Kawatani T, Endo A, Kawasaki H. Natural killer cell activity and cytokine production as prognostic factors in adult acute leukemia. Leukemia. 1996;10:478–482. [PubMed] [Google Scholar]
- 12.Fauriat C, Just-Landi S, Mallet F, Arnoulet C, Sainty D, Olive D, Costello RT. Deficient expression of NCR in NK cells from acute myeloid leukemia: Evolution during leukemia treatment and impact of leukemia cells in NCRdull phenotype induction. Blood. 2007;109:323–330. doi: 10.1182/blood-2005-08-027979. [DOI] [PubMed] [Google Scholar]
- 13.Fujisaki H, Kakuda H, Shimasaki N, Imai C, Ma J, Lockey T, Eldridge P, Leung WH, Campana D. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res. 2009;69(9):4010–4017. doi: 10.1158/0008-5472.CAN-08-3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bryceson YT, March ME, Ljunggren HG, Long EO. Synergy among receptors on resting NK cells for the activation of natural cytotoxicity and cytokine secretion. Blood. 2006;107(1):159–166. doi: 10.1182/blood-2005-04-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oberlies J, Watzl C, Giese T, Luckner C, Kropf P, Müller I, Ho AD, Munder M. Regulation of NK cell function by human granulocyte arginase. J Immunol. 2009;182(9):5259–5267. doi: 10.4049/jimmunol.0803523. [DOI] [PubMed] [Google Scholar]
- 16.Carson WE, Fehniger TA, Haldar S, Eckhert K, Lindemann MJ, Lai CF, Croce CM, Baumann H, Caligiuri MA. A potential role for interleukin-15 in the regulation of human natural killer cell survival. J Clin Invest. 1997;99:937–943. doi: 10.1172/JCI119258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooper MA, Bush JE, Fehniger TA, VanDeusen JB, Waite RE, Liu Y, Aguila HL, Caligiuri MA. In vivo evidence for a dependence on interleukin 15 for survival of natural killer cells. Blood. 2002;100:3633–3638. doi: 10.1182/blood-2001-12-0293. [DOI] [PubMed] [Google Scholar]
- 18.Prlic M, Blazar BR, Farrar MA, Jameson SC. In vivo survival and homeostatic proliferation of natural killer cells. J Exp Med. 2003;197:967–976. doi: 10.1084/jem.20021847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boyiadzis M, Memon S, Carson J, Allen K, Szczepanski MJ, Vance BA, Dean R, Bishop MR, Gress RE, Hakim FT. Up-regulation of NK cell activating receptors following allogeneic hematopoietic stem cell transplantation under a lymphodepleting reduced intensity regimen is associated with elevated IL-15 levels. Biol Blood Marrow Transplant. 2008;14:290–300. doi: 10.1016/j.bbmt.2007.12.490. [DOI] [PubMed] [Google Scholar]
- 20.Whiteside TL, Bryant J, Day R, Herberman RB. Natural killer cytotoxicity in the diagnosis of immune dysfunction: criteria for a reproducible assay. J Clin Lab Anal. 1990;4:102–114. doi: 10.1002/jcla.1860040207. [DOI] [PubMed] [Google Scholar]
- 21.Romagnani C, Juelke K, Falco M, Morandi B, D’Agostino A, Costa R, Ratto G, Forte G, Carrega P, Lui G, Conte R, Strowig T, Moretta A, Munz C, Thiel A, Moretta L, Ferlazzo G. CD56brightCD16− killer Ig-like receptor-NK cells display longer telomeres and acquire features of CD56dim NK cells upon activation. J Immunol. 2007;178:4947–4955. doi: 10.4049/jimmunol.178.8.4947. [DOI] [PubMed] [Google Scholar]
- 22.Nowbakht P, Ionescu MC, Rohner A, Kalberer CP, Rossy E, Mori L, Cosman D, DeLibero G, Wodnar-Filipowicz A. Ligands for natural killer cell-activating receptors are expressed upon the maturation of normal myelomonocytic cells but at low levels in acute myeloid leukemias. Blood. 2005;105:3615–3622. doi: 10.1182/blood-2004-07-2585. [DOI] [PubMed] [Google Scholar]
- 23.Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419:734–738. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- 24.Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, Posati S, Rogaia D, Frassoni F, Aversa F, Martelli MF, Velardi A. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 25.Chik KW, Li K, Pong H, Shing MM, Li CK, Yuen PM. Elevated serum interleukin-15 level in acute graft-versus-host disease after hematopoietic cell transplantation. J Pediatr Hematol Oncol. 2003;25:960–964. doi: 10.1097/00043426-200312000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, McKenna D, Le C, Defor TE, Burns LJ, Orchard PJ, Blazar BR, Wagner JE, Slungaard A, Weisdorf DJ, Okazaki IJ, McGlave PB. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]