Abstract
Adaptive responses can be induced in cells by very low doses of ionizing radiation resulting in an enhanced resistance to much larger exposures. The inhibitor of apoptosis (IAP) protein, survivin, has been implicated in many adaptive responses to cellular stress. Computerized axial tomography (CAT) used in image guided radiotherapy to position and monitor tumor response utilizes very low radiation doses ranging from 0.5 to 100 mGy. We investigated the ability of these very low radiation doses administered along with two 2 Gy doses separated by 24 h, a standard conventional radiotherapy dosing schedule, to initiate adaptive responses resulting in the elevation of radiation resistance in exposed cells. Human colon carcinoma (RKO36), mouse sarcoma (SA-NH), along with transformed mouse embryo fibroblasts (MEF), wild type (WT) or cells lacking functional tumor necrosis factor receptors 1 and 2 (TNFR1−R2−) were used to assess their relative ability to express an adaptive response when grown either to confluence in vitro or as tumors in the flank of C57BL/6 mice. The survival of each of these cells was elevated from 5 to 20% (P ≤ 0.05) as compared to cells not receiving a 100 mGy or lesser dose. Additionally, the cells exposed to 100 mGy exhibited elevations in survivin levels, reductions in apoptosis frequencies, and loss of an adaptive response if transfected with survivin siRNA. This survivin-mediated adaptive response has the potential for affecting outcomes if regularly induced throughout a course of image guided radiation therapy.
Keywords: Adaptive-response, Survivin, Computed Tomography, TNF receptors
Introduction
The adaptive response, defined as the ability of a very low dose of ionizing radiation (≤ 100 mGy) to confer enhanced resistance to cells or organisms subsequently exposed to a much larger dose of a deleterious agent, was initially observed in the context of elevating the inherent resistance of human lymphocytes against the toxicity of high doses of ionizing radiation and chemical mutagens (1). This phenomenon has garnered interest again given the expanding use of computerized axial tomography (CAT) as an essential and pervasive tool for rapid and highly sensitive analysis in both diagnostic- and therapeutic-associated medical procedures. In 2007 72 million CAT scans were performed in the United States alone (2). The dose range of most CAT procedures falls within the range of 0.5 to 100 mGy that overlaps the inductive dose range of the adaptive response (3). CAT imaging to monitor tumor responses as a function of therapy may initiate adaptive responses that may affect therapeutic outcomes (4). It is important to consider this possibility as CAT monitoring of tumor response in image guided radiotherapy is now routine and has become a standard of care.
An adaptive response that has been studied extensively is attributed to tumor necrosis factor (TNF) signaling (5,6). Stimulation of this pathway leads to the activation of nuclear factor κB (NFκB), enhanced expression of the manganese superoxide dismutase (SOD2) gene and elevated enzymatic activity (7–9). Another more tumor cell specific adaptive response is known to involve the inhibitor of apoptosis (IAP) protein survivin (10,11). Survivin is recognized as an important factor in tumor cell resistance. Its overexpression has been correlated with elevated resistance to radiation- and chemotherapy-induced cell killing and reduced frequencies of apoptosis (12–15). Ionizing radiation at doses of 1 to 8 Gy is known to significantly elevate survivin levels in malignant cells (14–16). We have focused this study on the effectiveness of very low radiation doses in the range of 5 to 100 mGy as inducers of an adaptive response when interspersed with two 2 Gy doses separated by 24 h, an irradiation scheme reflective of a standard image guided radiotherapy protocol. RKO36 human colon carcinoma, SA-NH mouse sarcoma, and transformed mouse embryo fibroblast (MEF) cells were used in this study. MEF, both WT and those engineered with germ line deletions of TNF receptors 1 and 2 were used to test the hypothesis that TNF signaling is an important factor in the expression of any adaptive response (7,9). Furthermore, these cells grow both in vitro as monolayers and as solid tumor growths when inoculated s.c. into C57BL/6 mice.
Materials and Methods
Cells and Culture Conditions
RKO human colorectal carcinoma cells (CRL-2577), obtained from the American Type Culture Collection (ATCC, Manasses, VA), were grown in Eagle’s minimum essential medium (Invitrogen Life Technologies, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS, Atlantic Biologicals, Lawrenceville, GA). A subclone of these cells transfected with the pCMV-EGFP2Xho vector allowing for the analysis of delayed hyper-recombination and/or deletion/mutation events in the progeny of surviving cells, designated RKO36, was generated and supplied to us by the laboratory of Dr. William F Morgan, University of Maryland, Baltimore as described in detail elsewhere (17). SA-NH mouse sarcoma cells, supplied by Dr. Luka Milas, the University of Texas MD Anderson Cancer Institute, were MAP tested and then frozen down for future use. Cells used from the fifth generation passage were adapted for in vitro and in vivo growth and were used in these experiments (18–20). Mouse embryo fibroblasts (MEF) were isolated from either 14–16 day old pregnant female C57BL/6 WT or TNFR1−R2− C57BL/6 knockout mice (9). Mice were euthanized, and the uterus was removed and placed in a culture dish containing sterile PBS. Organs, tail, limbs and head were removed for genotyping. Carcasses were placed in PBS with 0.25% trypsin and finely minced with scissors. Minced tissues were incubated for 15 min at 37 °C and pipetted to dissociate the tissue. This process was repeated 2–3 times after which supernatants were collected and centrifuged. Cells were re-suspended in culture medium containing (1:1) DMEM:F12 (Invitrogen Life Technologies), 10% FBS, 100 units/ml penicillin and 100 mg/ml streptomycin (Invitrogen Life Technologies) and plated in 60 mm dishes at a density of 106 cells/dish. All cell cultures were maintained at 37 °C in a humidified environment containing 5% CO2.
Generation of transformed MEF cell lines was as follows: The pLXSN vector designed for co-expression of c-myc and H-RasVal12 with an internal ribosomal entry site (a gift from Dr. A. Gudkov) was co-transfected with packaging plasmids in 293T cells using ProFection Mammalian Transfection calcium phosphate system (Promega, Madison, WI). Supernatants containing infectious retrovirus were harvested 48 hours post-transfection, pooled, and filtered through a 0.45-mm membrane, and exponentially growing MEFs were transduced.
RKO36 and MEF cells were grown in Dulbecco’s modified Eagle’s medium. SA-NH cells were grown in McCoy’s 5A medium (Invitrogen Life Technologies). In all experiments cells were grown to confluence and then re-fed with fresh medium and maintained for an additional 3 days. Cultures were again re-fed with fresh medium 1 day prior to each experiment.
Cell Survival Assay
All cells were irradiated with very low doses of 5 to 100 mGy at times 30 min to 6 h before or after exposure to the first of two 2 Gy doses, each separated by 24 h. Unirradiated cells served as controls. Immediately following the second 2 Gy dose, cells were counted, diluted, and known numbers seeded into 100-mm tissue culture dishes to allow the development of 100–200 colonies per dish. Colonies were stained with 20% crystal violet and scored. Five dishes per experimental point were used and experiments were repeated three times.
Single-cell Suspensions from Tumors
Viable MEF WT or TNFR1−R2− cells (1 × 107) were injected into the right hind leg of C57BL/6 mice and grown as tumors to 8 mm in diameter. Tumors were irradiated with 2 Gy followed 30 min or 3 h later by exposure to 100 mGy. Mice were sacrificed 24 h later and tumors were aseptically harvested, minced, and incubated in 4 ml PBS containing 5 mg/ml collagenase and 10 μg/ml DNase (Sigma-Aldrich, St. Louis, MO) for 1 h on a rotary shaker at room temperature. Cells were passed through sterile #200 nylon mesh and pelleted by centrifugation at 1000 rpm for 5 min at room temperature and counted using a hemocytometer. From each suspension cells were removed to determine plating efficiency. The remaining cell suspension was exposed to 2 Gy and plated for survival in 100-mm tissue culture dishes. Colonies were fixed and stained 10 days later. Five dishes per experimental point were used and experiments were repeated three times.
Animal Models
Female C57BL/6 mice 6–8 weeks of age were purchased from Harlan Laboratories (Indianapolis, IN). The care and treatment of experimental animals was in accordance with Institutional guidelines and adherence to the NIH Guide for the Care and Use of Laboratory Animals.
Survivin siRNA Transfection
MEF WT and TNFR1−R2−, RKO36 and SA-NH cells were grown to confluence and transfected with 100 nM survivin (Birc5) or negative control (NC) short interfering RNA (siRNA, Ambion by Life Technologies, Foster City, CA) using Lipofectamine 2000 reagent (Invitrogen Life Technologies). Two different human survivin siRNA sequences were used: (seq 1) 5′-UAGCAAAAGGGACACUGCCtt-3′, and (seq 2) 5′UGUAGAGAUGCGGUGGUCCtt-3′. Mouse survivin siRNA was 5′-UGUCUGUCCAGUUUCAAGAat-3′. siRNA oligomer and Lipofectamine 2000 were diluted in serum-free media and incubated for 5 min at room temperature. The siRNA oligomer and Lipofectamine 2000 were mixed and incubated for 20 min. Growth medium was aspirated from the dishes and cells were washed with PBS at 37 °C. siRNA Lipofectamine 2000 complexes were added to the dishes and incubated for 24 h with cells under their normal growth conditions. The medium was then aspirated, the cells washed with PBS at 37 °C, and fresh complete growth medium was added.
TUNEL Apoptosis Assay
Apoptosis was monitored using the TACS 2 TdT-Blue Label in Situ Apoptosis Detection Kit from Trevigen following the manufacturer’s instructions. Cells were grown to confluence, trypsinized and pelleted by centrifugation at 1000 rpm for 5 min at 4 °C. Cells, fixed in 70% ethanol, were stored at 4 °C overnight. Cells, dropped onto Trevigen Three Sample treated glass microscope slides and air dried overnight were sequentially immersed in 100%, 95% and then 70% ethanol for 5 min each. After rehydration, slides were immersed in PBS for 10 min followed by addition of 50 μl of Cytonin for 30 min at room temperature, washed twice in apoptosis grade water for 2 min each, immersed in quenching solution for 4.5 min, washed once in PBS for 1 min at room temperature and then immersed in TdT labeling buffer for 5 min at room temperature. 50 μl of labeling reaction mixture was applied to each cell sample, incubated at 37° C for 1 h, then immersed in TdT stop buffer, 5 min, and washed twice in PBS, 5 min, at room temperature. 50 μl of Strep-HRP solution was added for 10 min at 37 °C. Slides were washed 2x in PBS for 5 min and TACS-Blue label solution added for 7 min at room temperature, washed 3x in apoptosis grade water for 2 min, immersed in Nuclear Fast Red and then sequentially washed 10x in: apoptosis grade water, 70% ethanol (2x), 95% ethanol (2x), 100% ethanol (2x) and o-xylene (2x). Slides were placed in a light-tight container, mounting medium allowed to harden overnight at room temperature and apoptotic cells then scored using an Axioplan fluorescence microscope, 63X oil immersion objective, using GFP and Texas Red filters. At least 1000 cells were scored and apoptosis frequency calculated as the ratio of the number of apoptotic cells relative to the total number counted.
Western Blotting
Cell lysates, prepared from MEF WT, TNFR1−R2−, SA-NH, and RKO36 cells, were washed with cold PBS, harvested on ice, transferred to 50 ml tubes and pelleted, 1000 rpm for 5 min at 4 °C, then resuspended in 350 μl 50 mM potassium phosphate buffer, pH 7.8 and sonicated on ice (18). Total cellular protein was quantified by the Bradford method and adjusted to 2 μg/μl with PBS. Survivin and α-Tubulin protein levels were assessed using the WesternBreeze Chemiluminescent Western Blotting Immunodetection System (Invitrogen Life Technologies). Total protein (10 μg) was electrophoresed on a 12% SDS polyacrylamide gel and transferred onto PVDF membranes. The blots, blocked for 30 min, were incubated with primary antibody (1:1000 dilution of rabbit anti-survivin, 1:1000 dilution of rabbit anti-α-Tubulin) for 1 h at room temperature, washed 4x for 5 min and incubated with goat anti-rabbit IgG conjugated with alkaline phosphatase for 30 min at room temperature. After 4 washes, 5 min each, the protein bands were visualized by applying 2.5 ml chemiluminescent substrate to the membranes for 5 min. Membranes were exposed to BioMax XAR film (Kodak, Rochester, NY), scanned using an HP ScanJet 8200 (Houston, TX) and band intensities quantified using NIH ImageJ 1.47b software.
Statistical Analysis
Means and standard errors were calculated for all data points from at least three independent experiments. Pairwise comparisons of cell survival and apoptosis frequencies between each of the experimental conditions were performed using a Student’s two-tailed t test (SigmaPlot software 11.0, SPSS, Chicago, IL).
RESULTS
The Effect of Very Low Radiation Doses on RKO36 Cell Survival in a Split Dose Paradigm
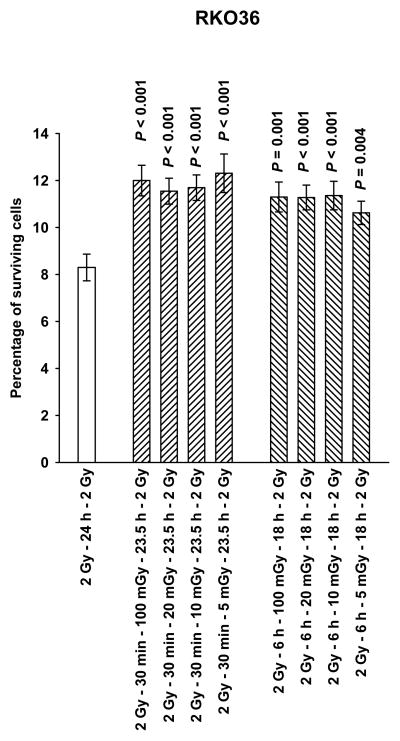
Presented in Fig. 1 are data describing the effects of 5, 10, 20, or 100 mGy, that if administered 30 min or 6 h following the first of two 2 Gy doses of radiation, induce a 35 to 40% increase in the baseline resistance of RKO36 cells to radiation-induced cell killing. Since the magnitude of the increase in survival was consistent throughout the dose range tested, all subsequent irradiations were performed using a dose of 100 mGy.
Figure 1.
The percentage of surviving RKO36 human colon carcinoma cells grown to confluence as a function of the timing of very low radiation doses of 5, 10, 20 and 100 mGy, administered 30 min or 6 h following the first of two 2 Gy doses separated by 24 h, are presented. P values were determined by comparing the survival of cells following two 2 Gy doses with those exposed to two 2 Gy doses along with the various very low mGy doses using a two-tailed Student’s t test with values ≤ 0.05 identified as significant. Each experiment was repeated 3x and error bars represent the standard error of the mean (SEM).
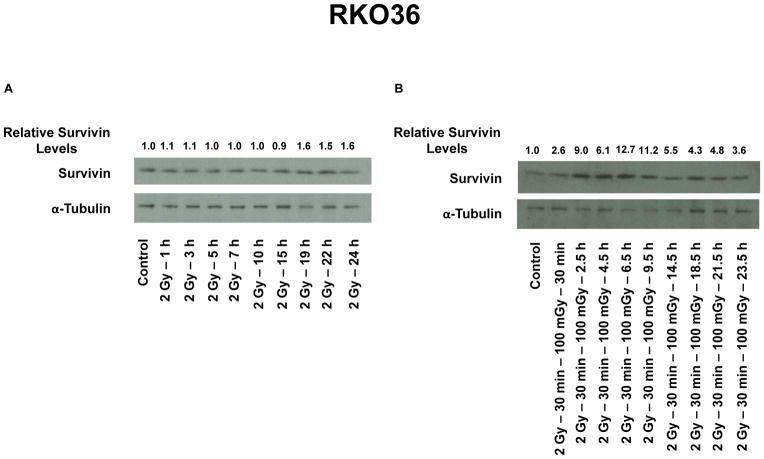
The Effect of a 100 mGy Exposure on Survivin Protein in RKO36 Cells Exposed to a Split Dose Paradigm
Western blots were performed to monitor survivin protein using samples isolated at various times up to 24 h following radiation exposure. Representative gels are presented in Fig. 2A and B. Following a single 2 Gy dose, survivin protein in RKO36 cells was observed to gradually increase to 60% over control levels by 24 h (see Fig. 2A). Exposure of cells to 100 mGy 30 min following a 2 Gy dose resulted in a more robust elevation of survivin levels ranging from 2.6- to 12.7-fold higher than levels in control cells (see Fig. 2B).
Figure 2.
Representative Western blots from 3x experiments describe changes in survivin protein levels in RKO36 cells over 24 h as a function of exposure to a single 2 Gy dose of ionizing radiation only (A), or a 2 Gy dose followed 30 min later by a 100 mGy exposure (B). Relative survivin levels were determined through densitometry measurements of survivin and corresponding α-Tubulin loading control band densities and normalized to unirradiated control values.
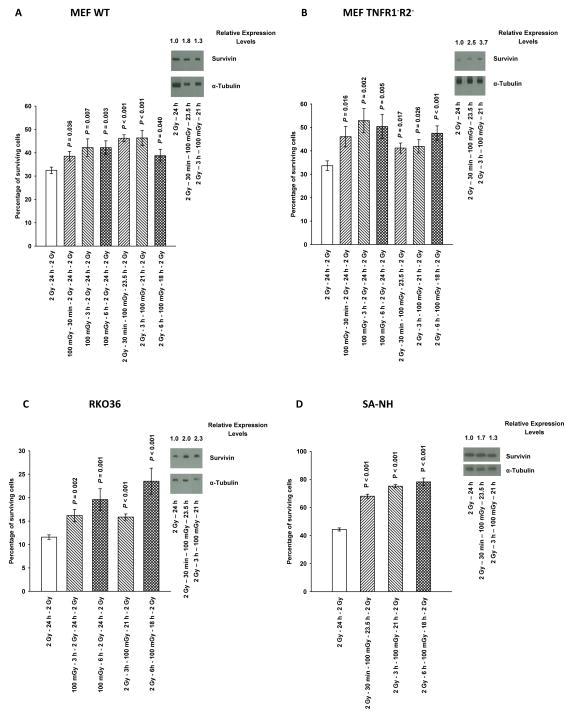
Comparative Adaptive Responses in MEF WT, MEF TNFR1−R2−, RKO36, and SA-NH cells
MEF WT, MEF TNFR1−R2−, RKO36, and SA-NH tumor cells, were exposed to 100 mGy either 30 min, 3 h, or 6 h before or after the first of two 2 Gy doses separated by 24 h. As described in Fig. 3A, B, C and D, all four cell systems exhibited significant adaptive responses (P ≤ 0.04). MEF TNFR1− R2− knockout cells were used to determine whether TNF signaling was an important factor in the expression of this adaptive response. Its robust development in MEF TNFR1−R2− cells indicates that this adaptive response is not dependent upon an intact TNF signaling pathway (see Fig. 3B).
Figure 3.
Cell survival as a function of the timing of a 100 mGy dose given prior to or following the first of two 2 Gy doses separated by 24 h is presented for: transformed mouse embryo fibroblast (MEF) wild type (WT) cells (A); transformed MEF cells lacking tumor necrosis factor receptors 1 and 2 (TNFR1−R2−) (B); human colon carcinoma RKO36 cells (C), and SA-NH mouse sarcoma cells (D). Western blots monitor the relative change in survivin band densities as a function of treatment as compared to cells exposed to a 2 Gy dose only. Times indicate when survivin analysis was performed following the 100 mGy dose and represent survivin levels at 24 h after the first 2 Gy dose. P values were determined by comparing the survival of cells following two 2 Gy doses with those also exposed to an additional 100 mGy dose using a two-tailed Student’s t test with values ≤ 0.05 identified as significant. Each experiment was repeated 3x and error bars represent the SEM.
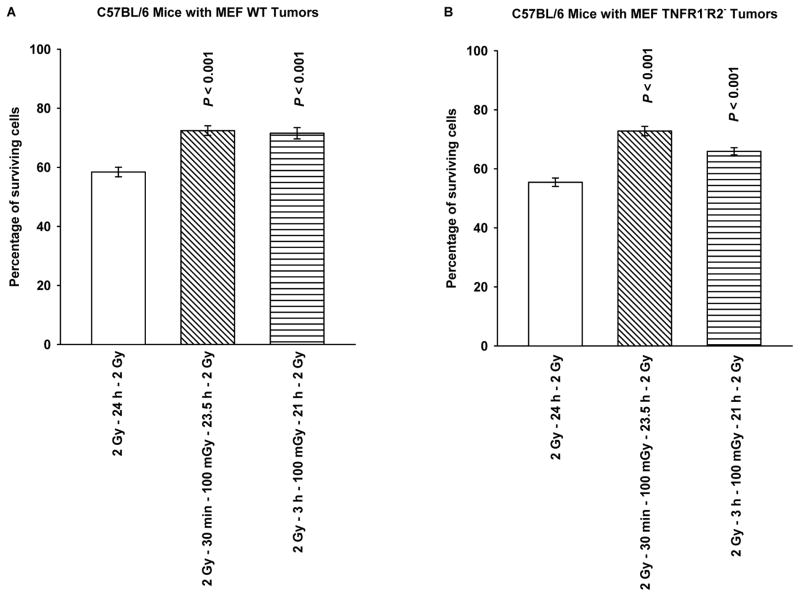
MEF WT and TNFR1−R2− knockout cells can grow both in vitro as assayable colonies and in vivo as growths in the flanks of C57BL/6 mice. MEF tumors 8 mm in size were irradiated with 2 Gy only or with 2 Gy plus 100 mGy 30 min or 3 h later and then excised 24 h following the first 2 Gy dose and made into single cell suspensions prior to irradiation with a second 2 Gy dose. This procedure was followed to allow for the use of a clonogenic assay in determining both the expression and the magnitude of any potential adaptive responses. As described in Fig. 4A and B, both MEF WT and TNFR1−R2− grown in vivo exhibited a highly significant adaptive response when compared to the 2 Gy only groups (P < 0.001). We conclude that TNF signaling is dispensable for development of an adaptive response under these conditions.
Figure 4.
Cell survival of MEF grown in the flanks of C57BL/6 mice as solid tumor growths, as a function of timing of a 100 mGy dose given 30 min or 3 h following the first of two 2 Gy doses separated by 24 h are presented for MEF WT (A) and MEF TNFR1−R2− (B). Both the first 2 Gy and subsequent 100 mGy doses were delivered to MEF growing in C57BL/6 mice. Tumors were removed just prior to the second 2 Gy dose, made into single cell suspension, and irradiated under in vitro conditions and surviving fraction assessed using an in vitro colony forming assay. P values were determined by comparing the survival of cells following two 2 Gy doses with those also exposed to a 100 mGy dose delivered 30 min or 3 h following the first 2 Gy dose using a Student’s two-tailed t test with values ≤ 0.05 identified as significant. Experiments were repeated 2x and error bars represent the SEM.
The Effect of Survivin siRNA Transfection on the Adaptive Response
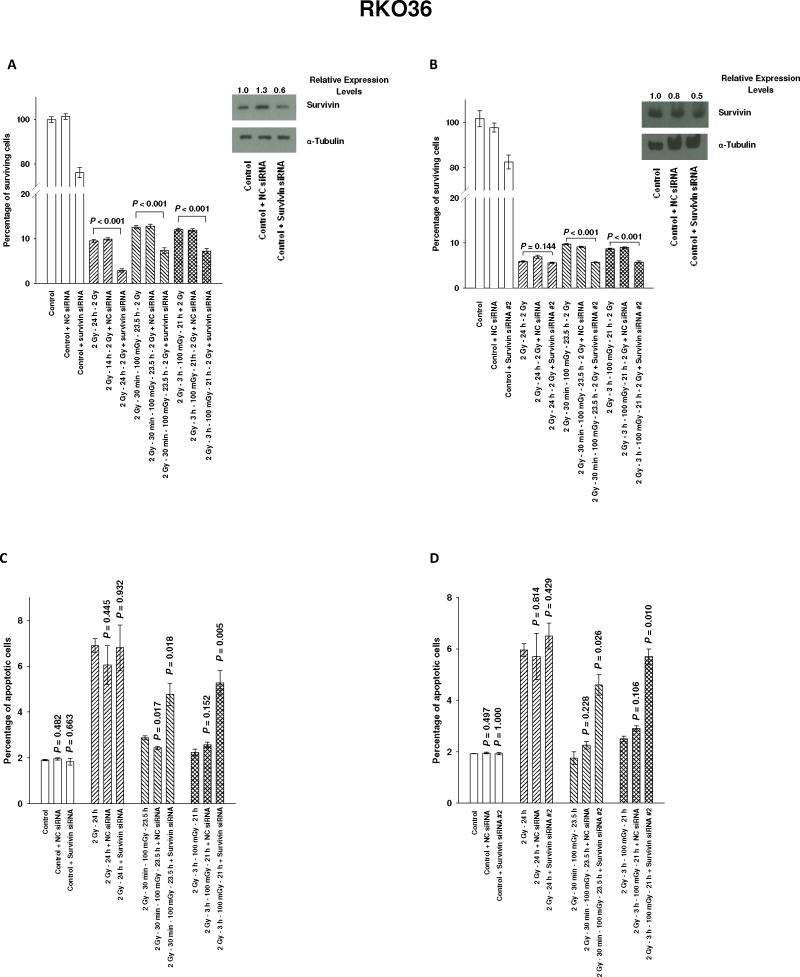
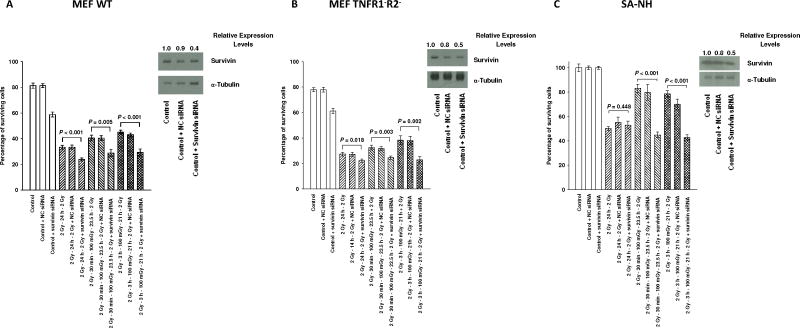
To assess the potential relationship between elevation in survivin protein levels and elevated resistance to ionizing radiation, RKO36 cells were exposed to these same radiation conditions following transfection with a negative control (NC) or two different survivin siRNA’s. As described in Fig. 5A and B, NC siRNA had no effect on cellular response to radiation exposure. Transfection with either of the survivin siRNA’s, seq 1 or 2, inhibited elevated survival following a 100 mGy exposure (P ≤ 0.001). Apoptosis frequencies presented in Fig. 5C and D were also significantly elevated in RKO36 cells transfected with either survivin siRNA (P ≤ 0.026). Transfection of MEF WT, MEF TNFR1−R2−, and SA-NH cells with survivin siRNA also inhibited 100 mGy induced adaptive responses (P ≤ 0.005) (See Fig. 6A, B and C). Western blotting, used to monitor the effects of survivin siRNA transfection on survivin protein levels at 24 h, demonstrated about 50% reduction in survivin.
Figure 5.
The effects of transfection of RKO36 cells with negative control (NC) and survivin siRNA sequences, 5′-UAGCAAAAGGGACACUGCCtt-3′ (A, C) or 5′-UGUAGAGAUGCGGUGGUCCtt-3′ (B, D) on survival and apoptosis responses, respectively, to two 2 Gy doses or 2 Gy followed 30 min or 3 h later with an additional 100 mGy exposure and relative survivin protein levels in control cells as determined by Western blotting. Survival and apoptosis experiments were repeated 3x and P values were determined by comparing the survival of each non-transfected cohort of cells following either two 2 Gy doses only or two 2 Gy doses with an additional 100 mGy exposure with their respective survivin siRNA transfected cohorts using a Student’s two-tailed t test. Error bars represent the SEM.
Figure 6.
Effects of transfection of MEF WT (A), MEF TNFR1−R2− (B), and SA-NH cells (C) with negative control (NC) or survivin siRNA, 5′-UAGCAAAAGGGACACUGCCtt-3′, on respective cell survival after two 2 Gy doses or 2 Gy followed 30 min or 3 h later with an additional 100 mGy exposure. Survivin protein levels in control cells were determined by Western blotting. Survival experiments were repeated 3x and P values were determined by comparing the survival of each non-transfected cohort of cells following either two 2 Gy doses only or two 2 Gy doses with an additional 100 mGy exposure with their respective survivin siRNA transfected cohorts using a Student’s two-tailed t test. Error bars represent the SEM.
The Effect of the Adaptive Response on Apoptosis
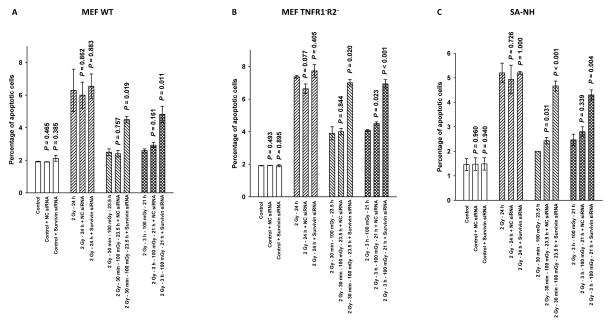
Described in Fig. 7A, B, C are the percentages of apoptotic cells appearing in the populations of MEF WT, TNFR1−R2−, and SA-NH cells respectively, as a function of survivin siRNA transfection and irradiation conditions at 24 h following the first 2 Gy dose. The 24 h time point was chosen to assess apoptosis frequencies since cells were irradiated with a second 2 Gy dose at this time and then counted and plated to assess clonogenic survival. Cells exposed to 100 mGy 30 min or 3 h following the first 2 Gy dose exhibited a much lower apoptotic frequency than did cells exposed only to 2 Gy. The magnitude of these differences at 24 h following the first 2 Gy dose indicated that an exposure to 100 mGy conferred a protection against the induction of apoptosis in all three cell lines. The reduced apoptotic frequencies associated with exposure to 100 mGy correlate with the elevated cell survival demonstrated in Fig. 6. An important endogenous inhibitor of apoptosis that is known to be both associated with enhanced tumor cell resistance and inducible by high doses of ionizing radiation, e.g. BIRC5 encoded inhibitor of apoptosis protein survivin, was identified as the potential effector of this adaptive response.
Figure 7.
The percentage of apoptotic cells as determined by the TUNEL assay are plotted as a function of radiation treatment and transfection with survivin siRNA, 5′-UAGCAAAAGGGACACUGCCtt-3′, for MEF WT (A), MEF TNFR1− R2− (B), and SA-NH cells (C). Comparisons were made in each cell system between the percentage of apoptotic cells counted 24 h after the first 2 Gy dose in the non-transfected group and its negative control (NC) or survivin siRNA transfected counterpart. Apoptosis was significantly elevated only in cells transfected with survivin siRNA and exposed to 100 mGy (P ≤ 0.019) as compared to no effect in transfected cells only exposed to 2 Gy (P > 0.05). Each experiment was repeated 3x and error bars represent the SEM.
DISCUSSION
Previous studies on the adaptive response have focused exclusively on a single exposure to a very low dose of radiation followed at some later time by a much larger radiation dose (1,7–9). Very low doses of radiation used to initiate these adaptive responses have been reported to range from 0.5 to 100 mGy, a dose range overlapping that currently received by patients from CAT scans used in diagnostic procedures (3,4). The rapid development of image guided radiotherapy protocols utilizing CAT scans or portal imaging for the treatment of cancer (21,22) has elevated the concern regarding the potential clinical relevance of such adaptive responses. We report here for the first time that delivering a very low dose of radiation, ≤ 100 mGy, between two much larger doses of 2 Gy separated by 24 h, simulating what is employed in a conventional fractionated radiation therapy paradigm, induces an adaptive response. The RKO human colon carcinoma cell line was chosen for study because it grows well in vitro both as a monolayer culture and as colonies in a standard cell survival assay. SA-NH mouse sarcoma cells can grow as tumors in C3H mice as well as cells in monolayers and colonies in vitro and have been used to investigate adaptive responses induced under both in vitro and in vivo conditions (18–20). Transformed mouse embryo fibroblast models were developed to facilitate the investigation of the role of TNF signaling in the low dose radiation-induced adaptive response. We previously reported using a BFS murine fibrosarcoma model, along with WT and TNFR1−R2− transformed MEF cells, that TNF signaling and associated increases in SOD2 enzymatic activity could be completely inhibited in cells defective in both TNF receptors 1 and 2 and that these cells failed to exhibit an adaptive response when exposed to a 100 mGy dose followed by a single 2 Gy exposure (8,9). We were able to develop transformed MEF WT and MEF TNFR1−R2− knockout cells capable of growth as monolayer cultures and colonies in standard survival assays, as well as tumors in C57BL/6 mice to test the role of TNF signaling in the expression of adaptive responses as a function of various irradiation conditions.
The magnitude of the adaptive response measured as an inducible increase in cell survival was significant for RKO36 colon carcinoma cells in the dose range tested, e.g., 5 to 100 mGy, and was accompanied by an increase in survivin protein levels (see Figs. 1 and 2, respectively). This enhancement in survivin protein level accompanied by elevated cell survival was also observed in MEF WT and TNFR1−R2− cells grown in culture (see Fig. 3) or as tumors in mice and then assayed for cell survival in vitro (see Fig. 4), demonstrating this adaptive response is both novel and independent of TNF receptor status or SOD2 activity (see supplemental data). The induction of this survivin-mediated adaptive response appears to occur over a wide range of time intervals, e.g., 30 min to 6 h either before or following a 2 Gy exposure. This suggests that the timing of the very low dose exposure is critical to the induction of this adaptive effect and that care should be taken to perform imaging procedures as close in time to each 2 Gy dose delivered in the treatment protocol as possible, most likely well within the 30 min period before or following the 2 Gy exposure.
Transfection of human RKO36, mouse SA-NH, and both WT and TNFR1−R2− mouse cells with survivin siRNA was sufficient to completely inhibit elevations in cellular radioresistance and the adaptive response, as well as reduce cell survival directly (see Figs. 5 and 6). We also monitored changes in apoptosis as a function of very low dose irradiation and transfection with survivin siRNA (see Figs. 5 and 7). The addition of a 100 mGy exposure to the 2 Gy dosing paradigm resulted in a reduction in apoptosis frequency that correlated with an elevation in survivin protein. Transfection with survivin siRNA resulted in decreased survivin levels and a concomitant increase in apoptotic frequencies, reduced cell survival, and enhanced radiation sensitivity as has been reported by others using various malignant cell systems (23,24). Human endometrial cancer cells, HL60 leukemia cells, renal clear cell carcinoma cells, and breast cancer cells following transfection with survivin siRNA alone, without the addition of any other deleterious agents, exhibited elevated apoptotic frequencies and lower cell survival (25–28).
Intact TNF signaling is not a requirement for the expression of this newly identified survivin-mediated adaptive response. NFκB activation and subsequent effects on survivin have been extensively characterized and have been identified as being controlled via the PI3K/Akt/NFκB signaling pathway (29–31). Conventional Radiation therapy protocols generally involve the use of multiple 2 Gy doses per fraction, each administered at 24 h intervals. For this reason the experimental design employed in this study utilized two 2 Gy doses separated by 24 h with or without a 100 mGy dose delivered from 30 min, 3 h, or 6 h following delivery of the first 2 Gy dose. The use of this wide range of time intervals for administration of the 100 mGy exposure allowed for an evaluation of the time dependence for the development of this adaptive response. All of the time points tested proved to be effective in the development of a survivin-mediated adaptive response strongly suggesting that the “safest” time interval for utilizing an imaging procedure during standard radiotherapy is well within a 30 min interval prior to or following each delivery of a 2 Gy dose. While survivin is known to be inducible by exposure to high radiation doses in the 1 to 8 Gy range (12,13), its elevation following exposure to very low doses of radiation, e.g., ≤ 100 mGy, in the range of doses used in image guided radiotherapy, was unexpected and represents a novel and important finding with clinical implications.
Survivin does not regulate apoptosis independently of other proteins which can modify its effects, but its role in elevating tumor cell resistance to radiation therapy is well documented and is generally associated with a reduction in apoptosis (11–13). Because survivin is overexpressed in malignant cells and can be elevated by both high and very low doses of ionizing radiation, it is recognized as an important risk factor associated with adverse outcomes in radiation therapy. The potential for very low doses of radiation delivered between intervals of high dose exposures used in standard radiotherapy treatment regimens to induce a survivin-mediated adaptive response is a novel observation and represents a unique identifiable adaptive response. While it is unclear whether such an adaptive response could be induced in a very high dose paradigm such as those used in Stereotactic Body Radiation Therapy, its potential for affecting therapy should be investigated. This study demonstrates the need to better recognize the potential treatment modifying effects of imaging techniques that utilize very low levels of radiation exposure in the context of their ability to induce adaptive responses capable of altering overall radiation responses. Increases in tumor cell survival as low as 5 to 20%, through a multi-treatment course of radiotherapy, could have serious adverse consequences regarding therapeutic outcomes. As a worst case scenario, a small increase in survival each day of treatment induced through a very low dose imaging-associated radiation exposure could lead to a persistent induction and maintenance of a survivin-mediated adaptive response. Such a response would be exponentially magnified by the number of treatments such that an increase in a theoretical survival of 0.45 to 0.50 for each of 32 treatments could result in a several orders of magnitude increase in overall tumor cell survival by the end of therapy.
Supplementary Material
Acknowledgments
Financial Support: (DJG, GEW, JJL) DOE Low Dose Program/Project Grant DE-SC0001271; (DJG) NIH R01-CA132998; (RRW) R01 CA111423.
We acknowledge the excellent technical assistance of Mr. Kenneth Baker in the scoring of TUNEL data and Mr. Michael Beckett for the isolation and development of the transformed wild type and TNFR1−R2− MEF’s. Both are members of the Department of Radiation and Cellular Oncology, The University of Chicago, Chicago IL 60637.
GRANT SUPPORT
This work was supported in part by the DOE Low Dose Program/Project Grant DE-SC0001271 awarded to Drs. Grdina, Li and Woloschak. Dr. Grdina was also supported by NIH NCI R01-CA132998 and Dr. Weichselbaum by NIH NCI R01-CA111423.
Footnotes
Conflict of Interest: Dr. David J. Grdina is a paid consultant to Pinnacle Biologics and Drs. Grdina and Murley are minority equity partners in Pinnacle Oncology LLC regarding the potential novel uses of amifostine. Dr. Weichselbaum is a consultant to Reflexion, a radiotherapy company.
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
Dr. David J. Grdina is a paid consultant to Pinnacle Biologics and Drs. Grdina and Murley are minority equity partners in Pinnacle Oncology LLC regarding the potential novel uses of amifostine. Dr. Weichselbaum is a consultant to Reflexion, a radiotherapy company.
References
- 1.Wolff S, Afzal V, Wiencke JK, Olivieri G, Michaeli A. Human lymphocytes exposed to low doses of ionizing radiations become refractory to high doses of radiation as well as to chemical mutagens that induce double strand breaks in DNA. Int J Radiat Biol Relat Stud Phys Chem Med. 1988;53(1):39–47. doi: 10.1080/09553008814550401. [DOI] [PubMed] [Google Scholar]
- 2.Dixon RG, Ogden K. Optimizing dose in computed tomographic procedures. Tech Vasc Interv Radiol. 2010;13(3):172–5. doi: 10.1053/j.tvir.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 3.Bonner WM. Low-dose radiation: Thresholds, bystander effects, and adaptive responses. PNAS USA. 2003;100(9):4973–5. doi: 10.1073/pnas.1031538100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phan N, De Lisio M, Parise G, Boreham DR. Biological effects and adaptive response from single and repeated computed tomography scans in reticulocytes and bone marrow of C57BL/6 mice. Radiat Res. 2012;177:164–175. doi: 10.1667/rr2532.1. [DOI] [PubMed] [Google Scholar]
- 5.Neta R. Modulation with cytokines of radiation injury: Suggested mechanisms of action. Environ Health Perspect. 1997;105(Suppl 6):1463–5. doi: 10.1289/ehp.97105s61463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh VK, Yadav VS. Role of cytokines and growth factors in radioprotection. Exp Mol Pathol. 2005;78:156–169. doi: 10.1016/j.yexmp.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Murley JS, Kataoka Y, Miller RC, Li JJ, Woloschak G, Grdina DJ. SOD2-mediated effects induced by WR1065 and low dose ionizing radiation on micronucleus formation in RKO human colon carcinoma cells. Radiat Res. 2011;175(1):57–65. doi: 10.1667/RR2349.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murley JS, Baker KL, Miller RC, Darga TE, Weichselbaum RR, Grdina DJ. SOD2-mediated adaptive responses induced by low-dose ionizing radiation via TNF signaling and amifostine. Free Radic Biol Med. 2011;51(10):1918–25. doi: 10.1016/j.freeradbiomed.2011.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grdina DJ, Murley JS, Miller RC, Mauceri H, Sutton HG, Thirman MJ, et al. A manganese superoxide dismutase (SOD2)-mediated adaptive response. Radiat Res. 2013;179:115–24. doi: 10.1667/RR3126.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones MK, Padilla OR, Webb NA, Norng M. The anti-apoptosis protein, survivin, mediates gastric epithelial cell cytoprotection against ethanol-induced injury via activation of the p34cdc2 cyclin dependent kinase. J Cell Physiol. 2008;215(3):750–64. doi: 10.1002/jcp.21358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marivin A, Berthelet J, Plenchette S, Dubrez L. The inhibitor of apoptosis (IAPs) in adaptive response to cellular stress. Cells. 2012;1:711–37. doi: 10.3390/cells1040711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu B, Mu Y, Cao C, Zeng F, Schneider S, Tan J, et al. Survivin as a therapeutic target for radiation sensitization in lung cancer. Cancer Res. 2004;64:2840–5. doi: 10.1158/0008-5472.can-03-3547. [DOI] [PubMed] [Google Scholar]
- 13.Capalbo G, Rodel C, Stauber RH, Knauer SK, Bache M, Kappler M, et al. The role of survivin for radiation therapy: Prognostic and predictive factor and therapeutic target. Strahlenther Onkol. 2007;11:593–9. doi: 10.1007/s00066-007-1800-4. [DOI] [PubMed] [Google Scholar]
- 14.Jin X, Gong L, Guo C, Hao JF, Wei W, Dai ZY, et al. Survivin expressions in human hepatoma HepG2 cells exposed to ionizing radiation of different LET. Radiat Environ Biophys. 2008;47:399–04. doi: 10.1007/s00411-008-0165-0. [DOI] [PubMed] [Google Scholar]
- 15.Rodel F, Reichert S, Sprenger T, Gaipl US, Mirsch J, Liersch T, et al. The role of survivin for radiation oncology: Moving beyond apoptosis inhibition. Curr Med Chem. 2011;18:191–9. doi: 10.2174/092986711794088362. [DOI] [PubMed] [Google Scholar]
- 16.Rodel C, Haas J, Groth A, Grabenbauer GG, Sauer R, Rodel F. Spontaneous and radiation-induced apoptosis in colorectal carcinoma cells with different intrinsic radiosensitivities: Survivin as a radioresistance factor. Int J Radiat Oncol Biol Phys. 2003;55(5):1341–1347. doi: 10.1016/s0360-3016(02)04618-7. [DOI] [PubMed] [Google Scholar]
- 17.Huang L, Kim PM, Nickoloff JA, Morgan WF. Targeted and nontargeted effects of low-dose ionizing radiation on delayed genomic instability in human cells. Cancer Res. 2007;67(3):1099–1104. doi: 10.1158/0008-5472.CAN-06-3697. [DOI] [PubMed] [Google Scholar]
- 18.Murley JS, Kataoka Y, Weydert CJ, Oberley LW, Grdina DJ. Delayed cytoprotection after enhancement of Sod2 (MnSOD) gene expression in SA-NH mouse sarcoma cells exposed to WR-1065, the active metabolite of amifostine. Radiat Res. 2002;158:101–09. doi: 10.1667/0033-7587(2002)158[0101:dcaeos]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 19.Murley JS, Nantajit D, Baker KL, Kataoka Y, Li JJ, Grdina DJ. Maintenance of manganese superoxide dismutase (SOD2)-mediated delayed radioprotection induced by repeated administration of the free thiol form of amifostine. Radiat Res. 2008;169:495–05. doi: 10.1667/RR1194.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grdina DJ, Murley JS, Kataoka Y, Baker KL, Kunnavakkam R, Coleman MC, et al. Amifostine induces antioxidant enzymatic activities in normal tissues and a transplantable tumor that can affect radiation response. Int J Radiat Oncol Biol Phys. 2009;73(3):886–96. doi: 10.1016/j.ijrobp.2008.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bujold A, Craig T, Jaffray D, Dawson LA. Image-guided radiotherapy: Has it influenced patient outcomes? Sem Radiat Oncol. 2012;22:50–61. doi: 10.1016/j.semradonc.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz DL. Current progress in adaptive radiation therapy for head and neck cancer. Curr Oncol Rep. 2012;14:139–47. doi: 10.1007/s11912-012-0221-4. [DOI] [PubMed] [Google Scholar]
- 23.Sah NK, Munshi A, Hobbs M, Carter BZ, Andreeff M, Meyn RE. Effect of down regulation of survivin expression on radiosensitivity of human epidermoid carcinoma cells. Int J Radiat Oncol Biol Phys. 2006;66(3):852–9. doi: 10.1016/j.ijrobp.2006.06.049. [DOI] [PubMed] [Google Scholar]
- 24.Asanuma K, Moriai R, Yajima T, Yagihashi A, Yamada M, Kobayashi D, Watanabe N. Survivin as a radioresistance factor in pancreatic cancer. Jpn J Cancer Res. 2000;91:1204–9. doi: 10.1111/j.1349-7006.2000.tb00906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ai Z, Yin L, Zhou X, ZHU Y, Zhu D, Yu Y, et al. Inhibition of survivin reduces cell proliferation and induces apoptosis in human endometrial cancer. Cancer. 2006;107:746–56. doi: 10.1002/cncr.22044. [DOI] [PubMed] [Google Scholar]
- 26.Lu YH, Luo XG, Tao X. Survivin gene RNA interference induces apoptosis in human HL60 leukemia cell lines. Cancer Biother Radiopharm. 2007;22:819–25. doi: 10.1089/cbr.2007.0401. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Chen Z, Du C, Xu G, Luo W. siRNA targeting survivin inhibits growth and induces apoptosis in human renal clear cell carcinoma 786-0 cells. Path Res Pract. 2009;205:823–7. doi: 10.1016/j.prp.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 28.Aliabadi HM, Landry B, Mahdipoor P, Uludag H. Induction of apoptosis by survivin silencing through siRNA delivery in a human breast cancer cell line. Mol Pharm. 2011;8:1821–30. doi: 10.1021/mp200176v. [DOI] [PubMed] [Google Scholar]
- 29.Lin J, Guan Z, Wang C, Feng L, Zheng Y, Caicedo E, et al. Inhibitor of differentiation 1 contributes to head and neck squamous cell carcinoma survival via the NF-κB/survivin and phosphoinositide 3-kinase/akt signaling pathways. Clin Cancer Res. 2009;16(1):77–87. doi: 10.1158/1078-0432.CCR-08-2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawakami H, Tomita M, Matsuda T, Ohta T, Tanaka Y, Fujii M, et al. Transcriptional activation of survivin through the NF-κB pathway by human T-cell leukemia virus type I Tax. Int J Cancer. 2005;115:967–74. doi: 10.1002/ijc.20954. [DOI] [PubMed] [Google Scholar]
- 31.Li W, Wang H, Kuang C, Zhu J, Yu Y, Qin Z, et al. An essential role for the Id1/PI3K/Akt/NFκB/survivin signaling pathway in promoting the proliferation of endothelial progenitor cells in vitro. Mol Cell Biochem. 2012;363:135–45. doi: 10.1007/s11010-011-1166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.