Abstract
The binding motif of human CTLA-4 is well known to be MYPPPY and for porcine CTLA-4 the binding motif is LYPPPY. Is this single amino acid difference of methionine (M) versus leucine (L) critical for the CTLA-4 binding? Recently, we have reported that the recombinant soluble porcine CTLA-4 was incapable of binding to human CD80. In this study we mutated L to M in the binding motif of the soluble porcine CTLA-4 and mutated M to L in the binding motif of the soluble human CTLA-4. We then analyzed how these mutations affected the binding affinity of the mutants to both porcine and human CD80+ cells. The soluble porcine CTLA-4-L97M mutant partially lost its binding affinity to porcine CD80 compared to the wild-type and conferred very weak binding ability to human CD80. These results indicate that the L in the binding motif of porcine CTLA-4 is important for determining its binding ability to porcine CD80. Wild-type soluble human CTLA-4 binds to both human and porcine CD80 with comparable affinity, however, the soluble human CTLA-4-M97L mutant almost lost its binding ability to human CD80 and increased its binding ability to porcine CD80. These results indicate that M in the human CTLA-4 binding motif is extremely critical for its binding to human CD80. Those data suggest that the human CTLA-4 based recombinant protein drugs such as human CTLA-4-Ig can be used and/or tested in a porcine model. Conversely, the use of porcine CTLA-4 based recombinant protein drugs such as porcine CTLA-4-Ig is restricted to swine models. The difference in binding specificity of CTLA-4 observed in this study may be useful for studies such as pig to nonhuman primate xeno-transplantation. Porcine CTLA-4- and human CTLA-4-M97L mutant-based recombinant protein drugs can be used to specifically block the direct presentation by donor antigen presenting cells in pig to nonhuman primate xeno-transplantation. Human CTLA-4-M97L mutant-based recombinant protein drugs will be more ideal as it is without immunogenicity to human being.
1. Introduction
T cell activation occurs following two molecular interactions commonly referred to as signal one and signal two. Signal one comes from the antigenic peptide-MHC complex interacting with the T cell receptor (TCR) and signal two describes a series of costimulatory receptors on antigen presenting cells (APCs) such as the CD28/CTLA-4-CD80/CD86 co-stimulation pathway. CTLA-4 is expressed on activated T cells and down regulates T cell proliferation and differentiation. The conserved mammalian CTLA-4 binding motif, MYPPPY (position 97–102) plays a critical role in binding to B7 [1–3]. Site directed mutagenesis demonstrated that while five single amino acid changes within this sequence partially inhibited the ability of CTLA-4 to bind to CD80, they completely abolished binding to CD86 [4]. Consistent with this, the mutant CTLA-4 sequence MYPPAA was unable to bind to CD80/CD86 in transgenic mice [5] and a similar sequence, MYPPPA, could not bind to CD80-expressing CHO cells [6]. However, further mutagenesis studies indicated that domains outside of this sequence also play a role in CTLA-4 binding. Single amino acid substitutions within the CDR1-like region of CTLA-4 that is located N-terminal to the MYPPPY prevented CTLA-4 from binding to CD80 and CD86 [4].
The porcine CTLA-4 binding motif is LYPPPY. It was reported that substituting L for M at position 134 (position 97 of the mature protein) inhibited the ability of porcine CTLA-4-Ig to bind to human CD80/CD86 [7]. The same group also showed that human and porcine CTLA-4-Ig were equally effective in binding to porcine CD86, suggesting that the leucine at position 97 is not necessary for the interaction of CTLA-4 and porcine CD86 [7].
Recently we have expressed and purified glycosylated and non-N-glycosylated soluble porcine CTLA-4 in yeast Pichia pastoris. While both isoforms bind to porcine CD80 on a porcine B cell lymphoma cell line LCL13271 with equal affinity (KD = 13 nM), neither was able to bind to human CD80 [8]. In this study, we mutated the L located within the binding motif of porcine CTLA-4 to the mammalian conserved M and mutated the M within the binding motif of human CTLA-4 to L. We evaluated the effects of these single amino acid substitutions on the ability of these mutants to bind to porcine and human CD80 by flow cytometry.
2. Materials and methods
The leucine at position 97 of the non-N-glycosylated wild-type porcine CTLA-4 (pCTLA-4-Non-N-Gly in pwPICZalpha) [8] was replaced with methionine using the QuickChange site-directed mutagenesis kit (Stratagene). The site-directed mutagenesis primers are L97M For (5′ TAC ATC TGT AAG GTC GAA TTG ATG TAC CCA CCT CCA TAC TAC GTT 3′) and L97M Rev (5′ AAC GTA GTA TGG AGG TGG GTA CAT CAA TTC GAC CTT ACA GAT GTA 3′). The mutant construct was confirmed by DNA sequencing analysis. Using the exact same strategy as described above the methonine at position 97 of the non-N-glycosylated wild-type human CTLA-4 (hCTLA-4-Non-N-Gly in pwPICZalpha, which was constructed using the procedure previously described for pCTLA-4-Non-N-Gly in pwPICZalpha [8]) was replaced with leucine. The forward PCR primer is M97L For (5′ TAC ATT TGT AAG GTT GAG TTG TTG TAC CCA CCT CCA TAC TAC TTG 3′) and the reverse PCR primer is M97L Rev (5′ CAA GTA GTA TGG AGG TGG GTA CAA CAA CTC AAC CTT ACA AAT GTA 3′).
Protein expression and purification in P. pastoris and Western blot analysis were performed as previously described [8]. FACS binding and blocking analysis were performed as previously described for the wild-type porcine CTLA-4 [8] using a porcine CD80-expressing B-cell lymphoma line LCL13271 [9] and human CD80-expressing acute myelogenous leukemia cell line (Cat# CRL-2740, ATCC, Manassas, VA).
3. Results and discussion
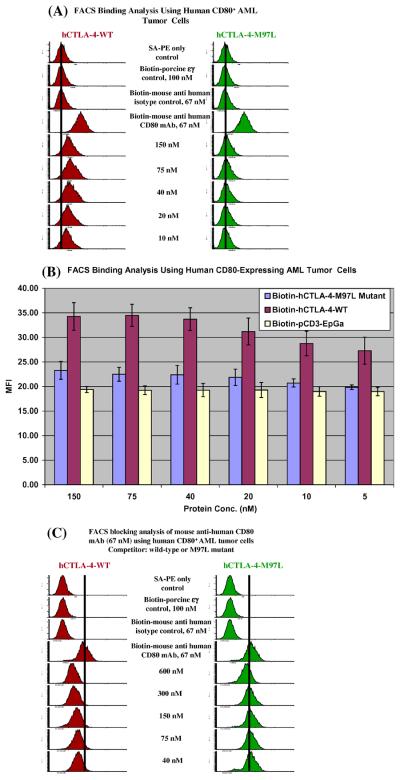
3.1. Soluble human CTLA-4-M97L mutant almost lost its binding to human CD80
The porcine CTLA-4 binding motif is LYPPPY and the soluble porcine CTLA-4 is unable to bind to human CD80 [8], therefore, we hypothesized that substituting M for L will decrease or abolish the binding ability of the soluble human CTLA-4 to human CD80. As shown in Fig. 1(A) and (B), the wild-type soluble human CTLA-4 bound to human CD80 very well. In contrast the human CTLA-4-M97L mutant almost lost its binding ability to human CD80 following the single amino acid mutation. These results demonstrated that M at position 97 is extremely critical in determining the ability of human CTLA-4 to bind to human CD80. To confirm the binding specificity, a human CD80 blocking assay was performed in which the unlabeled mutant or wild-type soluble human CTLA-4 were added to the cells first followed by addition of a biotinylated anti-human CD80 mAb. As shown in Fig. 1(C), the wild-type soluble human CTLA-4 blocked the binding of an anti-human CD80 mAb to human CD80 in a dose dependent manner. However the M97L mutant almost failed to block the binding of the same mAb suggesting that the single amino acid mutation caused almost loss in binding function to human CD80. The results from this blocking analysis confirmed that the binding of the wild-type human CTLA-4 is specific to human CD80.
Fig. 1.
Human CTLA-4-M97L mutant almost lost the ability to bind to human CD80. (A) Flow cytometry binding analysis of human CTLA-4 wild-type (left panel) and human CTLA-4-M97L mutant (right panel) to the human CD80-expressing acute myelogenous leukemia cell line. Immunofluorescence staining was performed using biotinylated wild-type, mutant or anti-human CD80 mAb as the primary stain and PE-conjugated streptavidin (PE-SA) for the second stain. PE-SA only; biotinylated protein control [porcine CD3εγ ectodomain single-chain fusion protein (pCD3εγ)] [10]; isotype control [biotinylated mouse (BALB/c) IgG1, κ, clone# MOPC-21, Biolegend] were included. (B) Bar graph presentation of the mean fluorescence intensity (MFI) from flow cytometry binding analysis data as described in (A). Different concentrations of biotinylated pCD3εγ were used as non-specific fluorescence control. Error bars are included based on the calculated standard deviation. The hCTLA-4-M97L mutant's ability to bind to human CD80 was significantly decreased compared to the hCTLA-4-WT, p < 0.001. (C) Human CD80 blocking analysis by flow cytometry of the wild-type human CTLA-4 (left panel) and human CTLA-4-M97L mutant (right panel) with anti-human CD80 mAb (clone 2D10) to the human CD80-expressing acute myelogenous leukemia cell line. The concentration of the competitor is indicated. All figures of (A)–(C) are representatives of multiple individual experiments.
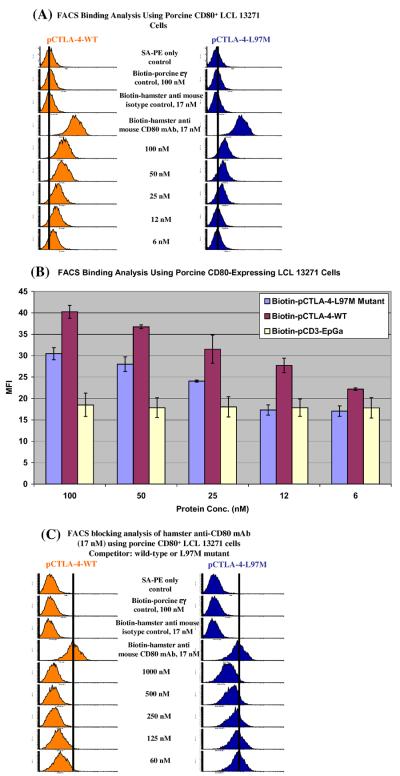
3.2. Soluble human CTLA-4-M97L mutant improved its binding ability to porcine CD80
We hypothesized that the soluble human CTLA-4-M97L mutant will bind to porcine CD80 stronger than the wild-type. As shown in Fig. 2(A) and (B), both wild-type and the M97L mutant bound to porcine CD80 very well with the mutant showing a stronger affinity. FACS blocking analysis of the binding for the hamster anti-mouse CD80 mAb to porcine CD80 demonstrated that both wild-type and the M97L mutant blocked the binding in a dose dependent manner (Fig. 2(C)).
Fig. 2.
Human CTLA-4-M97L mutant bound stronger to porcine CD80 compared to the wild-type. (A) Flow cytometry binding analysis of human CTLA-4 (left panel) and human CTLA-4-M97L mutant (right panel) to the porcine CD80-expressing B-cell lymphoma line LCL 13271. PE-SA only; biotinylated protein control (pCD3εγ) [10]; isotype control [biotinylated Armenian hamster IgG, clone# HTK888, Biolegend] were included. (B) Bar graph presentation of the MFI from flow cytometry binding analysis data as described in (A). Different concentrations of biotinylated pCD3εγ were used as non-specific fluorescence control. Error bars are included based on the calculated standard deviation. The hCTLA-4-M97L mutant conferred stronger binding to porcine CD80 following the M97L mutation. (C) CD80 blocking analysis by flow cytometry for human CTLA-4 (left panel) and human CTLA-4-M97L mutant (right panel) with anti-CD80 mAb (clone 16-10A1) to the porcine CD80-expressing B-cell lymphoma line LCL 13271. The concentration of the competitor is indicated. All data in this figure are representatives of multiple individual experiments.
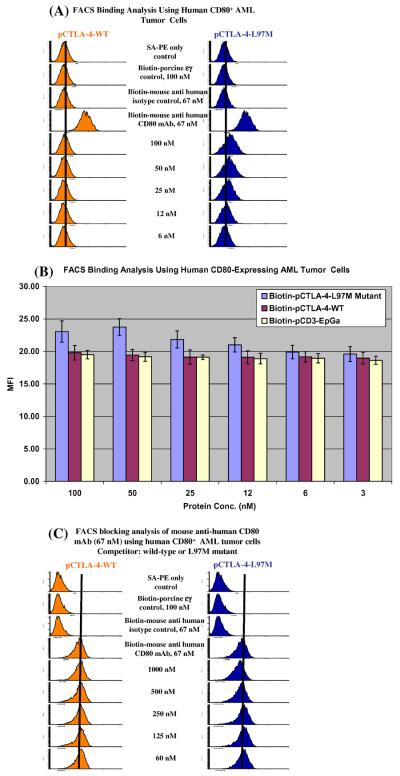
3.3. Soluble porcine CTLA-4-L97M mutant obtained weak binding ability to human CD80
Recently we have reported that the soluble porcine CTLA-4 was incapable of binding to human CD80 (left panel of Fig. 3(A) and [8]). However, as shown in right panel of Fig. 3(A) and (B), the soluble porcine CTLA-4-L97M mutant was able to bind to human CD80, although with very low affinity. Blocking analysis by flow cytometry also showed that the soluble porcine CTLA-4-L97M mutant was capable of, albeit very weakly, blocking the binding of an anti-human CD80 mAb to human CD80 (Fig. 3(C)). These results indicate that the soluble porcine CTLA-4-L97M mutant obtained the ability to very weakly bind to human CD80 following the single amino acid mutation.
Fig. 3.
Porcine CTLA-4-L97M mutant obtained weak binding to human CD80. (A) Flow cytometry binding analysis of porcine CTLA-4 (left panel) and porcine CTLA-4-L97M mutant (right panel) to the human CD80-expressing acute myelogenous leukemia cell line. PE-SA only; biotinylated protein control (pCD3εγ) [10]; isotype control [biotinylated mouse (BALB/c) IgG1, κ, clone# MOPC-21, Biolegend] were included. (B) Bar graph presentation of the MFI from flow cytometry binding analysis data from (A). Different concentrations of biotinylated pCD3εγ were used as non-specific fluorescence control. Error bars are included based on the calculated standard deviation. The pCTLA-4-L97M mutant conferred a weak but significant ability to bind to human CD80 compared to the pCTLA-4-WT, p < 0.05. (C) CD80 blocking analysis by flow cytometry for porcine CTLA-4 (left panel) and porcine CTLA-4-L97M mutant (right panel) with anti-human CD80 mAb (clone 2D10) to the human CD80-expressing acute myelogenous leukemia cell line. The concentration of the competitor is indicated. All figures in (A)–(C) are representatives of multiple individual experiments.
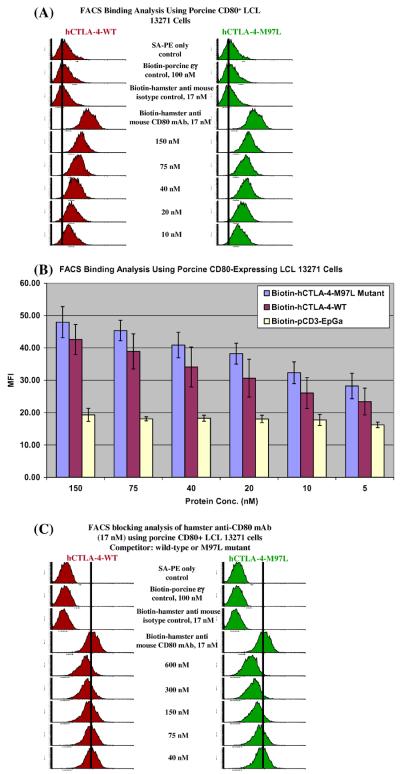
3.4. Soluble porcine CTLA-4-L97M mutant partially lost the binding affinity to porcine CD80
As shown in Fig. 4(A) and (B), the soluble porcine CTLA-4-L97M mutant bound to porcine CD80 with lower affinity than its wild-type. Blocking analysis by flow cytometry also showed a decreased ability of the L97M mutant to block the binding of a hamster anti-mouse CD80 mAb to porcine CD80 (Fig. 4(C)). These data demonstrated that the L at position 97 of porcine CTLA-4 is important for determining the binding to porcine CD80.
Fig. 4.
Porcine CTLA-4-L97M mutant partially lost the binding ability to porcine CD80. (A) Flow cytometry binding analysis of porcine CTLA-4 (left panel) and porcine CTLA-4-L97M mutant (right panel) to the porcine CD80-expressing B-cell lymphoma line LCL 13271. PE-SA only; biotinylated protein control (porcine CD3εγ) [10]; isotype control (biotinylated Armenian hamster IgG, clone # HTK888, Biolegend) were included. (B) Bar graph presentation of the MFI from flow cytometry binding analysis data as described in (A). Different concentrations of biotinylated pCD3εγ were used as non-specific fluorescence control. Error bars are included based on the calculated standard deviation. The pCTLA-4-L97M mutant's ability to bind to porcine CD80 was significantly decreased compared to the pCTLA-4-WT, p < 0.05. (C) Porcine CD80 blocking analysis by flow cytometry of porcine CTLA-4 (left panel) and porcine CTLA-4-L97M mutant (right panel) with anti-CD80 mAb (clone 16-10A1) to the porcine CD80-expressing B-cell lymphoma line LCL 13271. The concentration of the competitor is indicated. All figures in (A)–(C) are representatives of multiple individual experiments.
In summary the M at position 97 of human CTLA-4 is extremely critical for its binding to human CD80 and the L at position 97 of porcine CTLA-4 considerably influences the ability of porcine CTLA-4 bind to porcine CD80 (Table 1). These data suggest that human CTLA-4-based recombinant protein drugs can be utilized in porcine models. Porcine CTLA-4 and human CTLA-4-M97L mutant based recombinant protein drugs can be used to specifically block the direct presentation by donor antigen presenting cells in pig to nonhuman primate (or human in the future) xeno-transplantation. Human CTLA-4-M97L mutant based recombinant drugs will be more ideal as it is without immunogenicity to human being.
Table 1.
Cross-species binding of the soluble human CTLA-4 versus soluble porcine CTLA-4.
| Soluble human CTLA-4 |
Soluble porcine CTLA-4 |
|||
|---|---|---|---|---|
| WT | M97L | WT | L97M | |
| Human CD80 | ++++ | ≤+ | − | + |
| Porcine CD80 | ++++ | +++++ | ++++ | ++ |
Note: Binding percentage of the wild-type was presented as +++++ (125%), ++++ (100%), +++ (75%), ++ (50%), +(25%), −(no binding).
Acknowledgments
The work was supported in part by Dana Farber/Harvard Cancer Center Core development grant. We acknowledge the support, guidance and intensive manuscript review of Dr. David H. Sachs, Scientific Director of MGH-DF/HCC Recombinant Protein Expression and Purification Core. We thank Dr. Isabel Hanekamp and Sharon Germana for manuscript review.
References
- [1].Riha P, Rudd CE. CD28 co-signaling in the adaptive immune response. Self Nonself. 2010;1:231–40. doi: 10.4161/self.1.3.12968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Sansom DM. CD28, CTLA-4 and their ligands: who does what and to whom? Immunology. 2000;101:169–77. doi: 10.1046/j.1365-2567.2000.00121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bour-Jordan H, Esensten JH, Martinez-Llordella M, Penaranda C, Stumpf M, Bluestone JA. Intrinsic and extrinsic control of peripheral T-cell tolerance by costimulatory molecules of the CD28/B7 family. Immunol Rev. 2011;241:180–205. doi: 10.1111/j.1600-065X.2011.01011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Morton PA, Fu XT, Stewart JA, Giacoletto KS, White SL, Leysath CE, et al. Differential effects of CTLA-4 substitutions on the binding of human CD80 (B7-1) and CD86 (B7-2) J Immunol. 1996;156:1047–54. [PubMed] [Google Scholar]
- [5].Chikuma S, Abbas AK, Bluestone JA. B7-independent inhibition of T cell0s by CTLA-4. J Immunol. 2005;175:177–81. doi: 10.4049/jimmunol.175.1.177. [DOI] [PubMed] [Google Scholar]
- [6].Pentcheva-Hoang T, Egen JG, Wojnoonski K, Allison JP. B7-1 and B7-2 selectively recruit CTLA-4 and CD28 to the immunological synapse. Immunity. 2004;21:401–13. doi: 10.1016/j.immuni.2004.06.017. [DOI] [PubMed] [Google Scholar]
- [7].Vaughan AN, Malde P, Rogers NJ, Jackson IM, Lechler RI, Dorling A. Porcine CTLA4-Ig lacks a MYPPPY motif, binds inefficiently to human B7 and specifically suppresses human CD4+ T cell responses costimulated by pig but not human B7. J Immunol. 2000;165:3175–81. doi: 10.4049/jimmunol.165.6.3175. [DOI] [PubMed] [Google Scholar]
- [8].Peraino J, Zhang H, Hermanrud CE, Li G, Sachs DH, Huang CA, et al. Expression and purification of soluble porcine CTLA-4 in yeast Pichia pastoris. Protein Expr Purif. 2012;82:270–8. doi: 10.1016/j.pep.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cho PS, Lo DP, Wikiel KJ, Rowland HC, Coburn RC, McMorrow IM, et al. Establishment of transplantable porcine tumor cell lines derived from MHC-inbred miniature swine. Blood. 2007;110:3996–4004. doi: 10.1182/blood-2007-02-074450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Peraino JS, Hermanrud CE, Springett L, Zhang H, Li G, Srinivasan S, et al. Expression and characterization of recombinant soluble porcine CD3 ectodomain molecules: mapping the epitope of an anti-porcine CD3 monoclonal antibody 898H2-6-15. Cell Immunol. 2012;276:162–7. doi: 10.1016/j.cellimm.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]