Abstract
Ion pairing is one of the most fundamental chemical interactions and is essential for molecular recognition by biological macromolecules. From an experimental standpoint, very little is known to date about ion-pair dynamics in biological macromolecular systems. Absorption, infrared, and Raman spectroscopic methods were previously used to characterize dynamic properties of ion pairs, but these methods can be applied only to small compounds. Here, using NMR 15N relaxation and hydrogen-bond scalar 15N-31P J-couplings (h3JNP), we have investigated the dynamics of the ion pairs between lysine side-chain NH3+ amino groups and DNA phosphate groups at the molecular interface of the HoxD9 homeodomain-DNA complex. We have determined the order parameters and the correlation times for C-N bond rotation and reorientation of the lysine NH3+ groups. Our data indicate that the NH3+ groups in the intermolecular ion pairs are highly dynamic at the protein-DNA interface, which should lower the entropic costs for protein-DNA association. Judging from the C-N bond-rotation correlation times along with experimental and quantum-chemically derived h3JNP hydrogen-bond scalar couplings, it seems that breakage of hydrogen bonds in the ion pairs occurs on a sub-nanosecond timescale. Interestingly, the oxygen-to-sulfur substitution in a DNA phosphate group was found to enhance the mobility of the NH3+ group in the intermolecular ion pair. This can partially account for the affinity enhancement of the protein-DNA association by the oxygen-to-sulfur substitution, which is a previously observed but poorly understood phenomenon.
Introduction
Ion pairing is one of the most fundamental atomic interactions in both chemistry and biology. In solution, one distinguishes two major states of ion pairs: contact ion pairs (CIP) and solvent-separated ion pairs (SIP).1–4 In the CIP state, a cation and an anion are in direct contact with each other, whereas, in the SIP state, there is one or more solvent molecules between the electrostatically interacting cation and anion. In previous studies, ion-pair dynamics of small organic compounds were characterized experimentally using time-resolved absorption spectroscopy, infrared (IR) spectroscopy, and Raman spectroscopy.5–8 The transitions between the CIP and SIP states were found to occur on a ps – ns timescale for the ion pairs of these small organic compounds, and the free energy differences between their CIP and SIP states were found to be ~1–2 kcal/mol.5–9 Despite the wealth of information available for small organic compounds, very little is currently known about the dynamics of ion pairs tethered to biological macromolecules because their complexity renders application of the above-mentioned methods impractical.
The importance of ion pairs for protein function is evident from X-ray crystal structures. However, crystallographic data do not provide adequate information about dynamic properties of the ion pairs. For example, the presence of transitions between CIP and SIP states in macromolecules and their timescale are inaccessible by crystallography. Currently, ion-pair dynamics and its role in biological macromolecular systems is not understood by experimental means, which represents a bottleneck for understanding the relationship between structural dynamics and protein functions.
Here we present experimental data on ion-pair dynamics at protein-DNA interfaces. Formation of intermolecular ion pairs between protein and DNA along with release of counterions is the major driving force for many protein-DNA complexes (e.g. reviewed in Refs10–12). Our current work is based on the NMR methods developed recently for lysine (Lys) side-chain NH3+ groups.13–17 By analyzing the 15N relaxation of interfacial Lys NH3+ groups and hydrogen-bond scalar 15N-31P couplings (h3JNP) across the ion pairs between protein and DNA, we probe the ion-pair dynamics at the molecular interface in the HoxD9 homeodomain – DNA complex. 15N relaxation data provide motional information on reorientation and bond rotations of NH3+ groups,13 whereas h3JNP data reflecting orbital overlaps in hydrogen bonds provide unique information on hydrogen bonding.18–21 In the current case, sizable h3JNP couplings represent direct evidence for the CIP state of the intermolecular ion pairs. Owing to slower hydrogen exchange due to ion pairing, the NMR signals of the interfacial Lys side-chain NH3+ groups (Lys3, Lys55, and Lys57) in this complex can be clearly observed at 35 °C, a temperature at which signals from all the other NH3+ groups are broadened beyond detection.14 With this system, we demonstrate the highly dynamic nature of the interfacial ion pairs and discuss its biological significance. By comparing the ion-pair dynamics between normal phosphate (-O-PO2−-O-) and phosphorodithioate (-O-PS2−-O-) groups, this work also provides mechanistic insights into why the oxygen-to-sulfur substitution in DNA phosphate groups can enhance binding of proteins to DNA.22–26
Material and Methods
Sample preparation
2H/15N-labelel HoxD9 homeodomain (with C6S mutation) and unlabeled 24-bp DNA duplex containing a target sequence (TAATGG) were prepared as described previously.27–29 The protein was mixed with the 24-bp DNA duplex at a molar ratio of 1:1.4 (DNA in excess). Unmodified DNA strands were purchased from Integrated DNA Technologies. The DNA strand containing a phosphorodithioate group was synthesized on an Expedite 8909 DNA synthesizer with standard dA/dC/dG/dT-phosphoramidites (Glen Research) and dC-thiophosphoramidite (AM Biotechnologies / Glen Research). After reverse-phase HPLC purification via a 5’ dimethoxytrityl group, ESI-MS analysis confirmed oligo identity and incorporation of both sulfur atoms. Duplexes for NMR were prepared by annealing complementary strands and purified via anion-exchange chromatography to remove minor single-stranded DNA excess due to the uncertainty in measuring single strand concentrations. For fluorescence measurements, the DNA strand with a 5’ amine-linked rhodamine was purchased from Midland Certified Reagents and the annealed fluorescent DNA duplexes were purified using polyacrylamide gel electrophoresis. A 280-µl solution of 1.0 mM HoxD9 homeodomain–DNA complex, 20 mM NaCl, and 20 mM sodium phosphate (pH 5.8) was sealed into an inner tube of co-axial NMR tube with D2O separately sealed into the outer layer (to avoid isotopic isomers NDH2+, ND2H+, and ND3+).
NMR experiments
NMR experiments were performed using Bruker Avance III 600-MHz and 800-MHz spectrometers equipped with cryogenic probes. All 15N relaxation measurements were carried out at 35 °C. 15N longitudinal and transverse relaxation and 1H-15N heteronuclear NOE for Lys side-chain NH3+ groups were measured at 1H frequencies of 600 and 800 MHz as described in our previous work13. Order parameters S2axis, correlation times for C-N bond rotation, and reorientation of C-N bond were calculated from the relaxation data by nonlinear least-squares fitting as described previously13. In the fitting procedure, the heteronuclear NOE was calculated using Eq. 17 rather than Eq. 16 in Ref13, because relaxation rates of 4NzHzHz terms were found to be greater than 60 s−1 (which makes the two equations virtually identical). Uncertainties in calculated parameters were estimated with the Monte Carlo method. The effective rotational correlation time and diffusion anisotropy at 35 °C were determined from backbone 15N R1 and R2 rates as described30. 2-D H3(N)P spectra and 2-D spin-echo h3JNP–modulation constant-time HISQC spectra were recorded at 15 °C with a QCI (1H/13C/15N/31P) cryogenic probe at a 1H frequency of 600 MHz. Other details on the h3JNP analyses including DFT-based prediction are given in Supporting Information (SI).
Affinity measurements for homeodomain-DNA association
Affinities of HoxD9 homeodomain for the two 24-bp target DNA duplexes I and II were determined using the fluorescence anisotropy as a function of protein concentration (0.1 – 800 nM). Fluorescence arising from rhodamine conjugated at a 5’-terminus of DNA (4 nM) was monitored using an ISS PC-1 spectrofluorometer. Excitation and emission wavelengths used were 550 nm and 585 nm, respectively. The titration experiments were performed three times at 20°C using a buffer of 20 mM sodium acetate (pH 5.0) and 100 mM NaCl, in which aggregation of the free homeodomain was avoided.31
Results
Mobility of Lys NH3+ groups in ion pairs with DNA
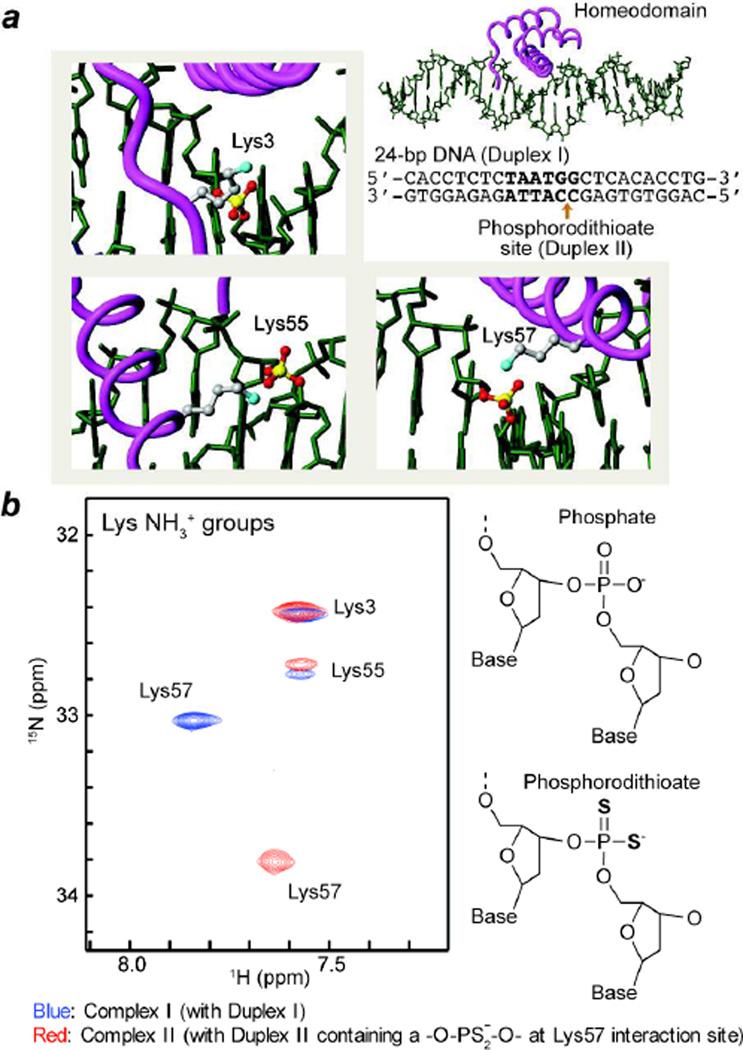
In this work, the ion-pair dynamics at protein-DNA interface were investigated for two DNA complexes of HoxD9 homeodomain: One with a normal 24-bp DNA duplex (‘Complex I’), the other with a modified 24-bp DNA duplex containing a phosphorodithioate group instead of the phosphate group that forms an ion pair with Lys57 (‘Complex II’) (Figure 1a). In Lys-selective two-dimensional (2D) heteronuclear in-phase single quantum coherence (HISQC) spectra14, 1H-15N cross-peaks of side-chain NH3+ groups of Lys3, Lys55, and Lys57 were observed for both complexes at pH 5.8 and 35 °C. As shown in Figure 1, the oxygen-to-sulfur substitution in the ion pair with Lys57 caused large perturbations in 1H and 15N resonances of Lys57 NH3+ group (|ΔδH|, 0.21 ppm; and |ΔδN|, 0.78 ppm). Signals from Lys3 and Lys55 NH3+ groups remained almost unchanged, which is not surprising because the location of the dithioation is specifically on the phosphate group interacting with Lys57. For the Lys NH3+ groups of Complexes I and II, we measured 15N relaxation rates R1, R2, and R(4NzHzHz) and 1H-15N nuclear Overhauser enhancement (NOE) as described previously13. From these relaxation data, we determined order parameters for the amino-group symmetry axis S2axis, C-N bond rotational correlation times τf, and reorientational correlation times τi for Lys NH3+ groups (Figure 2a). The results are summarized in Table I. Interestingly, the order parameters S2axis of Lys NH3+ groups interacting DNA were relatively low (<0.5), indicating that the ion pairs between Lys side-chain NH3+ and DNA phosphate groups are highly mobile. In previous work on ubiquitin, we found that Lys side-chain NH3+ groups involved in a hydrogen bond had order parameters S2axis < 0.5, an observation consistent with 1-µs molecular dynamics simulations13. Although one may expect that the hydrogen bonding and short-range electrostatic interactions in ion pairs may cause a higher degree of motional restriction, our data indicate that the ion pairs between protein and DNA are remarkably dynamic. The dynamic nature of the interfacial ion pairs should be favorable for protein-DNA association because it should minimize the loss of conformational entropy from immobilization of side chains upon the formation of the complex.
Figure 1.
(a) Intermolecular ion pairs involving Lys side-chain NH3+ groups (cyan spheres) and DNA phosphate or phosphorodithioate groups (yellow/red spheres) studied in this work. Two 24-bp DNA duplexes with the same sequence (the recognition site in bold) were used: Duplex I with no chemical modification; and Duplex II with a phosphorodithioate incorporated at the position of the ion pair with Lys57. (b) Overlaid HISQC spectra of the Lys NH3+ groups recorded for HoxD9 homeodomain complexes with Duplex I (blue) and with Duplex II (red) at 35 °C.
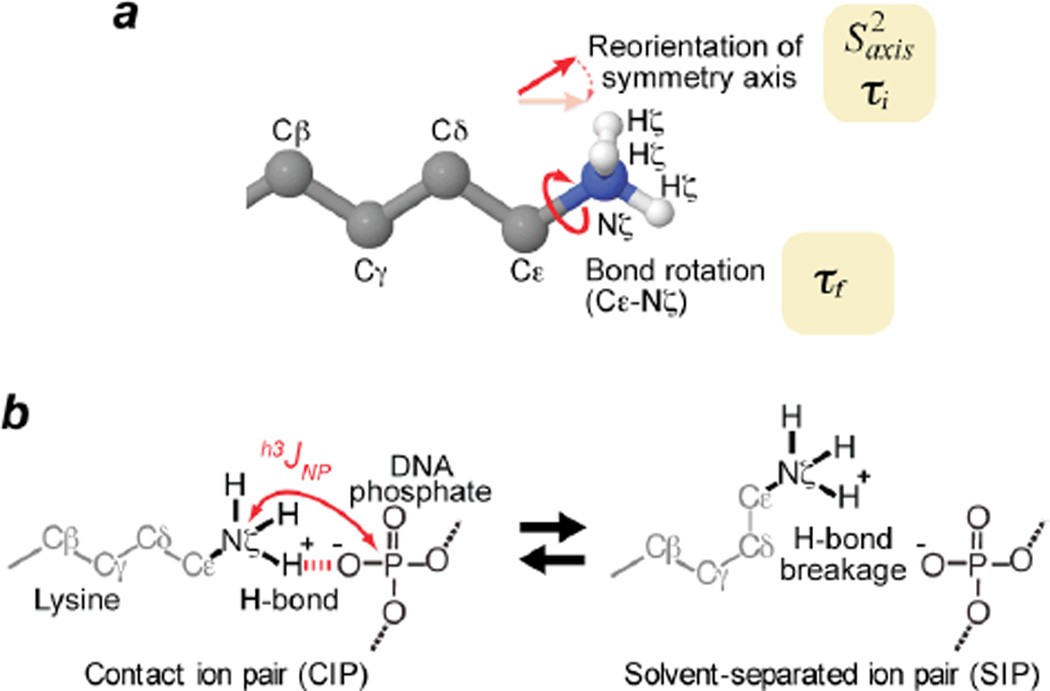
Figure 2.
Ion-pair dynamics involving Lys NH3+ and DNA phosphate group. (a) Reorientation of symmetry axis and C-N bond rotation studied by 15N relaxation for Lys side-chain NH3+ group. Determined values of the parameters describing these motions are shown in Table I. Based on the almost ideal tetrahedral geometry found for NH3+ groups,52 the order parameter for bond rotation was assumed to be 0.111.13 (b) Transitions between CIP and SIP states of intermolecular ion pairs. h3JNP coupling is present only for the CIP.
Table 1.
15N relaxation and dynamics parameters for the Lys side-chains NH3+ groups in the intermolecular ion pairs in the HoxD9 homeodomain-DNA complexes at 35 °C.
| Lys3 NH3+ | Lys55 NH3+ | Lys57 NH3+ | |
|---|---|---|---|
| Complex I a) | |||
| - 800 MHz - | |||
| 15N R1 (s−1) | 0.25 ± 0.01 | 0.43 ± 0.02 | 0.74 ± 0.01 |
| 15N R2,ini (s−1) c) | 0.98 ± 0.05 | 1.98 ± 0.21 | 2.27 ± 0.04 |
| 1H-15N NOE | −2.40 ± 0.03 | −2.72 ± 0.08 | −3.00 ± 0.03 |
| - 600 MHz - | |||
| 15N R1 (s−1) | 0.27 ± 0.01 | 0.59 ± 0.04 | 0.80 ± 0.02 |
| 1H-15N NOE | −3.07 ± 0.06 | −2.94 ± 0.17 | −3.17 ± 0.04 |
| - Dynamics -d) | |||
| S2axis | 0.22 ± 0.02 | 0.46 ± 0.06 | 0.48 ± 0.01 |
| τf (ps) | 3 ± 1 | 23 ± 2 | 113 ± 42 |
| τi (ps) | 222 ± 16 | 249 ± 42 | 82 ± 132 |
| Complex II b) | |||
| - 800 MHz - | |||
| 15N R1 (s−1) | 0.29 ± 0.02 | 0.43 ± 0.03 | 0.33 ± 0.02 |
| 15N R2,ini (s−1) c) | 1.16 ± 0.07 | 2.18 ± 0.26 | 1.76 ± 0.05 |
| 1H-15N NOE | −2.63 ± 0.06 | −2.72 ± 0.09 | −3.02 ± 0.13 |
| - 600 MHz - | |||
| 15N R1 (s−1) | 0.29 ± 0.02 | 0.54 ± 0.08 | 0.36 ± 0.02 |
| 1H-15N NOE | −3.24 ± 0.10 | −3.08 ± 0.20 | −3.08 ± 0.13 |
| - Dynamics -d) | |||
| S2axis | 0.26 ± 0.02 | 0.49 ± 0.06 | 0.39 ± 0.01 |
| τf (ps) | 8 ± 4 | 26 ± 12 | 27 ± 7 |
| τi (ps) | 163 ± 43 | 184 ± 87 | 36 ± 19 |
With Duplex I (see Figure 1).
With Duplex II that contains a phosphorodithioate group at the ion pair with Lys57.
The initial rate for intrinsically bi-exponential 15N transverse relaxation13 of NH3+.
Symbols are defined in Figure 2a. The molecular rotational correlation time and anisotropy were determined to be 10.6 ns and 2.1, respectively, from backbone 15N relaxation data.
Direct evidences for the CIP state
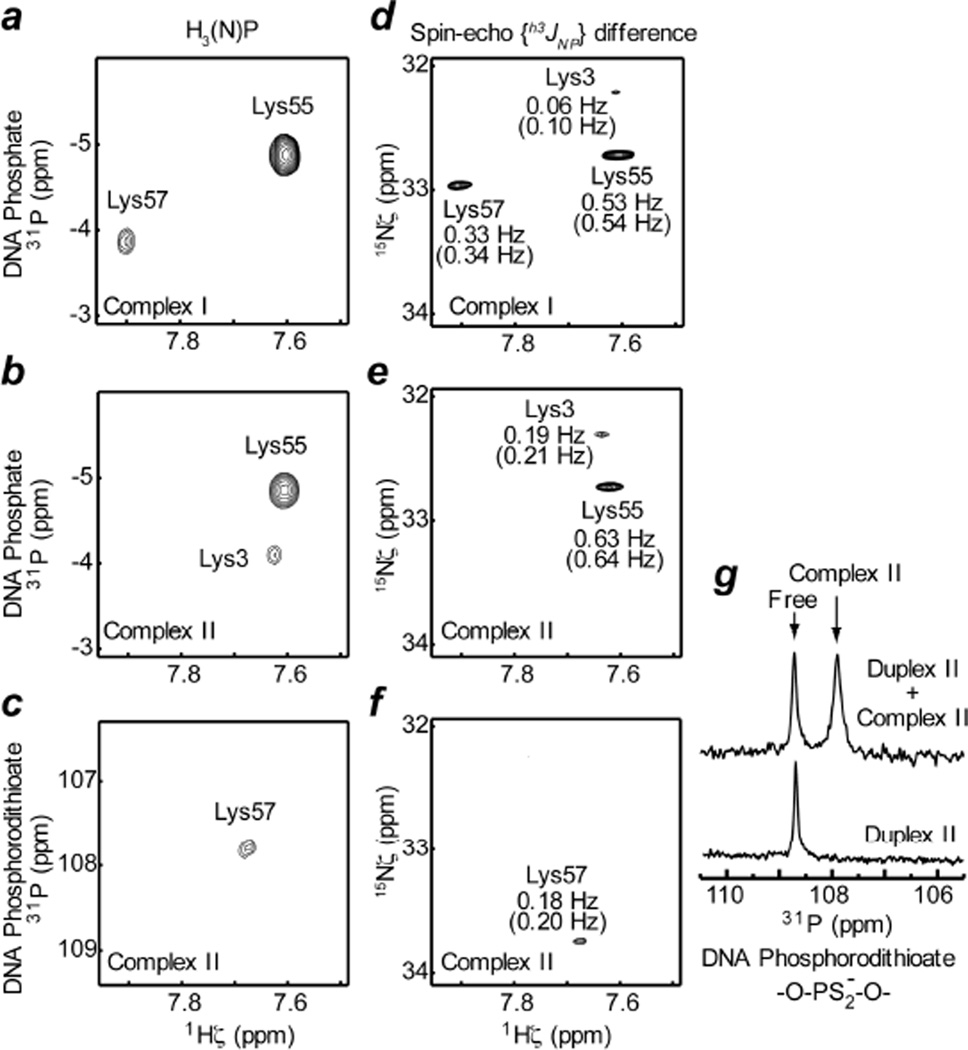
To obtain information on the CIP and SIP states, we studied hydrogen-bond scalar couplings h3JNP between Lys side-chain 15N and DNA 31P nuclei in the ion pairs at the protein-DNA interface. Note that the CIP state can exhibit h3JNP coupling whereas the SIP state cannot (Figure 2b). We designed the 2-D heteronuclear correlation H3(N)P experiment (the pulse sequence shown in Fig. S1A in SI) for observing the signals arising from coherence transfer via h3JNP coupling. Despite the small magnitude of this type of coupling (< 1 Hz), the very slow 15N transverse relaxation of NH3+ groups allowed us to observe the intermolecular correlation signals arising from h3JNP evolution by using relatively long periods for the coherence transfer.17 However, 31P longitudinal relaxation in the form of scalar relaxation of the second kind32 for 2NxPz and 2NyPz terms during the periods renders an additional loss in sensitivity (an effect akin to those described by Bax and coworkers33,34), which was estimated to be up to 10% in the current case (see SI for additional information). Spectra recorded by this experiment for Complexes I and II clearly show intermolecular 1H-31P correlation signals (Figures 3a, 3b, and 3c), indicating at least part-time presence of the CIP states for the ion pairs between Lys NH3+ and DNA phosphate / phosphorodithioate groups. Observed 31P chemical shifts for DNA phosphates in the ion pairs with Lys55 (−4.88 ppm) and Lys57 (−3.86 ppm) lie at the outer edges of typical 31P chemical shifts (−4.8 – −3.9 ppm) for B-form DNA,35 presumably due to ion pairing effects. 31P chemical shift of the phosphorodithioate group in the ion pair with Lys57 was found to be 117.78 ppm (Figure 3c), which was confirmed with 1D 31P NMR (Figure 3g).
Figure 3.
Hydrogen-bond scalar 15N-31P coupling h3JNP between 15N and 31P nuclei as evidence for the presence of CIP. (a, b, c) 2D H3(N)P spectra that give heteronuclear 1H-31P correlation cross peaks via h3JNP coupling. 31P chemical shifts are relative to trimethylphosphate (TMP). (d, e, f) Difference spectra recorded by the spin-echo h3JNP–modulation constant-time HISQC experiment. Measured absolute values of h3JNP are also indicated. Percentage errors were estimated to be less than 20%. Values in parentheses are h3JNP values taking account of the correction due to partial self-decoupling arising from 31P longitudinal relaxation (see SI). 31P carrier positions were set to −3 ppm for panels a, b, d, and e (for DNA phosphate); and 107 ppm for panels c and f (for DNA phosphorodithioate). Spectra in panels a and d were recorded for Complex I, and spectra in panels b, c, e, and f for Complex II. The pulse sequences and other relevant details are given in Figure S1 in SI. (g) Phosphorodithioate regions of 1D 31P spectra recorded for Duplex II in the free state and a mixture of Duplex II and Complex II. 31P chemical shift of the cross peak in the panel c agrees with 31P chemical shift of the signal from Complex II in the panel g.
To determine the absolute values of h3JNP coupling constants, we designed the spin-echo h3JNP-modulation constant-time HISQC experiment (the pulse sequence shown in Figure S1B in SI). Using signal intensities in the two sub-spectra recorded by this experiment, we determined the absolute values of the h3JNP coupling constants for the intermolecular ion pairs in Complex I and II to range from 0.06 to 0.63 Hz (Figures 3d, 3e, and 3f). The measured h3JNP constants are biased toward smaller values due to the partial self-decoupling effect36 arising from 31P longitudinal relaxation. But this effect can readily be corrected as described in SI. Differences between original and corrected values are less than 0.05 Hz. For Lys55 and Lys57 NH3+ groups, crystal structures of highly homologous protein-DNA complexes exhibit the CIP states of their ion pairs with DNA. For these NH3+ groups, we also predicted h3JNP coupling constants from the crystal structures by Density Functional Theory (DFT) method as described previously18. The results of the DFT calculations are given in Table S1 in SI. As shown in previous DFT studies (reviewed in Ref.19), a hydrogen bond scalar coupling is highly sensitive to subtle differences in hydrogen bonding geometry. This complicates the quantitative assessment of ion-pair dynamics accompanying geometrical change of hydrogen bonds. In fact, a large variation was found in h3JNP constants predicted from different crystal structures (Table S1 in SI). Nonetheless, the experimental h3JNP constants were comparable to the averages of the predicted constants, suggesting that the CIP state has a major presence in solution for the ion pairs of Lys55 and Lys57 NH3+ groups with a DNA phosphate group. The h3JNP coupling for phosphorodithioate – Lys57 NH3+ ion pair (0.18 Hz) was found to be significantly smaller than that for phosphate – Lys57 NH3+ ion pair (0.33 Hz). On the contrary, DFT calculations suggested that |h3JNP| of phosphorodithioate tends to be larger for the same hydrogen bonding geometry (see Tables S1, S2 in SI). The observed small |h3JNP| for the phosphorodithioate – Lys57 NH3+ ion pair could be partly due to the higher degree of dynamics that our 15N relaxation data indicate (see below).
Hydrogen-bonding dynamics of the intermolecular ion pairs
Our experimental and computational h3JNP coupling data suggest a major presence of CIP for the intermolecular ion pairs involving Lys55 and Lys57 NH3+ groups. This is consistent with our finding that C-N bond-rotation correlation times τf of these NH3+ groups are clearly slower than that of Lys3 NH3+ group (Table I). Hydrogen bonds with phosphate/phosphorodithioate in the CIP state should render slower bond rotations of Lys55 and Lys57 NH3+ groups. Transient breakage of the hydrogen bonds can be accompanied by rotational permutations of hydrogen atoms within the CIP state or a transition to SIP. Given the major presence of CIP, it is likely that the timescale of the transient hydrogen bond breakage for Lys55 and Lys57 NH3+ groups is faster than or comparable to their bond-rotation correlation times τf. In fact, such a relation between τf and hydrogen bonding lifetimes for Lys NH3+ groups in ubiquitin was previously found in molecular dynamics simulations.13 The τf and τi data in the current study suggest that the transient breakage of hydrogen bonds between Lys NH3+ and DNA phosphate / phosphorodithioate groups occurs on a sub-nanosecond timescale.
The faster C-N bond rotation of Lys3 NH3+ group implicates a higher population of the SIP state with no hydrogen bonds with DNA. The τf values for Lys3 NH3+ group in Complexes I and II are smaller than those (~ 25 ps) for Lys NH3+ groups that do not form any hydrogen bond in ubiquitin at 2 °C,13 but the higher temperature (35 °C) in the current study can explain this discrepancy. The Lys3 side chain is disordered in all of the crystal structures of homeodomain-DNA complexes except one (PDB 1IG7). The only structure available for the Lys3 side chain shows that the ion pair between Lys3 and DNA is in the SIP state with an Nζ…OP distance of 5.4 Å, although simple rotation about χ3 can reduce this distance to ~3.0 Å. On the other hand, weak h3JNP coupling was observed for Lys3 (Figures 3b, 3d and 3e), which indicates at least a part-time presence of the CIP. The small |h3JNP|, short τf, and small S2axis collectively suggest a low population of the CIP state for the ion pair of Lys3 NH3+ group. Thus the equilibrium between the CIP and SIP states (Figure 2b) for Lys3 appears to be shifted toward the SIP, while the corresponding equilibria for Lys55 and Lys57 appear to be shifted toward the CIP.
Impact of the oxygen-to-sulfur substitution on the ion-pair dynamics
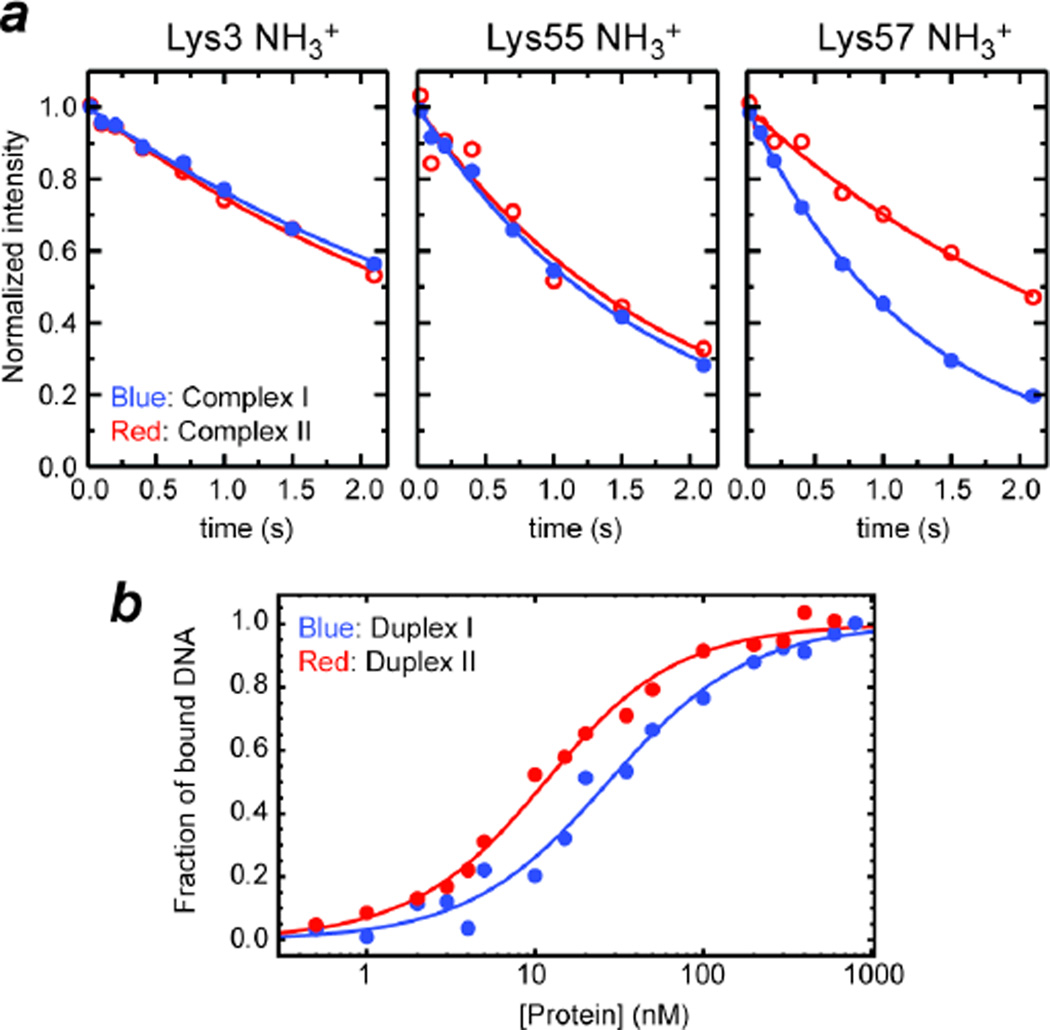
Our S2axis data indicate that Lys57 NH3+ group in the ion pair with a phosphorodithioate group is more mobile than in the ion pair with a phosphate group (Table I). The higher mobility is consistent with the two facts that 1) the effective ionic radius of sulfur (1.84 Å) is substantially larger than that of oxygen (1.40 Å), and that 2) the potential energy surface for an H•••S hydrogen bond is flatter than that for an H•••O hydrogen bond.37 Due to the flatter energy surface, on which a slight deviation from ideal hydrogen bond geometry causes only a marginal increase in enthalpy, a donor of a H•••S hydrogen bond should be able to take up a wider space without significant enthalpic loss. Order parameters for fixed-length bond vectors provide information only on orientational distributions, but do not provide the breadth of positional distributions or any information on translational motions.38 By assuming a motional model for the bond vectors, however, qualitative information on conformational entropy can be obtained from such order parameters.39–42 Although the entropy for a whole side chain can be empirically estimated for relatively short amino-acid side chains from order parameters measured only at the tip of the side chain, such relationships are less well-defined for longer amino-acid side chains such as lysine and arginine.40,43 Based on the experimental order parameters S2axis for Lys57 NH3+ groups in Complexes I and II along with Yang and Kay’s equation for the diffusion-in-a-cone model (i.e. entropic difference equals kB ln{[3 − (1 + 8Saxis,a)1/2 /[3 − (1 + 8Saxis,b)1/2]}, where kB is the Boltzmann constant),42 the increase in entropy for the symmetry axis of the NH3+ group by the oxygen-to-sulfur substitution was estimated to be 0.4 cal mol−1 K−1. We should mention that this is a rather crude estimate because the diffusion-in-a-cone model is likely to be too simplistic for the ion pairs undergoing the CIP-SIP transitions. Interestingly, the C-N bond rotation correlation time τf is significantly faster for Lys57 NH3+ group in the ion pair with a phosphorodithioate group. This causes substantially different 15N R1 rates of Lys57 NH3+ groups in Complexes I and II (Figure 4a; see also Table I). As considered previously for CH3 groups,44 the rotational entropy (Srot) of an NH3+ group is indirectly related to the bond rotation kinetics because the probability distribution function for rotation depends on the energy barrier for rotation. Provided that the Eyring equation in transition state theory is applicable to NH3+ rotation, experimental τf data along with the analytical expression44 of Srot suggest that NH3+ rotational entropy increases by 0.4 cal mol−1 K−1 upon the oxygen-to-sulfur substitution in the interacting DNA phosphate group. The overall increase of entropy (i.e. reorientational + rotational) by mobilizing the NH3+ group is thus estimated to be ~0.8 cal mol−1 K−1.
Figure 4.
Influence of the oxygen-to-sulfur substitution in the DNA phosphate group interacting with Lys57. (a) 15N longitudinal relaxation of Lys side-chain NH3+ groups at the protein-DNA interfaces of Complexes I and II. Lys57 NH3+ group exhibited substantially different relaxation upon the oxygen-to-sulfur substitution in the DNA phosphate group. 15N relaxation of the other NH3+ groups (interacting with normal phosphate group in both complexes) was virtually unaffected. (b) Binding isotherm as monitored with fluorescence arising from a rhodamine attached to the 5’-terminus of DNA. Fractions of bound DNA calculated from the fluorescence anisotropy data at varying concentrations of HoxD9 homeodomain are plotted for Duplexes I and II.
This mobilization of the ion pair can at least partially account for affinity enhancement of the protein-DNA association by the oxygen-to-sulfur substitution. The affinity of Duplex II was found to be 3-fold higher than that of Duplex I (Figure 4b). By fluorescence anisotropy-based titration experiments, the dissociation constants (Kd) were determined to be 31 ± 6 nM for Complex I and 12 ± 4 nM for Complex II. From the Kd data, the difference between the binding free energies (ΔΔG = ΔGII − ΔGI) is calculated to be −0.6 kcal mol−1. The major contributor to this ΔΔG should be the entropy term (i.e. −TΔΔS), because recent theoretical quantum chemical studies showed that the enthalpy ΔH for H•••S hydrogen bonds is slightly smaller than that for H•••O hydrogen bonds.37,45 In fact, the entropic term for mobilization of the NH3+ group alone corresponds to −0.24 kcal mol−-1 at 20°C. If the entropic effect for the other parts of the ion pair (e.g. methylene groups of the Lys side chain) is comparable to this, the observed ΔΔG can be explained in terms of the enhanced ion-pair dynamics, though other factors (e.g. different desolvation energies) may also contribute.
Discussion
Based on the counterion-condensation theory,46,47 macroscopic effects of counterion release due to ion pairing between protein and DNA were extensively studied by thermodynamic means in previous studies (e.g. reviewed in Refs10–12). On the other hand, the entropic effects for intermolecular ion pairs between protein side-chain and DNA phosphate groups was previously unknown by experimental means. Our experimental data on the ion-pair dynamics at the protein-DNA interface suggest that the entropic loss for basic side chains upon ion-pair formation is relatively small owing to remaining mobility as observed for the NH3+ groups in the ion pairs with DNA. Comparison of our results for Complexes I and II suggests that modulation of the ion-pair dynamics can directly impact binding affinity. It is worth mentioning that the importance of remaining mobility of DNA phosphate groups in protein-DNA recognition was suggested based on 1-D 31P NMR data two decades ago.48 The ion-pair dynamics seems entropically important for protein-DNA association.
The highly dynamic nature of the ion pairs between protein and DNA may also be kinetically advantageous. Due to extremely high DNA concentration in the nucleus and micromolar affinity for nonspecific DNA, transcription factors are mostly bound to nonspecific sites on chromosomal DNA in the search process before reaching their target DNA sites in vivo. In the case of the homeodomain, the structures of the nonspecific DNA complexes are very similar to that of the specific DNA complex 27,49. A nonspecific complex may form up to 6 CIPs involving Lys and Arg side chains as seen in crystal structures of the specific complexes. All CIPs with DNA need to be broken each time the protein moves from one nonspecific DNA site to another. Rapid CIP-SIP transitions should shorten the time necessary to break all CIPs, and thereby may facilitate the protein’s sliding on nonspecific DNA and to efficiently locate the target sites. If the mean lifetime of CIP is ~10−10 s and the population of SIP is ~10% (which corresponds to ~1.4 kcal/mol for a free energy difference between CIP and SIP, a reasonable value for small organic compounds5–9) for each ion pair, the time necessary for all 6 CIPs simultaneously to be broken is estimated to be ~10−5 s. This is compatible with the timescale of proteins’ sliding on DNA. One-dimensional coefficients for the sliding process, which were directly observed by single-molecule techniques,50,51 are typically between 10−3 and 10−1 µm2 s−1. These data together with a distance of 3.4 Å along the DNA axis for each sliding step from a nonspecific DNA site to an adjacent site suggest that the mean times for individual sliding steps are in the range of 10−6 – 10−4 s.
Conclusion
Our present work has provided the first experiment-based perspective of the ion-pair dynamics in a biological macromolecular system. Despite the simultaneous presence of the short-range electrostatic interaction and hydrogen bonding, the ion pairs between protein and DNA are highly dynamic on the sub-nanosecond timescale. The bond-rotation and reorientation correlation times suggest that breakage of hydrogen bonds in the CIP states of Lys NH3+-phosphate/phosphorodithioate ion pairs occurs on a sub-nanosecond timescale. The high degree of flexibility of the intermolecular ion pairs, as reflected in the low order parameters of the Lys side chain cations, should minimize the side-chain conformational entropic costs for protein-DNA association. The oxygen-to-sulfur substitution in DNA phosphate makes the intermolecular ion pair more dynamic, which seems to contribute to the affinity enhancement by this type of substitution. The experimental data on the ion-pair dynamics in the relatively small protein-DNA complex may allow the computational biology community to validate and even improve the molecular dynamics protocols and force fields relevant for the simulation of ion pairs. This may ultimately lead to advances in the engineering of proteins that target specific DNA/RNA binding sites, the design of oligonucleotide-based scaffold structures, and the improvement of in silico screening of drugs involving ion pairs.
Supplementary Material
Acknowledgments
This work was supported by Grants MCB-0920238 (to J.I.) and MCB-0918362 (to R.B.) from the National Science Foundation and by Grant 12BGIA8960032 from the American Heart Association (to J.I.). Additional support was provided by the Welch Foundation (AU-1296), National Cancer Institute (U54CA151668) and National Heart Lung and Blood Institute (HHSN268201000037C) (to D.G.G.). We thank Dr. Tianzhi Wang for technical support of UTMB’s NMR instruments; and Drs. Montgomery Pettitt, James Stivers, David Volk and John Ladbury for useful discussion.
Footnotes
Supporting Information
NMR pulse sequences used for investigating hydrogen-bond coupling constants h3JNP for lysine NH3+-DNA phosphate/phosphorodithoate ion pairs; Effect of 31P longitudinal relaxation on h3JNP coupling analysis; and DFT-based prediction of h3JNP constants from crystal structures. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Collins KD. Biophys J. 1997;72:65. doi: 10.1016/S0006-3495(97)78647-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Macchioni A. Chem Rev. 2005;105:2039. doi: 10.1021/cr0300439. [DOI] [PubMed] [Google Scholar]
- 3.Marcus Y, Hefter G. Chem Rev. 2006;106:4585. doi: 10.1021/cr040087x. [DOI] [PubMed] [Google Scholar]
- 4.Szwarc M. Acc Chem Res. 1969;2:87. [Google Scholar]
- 5.Simon JD, Peters KS. J Am Chem Soc. 1982;104:6542. [Google Scholar]
- 6.Masnovi JM, Kochi JK. J Am Chem Soc. 1985;107:7880. [Google Scholar]
- 7.Yabe T, Kochi JK. J Am Chem Soc. 1992;114:4491. [Google Scholar]
- 8.Peters KS, Li BL. J Phys Chem. 1994;98:401. [Google Scholar]
- 9.Lü JM, Rosokha SV, Lindeman SV, Neretin IS, Kochi JK. J Am Chem Soc. 2005;127:1797. doi: 10.1021/ja043998x. [DOI] [PubMed] [Google Scholar]
- 10.Record MT, Jr, Ha JH, Fisher MA. Methods Enzymol. 1991;208:291. doi: 10.1016/0076-6879(91)08018-d. [DOI] [PubMed] [Google Scholar]
- 11.Record MT, Jr, Zhang W, Anderson CF. Adv Protein Chem. 1998;51:281. doi: 10.1016/s0065-3233(08)60655-5. [DOI] [PubMed] [Google Scholar]
- 12.Privalov PL, Dragan AI, Crane-Robinson C. Nucleic Acids Res. 2011;39:2483. doi: 10.1093/nar/gkq984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esadze A, Li DW, Wang T, Brüschweiler R, Iwahara J. J Am Chem Soc. 2011;133:909. doi: 10.1021/ja107847d. [DOI] [PubMed] [Google Scholar]
- 14.Iwahara J, Jung YS, Clore GM. J Am Chem Soc. 2007;129:2971. doi: 10.1021/ja0683436. [DOI] [PubMed] [Google Scholar]
- 15.Takayama Y, Castañeda CA, Chimenti M, García-Moreno B, Iwahara J. J Am Chem Soc. 2008;130:6714. doi: 10.1021/ja801731g. [DOI] [PubMed] [Google Scholar]
- 16.Takayama Y, Sahu D, Iwahara J. J Magn Reson. 2008;194:313. doi: 10.1016/j.jmr.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zandarashvili L, Li DW, Wang T, Brüschweiler R, Iwahara J. J Am Chem Soc. 2011;133:9192. doi: 10.1021/ja202219n. [DOI] [PubMed] [Google Scholar]
- 18.Czernek J, Brüschweiler R. J Am Chem Soc. 2001;123:11079. doi: 10.1021/ja011618r. [DOI] [PubMed] [Google Scholar]
- 19.Grzesiek S, Cordier F, Jaravine V, Barfield M. Prog in NMR Spect. 2004;45:275. [Google Scholar]
- 20.Löhr F, Mayhew SG, Rüterjans H. J Am Chem Soc. 2000;122:9289. [Google Scholar]
- 21.Mishima M, Hatanaka M, Yokoyama S, Ikegami T, Walchli M, Ito Y, Shirakawa M. J Am Chem Soc. 2000;122:5883. [Google Scholar]
- 22.Cummins L, Graff D, Beaton G, Marshall WS, Caruthers MH. Biochemistry. 1996;35:8734. doi: 10.1021/bi960318x. [DOI] [PubMed] [Google Scholar]
- 23.King DJ, Ventura DA, Brasier AR, Gorenstein DG. Biochemistry. 1998;37:16489. doi: 10.1021/bi981780f. [DOI] [PubMed] [Google Scholar]
- 24.King DJ, Bassett SE, Li X, Fennewald SA, Herzog NK, Luxon BA, Shope R, Gorenstein DG. Biochemistry. 2002;41:9696. doi: 10.1021/bi020220k. [DOI] [PubMed] [Google Scholar]
- 25.Marshall WS, Caruthers MH. Science. 1993;259:1564. doi: 10.1126/science.7681216. [DOI] [PubMed] [Google Scholar]
- 26.Yang X, Bassett SE, Li X, Luxon BA, Herzog NK, Shope RE, Aronson J, Prow TW, Leary JF, Kirby R, Ellington AD, Gorenstein DG. Nucleic Acids Res. 2002;30:e132. doi: 10.1093/nar/gnf132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iwahara J, Clore GM. Nature. 2006;440:1227. doi: 10.1038/nature04673. [DOI] [PubMed] [Google Scholar]
- 28.Iwahara J, Clore GM. J Am Chem Soc. 2006;128:404. doi: 10.1021/ja056786o. [DOI] [PubMed] [Google Scholar]
- 29.Sahu D, Clore GM, Iwahara J. J Am Chem Soc. 2007;129:13232. doi: 10.1021/ja074604f. [DOI] [PubMed] [Google Scholar]
- 30.Tjandra N, Feller SE, Pastor RW, Bax A. J Am Chem Soc. 1995;117:12562. [Google Scholar]
- 31.Dragan AI, Li Z, Makeyeva EN, Milgotina EI, Liu Y, Crane-Robinson C, Privalov PL. Biochemistry. 2006;45:141. doi: 10.1021/bi051705m. [DOI] [PubMed] [Google Scholar]
- 32.Abragam A. The Principle of Nuclear Magnetism. Oxford: Carendon Press; 1961. p. 264. [Google Scholar]
- 33.Kuboniwa H, Grzesiek S, Delaglio F, Bax A. J Biomol Nmr. 1994;4:871. doi: 10.1007/BF00398416. [DOI] [PubMed] [Google Scholar]
- 34.Vuister GW, Bax A. J Am Chem Soc. 1993;115:7772. [Google Scholar]
- 35.Gorenstein DG. Chem Rev. 1994;94:1315. [Google Scholar]
- 36.Harbison GS. J Am Chem Soc. 1993;115:3026. [Google Scholar]
- 37.Wennmohs F, Staemmler V, Schindler M. J Chem Phys. 2003;119:3208. [Google Scholar]
- 38.Iwahara J, Clore GM. J Am Chem Soc. 2010;132:13346. doi: 10.1021/ja1048187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akke M, Brüschweiler R, Palmer AG. J Am Chem Soc. 1993;115:9832. [Google Scholar]
- 40.Li DW, Brüschweiler R. J Am Chem Soc. 2009;131:7226. doi: 10.1021/ja902477s. [DOI] [PubMed] [Google Scholar]
- 41.Li ZG, Raychaudhuri S, Wand AJ. Protein Sci. 1996;5:2647. doi: 10.1002/pro.5560051228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang DW, Kay LE. J Mol Biol. 1996;263:369. doi: 10.1006/jmbi.1996.0581. [DOI] [PubMed] [Google Scholar]
- 43.Trbovic N, Cho JH, Abel R, Friesner RA, Rance M, Palmer AG., 3rd J Am Chem Soc. 2009;131:615. doi: 10.1021/ja806475k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krishnan M, Smith JC. J Am Chem Soc. 2009;131:10083. doi: 10.1021/ja901276n. [DOI] [PubMed] [Google Scholar]
- 45.Howard DL, Kjaergaard HG. Phys Chem Chem Phys. 2008;10:4113. doi: 10.1039/b806165c. [DOI] [PubMed] [Google Scholar]
- 46.Manning GS. Q Rev Biophys. 1978;11:179. doi: 10.1017/s0033583500002031. [DOI] [PubMed] [Google Scholar]
- 47.Record MT, Anderson CF, Lohman TM. Q Rev Biophys. 1978;11:103. doi: 10.1017/s003358350000202x. [DOI] [PubMed] [Google Scholar]
- 48.Karslake C, Botuyan MV, Gorenstein DG. Biochemistry. 1992;31:1849. doi: 10.1021/bi00121a038. [DOI] [PubMed] [Google Scholar]
- 49.Iwahara J, Zweckstetter M, Clore GM. Proc Natl Acad Sci U S A. 2006;103:15062. doi: 10.1073/pnas.0605868103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blainey PC, Luo G, Kou SC, Mangel WF, Verdine GL, Bagchi B, Xie XS. Nat Struct Mol Biol. 2009;16:1224. doi: 10.1038/nsmb.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gorman J, Greene EC. Nat Struct Mol Biol. 2008;15:768. doi: 10.1038/nsmb.1441. [DOI] [PubMed] [Google Scholar]
- 52.Lehmann MS, Koetzle TF, Hamilton WC. J Am Chem Soc. 1971;94:2657. doi: 10.1021/ja00763a016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.