Abstract
Immune responses to tumor-associated antigens (TAs) are often detectable in tumor-bearing hosts, but they fail to eliminate malignant cells or prevent the development of metastases. Patients with cancer generate robust immune responses to infectious agents (bacteria and viruses) perceived as a “danger signal” but only ineffective weak responses to TAs, which are considered as “self.” This fundamental difference in responses to self versus nonself is further magnified by the ability of tumors to subvert the host immune system. Tumors induce dysfunction and apoptosis in CD8+ antitumor effector cells and promote expansion of regulatory T cells, myeloid-derived suppressor cells, or both, which downregulate antitumor immunity, allowing tumors to escape from the host immune system. The tumor escape is mediated by several distinct molecular mechanisms. Recent insights into these mechanisms encourage expectations that a more effective control of tumor-induced immune dysfunction will be developed in the near future. Novel strategies for immunotherapy of cancer are aimed at the protection and survival of antitumor effector cells and also of central memory T cells in the tumor microenvironment.
Keywords: Cancer, immunity, tumor escape, immune suppression, effector T cells
Evidence accumulated over the last few years convincingly shows that the host immune system is involved in cancer development and progression, as well as control of metastasis. The presence of antitumor cellular responses, humoral responses, or both to tumor-associated antigens (TAs) has been observed in many, but not all, patients with cancer.1,2 The evidence for such pre-existing antitumor immunity in patients with cancer confirms that the tumor-bearing host is capable of mounting an immune response to TAs. Tumor progression from a single transformed cell to a mass of malignant cells is a multistep process involving a series of genetic changes occurring in human subjects over a period of months or years and culminating in the established tumor.3 During this period, neither the host immune system nor the developing tumor are idle: those newly emerging tumor cells that are recognized by the immune system are eliminated only to be replaced by genetic tumor variants resistant to immune intervention and giving rise to a heterogenous population of malignant cells found in any tumor. Tumors are genetically unstable, and the emergence of new genetic variants, which is responsible for the tumor heterogeneity, ensures that the tumor survives in the face of the host immune system. Only the tumor cells that manage to avoid recognition escape and survive, whereas those that are recognized by the immune system are eliminated as soon as they arise. The tumor development involves a prolonged series of checks and balances between the host attempting to curtail tumor growth and the tumor benefiting from genetic changes, altering its microenvironment and avoiding immune elimination. Thus the tumor becomes resistant to immune effector cells.
The interactions between the host and the tumor have been referred to as “immune surveillance,” a concept that originated many years ago with F. M. Burnett and that introduced his vision of a vigilant host immune system able to spot, recognize, and eliminate tumor cells. A modern version of the immune surveillance theory not only emphasizes the ability of the host immune system to recognize and destroy tumor cells but also its contribution to “immune selection” of resistant tumor variants. Thus the “immune editing” hypothesis2,4 has been advanced to suggest that by means of elimination of tumor cells sensitive to immune intervention, the host immune system edits for survival of tumors that become resistant to immune cells. An alternative hypothesis allows for the progressing tumor to develop immunosuppressive mechanisms that will thwart any attempt of immune tumor elimination and in effect will induce a state of tumor-specific tolerance.5 In the first instance the immune system initiates the selection of resistant tumor variants, and in the second the tumor becomes a perpetrator of immune unresponsiveness. Central to the paradigms of immune selection or immune editing and immune suppression is the premise that the tumors acquiring new mutations are able to avoid immune intervention and are capable of both escaping and disabling the host immune system. Neither of the 2 hypotheses has been completely accepted today, and there are those who believe that tumors progress because of the genetic instability and others who favor tumor-specific tolerance of the immune system, which enables the tumor to take advantage of the tissue microenvironment regardless of the immune system and benefit from it. This controversy regarding the significance of the immune system in tumor development and progression underscores the complexity of interactions between the tumor and the immune cells. It surmises that these interactions might be bidirectional, are influenced by the local microenvironment, and not infrequently might result in demise not of the tumor but of tumor-reactive immune cells.
In this chapter the nature and components of the host immune response against tumors will be discussed, including the reasons for the failure of the immune system to contain tumor growth and metastasis. It is this latter aspect of the immunobiology of human malignancies that will be emphasized, largely because it directly affects cancer immunotherapy. A relatively recent realization that tumors have devised multiple and remarkably effective mechanisms for disarming the host immune system has opened a way for the introduction of novel therapeutic strategies aimed at eliminating tumor escape. If the tricks tumors use for protection from immune intervention by the host are responsible for their progression, then it could be surmised that a limited success of current immune therapies for cancer can be reversed by therapies that target the escape mechanisms, and because these escape mechanisms might be unique for each tumor rather than generalized, the future challenge will be to identify the “immunologic signature” of each tumor and then use selective therapies to eliminate the tricks and restore vigorous antitumor immunity.
TUMOR PROGRESSION AND THE HOST IMMUNE RESPONSE
There are several lines of evidence that point to an early, as well as late, involvement of the immune system in tumor development. Early tumor lesions, and even premalignant foci, such as melanocytic nevi, are frequently infiltrated with hematopoietic cells, including lymphocytes, macrophages, and occasionally granulocytes.6,7 The presence of immune cells in the tumor at later stages of development (ie, the abundance of tumor-infiltrating lymphocytes [TILs]) has been associated with improved patient survival in several early studies (reviewed in Whiteside8). More recently, studies by Fridman’s group performed a comprehensive multivariate analysis of cellular interactions in the tumor microenvironment based on the type, density, localization, and function of immune cells present within human colorectal cancer and demonstrated that immune reactivity at the tumor site influences clinical outcome.9–11 Thus increased densities of T-cell infiltrates with a high proportion of CD8+ T cells within primary colorectal carcinomas were associated with a significant protection against tumor recurrence.11 Furthermore, the same group also showed that coexpression of genes mediating cytotoxicity and TH1 adaptive immune responses accurately predicted survival in patients with colorectal carcinoma independently of the metastatic status.12 In aggregate these multiparameter analyses of tumor-infiltrating cells in situ suggest that immune cells can and indeed often do play a role in tumor control but that both intrinsic and extrinsic factors in the tumor microenvironment alter the balance required for optimal control.12
In many patients with cancer, it is possible to expand in culture and in vitro test functions of tumor-specific cytolytic T lymphocytes (CTLs) from the peripheral blood or TILs.8 This finding, which has been reproduced in many laboratories, suggests that precursors of such CTLs exist in the circulation or at the tumor site in patients with cancer and can be induced to proliferate when autologous dendritic cells (DCs) pulsed with relevant tumor epitopes and used as antigen-presenting cells (APCs). More recent experiments, using tetramers and flow cytometry, have directly demonstrated the presence of tumor peptide–specific T cells in the circulation of patients with cancer.1,13,14 Furthermore, the frequency of such peptide-specific T cells appears to be higher in the circulation of patients with cancer than in healthy subjects.15 Finally, the SEREX technology, based on the presence of tumor-specific antibodies in sera of patients with cancer, has been successfully used for tumor-antigen discovery in many laboratories.16 These findings, as well as recent identification of numerous TAs that appear to be immunogenic in that they induce humoral immune responses, cellular immune responses, or both in vitro by using human immune cells and in vivo in animal models of tumor growth, strongly support the notion that the host immune system recognizes the presence of the tumor and responds to it by generating both local and systemic immune responses.
If the tumors are not ignored by the immune system, why do they progress? Several answers to this question can be considered. First, there is the old argument for the lack of a “danger signal”17 in tumors akin to those presented by pathogens invading tissues during an infection. Recognition by DCs of pathogen-associated molecular patterns through the ubiquitous Toll-like receptors leads to efficient DC activation and maturation. It promotes generation of vigorous cellular and antibody responses to bacterial or viral antigens, presumably because the immune system perceives an infection as a danger signal17 benefiting the host. However, functional Toll-like receptors are known to be expressed by many human solid tumors,18 and recent data indicate that tumors use them to promote their own growth; for protection from spontaneous, immune-mediated, or drug-induced apoptosis; or both.18,19
Second, TAs are perceived by the immune system as “self” or “altered self” antigens, which evoke weak immune responses because tolerance prevents generation of immune responses to self. The only “unique” TAs are mutated antigens, and these are strongly immunogenic and elicit robust immune responses.20 However, only a handful of such mutated TAs are known, and the vast majority of TAs are poorly immunogenic or simply tolerogenic. In this context cancer can be viewed as an autoimmune phenomenon in which tolerance to self prevents effective immune responses to TAs Patients with cancer who have not been treated with chemotherapy or radiotherapy generally have normal immune responses to viral or bacterial antigens, yet they are unable to respond to their own TAs. Except for late-stage disease, they generally have normal delayed-type hypersensitivity responses to recall antigens but are anergic to autologous TAs. Although tolerance to self is a detriment to the generation of antitumor responses in patients with cancer, another factor that exerts an overwhelming effect on these responses is the tumor microenvironment. Each tumor creates its own milieu characterized by the presence of immunosuppressive factors and by the excess of TAs produced and released by the growing tumor. Evidence suggests that tumors produce a broad array of immunoinhibitory factors, which exert either local or systemic effects on the host antitumor immune responses.5 Therefore it is not surprising that antitumor immunity might be weak, inefficient, or even absent in patients with cancer, depending on the nature of tumor-host interactions, as well as the robustness of regulatory mechanisms in control of immune tolerance.
Immune antitumor responses could be influenced by the gradual deterioration of the immune system with age.21 The increased incidence of cancer present in the elderly might be due to immunosenescence (ie, progressive remodeling of the immune system with a reduced ability of immune cells to respond to activating stimuli and increased responsiveness to tolerogenic signals).21 Immunosenescence can significantly interfere with the effectiveness of cancer immunotherapies, and it has been suggested that clinical trials testing immunopotentiating agents in patients with cancer should be conducted in elderly subjects.21
Recent multiparameter analyses of primary and metastatic human tumors (eg, colorectal carcinoma) recognize several major immune “coordination profiles,” the presence of which is influenced by the balance between tumor escape and immune antitumor responses and that are subject to host-tumor cross-talk.12 In this context it is important to consider differences between primary and metastatic tumors. Not only are metastatic tumors more immunosuppressive, but also they appear to be less readily recognized by TA-specific immune effector cells. The latter could be due to defects in the expression levels of antigen-processing machinery (APM) components, MHC molecules, or both in the tumor and its metastases.22 Because different copy numbers of distinct trimolecular peptide–β2-microglobulin (β2 m)–MHC complexes presented on the tumor surface might lead to differential T-cell recognition, this aspect of tumor–immune cell interactions is critical.22,23 A recent comparison of primary renal cell carcinoma, renal cell carcinoma metastases, and normal renal tissue with respect to HLA ligand presentation and gene expression demonstrated a greater similarity between primary tumor and metastasis than between the tumor and normal tissue.24 This observation provides a good rationale for peptide-based immunotherapy because it is likely to preferentially target the tumor and its metastases and not the normal tissue.
NATURAL VERSUS ADAPTIVE IMMUNE RESPONSES TO MALIGNANCIES
Antitumor immune responses can be innate (natural) or acquired (adaptive). Innate immunity is mediated by cells or soluble factors that naturally exist in tissues or body fluids and can interfere with tumor growth or survival. Among hematopoietic cells, macrophages, granulocytes, natural killer (NK) cells (CD3−CD56+), non–MHC-restricted T cells (CD3+CD56−), and γδ T cells have the natural capability to eliminate tumor cell targets.21 In addition, natural antibodies with specificities directed at surface components of tumor cells might be present in the sera of patients with cancer.16 Other serum factors, including complement components, C-reactive protein, mannose-binding protein, and serum amyloid protein, also play a role in innate immunity.25 Adaptive immune responses to tumors are mediated by CD3+T-cell receptor (TCR+) T cells when they recognize tumor-derived peptides bound to self-MHC molecules expressed on APCs. Little is currently known about the molecular signals and cellular steps involved in directing APCs, such as DCs, to execute a tolerogenic versus immunogenic program in response to antigens. As indicated above, tumors can also serve as APCs, although low levels of MHC class I molecule expression, MHC class II molecule expression, or both on the surface of tumor cells makes this an inefficient process.22 More likely, TAs are taken up by DCs present at the tumor site, processed, and cross-presented to T cells in the tumor-draining lymph nodes in the form of the trimolecular peptide–β2m–MHC complexes.23 For adaptive immune response to occur, T cells expressing correct (cognate) TCRs have to be present. Recognition of the peptide and its binding to the variable domains of the TCR initiates signaling (signal 1) that leads to T-cell activation.26 This requirement implies prior sensitization and a clonal expansion of memory T cells in response to a cognate tumor epitope (anamnestic or recall responses). Alternatively, precursor T cells expressing the TCR can be primed by the cognate peptide–MHC ligands presented on APCs, and the subsequent development of antitumor effector cells is viewed as a primary immune response. In either case costimulatory molecules (signal 2) are necessary for an immune response to proceed,27 and once T-cell proliferation is initiated, appropriate cytokines (signal 3) become essential for sustaining the response.28 Recent findings stress the key importance of signal 3 for the development of immune responses and for their contraction.28 Like all immune responses, those that are TA specific do not go on forever but peak and then contract, restoring the preactivation balance. The precise mechanisms responsible for immune contraction are not yet defined, and regulatory T (Treg) cells, as well as other mechanisms, have been proposed to regulate immune reactivity, but it is clear that events in the environment play a dominant role in this respect.
Immune responses to malignant cells can be categorized as locoregional or systemic. In situ or local responses refer mainly to TILs, which accumulate in most human solid tumors and the role of which in tumor progression remains highly controversial. Long considered by some an effector arm of antitumor responses, TILs are viewed by others as victims of the tumor microenvironment because their effector functions are often impaired, presumably by tumor-derived factors.29 A failure of local antitumor responses mediated by TILs is thought to contribute to tumor progression. Systemic immunity to tumors, as measured by delayed-type hypersensitivity responses or by various ex vivo assays of T-cell responses in the peripheral circulation of patients with cancer, are difficult to demonstrate, and TA-specific responses have been particularly elusive. Nevertheless, by using highly sensitive multicolor flow cytometry, it has been possible to detect and measure the frequency of TA-specific CD8+ and CD4+ T cells in the peripheral circulation of patients with cancer.1 Although the response levels vary, TA-specific and nonspecific proliferative or cytotoxic responses of peripheral lymphocytes in patients with cancer appear to be at least partially impaired.29–31 Data indicate that the same functional impairments seen in TILs are found in both circulating and lymph node lymphocytes of patients with cancer.29,32 Thus it has been concluded that, in general, human tumors exert profound suppressive effects on both local and systemic antitumor immunity in these patients.
In contrast to the failure of antitumor immune responses to control tumor progression in human subjects, a large body of experimental evidence derived from preclinical animal models of cancer suggests that the immune system can prevent tumor growth or cause its rejection.33 In the prevention setting vaccination of animals with TAs plus adjuvant protects them from rechallenge with tumor,34 whereas immunotherapy of established tumors with vaccines, cytokines, adoptively transferred immune cells, or immunomodulatory agents results in tumor rejection, provided the tumor is not in an advanced stage. Remarkably, this has been a consistent pattern seen with carcinogen-induced, virally induced, and spontaneously arising tumors in mice, suggesting a fundamental difference in immune responses to tumor antigens between mice and human subjects. Indeed, it appears that the difference might be due to appreciably greater immunogenicity of murine TAs, which in most cases are virus- or carcinogen-related epitopes and thus foreign rather than self-epitopes. Alternatively, the answer might be that experimental murine tumors are established, grow, progress, and are eliminated by therapy in the very short time required for the completion of the experiment, leaving no time for the development of tumor escape mechanisms. In contrast, human tumors are diagnosed and treated after many years of coexistence with the host. An introduction or establishment of the tumor in mice is a dramatic event that mobilizes host defenses in contrast to a silent coexistence of tumor cells with the immune system for many years in human subjects. To minimize this difference, transgenic murine models have been developed, allowing for ensured, genetically driven tumor development in a “spontaneous” environment.35 Transgenic mice have been especially useful in the design of preventive cancer vaccines,34 and information they provide is encouraging for the development of immunoprophylaxis of cancer in human subjects. Nevertheless, to date, it has been difficult to translate the positive results seen in mice to immunotherapy of established human tumors. It is plausible that numerous and varied mechanisms of escape developed by the latter during the prolonged residence and interactions with the host provide human tumors with advantages not afforded to murine tumors established in an experimental setting.
TUMOR ASSOCIATED ANTIGENS
Recent progress in the development of cancer vaccines has been greatly facilitated by the availability of well-defined TAs, many of which have been characterized in the last decade.36 Most of these TAs are derived from self-proteins that are either mutated or otherwise differentially expressed in normal and tumor cells, as exemplified by oncogenes or oncofetal or cancer testis antigens. The major categories of TAs that have been often used as candidates for immune therapies are listed in Table I.36,37 A recent report provides a much longer prioritized list of well-characterized cancer antigens best suited for use in cancer vaccines.38 The list is based on criteria generated by a panel of experts convened by the National Cancer Institute38 and is designed to assist investigators in the field of immunotherapy in the selection of the most promising TAs for further testing in clinical trials.
TABLE I.
Human TAs that are candidates for immune therapies*
| TA category | Examples |
|---|---|
| Oncofetal | Oncofetal antigen/immature laminin receptor (OFA/iLRP) |
| Glypican 3 (heparan sulfate protoglycan) | |
| α-Fetoprotein (AFP) | |
| Carcinoembryonic antigen (CEA) | |
| Oncogenes | The RAS family: p53, Her2 neu |
| Cancer testis (CT) antigens: | MAGE-1 |
| BAGE | |
| GAGE | |
| NY-ESO-1/LAGE | |
| SAGE | |
| Other 35–40 CT antigens mapping to chromosome X (CT-X) or distributed throughout the genome (non-X CT) | |
| Human melanoma antigens | MART-1/MELAN-A |
| Gp100/pmel 17 | |
| Tyrosinase | |
| Tyrosinase related proteins (TRP) 1 and 2 | |
| Chondroitin sulfate proteoglycan (CSPG4) | |
| Human glioma antigens | IL-13 receptor α2 |
| Eph A2 | |
| Survivin | |
| EGFR variant III (EGFRvIII) | |
| Head and neck cancer antigens | EGFR |
| Human papilloma virus (HPV 16 or 18) | |
| Aldehyde dehydrogenase A1 (ALDHA1) | |
| CSPG4 | |
| Normal overexpressed or modified antigens | MUC-1 |
| Cyclin-B1 | |
| Prostate-specific antigen (pSA) | |
| Prostate membrane-specific Ag (PMSA) |
As already indicated, immune responses to TAs, even to those representing altered self-antigens, are detectable in tumor-bearing hosts, although in most cases no correlations between the presence of in vitro responses to TAs and prognosis have been documented. This is in contrast to numerous animal tumor models, which have provided strong evidence that in the presence of effective antitumor immunity, tumors fail to progress and established tumors regress.39 Nevertheless, human cancer vaccine trials in patients with cancer have made use of many well-characterized TAs in the hope that their presentation on appropriately polarized DCs will overcome difficulties with the generation of a strong immune response in the therapeutic setting. The most recent reports of such clinical trials in patients with cancer indicate that multiple subcutaneous injections of an immunogenic tumor peptide, such as NY-ESO-1, plus a mix of 2 potent adjuvants, such as Montanide ISA-51 and CpG7909, can be effective in inducing sustained peptide-specific immune responses and significantly prolong survival, even in patients with advanced disease, including solid tumors other than melanoma.40 These reports, demonstrating that antitumor, antivaccine, or both immune responses correspond to clinical outcome, suggest that the optimization of vaccination strategies is likely to overcome tumor-induced suppression and to restore the immune balance altered by cancer development.
IMMUNE CELLS IN THE TUMOR MICROENVIRONMENT
Immune cells that are most frequently found in the human microenvironment are lymphocytes, which are capable of mediating both innate and adaptive immunity, although monocytes, tumor-associated macrophages (TAMs), and DCs are also commonly seen.41 Inflammatory cells present in the tumor are in intimate contact with tumor cells, stromal fibroblasts, extracellular matrix components, and blood vessels. Proinflammatory cytokines secreted by inflammatory cells can contribute to tumor progression, and soluble factors produced by the tumor in response to nonspecific or tumor-specific signals, such as prostaglandin E2 (PGE2), adenosine, or TGF-β, downregulate functions of immune cells. The tumor microenvironment is created by the tumor, and it is continuously shaped and dominated by the tumor, which directs all cellular and molecular events taking place in the surrounding tissue.
Immune cells recruited to the tumor include T cells (CD3+TCR+), which are by far the largest component of mono-nuclear tumor infiltrates41 and have received the most attention. Although their accumulation in the tumor might be considered evidence of immune surveillance by the host, they are largely ineffective in arresting tumor growth, although they can proliferate and mediate antitumor cytotoxicity on their removal from the tumor bed and ex vivo IL-2 activation.42
Phenotypic and functional characteristics of human TILs are listed in Table II. More current data on the status of T cells found in human tumors suggest that their phenotypic and functional profile varies depending on the microenvironment created by the tumor and that this profile or “immune signature” can influence prognosis and disease outcome.9,12 It appears that TILs obtained from advanced or metastatic lesions are more functionally impaired than those from early lesions, suggesting that tumor burden or the potential of a tumor to suppress immune cells might determine the functional status of infiltrating T cells. Among CD4+ T cells present in the tumor, a subset of CD4+CD25high forkhead box protein 3 (FOXP3)–positive Treg cells is expanded to constitute from 5% to 15% of CD4 T cells in the infiltrate. Their frequency is higher in the tumor than in the peripheral circulation.43,44 These cells suppress functions of other immune cells in the microenvironment by mechanisms that might be cell contact dependent or might involve the production of inhibitory cytokines or adenosine.43–46 Recently, a potent proinflammatory T-cell subset, IL-17–producing TH17 cells, were observed among CD4+ cells in patients with ovarian carcinoma. The presence of these cells was significantly correlated to enhanced survival in these patients and was found to inversely correlate with the number of FOXP3+ Treg cells.47
TABLE II.
Morphologic, phenotypic, and functional characteristics of TILs found in human solid tumors
| Morphology: small to large lymphocytes |
| Phenotype: CD3+TCR-α/β+ T cells; few (<5%) CD3−CD56+ NK cells |
| Mix of CD4+ and CD8+ cells; variable CD4/CD8 ratio |
| Largely CD45RO+CCR7− memory T cells |
| Express activation markers (CD25, HLA-DR) |
| Nearly all are CD95+ |
| Accumulations of Treg cells (CD4+CD39 + TGF-β+) and CD4+IL-17+ TH17 cells |
| Clonality: oligoclonal, as determined based on TcR Vβ gene expression |
| Specificity: autotumor-specific T cells detectable in some tumors at a low frequency |
| Functions: Low or absent ζ chain expression: inefficient TCR signaling |
| Suppressed nuclear factor κB activation |
| Decreased locomotion, proliferation, cytotoxicity |
| Cytokine profile: TH2 type with IL-4, IL-5, and IL-13 production and no/little IL-2 or IFN-γ production; excess of IL-10 or TGF-β |
| In vitro response to IL-2 variable but more decreased in TILs recovered from metastatic rather than primary lesions |
| Increased levels of caspase-3 activity |
| Apoptosis of CD8+ T cells (TUNEL+; Anx+) |
TUNEL, Terminal deoxynucleotidyl transferase–mediated dUTP nick end labeling.
Macrophages (CD14+) present in tumors are referred to as TAMs. Although normal macrophages uptake antigens and play an important role in control of infections, TAMs are reprogrammed to inhibit functions of immune cells through the release of inhibitory cytokines, such as IL-10, PGE2, or reactive oxygen species (ROS).48 It is hypothesized that reprogramming of TAMs occurs in the tumor microenvironment as a result of tumor-driven activation. Evidence has accumulated indicating that invasiveness of tumors, such as human primary colon carcinomas, is directly related to the number of TAMs detected in the tumor. In patients with invasive breast cancer, an increased TAM count is an independent predictor of reduced relapse-free survival, as well as reduced overall survival.49 The available data support the active role of TAMs in tumor-induced immunosuppression on the one hand and in the promotion of tumor growth on the other. Furthermore, preliminary evidence suggests that the reciprocal differentiation of Treg and TH17 cells from an uncommitted common CD4+ precursor along either a suppressive or proinflammatory pathway, respectively, is biased by TAMs.47 Thus TAMs appear to significantly contribute to shaping of the tumor microenvironment.
A subset of myeloid-derived cells equivalent to CD11b+/Gr1+ cells in mice, which are CD34+CD33+CD13+CD15− and called myeloid-derived suppressor cells (MDSCs), accumulate in human tumors.50 They are recruited from the bone marrow by means of tumor-derived soluble factors, such as GM-CSF, vascular endothelial growth factor (VEGF), and IL-10; migrate to lymph nodes, where DCs cross-prime T cells; and interfere with this process. They also migrate to tumors, become tumor-associated MDSCs, and inhibit immune cell functions through the production of arginase 1, an enzyme involved in the L-arginine metabolism. Arginase 1 synergizes with inducible nitric oxide synthase (iNOS) to increase superoxide and nitric oxide production, inhibiting lymphocyte responses by the induction of iNOS in surrounding cells.51 Current data support the active role of MDSCs in tumor-induced immune suppression that contributes to functional dysfunction of immune cells in the tumor, as well as the peripheral circulation of patients with cancer.
DCs (HLA-DR+CD86+CD80+CD14−) are nature’s best APCs. They are a common component of tumor immune infiltrates and are responsible for the uptake, processing, and cross-presentation of TAs to naive or memory T cells, thus playing a crucial role in the generation of tumor-specific effector T cells.52 In addition, DCs control the induction of Treg cells. In patients with cancer, cellular interactions between antigen-presenting DCs and T cells lead to expansion and accumulation of Treg cells at the tumor site and in the periphery.52 The DC-derived signals that determine the outcome of DC–T-cell interactions operate at the levels of (1) antigen presentation (signal 1); (2) display of cos-timulatory molecules (signal 2); and (3) the presence of immunomodulatory cytokines (signal 3). Stimuli that lead to upregulation of signals 1 and 2 in the absence of signal 3 might facilitate peripheral tolerance induction.52 At the same time, newer evidence suggests that many conditions relevant to signal 1, such as antigen dose, determine whether Treg or TH2 effector (Teff) cells are induced, irrespective of the maturation state of DCs.52 In addition, insights into the APM in DCs and evidence that some of the components of APM, including MHC class II molecules, might be downregulated or altered in patients with cancer,23 suggest that Treg cell induction might be influenced not only by the nature and dose of the antigen but also by its processing and its presentation to T cells.
Tumor-associated DCs directly exposed to tumor cells, tumor-derived factors, or both have been shown to readily undergo apoptosis and to have impaired maturation.53 Specifically, tumor-derived factors, such as gangliosides, were shown to inhibit DC generation and their function in vitro.54 This suppressive effect of gangliosides on DCs was found to be mediated by tumor-derived VEGF, a known antidendropoietic factor.53 The data on functional impairments of tumor-associated DCs have to be balanced by numerous reports in the literature, which suggest that the presence of DCs in tumors is associated with improved prognosis and prolonged patient survival, as well as a reduced incidence of recurrent or metastatic disease.55 In contrast, patients with lesions reported to be scarcely infiltrated with DCs have a relatively poor prognosis.56 Fewer DCs were observed in meta-static than in primary lesions. In one study it was shown that the number of DCs present in the tumor was by far the strongest independent predictor of overall survival, as well as disease-free survival and time to recurrence, in a large cohort (n = 132) of patients with oral carcinoma compared with such well-established prognostic factors as disease stage or lymph node involvement.55 It appears that not only the number of DCs but also the presence of functionally unimpaired, normally signaling T cells in the tumor microenvironment are important for overall survival of patients with cancer.55
NK cells (CD3−CD56+CD16+), which mediate innate immunity and contain both perforin-rich and granzyme-rich granules, are well equipped to mediate lysis of tumor cells. Although NK cells represent “the first line of defense” against pathogens,57 most human tumor cells are resistant to perforin-mediated NK cell lysis, and NK cells are rarely found among TILs.41 This is despite the fact that tumor cells often downregulate MHC antigen expression and are enriched in MICA and MICB molecules.58 There might be several reasons for the paucity of NK cells in tumors, including the possibility that NK cells are present in premalignant or early lesions and absent from advanced tumors, which is consistent with their role in immune surveillance rather than killing of cancer cells at the tumor site.41 More recent data suggest that the primary biologic role of NK cells in tumor-bearing hosts might not be the elimination of tumor targets but rather the facilitation of DC–T-cell interactions and driving the immune responses to TAs.59 Because the tumor site is not likely to be the optimal milieu for this type of immune interaction, the paucity of NK cells in tumors might fit with their physiologic functions. The in vivo role of NK cells in antitumor immune defense is not yet clear, and work continues to define it further.
Polymorphonuclear leukocytes are infrequently seen in infiltrates of human solid tumors, with the exception of nests of eosinophils that might be present in association with tumor cells in some cases. In human tumors granulocytes, which are a major cellular component of many murine tumors, are rare, being largely replaced by TAMs or MDSCs. This could be explained by the fact that most inflammatory infiltrates into human tumors are chronic rather than acute, with granulocytes long gone by the time human tumors are diagnosed, biopsied, and examined.
B cells (CD19+, CD20+) are also rare in most human tumors, with the exception of breast cancer and melanoma.6,60 The primary function of B cells is differentiation into antibody-producing plasma cells. Although TA-specific antibodies are frequently detected in the circulation of patients with cancer, these antibodies are made and secreted in the tumor-draining lymph nodes, spleen, or other lymphoid tissues. From these sites, IgG molecules can readily be transported through plasma or lymph to tissue sites. Therefore the presence of B cells or plasma cells in tumors is not expected a priori, although it might be that the ability to make antibodies in situ could be an important aspect of host defense.
Inflammatory infiltrates present in human tumors change in composition and intensity during tumor progression. The initial acute inflammation involving the recruitment and influx of antitumor effector cells is replaced by chronic inflammation in later stages of tumor progression. Tissue hypoxia plays a major role in shaping the nature of immune infiltrates in tumors. It is created by activation of hypoxia-responsive genes in tumor cells61 and favors the influx of granulocytes and phagocytic macrophages, which depend on the glycolytic pathway for survival.62 These cells take up and process dying tumor cells, producing an abundance of ROS. The subsequent reoxygenation of the microenvironment is accompanied by activation of the nuclear factor κB pathway in both tumor cells and infiltrating immune cells, leading to the excessive secretion of proinflammatory cytokines.5 Responding to this nuclear factor κB–driven cascade of proinflammatory cytokines, the tumor and stromal cells produce a variety of soluble factors with wide-ranging biologic effects, including the promotion of tumor cell proliferation.5 In the tumor microenvironment cellular expansion, differentiation, or activation, as well as cell migration, matrix remodeling, and blood vessel growth, are reprogrammed to benefit the tumor. Thus the nature of chronic inflammatory infiltrates and functions of the tumor-infiltrating immune cells depend on how aggressively a given tumor remodels its microenvironment.
IMMUNE EFFECTOR CELLS IN THE CIRCULATION OF PATIENTS WITH CANCER
In human subjects peripheral blood is the major source of cells for studies of their antitumor functions. T lymphocytes, NK cells, monocytes, DCs, and B cells and their subsets have all been extensively evaluated in the peripheral circulation of patients with cancer by using conventional phenotypic and functional in vitro assays. Results indicate that signaling abnormalities, functional impairments, and apoptosis seen in immune cells obtained from the tumor microenvironment are likewise present in peripheral blood cells of patients with cancer.63,64 The finding of CD8+ T-cell apoptosis in the circulation of these patients is perhaps the most convincing evidence that all is not well with immune effector cells in cancer.65 The proportion of CD8+CD95+ T cells that bind Annexin V (Anx) and yet are 7-amino-actinomycin D negative (7AAD−) or propidium iodide (PI) negative is significantly greater in the peripheral circulation of patients with cancer, including those with head and neck, breast, and ovarian carcinomas and melanoma, than in age- or sex-matched healthy donors.65 As indicated in Table III,66–68 T cells that undergo spontaneous apoptosis in the circulation of these patients are CD3+CD95+, bind Anx, and have increased levels of caspase-3 activity and decreased expression of the TCR-associated ζ chain.63,69,70 Circulating CD8+ T cells, especially the effector subpopulations (CD8+CD45RO+CCR7−CD27− and CD8+CD28−), have a significantly greater propensity to undergo spontaneous apoptosis than CD4+ T cells in patients with cancer. This could explain the functional deficits found in CD8+ effector cells, such as the downregulation in expression of signaling molecules, specifically the ζ chain. The available data suggest that functional defects in T cells might be linked to their increased sensitivity to apoptosis and that the tumor participates in engineering spontaneous or activation-induced cell death of T cells.65 The highest proportions of Fas+Anx+CD8+ T cells are generally seen in a subset of patients with advanced active disease.70 In patients with cancer, the vast majority of circulating CD8+ T cells are CD95+, and the Fas/Fas ligand (FasL) pathway contributes to their apoptosis because human solid tumors express FasL and export it to the periphery in the form of FasL+ exosomes.71,72 However, tumor-induced apoptosis of immune cells engaging death ligand/receptor interactions is only one of many mechanisms used by tumors to engineer an immune escape.65 Based on increasing insights into these mechanisms, it is possible to speculate that the presence of the constellation of immune defects might allow for the identification of a subset of patients with cancer who have poor prognosis because their tumors create a particularly immunosuppressive environment.
TABLE III.
Characteristics of T lymphocytes in the peripheral circulation of patients with cancer*
| Predominant phenotype | |
| T lymphocytes: % CD3+CD95+Anx+ (increased vs NC) | |
| % CD3+CD25+ (increased vs NC) | |
| % CD3+HLA-DR+ (increased vs NC) | |
| CD8+ subset: | % CD8+CD95+Anx+ (increased vs NC) |
| CD8+ naive: | % CD8+CD45RO−CCR7+ (decreased vs NC) |
| CD8+ central memory: | % CD8+CD45RO+CCR7+ (decreased vs NC) |
| CD8+ peripheral memory: | % CD8+CD45RO+CCR7− (increased vs NC) |
| CD8+ effector cells: | % CD8+CD45RO−CCR7− (increased vs NC) |
| CD4+ subset: | % CD4+CCR7+ (decreased vs NC) |
| CD4+ naive: | % CD4+CD45RO−CCR7+ (decreased vs NC) |
| CD4+ memory cells: | % CD4+RO45RO+CCR7+ (decreased vs NC) |
| CD4+ Treg cells: | % CD4+CD25+ (increased proportions vs NC) |
| Clonality: Polyclonal with various restricted TCR Vβ specificities | |
| Specificity: TA-specific/tetramer+ T cells detectable in many cases | |
| Functions | |
| Low ζ chain expression in T and NK cells: inefficient TCR signaling | |
| Decreased proliferation in response to anti-CD3 antibody, PMA/ionomycin, mitogens | |
| Decreased antitumor cytotoxicity and NK/lymphokine-activated killer activity | |
| Cytokine profile: highly variable | |
| Apoptosis of CD8+ T cells and NK cells (Anx+) | |
| Increased caspase-3 activity in T cells | |
| Increased lymphocyte turnover | |
Apoptosis of Fas+, activated CD8+T cells in the circulation of patients with cancer leads to a rapid turnover of T lymphocytes, contributing to a loss of antitumor effector cells and an aberrant lymphocyte homeostasis.66,73 Recent data indicate that circulating Vβ-restricted CD8+ T cells and tumor peptide–specific tetramer–positive CD8+T cells are especially sensitive to apoptosis.74 By using T-cell receptor excision circle (TREC) analysis, a PCR-based technique that allows for quantification of recent thymic emigrants in the peripheral circulation, it has been determined that patients with cancer had significantly fewer recent thymic emigrants than healthy age-matched donors.67 The results suggest that the lymphocyte turnover is faster in patients with cancer than in healthy control subjects, either because the thymic output in patients is lower or the peripheral expansion of T cells is greater, diluting T-cell receptor excision circles and enhancing the maturation rate of naive T cells.66,73 Such rapid turnover of T cells could have detrimental effects on antitumor responses. A loss of effector subpopulations of CD8+ T cells, which appear to be targeted for apoptosis in patients with cancer, might severely compromise antitumor functions of the host and contribute to tumor progression.73
The clinical significance of spontaneous apoptosis of CD8+effector cells in patients with cancer is currently unknown. A search for surrogate markers of prognosis or a response to therapy in patients with cancer has led to further studies of CD8+ T-cell apoptosis. The level of spontaneous apoptosis discriminates between patients with cancer and healthy control subjects but not between patients with active disease versus those who are NED after oncologic therapies.67 However, expression of CCR7, which is also a differentiation marker for T cells, by CD8+ T cells was observed to protect the CD8+ effector cells from apoptosis because CCR7 signaling correlated with higher Bcl-2 expression but lower Bax and Fas expression and phosphoinositide 3-kinase pathway activation in CD8+ T cells.68 The frequency of circulating CD8+CCR7+ T cells now emerges as an immune biomarker that might be predictive of survival benefits in patients with cancer. Pending validation, this immunologic biomarker that is simply defined by flow cytometry could acquire substantial clinical usefulness in the future.
Another subset of antitumor effector cells, NK cells, representing 8% to 10% of lymphocytes in the peripheral circulation, has been credited with the ability to eliminate tumor cells in the circulation and thus prevent establishment of distant metastases.75 Recent data suggest that in addition to mediating perforin-mediated lysis, NK cells constitutively express several ligands of the TNF family and can therefore induce apoptosis in a broad variety of tumor cell targets.76 This mechanism of tumor cell elimination might be of greater biologic importance than secretory, granule-mediated killing, largely because most tumor cells express receptors for the TNF family ligands and are sensitive to death by apoptosis.76 NK cells, which are able to discriminate between normal and abnormal cells based on the presence and expression levels of MHC class I molecules, are considered to play a major role in early stages of tumor development. They express receptors that enable them to survey the target for the respective ligands. These receptors are of 2 types: killer inhibitory receptors, killer activating receptors, or both.57 NK cell functions and their interactions with other cells or extracellular matrix molecules are regulated through these receptors and Fcγ receptors.57 In the peripheral circulation of patients with cancer, NK cells, like CD8+T cells, can also be dysfunctional. On a per-cell basis, these NK cells mediate lower levels of cytotoxicity.77 Furthermore, some studies suggest that NK cells are also sensitive to apoptosis.78 Among circulating NK cells in patients with breast cancer, a subset of CD56brightCD16dim NK cells, which represents about 95% of all NK cells and is responsible for effector functions, preferentially binds Anx and thus is primed for apoptosis.79 These patients also had significantly lower NK activity than the age-and sex-matched healthy control subjects tested in parallel. These and other data suggest that endogenous circulating NK cells have the potential to play a role in tumor surveillance, but in the presence of the tumor, their antitumor functions are subverted, and no longer control metastasis dissemination. Once the tumor is established, it especially subverts the subsets of NK cells found at the sites of metastasis and those responsible for cytotoxic functions.
In addition to NK cells, another category of nonspecific effector cells, CD3+CD56+NK/T cells, can potentially eliminate tumor targets. They represent a very minor subset of circulating lymphocytes in healthy subjects but have been reported to be expanded in patients with cancer, as well as tumor-bearing rodents.80 NK/T cells are also a minor component of TILs. In the presence of IL-2, NK/T cells, like CD3−CD56+ NK cells, readily differentiate into lymphokine-activated killer cells containing numerous granzyme- and perforin-containing granules and are able to mediate tumor cell lysis.77 Both NK and NK/T cells express receptors for IL-18 and thus are activated in the presence of this cytokine as well.
REGULATORY IMMUNE CELLS IN PATIENTS WITH CANCER
The presence in the circulation of patients with cancer of suppressor lymphocytes capable of downregulating functions of other immune cells was described many years ago.81 Today such cells are phenotypically identified as CD4+CD25highFOXP3+ T cells and referred to as Treg cells.82 They can be isolated from PBMCs or tumor sites by means of immunoselection on magnetic beads coated with antibodies to surface antigens expressed on Treg cells, such as CD25 or CD39. In mice depletion of CD4+CD25+T cells results in the development of autoimmunity, and in tumor-bearing animals it promotes immune responses to autologous tumor. In patients with cancer, tumor-associated lymphocytes are enriched in CD3+CD4+CD25high T cells.83 On sorting by flow, these T cells have been shown to secrete TGF-β or IL-10 and to enzymatically cleave ATP to adenosine.45,46 The mechanisms through which these T cells regulate antitumor immune responses are being intensively investigated, and because Treg cells come in different flavors (eg, natural Treg cells, inducible TR1 cells, CD39+ Treg cells, or cytotoxic T lymphocyte–associated antigen–positive Treg cells), these mechanisms vary, likely depending on the microenvironmental context. Similarly, the microenvironment influences the induction of Treg cells; for example, TR1 cells are preferentially induced at the tumor site, which is rich in IL-10, TGF-β, and PGE2, all of which have been shown to promote TR1 cell generation.43,44 The prognostic significance of Treg cells in patients with cancer has been controversial, with many reports linking their accumulations to poor prognosis, presumably as a result of suppressed antitumor immunity,84 and others reporting better survival in the presence of increased Treg cell frequencies,85 possibly because of their ability to suppress tumor-promoting mechanisms or induce tumor cell death. The controversy arises because in human subjects no definite identity marker for Treg cells exists, and their functional repertoire is broad and varied. Nevertheless, their responsibility for the contraction of immune responses is critical for health.
Another subset of CD4+T cells with an origin shared with Treg cells has recently been identified. Like Treg cells, CD4+ TH17-producing T cells originate from uncommitted CD4+ T-cell precursors, and the participation of TGF-β in their differentiation links them to Treg cells.86 However, TH17 cells produce IL-17, IL-21, and IL-IL-22, promoting tissue inflammation, and require the presence of IL-6, as well as the transcription factors signal transducer and activator of transcription 3 (STAT3), RORγ, and RORα, for differentiation.87 Although the presence of TH17 cells has been documented in several human carcinomas,86 their function in tumors remains controversial. Recent reports show that CD4+FOXP3+CCR6+ Treg cells can produce IL-17 on activation and can inhibit proliferation of CD4+ responder T cells,87 confirming a relationship between Treg and TH17 cells that can be modulated by cytokines in the tumor microenvironment. It also emphasizes the plasticity of T-suppressor and T-effector subsets of CD4+ lymphocytes.
The second major subset of regulatory cells in cancer are MDSCs (CD34+CD33+CD13+CD11b+CD15−).50 Tumors recruit MDSCs from the bone marrow through tumor-derived soluble factors, such as GM-CSF, TGF-β, IL-10, and VEGF.5 Immature myeloid cells migrate to lymph nodes, where DCs cross-prime T cells and interfere with this process, thus suppressing CTL generation. They also migrate to the tumor site and become MDSCs able to produce arginase I and promote iNOS activation.5,51 MDSCs also produce high levels of ROS and indo-leamine-2,3-dioxygenase, an enzyme involved in the catabolism of tryptophan, an essential amino acid for T-cell proliferation and differentiation.88 In tumor-bearing mice MDSCs accumulate in the spleen, reaching a very high frequency and exerting potent immune suppression, thereby favoring tumor growth. GM-CSF, often used as an immune adjuvant,89 is also a product of tumor cells, which recruits MDSCs from the bone marrow and is responsible for their accumulation in patients with cancer.90 In patients with cancer, normal physiologic functions of GM-CSF and MDSCs are subverted by the tumor to promote its development.
The tumor uses a variety of mechanisms and produces various factors and enzymes that enable it to suppress the host antitumor immune responses. Some of these factors are listed in Table IV. Among these factors, 2 have recently been in the limelight. B7-H1 is an immunoglobulin-like immunosuppressive molecule broadly expressed in tumor cells, which signals to its counterreceptor, programmed death 1 (PD-1), on T cells.91 Signaling delivered to T cells through B7-H1 (programmed death ligand 1 [PD-L1]) inhibits their proliferation, cytokine production, and effector functions.92 Also, triggering by the PD-L1+ tumors of PD-1 on T cells increases tumor cell resistance to immune and drug-induced death,91 demonstrating that cancer cells can use receptors on immune cells as signals to induce resistance to therapy. Blockade of PD-L1/PD-1 interactions promotes generation of TA-specific T cells and attenuates their inhibition by Treg cells.93 Therefore PD-1 antagonists, which are expected to augment TA-specific immune responses, might be useful in therapy of cancer.94 Levels of the cytokine IL-17 have been shown to be increased in the tumor microenvironment.95 Adoptive transfer studies and examination of the tumor microenvironment suggest that CD4+ T cells accumulating in the tumor are the main source of IL-17 and that the enhancement of tumor growth by IL-17 is mediated by its binding to IL-17 receptors expressed on tumor cells, initiating IL-6 production, which in turn activates oncogenic STAT3, upregulating prosurvival and proangiogenic genes.95 Thus TH17 seems to promote tumor growth, in part through activation of an IL-6/STAT3 pathway in tumor cells. These data are contradictory to the recently reported improved survival of those patients with ovarian cancer whose tumors contained large numbers of TH17+TILs.47 This discrepancy illustrates the difficulty of dissecting the role of TH17 in human cancer and of interpreting environmental interactions occurring in different tumor types.
TABLE IV.
Molecularly defined immunoinhibitory factors produced by human tumors*
| TNF family ligands | Induce apoptosis through the TNF family receptors |
| FasL | Fas |
| TRAIL | TRAIL-R |
| TNF | TNF-R1 |
| B7-H1 (PD1L) | Binds PD1 and inhibits lymphocyte and DC functions |
| Cytokines | |
| TGF-β | Inhibits lymphocyte proliferation and perforin and granzyme mRNA expression; promotes Treg cell expansion |
| IL-10 | Inhibits cytokine production, including that of IL-12; promotes Treg cell expansion |
| GM-CSF | Promotes expansion of immunosuppressive tumor- associated macrophages; recruits MDSCs |
| IL-17 | Largely produced by CD4+ T cells in the tumor; binds to IL-17 receptor on tumor cells, initiating the IL-6/STAT3 cascade |
| Enzymes | |
| Indoleamine-2,3-dioxygenase (IDO) | Inhibits T-cell activation |
| Arginase I | Metabolizes L-arginine, another amino acid for essential T cell proliferation |
| iNOS | Produces immunosuppressive nitric oxide |
| COX2 | Produces immunosuppressive PGE2 |
| Small molecules | |
| PGE2 | Inhibits leukocyte functions through increased cyclic AMP levels |
| Epinephrine | Inhibits leukocyte functions through increased cyclic AMP levels |
| Adenosine | Inhibits leukocyte functions through increased cyclic AMP levels |
| ROS | Inhibits leukocyte functions through superoxide generation |
| Viral-related products | |
| p15E (CKS-17, synthetic peptide) | Inhibits production of type I cytokines, upregulates IL-10 synthesis |
| EBI-3 (homologue of IL-12 p40) | Inhibits IL-12 production |
| Tumor-associated gangliosides | Inhibit IL-2–dependent lymphocyte proliferation, induce apoptotic signals, suppress nuclear factor κB activation, interfere with DC generation |
FasL, Fas ligand; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand.
This partial listing of tumor-derived immunoinhibitory factors demonstrates the diversity of mechanisms that human tumors are known to have evolved to incapacitate the host immune system.
NEW INSIGHTS INTO ANTITUMOR IMMUNITY
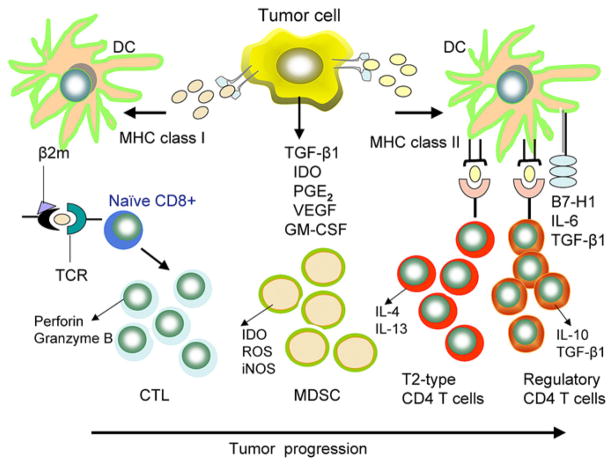
The field of tumor immunity has long suffered from a misconception that cancer cells are ignored by the immune system and that tumors are passive targets for antitumor responses. It is now certain that growing tumors attract components of both innate and adaptive host immunity.96 Although most TAs are self-antigens that are overexpressed or altered posttranscriptionally, immune responses to TAs, including those listed in Table I, are clearly made. A growing tumor releases TAs and produces numerous cytokines/chemokines, which attract immune cells, including DCs, to the tumor site and tumor-draining lymph nodes. These DCs take up TAs, maturing into IL-12–secreting cells, and process the TAs by using the APM components for the presentation to T cells as peptide–MHC class I–β2 m complexes. These T cells develop into TH1-type CD8+ CTLs (Fig 1). DCs can also take up and process another set of TAs through the MHC class II pathway, generating TH1-type CD4+ TH cells that produce IFN-γ and IL-2. These cells help to expand the population of TA peptide–specific CTLs, which are capable of eliminating the tumor through cytotoxic molecules, perforin, and granzymes. TH1-type help is essential for the generation of effective CTL responses. However, DCs taking up the same MHC class II–restricted TAs can also promote the development of Treg cells (Fig 1). Mechanisms involved in DC-mediated expansion of Treg cells, as opposed to TH1 (effector) cells or TH17 cells, are currently not understood, yet Treg cell accumulations at the tumor site and suppression by Treg cell of antitumor specific immunity appear to have adverse effects on the host’s ability to eliminate cancer and might influence prognosis.84 In contrast, accumulations of CD4+ TH17+ cells seem to predict a better survival in some cancers but in others correlate with tumor progression.47 In patients with cancer, cellular interactions between TA-presenting DCs and T cells preferentially lead to expansion and accumulation Treg cells and MDSCs at the tumor site and in the periphery.52 It appears that tumors have the capability to enhance the maturation of a distinct type of DC that does not promote the generation of TA-specific TH1 cells but instead is programmed to induce Treg cells and to recruit MDSCs (Fig 1). The proinflammatory cytokines IL-6 and TNF-α produced by these DCs in combination with tumor-derived soluble immunoinhibitory factors appear to be important for shifting the balance of immune response from immunogenic to tolerogenic.
FIG 1.
Effects of the tumor on immune cells. In the tumor microenvironment an excess of immunoinhibitory factors favors the generation and expansion of TH2-type T cells and Treg cells ather than CTLs and TH1-type effector cells. The downregulation of MHC molecules and defects in the APM components in DCs, as well as the immunosuppressive effects of accumulating MDSCs on DC maturation and function, contributes to the polarization of immune responses toward tolerance and away from immunity. The balance between stimulatory and suppressive responses shifts in favor of suppression as the tumor grows. Immune therapies are expected to shift this balance back to TH1-type responses, which promote expansion of CD4+ TH1 cells producing IFN-γ and IL-2, as well as CD8+ CTLs. IDO, Indoleamine 2,3-deoxygenase.
Thus signals delivered to T cells by DCs in the tumor microenvironment determine whether these T cells will develop into Treg or TH1 cells. These signals might be influenced by (1) the dose and type of TA processed by DCs, (2) the DC maturation status because immature DCs are known to induce tolerance rather than immunity, (3) the expression of costimulatory molecules on DCs, and (4) the effects of cytokines produced by interacting DCs and T cells on the induction of Treg versus TH1 cells.
At the time human tumors are diagnosed, the balance between immunogenic and tolerogenic signals delivered to immune cells is strongly skewed toward tolerance, mainlybecause of tumor-induced suppression. Therefore immune therapies administered in the minimal residual disease setting and designed to augment antitumor TH1-type CD4+T cells and CTLs are expected to tip the balance in favor of immunostimulation and away from immunosuppression. For this reason, therapeutic antitumor vaccination strategies are considered a promising addition to conventional therapies for cancer. However, complexities of the tumor-induced immune suppression, which engages numerous molecular mechanisms, present a formidable challenge to antitumor therapies, including vaccines. Novel approaches targeting these mechanisms of immune suppression (Table V) are needed to improve the treatment of cancer.
TABLE V.
Potential strategies for the design of more effective antitumor therapies
| Induce and sustain activity and survival of CTLs and of nonspecific antitumor effector cells: passive or active immunotherapy with antibodies, immune cells, or antitumor vaccines |
| Prevent immune suppression |
| Inhibit production or activity of tumor-derived suppressive factors |
| Inhibit generation or functions of Treg cells and MDSCs |
| Alter tumor microenvironment |
| Optimize lymphocyte/DC functions in the tumor microenvironment to enhance TH1-type responses |
| Combine therapeutic antitumor vaccines with chemotherapy |
| Treat early disease or in an adjuvant setting |
CONCLUSIONS
The existing evidence for dysfunction and death of antitumor effector cells in tumor-bearing hosts introduces a new paradigm for immunotherapy of cancer. Although previous emphasis has been on activation of immune cells and upregulation of their antitumor functions, the current concept is to consider therapies that could block or reverse tumor escape, at the same time protecting immune cells from the influence of immunosuppressive factors present in the tumor microenvironment. These novel therapeutic strategies take advantage of the tremendous progress made recently in our basic understanding of interactions between the tumor and the host immune system. Current insights into these interactions suggest that combinations of conventional cancer therapies with newly designed DC-based vaccines and survival cytokines (eg, IL-2, IL-7, and IL-15) offer therapeutic benefits. Some of the other promising strategies under consideration for improvements in the effects of immune therapies are listed in Table V. It is expected that as molecular mechanisms used by tumors to avoid, bypass, or subvert the immune system of the host are becoming clear, novel and more effective antitumor therapies targeting these mechanisms will emerge in the near future.
Acknowledgments
Supported in part by National Institutes of Health grant PO1-CA109688 to Theresa L. Whiteside.
Abbreviations used
- Anx
Annexin V
- APC
Antigen-presenting cell
- APM
Antigen-processing machinery
- β2 m
β2-microglobulin
- CTL
Cytolytic T lymphocyte
- DC
Dendritic cell
- FasL
Fas ligand
- FOXP3
Forkhead box protein 3
- iNOS
Inducible nitric oxide synthase
- MDSC
Myeloid-derived suppressor cell
- NK
Natural killer
- PD-1
Programmed death 1
- PD-L1
Programmed death ligand 1
- PGE2
Prostaglandin E
- ROS
Reactive oxygen species
- STAT3
Signal transducer and activator of transcription 3
- TA
Tumor-associated antigen
- TAM
Tumor-associated macrophage
- TCR
T-cell receptor
- TIL
Tumor-infiltrating lymphocyte
- Treg
Regulatory T
- VEGF
Vascular endothelial growth factor
Footnotes
Disclosure of potential conflict of interest. T. L. Whiteside has declared that she has no conflict of interest.
References
- 1.Romero P, Cerottini JC, Speiser DE. The human T cell response to melanoma antigens. Adv Immunol. 2006;92:187–224. doi: 10.1016/S0065-2776(06)92005-7. [DOI] [PubMed] [Google Scholar]
- 2.Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 2006;6:836–48. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- 3.Zhang L, Zhou W, Velculescu VE, Kern SE, Hruban RH, Hamilton SR, et al. Gene expression profiles in normal and cancer cells. Science. 1997;276:1268–72. doi: 10.1126/science.276.5316.1268. [DOI] [PubMed] [Google Scholar]
- 4.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–8. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 5.Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27:5904–12. doi: 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kornstein MJ, Brooks JS, Elder DE. Immunoperoxidase localization of lymphocyte subsets in the host responses to melanoma and nevi. Cancer Res. 1983;43:2749–53. [PubMed] [Google Scholar]
- 7.Von Kleist S, Berling J, Bohle W, Wittekind C. Immunohistochemical analysis of lymphocyte subpopulations infiltrating breast carcinomas and benign lesions. Int J Cancer. 1987;40:18–23. doi: 10.1002/ijc.2910400105. [DOI] [PubMed] [Google Scholar]
- 8.Whiteside TL. Tumor infiltrating lymphocytes in human malignancies. Austin (TX): R.G. Landes Co; 1993. [Google Scholar]
- 9.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–4. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 10.Galon J, Fridman W-H, Pages F. The adaptive immunologic microenvironment in colorectal cancer: a novel perspective. Cancer Res. 2007;67:1883–6. doi: 10.1158/0008-5472.CAN-06-4806. [DOI] [PubMed] [Google Scholar]
- 11.Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, et al. Effector memory T cells, early metastasis and survival in colorectal cancer. N Engl J Med. 2005;353:2654–66. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 12.Camus M, Tosolini M, Mlecnik B, Pages F, Kirilovsky A, Berger A, et al. Coordination of intratumoral immune reaction and human colorectal cancer recurrence. Cancer Res. 2009;69:2685–93. doi: 10.1158/0008-5472.CAN-08-2654. [DOI] [PubMed] [Google Scholar]
- 13.Lee PP, Yee C, Savage PA, Fong L, Brockstedt D, Weber JS, et al. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat Med. 1999;5:677–85. doi: 10.1038/9525. [DOI] [PubMed] [Google Scholar]
- 14.Pittet MJ, Speiser DE, Lienard D, Valmore D, Guillaume P, Dutoit V, et al. Expansion and functional maturation of human tumor antigen-specific CD8 + T cells after vaccination with antigenic peptide. Clin Cancer Res. 2001;7:796s–803. [PubMed] [Google Scholar]
- 15.Hoffmann TK, Donnenberg AD, Finkelstein SD, Donnenberg VS, Friebe-Hoffmann F, Myers EN, et al. Frequencies of tetramer+ T cells specific for the wild-type sequence p53264–272 peptide in the circulations of patients with head and neck cancer. Cancer Res. 2002;62:3521–9. [PubMed] [Google Scholar]
- 16.Sahin U, Tureci O, Pfreundschuh M. Serologic identification of human tumor antigens. Curr Opin Immunol. 1997;9:709–16. doi: 10.1016/s0952-7915(97)80053-2. [DOI] [PubMed] [Google Scholar]
- 17.Matzinger P. An innate sense of danger. Semin Immunol. 1998;10:399–415. doi: 10.1006/smim.1998.0143. [DOI] [PubMed] [Google Scholar]
- 18.Szczepanski MJ, Czystowska M, Szajnik M, Harasymczuk M, Boyiadzis M, Kruk-Zagajewska A, et al. Triggering of toll-like receptor 4 expressed on human head and neck squamous cell carcinoma promotes tumor development and protects the tumor from immune attack. Cancer Res. 2009;69:3105–13. doi: 10.1158/0008-5472.CAN-08-3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uematsu S, Akira S. Toll-like receptors and innate immunity. J Mol Med. 2006;84:712–25. doi: 10.1007/s00109-006-0084-y. [DOI] [PubMed] [Google Scholar]
- 20.Hollstein M, Sidransky D, Vogelstein B, Harris C. p53 mutations in human cancers. Science. 1991;253:49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- 21.Provinciali M. Immunosenescence and cancer vaccines. Cancer Immunol Immunother. 2009;58:1959–67. doi: 10.1007/s00262-009-0665-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrone S, Whiteside TL. Tumor microenvironment and immune escape. Surg Oncol Clin North Am. 2007;16:755–74. doi: 10.1016/j.soc.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Ferris RL, Whiteside TL, Ferrone S. Immune escape associated with functional defects in antigen processing machinery in head and neck cancer. Clin Cancer Res. 2006;12:3890–5. doi: 10.1158/1078-0432.CCR-05-2750. [DOI] [PubMed] [Google Scholar]
- 24.Stickel JS, Weinzieri AO, Hillen N, Drews O, Schuler MM, Hennenlotter J, et al. HLA ligand profile of primary renal cell carcinoma maintained in metastases. Cancer Immunol Immunother. 2009;58:1407–17. doi: 10.1007/s00262-008-0655-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fearon DT, Locksley RM. The instructive role of innate immunity in the acquired immune response. Science. 1996;272:50–4. doi: 10.1126/science.272.5258.50. [DOI] [PubMed] [Google Scholar]
- 26.Irving BA, Weiss A. The cytoplasmic domain of the T cell receptor zeta chain is sufficient to couple to receptor-associated signal transduction pathways. Cell. 1991;64:891–901. doi: 10.1016/0092-8674(91)90314-o. [DOI] [PubMed] [Google Scholar]
- 27.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–48. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 28.Kalinski P, Hilkens CM, Wierenga EA, Kapsenberg ML. T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol Today. 1999;20:561–7. doi: 10.1016/s0167-5699(99)01547-9. [DOI] [PubMed] [Google Scholar]
- 29.Whiteside TL. Immune suppression in cancer: effects on immune cells, mechanisms and future therapeutic interventions. Semin Cancer Biol. 2006;16:3–15. doi: 10.1016/j.semcancer.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 30.Reichert TE, Strauss L, Wagner EM, Gooding W, Whiteside TL. Signaling abnormalities and reduced proliferation of circulating and tumor-infiltrating lymphocytes in patients with oral carcinoma. Clin Cancer Res. 2002;8:3137–45. [PubMed] [Google Scholar]
- 31.Uzzo RG, Clark PE, Rayman P, Bloom T, Rybicki L, Novick AC, et al. Alterations in NFκB activation in T lymphocytes of patients with renal cell carcinoma. J Natl Cancer Inst. 1999;91:718–21. doi: 10.1093/jnci/91.8.718. [DOI] [PubMed] [Google Scholar]
- 32.Reichert TE, Rabinowich H, Johnson JT, Whiteside TL. Human immune cells in the tumor microenvironment: mechanisms responsible for signaling and functional defects. J Immunother. 1998;21:295–306. doi: 10.1097/00002371-199807000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Cavallo F, Offringa R, van der Burg SH, Forni G, Melief CJ. Vaccination for treatment and prevention of cancer in animal models. Adv Immunol. 2006;90:175–213. doi: 10.1016/S0065-2776(06)90005-4. [DOI] [PubMed] [Google Scholar]
- 34.Lollini PL, De Giovanni C, Pannellini T, Cavallo F, Forni G, Nanni P. Cancer immunoprevention. Future Oncol. 2005;1:57–66. doi: 10.1517/14796694.1.1.57. [DOI] [PubMed] [Google Scholar]
- 35.Pannellini T, Forni G, Musiani P. Immunobiology of her-2/neu transgenic mice. Breast Dis. 2004;20:33–42. doi: 10.3233/bd-2004-20105. [DOI] [PubMed] [Google Scholar]
- 36.Parmiani G, De Filippo A, Novellino L, Castelli C. Unique human tumor antigens: immunobiology and use in clinical trials. J Immunol. 2007;178:1975–9. doi: 10.4049/jimmunol.178.4.1975. [DOI] [PubMed] [Google Scholar]
- 37.Finn OJ, Binder RJ, Brickner AG, Butterfield LH, Ferris RL, Kalinski P, et al. Human tumor antigens as targets of immunosurveillance and candidates for cancer vaccines. In: Gires O, Seliger B, editors. Tumor-associated antigens: identification, characterization, and clinical applications. Weinheim (Germany): Wiley-VCH; 2009. pp. 23–43. [Google Scholar]
- 38.Cheever M, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, et al. The prioritization of cancer antigens: a National Cancer Institute pilot project for the acceleration of translational research. Clin Cancer Res. 2009;15:5323–37. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smyth MJ, Dunn GP, Schreiber RD. Cancer immunosurveillance and immunoediting: the roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv Immunol. 2006;90:1–50. doi: 10.1016/S0065-2776(06)90001-7. [DOI] [PubMed] [Google Scholar]
- 40.Jager E, Karbach J, Gnjatic S, Neumann A, Bender A, Valmori D, et al. Recombinant vaccinia/fowlpox NY-ESO-1 vaccines induce both humoral and cellular NY-ESO-1-specific immune responses in cancer patients. Proc Natl Acad Sci U S A. 2006;103:14453–8. doi: 10.1073/pnas.0606512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whiteside TL. The local tumor microenvironment. In: Kaufmann H, Wolchok JD, editors. General principles of tumor immunotherapy: basic and clinical applications of tumor immunology. New York: Springer; 2007. pp. 145–67. [Google Scholar]
- 42.June CH. Adoptive T cell therapy for cancer in the clinic. J Clin Invest. 2007;117:1466–76. doi: 10.1172/JCI32446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bergmann C, Strauss L, Zeidler R, Lang S, Whiteside TL. Expansion of human T regulatory type 1 cells in the microenvironment of COX-2-overexpressing head and neck squamous cell carcinoma. Cancer Res. 2007;67:8865–73. doi: 10.1158/0008-5472.CAN-07-0767. [DOI] [PubMed] [Google Scholar]
- 44.Woo EY, Chu CS, Goletz TJ, Schlienger K, Yeh H, Coukos G, et al. Regulatory CD4 + CD25 + T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001;61:4766–72. [PubMed] [Google Scholar]
- 45.Strauss L, Bergmann C, Szczepanski M, Gooding W, Johnson TJ, Whiteside TL. A unique subset of CD4 + CD25highFOXP3 + T cells secreting IL-10 and TGF-β1 mediates suppression in the tumor microenvironment. Clin Cancer Res. 2007;13:4345–54. doi: 10.1158/1078-0432.CCR-07-0472. [DOI] [PubMed] [Google Scholar]
- 46.Mandapathil M, Szczepanski MJ, Szajnik M, Ren J, Lenzner DE, Jackson EK, et al. Increased ectonucleotidase expression and activity in Treg of patients with head and neck cancer. Clin Cancer Res. 2009;15:6348–57. doi: 10.1158/1078-0432.CCR-09-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, Wei S, et al. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor microenvironment. Blood. 2009;114:1141–9. doi: 10.1182/blood-2009-03-208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martinez O, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–61. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 49.Leek RD, Lewis CE, Whitehouse R, Geenall M, Clarke J, Harris AL. Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res. 1996;56:4625–9. [PubMed] [Google Scholar]
- 50.Serafini P, Borello I, Bronte V. Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Semin Cancer Biol. 2006;16:53–65. doi: 10.1016/j.semcancer.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 51.Ochoa AC, Zea AH, Hernandez C, Rodríguez PC. Arginase, prostaglandins, and myeloid supressor cells in renal cell carcinoma. Clin Cancer Res. 2007;13:721s–6s. doi: 10.1158/1078-0432.CCR-06-2197. [DOI] [PubMed] [Google Scholar]
- 52.Diebold SS. Determination of T-cell fate by dendritic cells. Immunol Cell Biol. 2008;86:389–97. doi: 10.1038/icb.2008.26. [DOI] [PubMed] [Google Scholar]
- 53.Gabrilovich DI, Chen HL, Girgis KR, Cunningham HT, Meny GM, Nadaf S, et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med. 1996;2:1096–103. doi: 10.1038/nm1096-1096. [DOI] [PubMed] [Google Scholar]
- 54.Shurin GV, Shurin MR, Bykovskaja S, Shogan J, Lotze MT, Barksdale EM. Neuroblastoma-derived gangliosides inhibit dendritic cell generation and function. Cancer Res. 2001;61:363–9. [PubMed] [Google Scholar]
- 55.Reichert TE, Scheuer C, Day R, Wagner W, Whiteside TL. The number of intra-tumoral dendritic cells and ζ-chain expression in T cells as prognostic and survival biomarkers in patients with oral carcinoma. Cancer. 2001;91:2136–47. [PubMed] [Google Scholar]
- 56.Murphy GF, Radu A, Kaminer M, Berg D. Autologous melanoma vaccine induces inflammatory responses in melanoma metastases: relevance to immunologic regression and immunotherapy. J Invest Dermatol. 1993;100(suppl):335S–41. doi: 10.1111/1523-1747.ep12470236. [DOI] [PubMed] [Google Scholar]
- 57.Lanier LL. Natural killer cell receptor signaling. Curr Opin Immunol. 2003;15:308–14. doi: 10.1016/s0952-7915(03)00039-6. [DOI] [PubMed] [Google Scholar]
- 58.Chang CC, Campoli M, Ferrone S. Classical and nonclassical HLA class I antigen and NK cell-activating ligand changes in malignant cells: current challenges and future direction. Adv Cancer Res. 2005;93:189–234. doi: 10.1016/S0065-230X(05)93006-6. [DOI] [PubMed] [Google Scholar]
- 59.Kelly JM, Darcy PK, Markby JL, Godfrey DI, Takeda K, Yagitab H, et al. Induction of tumor-specific T cell memory by NK cell-mediated tumor rejection. Nat Immunol. 2002;3:83–90. doi: 10.1038/ni746. [DOI] [PubMed] [Google Scholar]
- 60.Coronella JA, Spier C, Welch M, Trevor KT, Stopeck AT, Villar H, et al. Antigen-driven oligoclonal expansion of tumor-infiltrating B cells in infiltrating ductal carcinoma of the breast. J Immunol. 2002;169:1829–36. doi: 10.4049/jimmunol.169.4.1829. [DOI] [PubMed] [Google Scholar]
- 61.Denko NC, Fontana LA, Hudson KM, Sutphin PD, Raychaudhuri S, Altman R, et al. Investigating hypoxic tumor physiology through gene expression patterns. Oncogene. 2003;22:5907–14. doi: 10.1038/sj.onc.1206703. [DOI] [PubMed] [Google Scholar]
- 62.Aller MA, Arias JL, Nava MP, Arias J. Posttraumatic inflammation is a complex response based on the pathological expression of the nervous, immune and endocrine function systems. Exp Biol Med. 2004;229:170–81. doi: 10.1177/153537020422900206. [DOI] [PubMed] [Google Scholar]
- 63.Dworacki G, Meidenbauer N, Kuss I, Hoffmann TK, Gooding MS, Lotze M, et al. Decrease ζ chain expression and apoptosis in CD3+ peripheral blood T lymphocytes of patients with melanoma. Clin Cancer Res. 2001;7(suppl):947s–57. [PubMed] [Google Scholar]
- 64.Finke JH, Rayman P, George R, Tannenbaum CS, Kolenko V, Uzzo R, et al. Tumor-induced sensitivity to apoptosis in T cells from patients with renal cell carcinoma: role of nuclear factor kappa B suppression. Clin Cancer Res. 2001;7(suppl):940s–6. [PubMed] [Google Scholar]
- 65.Whiteside TL. Tumor-induced death of immune cells: its mechanisms and consequences. Semin Cancer Biol. 2001;12:43–50. doi: 10.1006/scbi.2001.0402. [DOI] [PubMed] [Google Scholar]
- 66.Kuss I, Schaefer C, Godfrey TE, Ferris RL, Harris J, Gooding W, et al. Recent thymic emigrants and subsets of naïve and memory T cells in the circulation of patients with head and neck cancer. Clin Immunol. 2005;116:27–36. doi: 10.1016/j.clim.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 67.Kim W-J, Tsukishiro T, Johnson JT, Whiteside TL. Expression of pro- and anti-apoptotic proteins in circulating CD8 + T cells of patients with squamous cell carcinoma of the head and neck (SCCHN) Clin Cancer Res. 2004;10:5101–10. doi: 10.1158/1078-0432.CCR-04-0309. [DOI] [PubMed] [Google Scholar]
- 68.Kim J-W, Ferris RL, Whiteside TL. Chemokine receptor 7 (CCR7) expression and protection of circulating CD8 + T lymphocytes from apoptosis. Clin Cancer Res. 2005;11:7901–10. doi: 10.1158/1078-0432.CCR-05-1346. [DOI] [PubMed] [Google Scholar]
- 69.Kuss I, Saito T, Johnson JT, Whiteside TL. Clinical significance of decreased ζ chain expression in peripheral blood lymphocytes of patients with head and neck cancer. Clin Cancer Res. 1999;5:329–34. [PubMed] [Google Scholar]
- 70.Hoffmann TK, Dworacki G, Meidenbauer N, Gooding W, Johnson JT, Whiteside TL. Spontaneous apoptosis of circulating T lymphocytes in patients with head and neck cancer and its clinical importance. Clin Cancer Res. 2002;8:2553–62. [PubMed] [Google Scholar]
- 71.Whiteside TL. The role of death receptor ligands in shaping tumor microenvironment. Immunol Invest. 2007;36:25–46. doi: 10.1080/08820130600991893. [DOI] [PubMed] [Google Scholar]
- 72.O’Connell J, O’Sullivan GD, Collins JK, Shanahan F. The Fas counterattack: Fas-mediated T cell killing by colon cancer cells expressing Fas ligand. J Exp Med. 1996;184:1075–82. doi: 10.1084/jem.184.3.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Whiteside TL. Lymphocyte homeostasis and the antitumor immune response. Exp Rev Clin Immunol. 2006;1:369–78. doi: 10.1586/1744666X.1.3.369. [DOI] [PubMed] [Google Scholar]
- 74.Albers AE, Visus C, Tsukishiro T, Ferris RL, Gooding W, Whiteside TL, et al. Alterations in the T-cell receptor variable β gene-restricted profile of CD8 + T lymphocytes in the peripheral circulation of patients with squamous cell carcinoma of the head and neck. Clin Cancer Res. 2006;12:2394–403. doi: 10.1158/1078-0432.CCR-05-1818. [DOI] [PubMed] [Google Scholar]
- 75.Whiteside TL. Immune effector cells in the tumor microenvironment: their role in regulation of tumor progression. In: Yefenof E, editor. Innate and adaptive responses in the tumor microenvironment. New York: Springer; 2008. pp. 1–34. [Google Scholar]
- 76.Vujanovic NL, Nagashima S, Herberman RB, Whiteside TL. Non-secretory apoptotic killing by human natural killer cells. J Immunol. 1996;157:1117–26. [PubMed] [Google Scholar]
- 77.Whiteside TL, Vujanovic NL, Herberman RB. Natural killer cells and tumor therapy. Curr Topics Microbiol Immunol. 1998;230:221–44. doi: 10.1007/978-3-642-46859-9_13. [DOI] [PubMed] [Google Scholar]
- 78.Hansson M, Asea A, Ericsson U, Hermodsson S, Hellstrand K. Induction of apoptosis in NK cells by monocyte-derived reactive oxygen metabolites. J Immunol. 1996;156:42–7. [PubMed] [Google Scholar]
- 79.Whiteside TL. Apoptosis of immune cells in the tumor microenvironment and peripheral circulation of patients with cancer: implications for immunotherapy. Vaccine. 2002;20:A46–51. doi: 10.1016/s0264-410x(02)00387-0. [DOI] [PubMed] [Google Scholar]
- 80.Smyth MF, Godfrey DI. NKT cells and tumor immunity—a double-edged sword. Nat Immunol. 2000;1:459–60. doi: 10.1038/82698. [DOI] [PubMed] [Google Scholar]
- 81.Gershon RK. A disquisition on suppressor T cells. Transpl Rev. 1975;26:170–85. doi: 10.1111/j.1600-065x.1975.tb00179.x. [DOI] [PubMed] [Google Scholar]
- 82.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev. 2003;3:253–7. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 83.Bergmann C, Strauss L, Wang Y, Szczepanski MJ, Lang S, Johnson JT, et al. T regulatory type 1 cells (Tr1) in squamous cell carcinoma of the head and neck: mechanisms of suppression and expansion in advanced disease. Clin Cancer Res. 2008;14:3706–15. doi: 10.1158/1078-0432.CCR-07-5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma foster immune privilege and predicts reduced survival. Nat Med. 2004;10:942–9. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 85.Salama P, Phillips M, Grieu P, Morris M, Zeps N, Joseph D, et al. Tumor-infiltrating FOXP3 + T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol. 2008;27:186–92. doi: 10.1200/JCO.2008.18.7229. [DOI] [PubMed] [Google Scholar]
- 86.Martin-Orozco N, Dong C. The IL-17/IL23 axis of inflammation in cancer: friend or foe? Curr Opin Investig Drugs. 2009;10:543–9. [PubMed] [Google Scholar]
- 87.Voo KS, Wang Y-H, Santori FR, Boggiano C, Wang Yi-H, Arima K, et al. Identification of IL-17-producing FOXP+ regulatory T cells in humans. Proc Natl Acad Sci U S A. 2009;106:4793–8. doi: 10.1073/pnas.0900408106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Munn DH, Mellor AL. Indoleamine 2, 3-dioxygenase and tumor-induced tolerance. J Clin Invest. 2007;117:1147–54. doi: 10.1172/JCI31178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci U S A. 1993;90:3539–43. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Filipazzi F, Valenti R, Huber V, Pilla L, Canese P, Iero M, et al. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol. 2007;25:2546–53. doi: 10.1200/JCO.2006.08.5829. [DOI] [PubMed] [Google Scholar]
- 91.Azuma T, Yao S, Zhu G, Flies SJ, Chen L. B7-H1 is a ubiquitous antiapoptotic receptor on cancer cells. Blood. 2008;111:3635–43. doi: 10.1182/blood-2007-11-123141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Blank C, Mackensen A. Contribution of the PD-L1/PD-1 pathway to T-cell exhaustion: an update on implications for chronic infections and tumor evasion. Cancer Immunol Immunother. 2007;56:739–45. doi: 10.1007/s00262-006-0272-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang W, Lau R, Yu D, Zhu W, Korman A, Weber J. PD1 blockade reverses the suppression on melanoma antigen-specific CTL by CD4 + CD25hi regulatory T cells. Int Immunol. 2009;21:1065–77. doi: 10.1093/intimm/dxp072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hirano F, Kaneko K, Tamura H, Dong H, Wang S, Ichikawa M, et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005;65:1089–96. [PubMed] [Google Scholar]
- 95.Wang L, Yi T, Kortylewski M, Pardoll DM, Zeng D, Yu H. IL-17 can promote tumor growth through an IL-6-Stat3 signalling pathway. J Exp Med. 2009;206:1457–64. doi: 10.1084/jem.20090207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Finn OJ. Cancer immunology. N Engl J Med. 2008;358:2704–15. doi: 10.1056/NEJMra072739. [DOI] [PubMed] [Google Scholar]