Abstract
Background
Tumor treatment is the mainstay of therapy for paraneoplastic neurologic disorders (PNDs), but it is only effective in some cases and other treatment options are limited.
Objective
To evaluate the short-term use of a combination of prednisone and tacrolimus for acute neurologic worsening in PND in which intracellular antigens are targeted.
Design
Retrospective single-center case series of patients with PND treated with tacrolimus.
Setting
The Rockefeller University Hospital, a research hospital in New York, New York.
Patients
Twenty-six patients with PND with high titer (≥1:1000) anti-HuD, anti-Yo, or anti-CRMP5 auto-antibodies were enrolled. Patients were referred from Memorial Sloan-Kettering Cancer Center or self-referred. Two patients discontinued intervention owing to adverse events.
Interventions
Patients were treated with tacrolimus, 0.15–0.30 mg/kg per day, in 2 divided oral doses with 60 mg per day of oral prednisone tapered off during 1 to 4 weeks.
Main Outcome Measures
The primary outcome measure was median survival. Neurologic examinations before and after treatment as well as adverse events are described.
Results
Median survival time was 52 months from time of diagnosis. Some patients experienced neurologic improvement that was functionally meaningful. The incidence of adverse events was similar to that generally reported with tacrolimus.
Conclusions
A short course of prednisone and tacrolimus to target central nervous system T cells in patients with PND with acute neurologic decline in which intracellular antigens are targeted was well tolerated and warrants further study.
Paraneoplastic neurologic disorders (PNDs) are a rare group of disorders that are believed to develop when tumor cells express proteins normally restricted to neurons (onconeural proteins). Because neurons are immunologically privileged, aberrant onconeural protein expression in the periphery by the tumor can induce antitumor immune responses and these in turn can trigger autoimmune neurologic disease in the brain. The prognosis in PND is poor. Patients with the Hu syndrome survive 6 to 16 months after diagnosis.1–4 Although survival of patients with paraneoplastic cerebellar degeneration (PCD) associated with breast cancer is longer, reports of median survival of patients with PCD associated with gynecologic cancer is also poor, ranging from 5 to 22 months.3–5
Paraneoplastic neurologic disorders were originally defined as autoimmune by the detection of autoantibodies in patients’ serum and cerebrospinal fluid (CSF), and most treatment strategies to date have targeted the humoral immune response. One trial using intravenous immunoglobulin, methylprednisolone, and cyclophosphamide demonstrated a decrease in antibody titer but no clinical or survival benefit.3 Rituximab treatment was associated with neurologic improvement in one small study, with no correlation seen between clinical response and change in autoantibody titer.6 In another trial of plasma exchange plus either cyclophosphomide or conventional chemotherapy, 6 of 20 patients had an improvement in Rankin score, although 3 patients worsened clinically despite decreased serum antibody titer.7 The lack of correlation between change in antibody titer and clinical response,8 brain autopsy studies showing prominent CD8 T-cell infiltrates,9 and the demonstration of antigen-specific cytotoxic T cells in the blood and CSF of patients with PND10–12 indicate a role for T cells in the pathophysiology of some PNDs,9 specifically those in which the target antigen is intracellular.13
Tacrolimus (FK506) is a potent inhibitor of T cells, widely used for the prevention and treatment of transplant rejection. We hypothesized that tacrolimus may be useful in PND because it effectively crosses the blood brain barrier and T cells likely play a critical role in disease pathogenesis. However, when used long term, tacrolimus is associated with a 3- to 5-fold increased incidence of malignancy.14 Because of concerns about tumor outgrowth with prolonged immunosuppression in patients harboring antitumor immune responses, we developed a protocol involving a short-term treatment strategy, aimed at treating acute neurologic worsening in PND. The goal of combination treatment with high-dose steroid and tacrolimus was to induce cell death of activated autoimmune T cells in the CSF. Glucocorticoids have been shown to induce apoptosis in mature human T cells,15 and FK506 has been shown to potentiate this effect16–18 and have good CSF penetration owing to its lipid solubility.19 We report here our experience using tacrolimus and prednisone as a treatment for patients with PND with immune responses to the intracellular antigens Hu, Yo, and CRMP5. In patients with CSF pleocytosis, this treatment reduced CSF white blood cell (WBC) counts, although 2 such patients developed recurrent pleocytosis requiring retreatment. These observations, together with our clinical results, suggest that this treatment strategy warrants further exploration in patients in which intra-cellular antigens are targeted, perhaps in combination with other agents that inhibit WBC entry into the CSF.
METHODS
PATIENTS
This was a retrospective case series conducted at The Rockefeller University Hospital, a research hospital in New York. Patients were referred from Memorial Sloan-Kettering Cancer Center or self-referred. Inclusion criteria included the presence of high titer antibodies (≥1:1000) to PND antigens and neurologic symptoms consistent with PND. Patients were treated if they were acutely flaring with worsening neurologic signs or symptoms (within 6 months) and were retreated if they subsequently worsened. Exclusion criteria included a creatinine level of 2 mg/dL or greater (to convert to micromoles per liter, multiply by 88.4); a WBC count of 3800/μL or less (to convert to ×109 per liter, multiply by 0.001); a platelet level of 120×103/μL or less (to convert to×109 per liter, multiply by 1.0); a hemoglobin level of 8.5 g/dL or less (to convert to grams per liter, multiply by 10.0); known central nervous system metastasis; known active additional malignancy other than non-melanoma skin cancer; known active infection with hepatitis B or C, human immunodeficiency virus, or syphilis; New York Heart Association classification 3 or 4 status; pulmonary disease that limits daily activities; or intravenous drug use.
Treatment consisted of tacrolimus, 0.15–0.30 mg/kg per day, in 2 divided oral doses in conjunction with 60 mg per day of oral prednisone tapered off during 1 to 4 weeks. Patients were usually treated as inpatients at The Rockefeller University Hospital for the first 10 days of treatment and followed up as outpatients. Treatment was given for no more than 4 weeks, given the theoretical concern that prolonged suppression of the PND T-cell response could lead to tumor outgrowth.20
This protocol was approved by The Rockefeller University institutional review board. Written informed consent was obtained from all patients. This study is registered on www.clinicaltrials.gov as NCT00378326.
DATA COLLECTION
Neurologic history and physical examination were documented before treatment and on the last day of inpatient care. Because of variability in duration of hospitalization, examinations were not completed at uniform points (range, 5–24 days; median, 14 days from initiation of therapy). Patients were monitored for adverse events (AEs) and events were graded using the Common Toxicity Criteria of the National Cancer Institute. Survival was assessed by follow-up phone calls, e-mails and queries to the Social Security Administration’s Death Master File through 2010. Patients also underwent pre- and post-treatment lumbar puncture for CSF analysis and leukapheresis for immunologic assessments.
STATISTICAL ANALYSIS
The Wilcoxon signed rank test was used to determine whether there was a difference between pre- and posttreatment CSF WBC counts in patients with pretreatment pleocytosis. P<.05 was considered statistically significant. The Kaplan-Meier curve was used to describe survival, with 95% confidence intervals. The Mann-Whitney U test was used to assess whether there was a relationship between the extent of disease or response to tumor treatment and median survival time. Ninety-five percent confidence intervals were calculated using the binomial exact method to determine whether the AEs that occurred in this study were within range of reported values for tacrolimus toxicity.
RESULTS
PATIENT CHARACTERISTICS
Between January 14, 1998, and May 20, 2008, 26 patients with PND with worsening neurologic symptoms were enrolled (eFigure, http://www.archneurol.com) (Table 1). There were 5 patients who initially experienced subjective improvement, then subsequently worsened after discharge and returned for retreatment. The treatment intervals in the 5 retreated patients ranged from 2 to 12 months, with an average of 7 months. All except 3 patients were treated with chemotherapy for their cancers. Five of 26 patients (19%) had a personal medical history of another autoimmune disorder as compared with 3% in the general American population,21 a difference that is statistically significant (exact binomial test; 95% CI, 0.07–0.39; P<.001).
Table 1.
Clinical Profile of Patients Treated With Tacrolimus and Prednisonea
| Age, y | Ab | Cancer | Neurologic Symptoms | Treatment With Chemotherapy | Treatment With Chemotherapy During or Within 3 mo of Tacrolimus Treatment |
Prior Immunotherapy | Time From PND Dx to Tacrolimus Treatment, mo |
Time From Most Recent Flare to Tacrolimus Treatment, mo |
Pretreatment Rankin Score | Survival Change in Neurologic Symptoms Posttreatment |
From Time of PND Dx, mo |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 52 | Yo | Breastb,c | PCD | Yes | Yes | None | 6 | 5 | 4 | Unchanged | 46 |
| 67 | Yo | Fallopian tubed,e | PCD | Yes | No | None | 8 | 8 | 4 | Unchanged | 53f |
| 56 | Yo | Fallopian tube and breastd,e | PCD | Yes | Yes | None | 4 | 1 | 4 | Worse, tremor | 44f |
| 31 | Yo | No known tumor, s/p myomectomy of benign fibroid tumorsg | PCD | No | NA | IVIG, prednisone | 2 | 1 | 4 | Improved, dysarthria | 50f |
| 65 | Yo | Ovarianc,d | PCD | Yes | Yes | None | 6 | 1 | 4 | Worse, tremor | 51 |
| 57 | Yo | Ovariand,e | PCD | Yes | Yes | None | 8 | Treatment 1: 2 Treatment 2: 5 |
4 | Treatment 1: worse, titubation, nystagmus, appendicular ataxia; Treatment 2 unchanged | 122 |
| 59 | Yo | Ovarianh,i | Cerebellar/brain stem syndrome | Yes | No | None | 8 | 1 | 4 | Improved, dysarthria, tongue movements | 29 |
| 59 | Yo | Ovarianc,d | PCD | Yes | Treatment 1: Yes Treatment 2: Yes Treatment 3: No Treatment 4: No |
None | 5 | Treatment 1: 4 Treatment 2: 3 Treatment 3: 1 Treatment 4: 6 |
4 | Treatment 1: improved, gait Treatment 2: improved, dysarthria Treatment 3: improved, appen- dicular ataxia Treatment 4: unchanged |
24j |
| 56 | Yo | Ovariand,i | PCD | Yes | Treatment 1: No Treatment 2: No Treatment 3: Yes |
None | 9 | Treatment 1: 4 Treatment 2: 1 Treatment 3: 1 |
4 | Treatment 1: improved, dysarthria Treatment 2: improved, dyarthria, gait, appen- dicular ataxia Treatment 3: improved, dysarthria |
37 |
| 67 | Yo | Ovarianc,h | PCD | Yes | No | IVIG | 1 | 4 | 4 | Unchanged | 56f |
| 45 | Yo | Ovarianc,h | PCD | Yes | Treatment 1: Yes Treatment 2: No |
IVIG, rituximab, post-tacrolimus treatment | 1 | Treatment 1: 1 Treatment 2: 1 |
Treatment 1:3 improved, Treatment 2: 4 |
Treatment 1: appen- dicular ataxia, nystagmus, resolved square wave jerks Treatment 2: improved, dysarthria, gait |
49 |
| 60 | Yo | Ovariand,i | PCD | Yes | No | IVIG, plasma- pheresis, steroids | 6 | 1 | 4 | Improved, dizziness | 27 |
| 50 | Yo | Ovarianh,i | PCD | Yes | No | None | 15 | 3 | 3 | Worse, peripheral neuropathy | 21 |
| 69 | Yo | Ovarianh,i | PCD | Yes | Yes | None | 0 | 1 | 4 | Worse, dysarthria, appen- dicular ataxia, weakness, tremor | 3 |
| 54 | Yo | Ovarianc,e | PCD | Yes | Yes | IVIG | 2 | 3 | 4 | Improved, dysarthria, truncal and appen- dicular ataxia | 2j |
| 75 | Yo | Ovarian and breastc,h | PCD | Yes | Yes | None | 1 | 1 | 4 | Improved, appen- dicular ataxia, nystagmus | 27 |
| 74 | Yo | Ovarian and breastc,d | PCD, sensory and motor neuropathy | Yes | Yes | None | 3 | 3 | 4 | Improved, gait, less pain | 59f |
| 52 | Yo | Unknown primary, groin LN with highly atypical cells favoring carci- nomac,d | PCD | Yes, post- tacrolimus treatment | No | IVIG, methylpred- nisolone | 3 | 1 | 4 | Improved, dysarthria | 83f |
| 60 | Yo | Unknown primary, high grade, serous, adenocar- cinoma, probable peritoneal originc,d | PCD | Yes | Yes | None | 1 | 1 | 4 | Improved; nystagmus | 64f |
| 56 | Hu | SCLCi,k | Sensory neuropathy | Yes | No | IVIG, prednisone, methotrexate, cyclosporin | 35 | 5 | 4 | Improved, peripheral neuropathy | 92 |
| 61 | Hu | SCLCc,k | Peripheral neuropathy, pan cerebellar syndrome | Yes | No | None | 12 | 1 | 4 | Improved, dysarthria | 73 |
| 73 | Hu | SCLCc,k | Sensory neuropathy | Yes | No | None | 6 | Treatment 1: 2 Treatment 2: 2 |
3 | Treatment 1: unchanged Treatment 2: not evaluable |
37j |
| 59 | Hu | SCLCc,k | Sensory neuropathy | Yes | No | None | 22 | 1 | 4 | Improved, peripheral neuropathy | 70f |
| 63 | Hu | SCLCc,k | Paraneo- plastic encephalo- myelitis | Yes | No | None | 1 | 1 | 5 | Unchanged | 3 |
| 78 | Hu | SCLCk,l | Autonomic dysfunction, GI dysmo- tility, sensory neuropathy | No | No | None | 27 | 2 | 4 | Unchanged | 16 |
| 71 | C5 | Small cell carcinoma of probable lung originl,m | PCD, limbic encepha- litis, frontal involvement | No | NA | None | 0 | 3 | 5 | Worse, confusion, agitation | 1 |
Abbreviations: Ab, antibody; C5, CRMP5; Dx, diagnosis; GI, gastrointestinal; IVIG, intravenous immunoglobulin; LN, lymph node; NA, not applicable; PCD, paraneoplastic cerebellar disease; PND, paraneoplastic neurologic disorder; SCLC, small cell lung cancer; s/p, status post.
For extent of disease, the Veterans Affairs Lung Study Group staging system was used for patients with SCLC; local, regional, or metastatic was used for patients with gynecological cancer; and TNM staging was used for the patient with breast cancer.
Stage IIB.
Complete response to tumor treatment.
Extent of disease was regional.
Response to tumor treatment was not known.
Alive at the time of analysis.
Extent of disease was not available.
Metastatic.
Other response.
Lost to follow-up.
Extent of disease was limited.
No treatment.
Extent of disease was extensive.
The median time from the most recent flair of neurologic symptoms to time of treatment with tacrolimus was 1.5 months (range, <1 to 11 months). Patients were treated with an initial tacrolimus dose of 0.15–0.32 mg/kg per day given for 2 to 32 days (median, 10 days). The median trough level measured after the fifth dose was 15.1 ng/mL (range, 5.3 to >60 ng/mL; target range, 5–20 ng/mL).
This case series was uncontrolled and not designed to quantify neurologic outcomes. While most neurologic symptom changes were minor, there were some anecdotal incidences of significant improvement (Table 1). For example, 2 patients (1 with PCD and 1 with Hu syndrome) who had garbled speech became able to communicate after treatment. One patient required full assistance with meals but was able to feed herself after treatment. Another patient who was wheelchair bound and unable to bear her own weight before treatment be- came able to walk 30 to 50 steps with a walker, mobility she did not previously have. These outcomes were functionally meaningful with significant effects on quality of life.
CSF PLEOCYTOSIS
In 19 of 34 treatment events (56%), both pre- and post-treatment CSF samples were available for analysis. The median WBC count was low and did not change after treatment (3 cells/mm3 for both pre- and posttreatment). In a subgroup analysis of 8 patients with pretreatment CSF pleocytosis (7 of which were sampled within 3 months of onset of symptoms), the median WBC count decreased from 9.5 to 5.5 cells/mm3 posttreatment and this difference was statistically significant (P = .03).
Although PND has been described as a relentlessly progressive disease, in our experience, some patients have a relapsing course. Five of 26 patients who initially improved clinically, subsequently developed recurrent flares of neurologic worsening and returned for retreatment. Repeated CSF studies were available for 2 of these patients. In both, CSF pleocytosis normalized after treatment (Figure 1). Clinical flares 1 and 10 months later, respectively, correlated with recurrent CSF pleocytosis. In 1 of the patients where repeat CSF studies were possible (Figure 1A), after treatment of this flair, CSF WBC values again returned to normal, and this was associated with another favorable clinical response. Thus, CSF WBCs can correlate with recurrent symptomatic neurologic flares in patients with PND, and decreases in CSF pleocytosis can be associated with neurologic improvement following tacrolimus treatment and retreatment.
Figure 1.
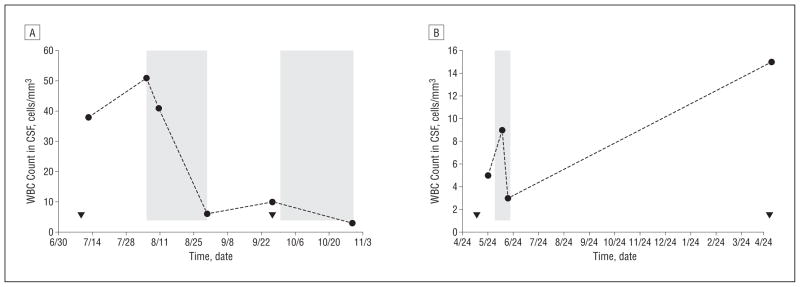
Cerebrospinal fluid (CSF) white blood cell (WBC) count correlates with onset of neurologic symptoms and treatment with tacrolimus. The CSF WBC counts of 2 patients with Yo antibodies with ovarian cancer are shown in the context of neurologic symptom development (triangles) and tacrolimus treatment duration (shaded areas) (A and B). The first CSF sample was obtained approximately 1 week prior to the onset of treatment (B).
SURVIVAL
One concern in immunosuppressing patients with PND with cancer is that their cancer, which may have been held partially in check by their immune system, might progress and cancer-related mortality would increase. The median survival from time of PND diagnosis for all patients was 52 months (Figure 2A). The median survival from initiation of treatment with tacrolimus was 48 months (Figure 2B). At the time of this writing, 8 patients were still alive, 3 were lost to follow-up, and 15 had died.
Figure 2.
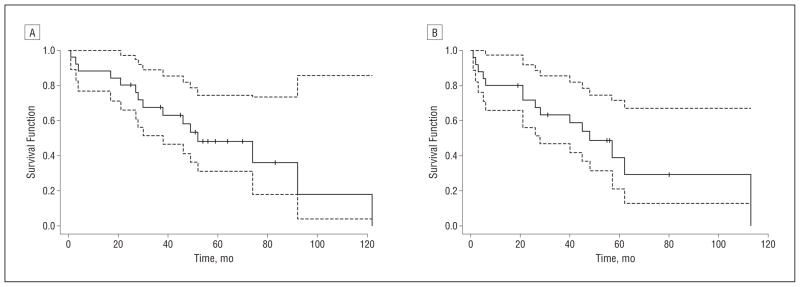
Kaplan-Meier survival curves. A, From time of diagnosis of paraneoplastic neurologic disorder. B, From start time of tacrolimus treatment. The dotted lines indicate 95% confidence intervals; the tick marks, censored patients; and the solid line, the survival curve.
Of the 26 patients enrolled in the study, the largest subgroup was 13 patients with Yo antibodies and ovarian cancer, all of whom received tumor treatment. The median survival from time of diagnosis in this subgroup was 38 months. Because tumor type has been demonstrated to be an independent predictor of survival in PCD,5 it stands to reason that the extent of disease and response to tumor treatment influence survival time. There was a trend toward shorter median survival times in patients with metastatic vs regional disease (27 and 44 months, respectively), but this difference was not statistically significant (P = .38). Similarly, there was a trend toward longer survival times in those with complete response to tumor treatment vs those with incomplete response (49 and 27 months, respectively), but this difference was not statistically significant (P = .29).
ADVERSE EVENTS
Twenty-three of 26 patients (88%) experienced at least 1 AE. Three of them were serious AEs resulting in hospitalization. One serious AE occurred in a Yo antibody–positive patient with PCD and ovarian cancer. She had been previously treated with numerous chemotherapy regimens, most recently within 2 months of tacrolimus treatment. After 6 days of treatment, her serum tacrolimus level was supratherapeutic (>60 ng/mL). She experienced worsening dysarthria, weakness, ataxia, and tremor. This did not abate even when levels normalized and she was transferred to an acute care hospital, where she continued treatment with tacrolimus for a total of 14 days with the addition of intravenous immunoglobulin. However, her neurologic status continued to deteriorate and she died 3 months later.
The second patient harbored a CRMP5 antibody and was encephalopathic at study entry. After only 5 days of tacrolimus treatment, her mental status further deteriorated and she began to refuse all food and medications. Tacrolimus treatment was discontinued and she was transferred to an acute care hospital to conduct a search for a primary tumor. At this hospital, she continued to have progressive encephalopathy and cerebellar degeneration. She died 33 days after discontinuation of tacrolimus treatment. At autopsy, she was found to have bacterial pneumonia as well as metastases of small cell carcinoma of probable lung origin in her brain, liver, adrenal glands, and thoracic lymph nodes. She was never treated with chemotherapy.
The third patient had Hu syndrome and small cell lung cancer. At study entry, she had a resolving productive cough and had just completed a course of levofloxacin. On the second day of treatment, she developed shortness of breath without a fever and was diagnosed as having pneumonia. This patient was not concurrently treated with chemotherapy. Tacrolimus treatment was discontinued and she was transferred to an acute care hospital. She responded to intravenous antibiotics and was discharged home 5 days later.
The incidence of all other AEs (Table 2), except low serum total protein and albumin levels and fatigue, was consistent with previous reports of toxicity in trials using oral tacrolimus for hepatic and renal transplants.22–24 Of the patients with Yo antibodies who had pre- and post- treatment CA125 levels available for analysis, the median pre- and posttreatment levels were 18 and 13 units/mL, respectively, both between normal limits (reference range, 0–35 units/mL).
Table 2.
Adverse Events
| Points, No. | Grade | ||
|---|---|---|---|
|
|
|
||
| Serious Adverse Events | 3 | ||
| Hospitalization, search for primary tumor | 1 | 4 | |
| Infection, lung | 1 | 4 | |
| Ataxia and weakness | 1 | 4 | |
| Infectious Adverse Events | 2 | ||
| Oral abscess | 1 | 3 | |
| Oral herpes simplex | 1 | 2 | |
|
Adverse Events (Grade 1 or 2) Occurring in >3 Patients |
Points, No. (%) | 95% CI | Reported Ranges, % |
| Tacrolimus, high | 13 (50) | 30–70 | |
| Headache | 12 (46) | 27–67 | 37–64 |
| BUN, high | 10 (38) | 20–59 | 30 |
| High blood pressure | 9 (35) | 17–56 | 38–50 |
| Glucose, high | 7 (27) | 12–48 | 22–47 |
| Nausea | 7 (27) | 12–48 | 32–46 |
| Total protein, low | 7 (27) | 12–48 | 3 |
| Albumin, low | 6 (23) | 9–44 | Reported |
| Dizziness | 6 (23) | 9–44 | 19 |
| Hematocrit, low | 5 (19) | 7–39 | 5–47 |
| Vomiting | 5 (19) | 7–39 | 14–29 |
| Fatigue | 4 (15) | 4–35 | Reported |
| Tremors | 4 (15) | 4–35 | 48–56 |
Abbreviation: BUN, blood urea nitrogen.
COMMENT
Owing to the rare incidence of PND (approximately 0.01% of patients with cancer), there are few controlled clinical trials to model evidence-based guidelines for effective treatment. Treatment decisions are often based on expert opinion and case series. The treatment of the underlying cancer is the mainstay of management, a prudent approach to reducing the burden of inciting antigen.2,25,26 Beyond treatment of the tumor, most efforts have aimed at suppression of humoral immune responses.27 Given the inadequate responses, we have attempted to align treatment strategies with our understanding of disease pathogenesis by undertaking a study targeting central nervous system T cells, treating 26 patients with PND who had autoimmunity to intracellular antigens with tacrolimus and prednisone.
In this retrospective case series, some subjective neurologic improvement was noted. We also found a statistically significant decrease in CSF WBCs in those patients with pretreatment pleocytosis, suggesting an acute treatment effect. Without a control arm, we cannot rule out the possibility that this represents the natural history of the disease, as one report notes that CSF T-cell counts decline over time,28 although in our experience this is typically a more protracted process without tacrolimus treatment. Moreover, 2 of 8 patients developed rebound pleocytosis between 1 and 10 months later, and in 1 case, we were able to again document an acute response to tacrolimus. These observations suggest that tacrolimus, a lipid soluble drug with good CSF penetration, may consistently and effectively reduce autoimmune CSF T cells. More generally, the lack of complete clinical response may relate to ongoing peripheral auto-immunity (eg, continuing immune response to residual PND antigen–expressing cancer cells), suggesting that combining tacrolimus with an agent that blocks entry of T cells into the CSF may be worth clinical consideration.
We assessed survival as a measure of long-term clinical response to tacrolimus. Statistically significant predictors of survival in PND include antibody type,4 tumor type,5 rate of tumor treatment,4,25 age older than 60 years,5,25 and poor baseline functional status.25 The cohort in this study was enriched with patients with those established risk factors for shorter survival. Seventeen of 19 patients with Yo antibodies (89%) in our cohort had a gynecologic cancer, a population that has significantly shorter survival times than patients with breast cancer.5 Moreover, the baseline performance status of our cohort of patients was very poor. Twenty-three of 26 patients could not walk without assistance or perform activities of daily living at enrollment. Finally, age older than 60 years has been shown to have a statistically significant effect on survival,5 and the median age of patients in our study was 60 years. Despite these poor risk factors, the survival in our cohort was higher than expected from the literature, suggesting the possibility that our acute intervention, directed at reducing CSF T cell–mediated autoimmunity, may have conferred a long-term survival advantage.
For example, the largest subgroup in our cohort, 13 patients with Yo antibodies and ovarian cancer (all of whom received tumor treatment, with a median age of 59 years), had a median survival from time of diagnosis of 38 months. Rojas et al5 reported a median survival time of 22 months in a similar subgroup with the same antibody type, tumor type, and rate of tumor treatment: 13 patients with ovarian cancer with Yo antibodies, all of whom were also given tumor treatment (with no reported mean age). Though these are well-matched groups, we recognize that our study did not include a control group. In addition, other factors may confound the longer survival time in this case series. For example, extent of disease and response to tumor treatment have not been shown to be independent predictors of survival, but approximately half of PND-related deaths are attributable to the underlying cancer.5,29 In our subgroup of patients with ovarian cancer, we did see a trend toward association between extent of disease or response to tumor treatment and survival, but these were not statistically significant; this may be owing to a small sample size. Another confounder of survival times in this study could be recruitment bias; half of the patients were self-referred, biasing this cohort toward families actively seeking aggressive treatment and research studies.
Tacrolimus was approved by the Food and Drug Administration in 1994; the long history of use of this widely prescribed drug means that its AE profile is well documented. The AEs experienced in this cohort were consistent with those in tacrolimus trials that led to its original approval. In conclusion, a brief trial of tacrolimus in combination with prednisone for PND offers little clinical downside, may confer an acute improvement in neurologic symptoms, and indeed may be associated with longer survival in patients with Yo antibodies and ovarian cancer. Future efforts might include combining tacrolimus with agents that target other components of cellular immunity such as B-cell depletion with rituximab or with inhibitors of lymphocyte trafficking such as natalizumab.
Acknowledgments
Funding/Support: This study was supported by grant R01 CA093507 from the National Institutes of Health, the American College of Rheumatology Research and Education Foundation Physician Scientist Development Award, and grants UL1RR024143 and 8 KL2 TR000151 from the National Institutes of Health’s National Center for Research Resources and National Center for Advancing Sciences to The Rockefeller University Hospital. Dr Orange is the Beth and Ravenel Curry clinical scholar, and Dr Darnell is a Howard Hughes Medical Institute investigator.
Footnotes
Financial Disclosure: None reported.
Additional Contributions: We are grateful to the patients with paraneoplastic neurologic disorders who participated in this study and their families as well as the entire staff of The Rockefeller University Hospital for facilitating patient studies. We thank Jerome Posner, MD, for helpful discussions and for referring many of the patients in this study. We also thank members of our laboratories for helpful discussions and review of the article.
Author Contributions: Drs Orange and Frank contributed equally to this article. Study concept and design: Darnell. Acquisition of data: Dousmanis, Marmur, Buckley, Parveen, and Graber. Analysis and interpretation of data: Orange, Frank, Tian, Dousmanis, Marmur, Graber, Blachère, and Darnell. Drafting of the manuscript: Orange, Frank, Tian, and Darnell. Critical revision of the manuscript for important intellectual content: Tian, Dousmanis, Marmur, Buckley, Parveen, Graber, Blachère, and Darnell. Statistical analysis: Frank and Tian. Obtained funding: Darnell. Administrative, technical, and material support: Orange, Frank, Marmur, Parveen, Graber, and Blachère. Study supervision: Darnell.
References
- 1.Dalmau J, Graus F, Rosenblum MK, Posner JB. Anti-Hu–associated paraneoplastic encephalomyelitis/sensory neuronopathy: a clinical study of 71 patients. Medicine (Baltimore) 1992;71(2):59–72. doi: 10.1097/00005792-199203000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Keime-Guibert F, Graus F, Broët P, et al. Clinical outcome of patients with anti-Hu-associated encephalomyelitis after treatment of the tumor. Neurology. 1999;53(8):1719–1723. doi: 10.1212/wnl.53.8.1719. [DOI] [PubMed] [Google Scholar]
- 3.Keime-Guibert F, Graus F, Fleury A, et al. Treatment of paraneoplastic neurological syndromes with antineuronal antibodies (Anti-Hu, anti-Yo) with a combination of immunoglobulins, cyclophosphamide, and methylprednisolone. J Neurol Neurosurg Psychiatry. 2000;68(4):479–482. doi: 10.1136/jnnp.68.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shams’ili S, Grefkens J, de Leeuw B, et al. Paraneoplastic cerebellar degeneration associated with antineuronal antibodies: analysis of 50 patients. Brain. 2003;126(pt 6):1409–1418. doi: 10.1093/brain/awg133. [DOI] [PubMed] [Google Scholar]
- 5.Rojas I, Graus F, Keime-Guibert F, et al. Long-term clinical outcome of paraneoplastic cerebellar degeneration and anti-Yo antibodies. Neurology. 2000;55 (5):713–715. doi: 10.1212/wnl.55.5.713. [DOI] [PubMed] [Google Scholar]
- 6.Shams’ili S, de Beukelaar J, Gratama JW, et al. An uncontrolled trial of rituximab for antibody associated paraneoplastic neurological syndromes. J Neurol. 2006;253(1):16–20. doi: 10.1007/s00415-005-0882-0. [DOI] [PubMed] [Google Scholar]
- 7.Vernino S, O’Neill BP, Marks RS, O’Fallon JR, Kimmel DW. Immunomodulatory treatment trial for paraneoplastic neurological disorders. Neuro Oncol. 2004;6(1):55–62. doi: 10.1215/S1152851703000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Furneaux HF, Reich L, Posner JB. Autoantibody synthesis in the central nervous system of patients with paraneoplastic syndromes. Neurology. 1990;40(7):1085–1091. doi: 10.1212/wnl.40.7.1085. [DOI] [PubMed] [Google Scholar]
- 9.Jean WC, Dalmau J, Ho A, Posner JB. Analysis of the IgG subclass distribution and inflammatory infiltrates in patients with anti-Hu-associated paraneoplastic encephalomyelitis. Neurology. 1994;44(1):140–147. doi: 10.1212/wnl.44.1.140. [DOI] [PubMed] [Google Scholar]
- 10.Albert ML, Darnell JC, Bender A, Francisco LM, Bhardwaj N, Darnell RB. Tumor-specific killer cells in paraneoplastic cerebellar degeneration. Nat Med. 1998;4(11):1321–1324. doi: 10.1038/3315. [DOI] [PubMed] [Google Scholar]
- 11.Albert ML, Austin LM, Darnell RB. Detection and treatment of activated T cells in the cerebrospinal fluid of patients with paraneoplastic cerebellar degeneration. Ann Neurol. 2000;47(1):9–17. [PubMed] [Google Scholar]
- 12.Roberts WK, Deluca IJ, Thomas A, et al. Patients with lung cancer and paraneoplastic Hu syndrome harbor HuD-specific type 2 CD8+T cells. J Clin Invest. 2009;119(7):2042–2051. doi: 10.1172/JCI36131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Darnell RB, Posner JB. Paraneoplastic Syndromes. Oxford, England: Oxford University Press; 2011. [Google Scholar]
- 14.Marcén R. Immunosuppressive drugs in kidney transplantation: impact on patient survival, and incidence of cardiovascular disease, malignancy and infection. Drugs. 2009;69(16):2227–2243. doi: 10.2165/11319260-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 15.Tuosto L, Cundari E, Gilardini Montani MS, Piccolella E. Analysis of susceptibility of mature human T lymphocytes to dexamethasone-induced apoptosis. Eur J Immunol. 1994;24(5):1061–1065. doi: 10.1002/eji.1830240508. [DOI] [PubMed] [Google Scholar]
- 16.Renoir JM, Mercier-Bodard C, Hoffmann K, et al. Cyclosporin A potentiates the dexamethasone-induced mouse mammary tumor virus-chloramphenicol acetyltransferase activity in LMCAT cells: a possible role for different heat shock protein-binding immunophilins in glucocorticosteroid receptor-mediated gene expression. Proc Natl Acad Sci U S A. 1995;92(11):4977–4981. doi: 10.1073/pnas.92.11.4977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tai PK, Albers MW, Chang H, Faber LE, Schreiber SL. Association of a 59-kilodalton immunophilin with the glucocorticoid receptor complex. Science. 1992;256(5061):1315–1318. doi: 10.1126/science.1376003. [DOI] [PubMed] [Google Scholar]
- 18.Migita K, Eguchi K, Kawabe Y, Origuchi T, Tominaga M, Nagataki S. FK506 potentiates steroid-induced T-cell apoptosis. Transplantation. 1997;64(9):1365–1369. doi: 10.1097/00007890-199711150-00022. [DOI] [PubMed] [Google Scholar]
- 19.Wallemacq PE, Reding R. FK506 (tacrolimus), a novel immunosuppressant in organ transplantation: clinical, biomedical, and analytical aspects. Clin Chem. 1993;39(11 pt 1):2219–2228. [PMC free article] [PubMed] [Google Scholar]
- 20.Darnell RB, Posner JB. Paraneoplastic syndromes involving the nervous system. N Engl J Med. 2003;349(16):1543–1554. doi: 10.1056/NEJMra023009. [DOI] [PubMed] [Google Scholar]
- 21.Jacobson DL, Gange SJ, Rose NR, Graham NM. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin Immunol Immunopathol. 1997;84(3):223–243. doi: 10.1006/clin.1997.4412. [DOI] [PubMed] [Google Scholar]
- 22.Randomised trial comparing tacrolimus (FK506) and cyclosporin in prevention of liver allograft rejection: European FK506 Multicentre Liver Study Group. Lancet. 1994;344(8920):423–428. [PubMed] [Google Scholar]
- 23.A comparison of tacrolimus (FK 506) and cyclosporine for immunosuppression in liver transplantation: the U S. Multicenter FK506 Liver Study Group. N Engl J Med. 1994;331(17):1110–1115. doi: 10.1056/NEJM199410273311702. [DOI] [PubMed] [Google Scholar]
- 24.Laskow DA, Vincenti F, Neylan J, Mendez R, Matas A. Phase II FK 506 multi-center concentration control study: one-year follow-up. Transplant Proc. 1995;27(1):809–811. [PubMed] [Google Scholar]
- 25.Graus F, Keime-Guibert F, Reñe R, et al. Anti-Hu-associated paraneoplastic encephalomyelitis: analysis of 200 patients. Brain. 2001;124(pt 6):1138–1148. doi: 10.1093/brain/124.6.1138. [DOI] [PubMed] [Google Scholar]
- 26.Sillevis Smitt P, Grefkens J, de Leeuw B, et al. Survival and outcome in 73 anti-Hu positive patients with paraneoplastic encephalomyelitis/sensory neuronopathy. J Neurol. 2002;249(6):745–753. doi: 10.1007/s00415-002-0706-4. [DOI] [PubMed] [Google Scholar]
- 27.Darnell RB, Posner JB. Paraneoplastic syndromes affecting the nervous system. Semin Oncol. 2006;33(3):270–298. doi: 10.1053/j.seminoncol.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 28.Psimaras D, Carpentier AF, Rossi C. PNS Euronetwork. Cerebrospinal fluid study in paraneoplastic syndromes. J Neurol Neurosurg Psychiatry. 2010;81(1):42–45. doi: 10.1136/jnnp.2008.159483. [DOI] [PubMed] [Google Scholar]
- 29.Giometto B, Grisold W, Vitaliani R, Graus F, Honnorat J, Bertolini G. PNS Euro-network. Paraneoplastic neurologic syndrome in the PNS Euronetwork database: a European study from 20 centers. Arch Neurol. 2010;67(3):330–335. doi: 10.1001/archneurol.2009.341. [DOI] [PubMed] [Google Scholar]


