Abstract
Fibromyalgia (FMS) is a chronic widespread pain and fatigue syndrome that affects 3 to 6 million adults in the United States. Core symptoms of fibromyalgia include pain, fatigue, and mood and sleep disturbances. To date, consensus has not been reached among researchers regarding the pathogenesis of FMS nor the specific role of cytokine activation on the neuroendocrine-immune response patterns in persons with FMS. The purpose of this article is to describe and synthesize the results of research studies focused on the relationship between cytokines and FMS and among cytokines and core symptoms of FMS. There is some support in the literature for relationships among FMS symptoms and cytokines; however, there are discrepant findings related to whether pro- and anti-inflammatory cytokines are elevated or reduced in persons with FMS and whether or not their levels correlate with the core symptoms of this disorder. Although the use of cytokine biomarkers must be considered exploratory at this time due to the lack of consistent empirical findings, biobehavioral research focused on understanding the relationship of FMS with cytokines may lead to a better understanding of this complex syndrome. This knowledge may ultimately contribute to the development of interventions for symptom management that address not only the symptom manifestation but also a biological mediator of symptoms.
Keywords: Fibromyalgia, Cytokines, Core Symptoms
Fibromyalgia (FMS) is a chronic widespread pain (CWP) and fatigue syndrome that affects three to six million adults in the United States and is one of the most common conditions seen in rheumatology clinics worldwide (Lawrence et al., 2008; Mease et al., 2007; National Institute of Arthritis and Musculoskeletal and Skin Diseases [NIAMS], 2004). The prevalence rate of FMS is approximately 2% (Lawrence et al., 2008). Although FMS affects individuals across the age span, more than 90% of FMS diagnoses are reported in women (Carpenter, 2006; Lawrence et al., 2008). With annual expenditures for the diagnosis and treatment of FMS at approximately U.S.$20 billion, FMS presents a significant burden to individuals, families, and society (Jones, Ross, Adams, & Bennett, 2006; Wallace, 2006). One of the major limitations in the research about and clinical care of individuals with FMS is the lack of objective markers to guide the diagnosis and progression of treatment or to predict response to treatment. Valid and reliable biomarkers would help to standardize the diagnosis of FMS, assist with monitoring the response to treatment, and improve the quality of outcomes research and clinical care (Mease et al., 2007).
Although there is much speculation about the etiology of FMS, one of the major theories is that cytokines may have a role in both the etiology of the syndrome and in the intensity of core symptoms. Core symptoms of FMS include pain, fatigue, and mood and sleep disturbances (Mease et al., 2007; Zautra, Fasman, Parish, & Davis, 2007). The purpose of this integrated literature review is to examine the current state of the science for the relationships between cytokines and FMS and its core symptoms and to discuss the potential usefulness of cytokines as biomarkers in FMS.
Background
The American College of Rheumatology’s (ACR) diagnostic criteria for FMS require concurrent presence of widespread pain in all four quadrants of the body for at least 3 months and the presence of at least 11 of 18 defined tender points with manual palpation using 4 kg of pressure (Katz, Wolfe, & Michaud, 2006; NIAMS, 2004; Wolfe et al., 1990). Originally developed for use in research, these criteria are not routinely used in clinical practice (Abeles, Pillinger, Solitar, & Abeles, 2007; Katz et al., 2006), where the diagnosis of FMS is often made without the formal tender-point examination (Katz et al., 2006). Experienced clinicians may not rely on the ACR criteria for diagnosis as tender points and widespread pain alone do not address associated psychosocial and distress features and the multiple symptom complaints associated with FMS (Katz et al., 2006; Wolfe, 2003). To date, there are no fully satisfactory explanations for the observed relationships among symptoms of FMS or accurate diagnoses leading to effective remedies (Smythe, 2006). Given the continuing debate about what measures should be used in the evaluation and diagnosis of FMS (Katz et al., 2006; Mease, 2005; Wolfe, 2003) and the absence of laboratory tests that may help establish FMS diagnosis (Bennett, 2008), the search for biomarkers of FMS is important for both clinical and research purposes.
There are multiple theories regarding the pathophysiology of FMS. One leading hypothesis suggests that FMS may involve aberrations of the hypothalamic-pituitary-adrenal (HPA) axis that are associated with or caused by cytokine imbalance (Abeles et al., 2007; Mease et al., 2007). Cytokines are immunomodulating proteins that have a wide range of biological activities. Generally classified into three categories (i.e., lymphokines and monokines, growth factors, and colony stimulating factors; Corwin, 2000), cytokines have traditionally been identified as proinflammatory or anti-inflammatory. The major proinflammatory cytokines are interleukin 1α (IL- 1α), interleukin 1β (IL-1β), interleukin 2 (IL-2), interleukin 6 (IL-6), and tumor necrosis factor α (TNF-α); the major anti- inflammatory cytokines are interleukin 4 (IL-4), interleukin 10 (IL-10), and interleukin 13 (IL-13). Cytokines affect the functions of the central nervous system (CNS) through autonomic, neuroendocrine, and behavioral mechanisms (Webster, Tonelli, & Sternberg, 2002).
Cytokine receptors are important modulators of the proinflammatory response. These receptors regulate inflammation by functioning as agonists or antagonists of cytokine signaling (Levine, 2004). Most cytokine receptors inhibit cytokine signaling, but a notable exception is the IL-6 receptor (soluble IL-6 receptor [sIL-6R]), which potentiates IL-6 signaling (Gustot et al., 2005). Measures of cytokine receptors are often done concurrently with circulating cytokine levels to give a more complete representation of the relative balance between a cytokine and its associated regulatory or counterregulatory mechanism.
In FMS, the interest in cytokines as potential mediators of core symptoms began when Wallace, Margolin, and Waller (1988) reported the acute onset of FMS in persons with cancer, who were recipients of IL-2, a cytokine that stimulates the immune system to produce CD4+ (T-helper) cells. A close resemblance of the clinical features (pain, fatigue, mood and sleep disorders) of the cancer patients receiving IL-2 therapy to patients with FMS-like symptoms led researchers to further explore the relationship among cytokines and FMS (Wallace, 2006). This line of research is consistent with the “sickness behavior” phenomenon (Miller, 2003), the constellation of symptoms and behaviors that accompany conditions associated with inflammation/immune activation. The sickness behaviors have characteristic symptoms including fatigue, pain, and depressed mood that are thought to be caused by cytokines acting in the brain (Kelley et al., 2003).
Method
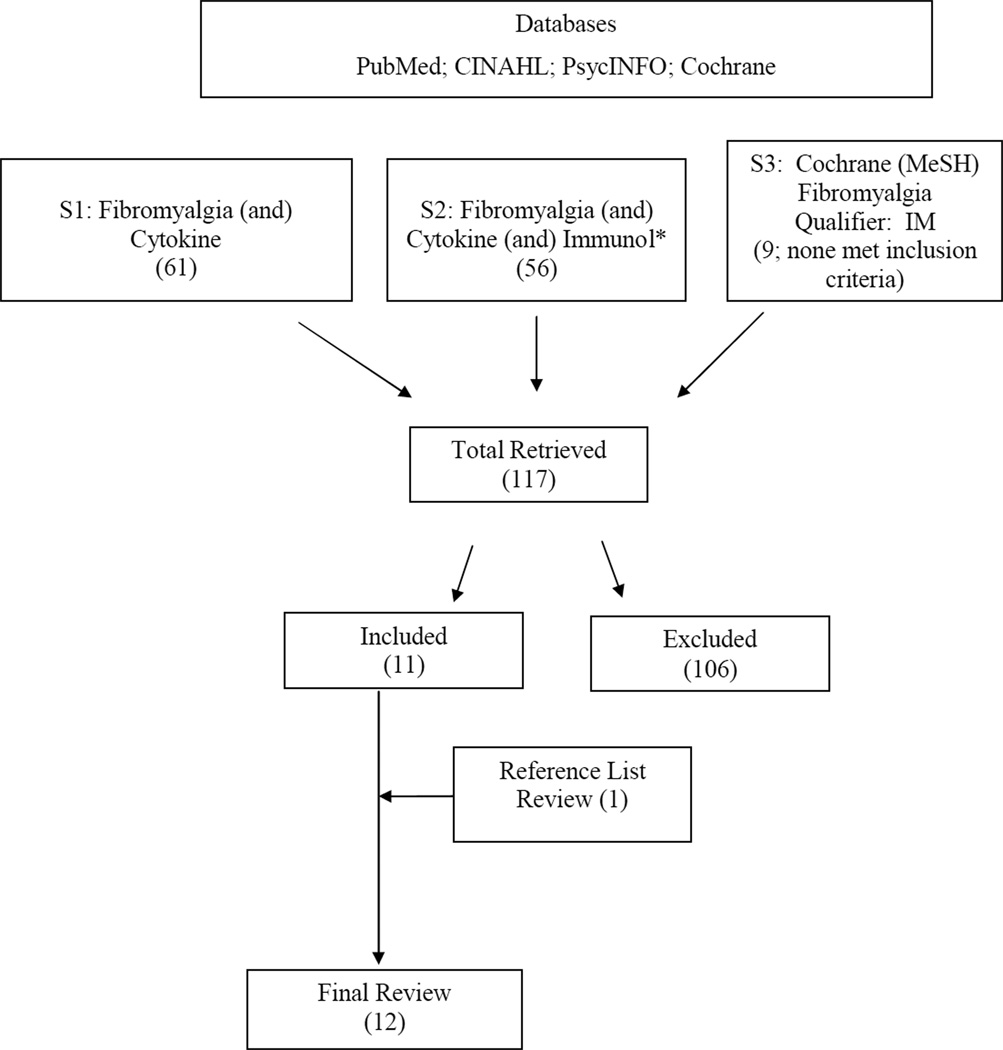
The electronic databases PubMed/Medline, CINAHL, and PsycINFO were searched from their date of inception to June 2008. Criteria for literature retrieval were quantitative and qualitative studies that emerged from meshing the key words “cytokine” and “fibromyalgia.” Within the (N=61) retrieved articles, the search term “immunol*” was added to “cytokine” and “fibromyalgia,” for a return of 56 articles. Articles were reviewed to examine whether they were research studies that included participants meeting the standard research-related diagnostic criteria for FMS and had a measure of cytokines derived from peripheral blood. Studies in which cytokines were administered as therapy to patients with FMS were excluded. Due to the limited number of retrieved articles that met the inclusion criteria, a MeSH search of the Cochrane Database of systematic reviews was conducted, using the key word “fibromyalgia” and the qualifier term “IM” (immunology). This search produced nine more articles; however, none met the original review criteria. To complete the literature search, the reference lists of qualifying articles were reviewed. From 117 articles retrieved via all of these search methods, a total of 12 articles met the inclusion criteria and are included in this review. This strategy is depicted in Figure 1.
Figure 1.
Literature Search Method
Cytokines and FMS
Table 1 summarizes findings related to changes in cytokines in persons with FMS. Each study explored levels of proinflammatory and/or anti-inflammatory cytokines derived from peripheral blood samples in persons diagnosed with FMS. Several studies (n = 7) examined the expression of cytokines in cultured serum or plasma. Cytokine expression was stimulated with different mitogens including phorbol myristic acetate (PMA), concanavalin A (Con A), and the lectin phytohemagglutinin (PHA).
Table 1.
Summary of Findings in the Literature Related to Changes in Cytokines in Persons with Fibromyalgia (FMS) by Method and Medium
| 1st Author/Date | Method | Medium | Cytokine Increased |
Cytokine Decreased |
|---|---|---|---|---|
| Wallace, 1989 | Radioimmunoassay | Serum | a | a |
| Hader, 1991 | Tridiated (3H) thymidine | PBMC-stimulated | IL-2 | |
| Maes, 1999 | ELISA | Serum | IL-1Ra* | |
| Wallace, 2001 | ELISA | PBMC-stimulated | IL-1Ra* | |
| IL-6* | ||||
| IL-8* | ||||
| Gür, 2002a | Immunoassay | Serum | IL-2r* | |
| IL-8* | ||||
| Gür, 2002b | Immunoassay | Serum | IL-2r* | |
| IL-8* | ||||
| Amel Kashipaz, 2003 | Flow cytometry | PBMC-stimulated | b | b |
| Üçeyler, 2007 | ELISA | Serum | IL-4 | |
| IL-10 | ||||
| Ardiç, 2007 | ELISA | Serum | IL-1α* | |
| Bazzichi, 2007 | ELISA | Plasma | TNF-α | IL-1 |
| IL-8 | IL-6 | |||
| IL-10 | ||||
| IL-10 | ||||
| Hesse-Husain, 2007 | Biochip Array | PBMC-stimulated | IL-1α* | |
| IL-1β* | ||||
| IL-2* | ||||
| IL-4* | ||||
| TNF-α* | ||||
| Macedo, 2007 | Biochip Array | PBMC-stimulated | IL-8* | IL-1α* |
| IL-1 β* | ||||
| IL-6* | ||||
| TNF-α* | ||||
| IL-2* | ||||
| IL-4* | ||||
| IL-10* |
NOTE: ELISA = enzyme-linked immunosorbent assay; IL = interleukin ( a generic term for cytokines produced by leukocytes); PBMC = peripheral blood mononuclear cells (lymphocytes and monocytes isolated from peripheral blood).
Levels of IL-1β, IL-2, IL-2R, and TNF-α were not significantly different between groups
Cytokine producing cells were not statistically different among groups in either unstimulated or IFN-γ stimulated conditions
p = .05
Hader, Rimon, Kinarty, and Lahat (1991) used the mitogen Con A to stimulate lymphocyte production in peripheral venous blood to test both the production (by CD4+ cells) and inhibition (by CD8+ cells) of the proinflammatory cytokine IL-2 in a sample of 12 adults diagnosed with FMS as compared to 10 healthy controls (participant age and gender not reported). In cells of participants with FMS, IL-2 production by Con A-stimulated T cells and CD4+ T cells differed from controls. Specifically, IL-2 levels of the FMS patient decreased com-pared to those obtained from control T cells. Noting that there was a need for a higher concentration of mitogen to achieve optimal IL-2 secretion and delay in the peak time of optimal IL-2 secretion, Hader et al. (1991) suggested a potential defect in the IL-2 secretion pathway in FMS patients.
In two separate studies, Wallace, Peter, Bowman, and Silverman (1989) and Wallace et al. (2001) investigated cytokine levels in persons diagnosed with FMS. Comparing a sample of 16 adults (15 females and 1 male) diagnosed with FMS to healthy controls (n = 55), Wallace et al. (1989) stimulated serum lymphocytes with PHA, Con A, and pokeweed mito- gens. There were no statistically significant differences between persons diagnosed with FMS and healthy controls. However, persons diagnosed with FMS demonstrated higher responses to PHA and Con A mitogens than healthy controls. In a later study, Wallace et al. (2001) examined the production of cytokines in a sample of 56 adults diagnosed with FMS (52 females and 4 males) compared to 39 healthy controls (38 females and 1 male). Measuring the cytokines IL-1β, interleukin 1 receptor antagonist (IL-1Ra), IL-2, IL-6, IL-8, IL-10, TNF-α and interferon α (IFN-α) in sera and supernatants of peripheral blood mononuclear cells (PBMC), some stimulated with lectins and PMA and some not stimulated, the authors reported that only serum IL-1Ra levels (p = .002 for those with FMS symptoms <2 years; p = .001 for those with FMS symptoms >2 years) and serum IL-8 levels (p = .01 for those with FMS symptoms >2 years) were significantly increased in FMS patients as compared to controls. When incubated in nonstimu-lated PBMC, production of IL-1Ra and IL-6 levels were also significantly elevated compared to controls. Statistical significance, however, was attained only in patients who reported over 2 years of symptoms (p = .01). Noting that IL-8 promotes sympathetic pain and IL-6 induces hyperalgesia, fatigue, and depression, Wallace et al. (2001) suggested that IL-1Ra may be released to balance the IL-6 and IL-8 inflammatory response. Although the study’s findings were intriguing, the small sample sizes, lack of significant findings in the first study (Wallace et al., 1989), post hoc analysis of cytokines by length of symptoms, and the complex laboratory procedures in the second study (Wallace et al., 2001) contributed to study limitations.
The association of cytokines and FMS was further explored in stimulated and unstimulated peripheral blood mononuclear cells (PBMC) in individuals (N = 22; 6 males and 16 females) diagnosed with chronic fatigue syndrome (CFS) and FMS (n = 14), chronic fatigue only (n = 2), FMS only (n = 4), and in age- and sex-matched healthy controls (N = 19; 5 males/14 females; Amel Kashipaz, Swinden, Todd, & Powell, 2003). PBMCs were cultured in fetal calf serum (FCS) in the presence or absence of a broad-spectrum antibiotic, polymyxin B sulfate, to inhibit the effect of endotoxins on cytokine production by monocytes. Cytokine production by CD14+ (monocytes) and CD14-negative cells (lymphocytes) was not statistically different among groups in either unstimulated or IFN-γ stimulated conditions. Findings of this study were limited by the small sample size (n = 4 persons with FMS), the potentially confounding influence of CFS on the relationship between FMS and cytokine levels and the multilevel laboratory procedures and agents used in preparation for the final flow cytometric analysis and reported results (Amel Kashipaz et al., 2003).
A more recent study investigated the effects of dexamethosone (DEX), a corticosteroid, in the supernatants of PHA-stimulated PBMC, on cytokine levels in 18 persons (16 females; 2 males) with FMS and 24 age- and gender-matched controls (Macedo et al., 2007). Levels of cytokines in the supernatant of PHA-stimulated PBMC cultures did not differ significantly between the two groups. There were significantly higher levels of IL-8 (p < .001) after DEX administration and reduced levels of IL-2, IL-4, IL-6, and IL-10 (p < .005). A treatment group interaction was observed for IL-2 (p = .001) in that IL-2 levels were higher before DEX treatment in the FMS group but lower after receiving DEX. Furthermore, there was a trend toward lower IL-4 levels observed in FMS patients compared to healthy controls (p = .07). Findings from this study are limited by the possible effects of multiplicity, given the small sample size of this study coupled with the high number of measures.
Cytokines and Core Symptoms of FMS
In addition to studies examining elevated or decreased levels of cytokines and cytokine expression in persons with FMS, several studies have examined the relationship of core symptoms of FMS (pain, fatigue, mood and sleep disorders) with cytokines. Table 2 lists associations that have been documented in the literature between cytokines and symptoms that may be relevant to FMS.
Table 2.
Cytokines and Associations Potentially Relevant to Fibromyalgia
| Cytokine | Association |
|---|---|
| IL-1β | Hyperalgesia, fatigue, fever, sleep, myalgias, substance P antinociception (increases GABA and decreases NDMA); norepinephrine and epinephrine stimulate its release |
| TNF-α | Stress; regulates substance P expression, rapid eye movement sleep, allodynia; increases excitatory amino acids; norepinephrine and epinephrine stimulate its release |
| IL-1Ra | Stress; inhibits IL-8 expression |
| IFN-γ | Stress, anxiety; lowers substance P; myalgias |
| IL-2 | Myalgias, cognitive dysfunction |
| IL-6 | Stress, fatigue, hyperalgesia, depression; norepinephrine, epinephrine and substance P stimulate its release; activates sympathetic nervous system |
| Il-8 | Substance P stimulates production, mediates sympathetic pain |
| IL-10 | Blocks pain |
Note: GABA = γ-aminobutryic acid; IFN-γ = interferon-γ; IL = interleukin; NMDA = N-methyl-D-aspartate; TNF-α = tumor necrosis factor-α.
SOURCE: Wallace, 2006, p. 18. Copyright 2006 by Bentham Science Publishers. Reprinted with permission.
Pain
Three of the research studies examined the relationship between cytokines and pain in persons with FMS. Each study demonstrated a statistically significant relationship between pain and increased cytokine levels.
Gür et al. (2002b) reported that IL-8 levels in FMS patients significantly correlated with pain intensity as measured by TPi (r = 0.35; p < .01). Gür et al. (2002a) reported finding a significant correlation between TPi and increased levels of IL-2r (p = .02) and IL-8 (p = .01) in FMS patients as compared to healthy controls. Bazzichi et al. (2007) explored the relationship among plasma levels of cytokines (IL-1, IL-6, IL-8, IL-10, TNF-α), TPi pain scores, and Fibromyalgia Impact Questionnaire (FIQ) scores in a convenience sample of 81 women diagnosed with FMS. The findings were compared to 45 age-and gender-matched healthy controls. In participants with FMS, IL-6 levels were positively correlated with TPi pain scores (r = .305, p = .02) and the total FIQ score (r = .298, p = .01), and TNF-α levels were positively correlated with the total FIQ scores (r = .234, p = .05). The FIQ is a well-validated measure of multiple symptom domains that has been used extensively in fibromyalgia research (Jones et al., 2008).
Pain and Depression
Six studies examined the relationship between cytokine levels and pain and depression. Two of these measured cytokines prior to and after a symptom management intervention. Findings regarding the associations among pain, depression and cytokines were inconsistent and in some cases, contradictory.
Using well-validated symptom measures in a sample of 21 persons with FMS (17 females and 4 males) compare to healthy controls (n = 33; 28 females and 5 males), Maes et al. (1999) examined the relationships among pain (visual analogue scale [VAS]; TPi with dolorimetry), depressive symptoms (Hamilton Depression Rating Scale [HDRS]; Hamilton, 1960), and cytokines (serum IL-6, sIL-6R, and sIL-1R antagonist [sIL-1RA]). There were no significant differences in serum IL-6, sIL-6R, and sIL-1RA between FMS patients and controls (Maes et al., 1999). However, in a sub-group analysis comparing those with an HDRS score > 16 (identified as moderate to severe depressive state) to those with an HDRS score 0–15 (none to mild depression) and healthy volunteers, there were significantly higher levels of sIL-6R (p = .003) and sIL-1RA (p = .02) in FMS patients with an HDRS score > 16. Although there was not a statistically significant relationship between pain and cytokine levels, 74.3% of the variance in the immune marker, sIL-6R was explained by the combined effects of HDRS scores and the severity of pain and stiffness in persons with FMS (Maes et al., 1999).
Different findings were reported in two studies led by Gür (Gür et al., 2002a, 2002b). In both studies, decreased, rather than increased, levels of depression were correlated with higher levels of pro-inflammatory cytokines in persons with FMS; however, the correlation coefficients were less than .10. In 81 FMS patients (gender not reported) compared to 32 age-matched healthy controls, FMS patients with mild depressive symptoms (HDRS score <16) had significantly higher serum IL-2r (p = .01) and IL-8 (p = .01) than healthy volunteers. IL-8 levels positively correlated with pain intensity (r = .35; p < .01; Gür et al., 2002b). In a study to determine differences in regional cerebral blood flow (rCBF), serum cytokine levels (IL-1β, IL-2r, IL-6, IL-8), and depressive symptoms in women with FMS (n = 19) compared to healthy, age- and gender-matched controls (n = 20), FMS patients with mild depressive symptoms had significantly higher serum IL-8 levels (p = .021) than FMS patients with more severe depressive symptoms (HDRS score > 16; Gür et al., 2002a).
Bazzichi et al. (2007) compared levels of cytokines (IL-1, IL-6, IL-8, IL-10, TNF-α), TPi scores, and FIQ scores with results from the Structured Clinical Interview for DSM-IV (Diagnostic and Statistical Manual for Mental Disorders[Fourth Edition]) Axis-1 Disorders (SCID-I/P; First, Spitzer, Gibbon, & Williams, 1997) in 81 female FMS patients and 45 healthy female controls. There were statistically significant correlations between IL-6 and the TPi score (r = .305, p = .02), the total FIQ score (r = .298, p = .01), and IL-10 levels (r =.354, p = .003). Although the researchers found no correlations between cytokine levels and current measures of depression and anxiety, in a sub-group analysis of FMS patients psychiatrically evaluated and diagnosed with depression, there were higher levels of the anti-inflammatory cytokine, IL-10 (p < .001), as compared to controls.
In two studies, researchers measured cytokine levels, pain, and depression scores following an intervention for pain (Ardiç et al., 2007; Üçeyler et al., 2006). In the first, a study to evaluate the clinical effect of balneotherapy (water therapy) and its influence on the expression of the inflammatory mediators IL-1, prostaglandin E2 (PGE2) and leukotriene (LTB4), 24 female FMS patients were randomly assigned to treatment group (Group 1, n = 12) or to a non-treatment control group (Group 2, n = 9; attrition = 3; Ardiç et al., 2007). Group 1 received daily 20-min sessions of balneotherapy for 3 weeks (total of 15 sessions). Group 2 maintained regular daily activities. There was an additional control group of 10, age-matched healthy females (Group 3). Pain (VAS), depression (Beck Depression Inventory [BDI]; Beck, Rush, Shaw, & Emery, 1979) and serum cytokines were measured at baseline (pretest) and at the end of treatment (posttest). After the treatment period, pain intensity scores (p < .001), BDI scores (p < .001), and IL-1α (p < .05) were significantly lower in the treatment group (Group 1) than in the two control groups (Groups 2 and 3). Findings of this study, while demonstrating a relationship between FMS symptoms (pain, depression) and pro-inflammatory cytokine levels, were limited by a small sample size and brief intervention period. In the second study, intravenous immunoglobulin (IVIG) was administered in 10 mg doses, once daily for 3 consecutive days to CWP patients (N = 40), and measures of IL-2, TNF-α, IL-8, IL-4, and IL-10 were collected at Day 3 (Üçeyler et al., 2006). Of the 40 CWP patients, 26 (23 females; 3 males) had a confirmed diagnosis of FMS. A control group of 40 age-and sex-matched healthy volunteers and an additional group of 15 CWP patients (9 females; 6 males) who served as an internal control group did not receive the IVIG treatment. Pain and mood were measured using numerical rating scales; for pain, 0 = no pain, and 10 = worst pain imaginable; for mood, 0 = deeply depressed, and 10 = even-tempered. Measuring serum IL-2, TNF-α, IL-8, IL-4, and IL-10 with ELISA, the investigators reported a significant reduction of anti-inflammatory cytokines IL-4 (p < .0001) and IL-10 (p = .03) in CWP patients who received the IVIG treatment compared with healthy controls (n = 40) and internal control group (n = 15). There were no other significant between group differences.
Fatigue
Four studies included an examination of the relationship between cytokines and fatigue in FMS. Generally, reported findings of the associations between fatigue and cytokines have been inconsistent.
In the first study, using a variety of lymphocyte mitogenic stimulation tests (Wallace et al., 1989), researchers compared serum concentrations of proinflammatory cytokines with severity of fatigue scores in persons with FMS (n = 16) as compared to healthy controls that were neither age- nor gender-matched (n = 55). The authors reported that levels of IL-1β, IL-2, IL-2 receptor (IL-2R), and TNF-α were not significantly different between groups. In a subsequent study, Wallace et al. (2001) compared cytokine profiles of FMS patients with a history of symptoms less than 2 years (n = 23), patients with symptoms for more than 2 years (n = 23), and age-, gender- and ethnically-matched healthy controls (n = 36). Cytokines were measured in PBMC that were incubated with and without stimulation by lectins and PMA. IL-8 levels were reported as three times higher in FMS patients compared to controls (p = .01). Although serum levels of IL-1Ra were elevated regardless of FMS symptom duration (< 2 years, p = .002; > 2 years, p = .001), there were statistically significantly elevated levels of PBMC-produced IL-1Ra and IL-6 only in FMS patients having symptom duration > 2 years (p = .01). In another study in which Üçeyler et al. (2006) measured fatigue with a numerical rating scale where 0 = no exhaustion, and 10 = insuperable exhaustion, the authors reported a significant reduction in anti-inflammatory cytokines IL-4 (p < .0001) and IL-10 (p = .03) in patients with CWP, including FMS patients compared with controls; no significant increase in pro-inflammatory cytokines and no significant correlations among FMS symptoms of fatigue and cytokine levels were found. Finally, in a study to examine the effect of depression level on serum cytokines in a sample of FMS (n = 81) patients compared to healthy controls (n = 32), Gür et al. (2002b) included a clinical examination of fatigue. Although not reporting the scoring mechanism for fatigue used in the study, the authors did report modest correlations between serum cytokines and fatigue (IL-1β, IL-2r, IL-6, IL-8); none were statistically significant.
Sleep
One study examined the association among cytokines and sleep (Hesse-Husain, 2007). The findings revealed increased levels of IL-2 and trends in the relationships among cytokines and sleep in FMS. Measuring cytokine levels (IL-1α, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IFN-γ and IFN-α) and sleep in 24 female FMS patients and 26 healthy, age-and gender-matched controls, Hesse-Husain explored levels of cytokines before and after the application of a psychosocial stressor, the Trier Social Stress Test (TSST; Kirschbaum, Pirke, & Hellhammer, 1993). Peripheral blood samples were taken 30 min before, 1 min after, and 120 min after the administration of the TSST. PBMCs were stimulated by the mitogen PHA with a biochip array used for simultaneous quantification of different proinflammatory and anti-inflammatory cytokines. FMS patients demonstrated significantly elevated IL-2 (p = .045) production and a trend toward a decreased IL-4 (p = .06) production. In a subgroup analysis, patients with the worst sleep quality had significantly higher levels of IL-2 (p = .05) concentrations than patients in the lowest and middle tertile.
Discussion
Given that there are no diagnostic laboratory tests for FMS and no gold standard for diagnosing FMS (Bennett, 2008; Katz et al., 2006; NIAMS, 2004), the search for biomarkers of the syndrome is important for both clinical and research purposes. Although there is theoretical support for the view that cytokines may be associated with the pathogenesis and core symptoms in FMS, this integrated review found limited empirical evidence to support these relationships. There are discrepant findings related to whether proinflammatory and anti-inflammatory cytokines are elevated or reduced in persons with FMS and whether they correlate with the core symptoms of this disorder. In addition to discrepant findings, there are several notable methodologic limitations in these reports. Sample sizes were generally small, the composition of most samples was heterogenous, and most designs were cross-sectional and failed to control for factors known to affect cytokine levels such as gender, length of disease, body mass index, and concomitant medications. Another notable limitation is that there was little replication of study designs.
In addition to the challenges described above, the findings of studies reviewed here are difficult to integrate because of the multiple ways that cytokines in peripheral blood were measured. There were differences in laboratory techniques, including differences in measurement platforms, cell type, and sample collection. In studies that measured cytokine expression under stimulated conditions, several different stimulants and methods of stimulation were used. For both stimulated and unstimulated cytokine measures, researchers used a variety of methods, including ELISA or radioimmunoassay, and commercial kits from different manufacturers. Furthermore, each study used a limited number of cytokine measures, with a few representative proinflammatory and/or anti-inflammatory cytokines measured per study and little consistency across studies in which cytokines were selected for measurement.
Biobehavioral Research Implications
The relationships of FMS and its core symptoms with cytokines are indeterminate yet potentially significant for further biobehavioral research. Although research findings to date have been inconsistent, biobehavioral researchers should continue to pursue this potentially promising avenue of research. In light of the potential importance of this line of research, there are several a priori design issues to consider in future research. Investigators must carefully consider using a set of measures across time. The advent of multiplex technologies, which permit the simultaneous measure of multiple cytokines in very small samples, provides an opportunity for investigators to measure panels of cytokines. This ability is useful due to growing recognition that the balance between proinflammatory and anti-inflammatory cytokines is important These panels may give a more complete representation of the proinflammatory and anti-inflammatory balance among cytokines as compared to traditional methods that have been limited to measuring only a few proinflammatory and/or anti-inflammatory cytokines per sample. Although multiplex platforms are a potentially useful innovation for measuring cytokines, a recent study of multiplex platforms found that cytokine measure in plasma was highly dependent on the platform, with the consistency of the detection of cytokines across platforms being dependent upon the cytokine being analyzed (Toedter, Hayden, Wagner, & Brodmerkel, 2008). Thus, the potential benefits and drawbacks of both traditional and more recent methods must be considered in future research.
Biobehavioral research focused on understanding the relationships of FMS with cytokines may lead to a better understanding of this complex syndrome. This knowledge may ultimately contribute to the development of interventions for symptom management that address not only the symptom manifestation but also a biological mediator of symptoms.
Acknowledgments
Funding: Components of the research were supported by the National Institute of Nursing Research through grant #P20 NR008988 (N. McCain, PI)
Contributor Information
Victoria Menzies, Virginia Commonwealth University, School of Nursing, 1100 East Leigh Street, P. O. Box 980567, Richmond, VA 23298-0567, Telephone: (804) 628-3381, FAX: (804) 828-7743, vsmenzies@vcu.edu.
Debra Lyon, Virginia Commonwealth University, School of Nursing, 1100 East Leigh Street, P. O. Box 980567, Richmond, VA 23298-0567, Telephone: (804) 828-5635, FAX: (804) 828-7743, delyon@vcu.edu.
References
- Abeles AM, Pillinger MH, Solitar BM, Abeles M. Narrative review: The pathophysiology of fibromyalgia. Annals of Internal Medicine. 2007;146:726–734. doi: 10.7326/0003-4819-146-10-200705150-00006. [DOI] [PubMed] [Google Scholar]
- Amel Kashipaz MR, Swinden D, Todd I, Powell RJ. Normal production of inflammatory cytokines in chronic fatigue and fibromyalgia syndromes determined by intracellular cytokine staining in short-term cultured blood mononuclear cells. Clinical and Experimental Immunology. 2003;132:360–365. doi: 10.1046/j.1365-2249.2003.02149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardiç F, Özgen M, Aybek H, Rota S, Çubukçu D, Gökgöz A. Effects of balneotherapy on serum IL-1, PGE2 and LTB4 levels. Rheumatology International. 2007;27:441–446. doi: 10.1007/s00296-006-0237-x. [DOI] [PubMed] [Google Scholar]
- Bazzichi L, Rossi A, Massimetti G, Giannaccini G, Giuliano T, De Feo F, et al. Cytokine patterns in fibromyalgia and their correlation with clinical manifestations. Clinical and Experimental Rheumatology. 2007;25:225–230. [PubMed] [Google Scholar]
- Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive therapy for depression. New York: Guilford Press; 1979. [Google Scholar]
- Bennett RM. Fibromyalgia and chronic fatigue syndrome. In: Goldman L, Ausiello D, editors. Cecil medicine. 23rd ed. Philadelphia, PA: Saunders Elsevier; 2008. pp. 2078–2082. [Google Scholar]
- Carpenter KJ. The mystery of fibromyalgia, part I - is fibromyalgia real pain, and what causes it? Psychopharmacology Educational Update. 2006;1(12):2–3. [Google Scholar]
- Corwin EJ. Understanding cytokines part I: Physiology and mechanism of action. Biological Research for Nursing. 2000;1:30–40. doi: 10.1177/109980040000200104. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV axis-I disorders (SCID-I) Washington, DC: American Psychiatric Press, Inc; 1997. [Google Scholar]
- Gür A, Karakoc M, Erdogan S, Nas K, Cevik R, Sarac AJ. Regional cerebral blood flow and cytokines in young females with fibromyalgia. Clinical and Experimental Rheumatology. 2002a;20:753–760. [PubMed] [Google Scholar]
- Gür A, Karakoc M, Nas K, Cevik R, Denli A, et al. Cytokines and depression in cases with fibromyalgia. The Journal of Rheumatology. 2002b;29:358–361. [PubMed] [Google Scholar]
- Gustot T, Lemmers A, Louis E, Nicaise C, Quertinmont E, Belaiche J, et al. Profile of soluble cytokine receptors in chrohn’s disease. Gut. 2005;54:488–495. doi: 10.1136/gut.2004.043554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hader N, Rimon D, Kinarty A, Lahat N. Altered interleukin-2 secretion in patients with primary fibromyalgia syndrome. Arthritis and Rheumatism. 1991;34:866–872. doi: 10.1002/art.1780340712. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse-Husain J. Fibromyalgia: A psychoneuroimmunological perspective[Electronic version] 2007 Retrieved March 5, 2008 from University of Trier, Department for Theoretical and Clinical Psychobiology Web site: http://ubt.opus.hbz-nrw.de/volltexte/2007/405/
- Jones KD, Burckhardt CS, Deodar AA, Perrin NA, Hanson GC, Bennett RM. A six-month randomized controlled trial of exercise and pyridostigmine in the treatment of fibromyalgia. Arthritis & Rheumatism. 2008;58:612–622. doi: 10.1002/art.23203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KD, Ross RL, Adams DG, Bennett RM. Fibromyalgia: Rational management in primary care. Clinician Reviews. 2006;16(5):42–48. Retrieved March 5, 2008 from http://www.clinicianreviews.com/index.asp?show=lesson&page=courses/105241/lesson.htm&lsn_id=105241. [Google Scholar]
- Kelley KW, Bluthe R-M, Dantzer R, Zhou J-H, Shen W-H, Johnson RW, et al. Cytokine-induced sickness behavior. Brain, Behavior, and Immunity. 2003;17:112–118. doi: 10.1016/s0889-1591(02)00077-6. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The 'trier social stress test'--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. part II. Arthritis and Rheumatism. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine SJ. Mechanisms of soluble cytokine receptor generation. Journal of Immunology. 2004;173:5343–5348. doi: 10.4049/jimmunol.173.9.5343. [DOI] [PubMed] [Google Scholar]
- Macedo JA, Hesse J, Turner JD, Ammerlaan W, Gierens A, Hellhammer DH, et al. Adhesion molecules and cytokine expression in fibromyalgia patients: Increased L-selectin on monocytes and neutrophils. Journal of Neuroimmunology. 2007;188:159–166. doi: 10.1016/j.jneuroim.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Maes M, Libbrecht I, Van Hunsel F, Lin AH, De Clerck L, Stevens W, et al. The immune-inflammatory pathophysiology of fibromyalgia: Increased serum soluble gp130, the common signal transducer protein of various neurotrophic cytokines. Psychoneuroendocrinology. 1999;24:371–383. doi: 10.1016/s0306-4530(98)00087-0. [DOI] [PubMed] [Google Scholar]
- Mease P. Fibromyalgia syndrome: Review of clinical presentation, pathogenesis, outcome measures, and treatment. The Journal of Rheumatology Supplement. 2005;75:6–21. [PubMed] [Google Scholar]
- Mease P, Arnold LM, Bennett R, Boonen A, Buskila D, Carville S, et al. Fibromyalgia syndrome. The Journal of Rheumatology. 2007;34:1415–1425. [PubMed] [Google Scholar]
- Miller AH. Cytokines and sickness behavior: Implications for cancer care and control. Brain, Behavior, and Immunity. 2003;17:132–134. doi: 10.1016/s0889-1591(02)00080-6. [DOI] [PubMed] [Google Scholar]
- National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) Questions and answers about fibromyalgia. 2004 Retrieved 2/12/2008, from http://www.niams.nih.gov/Health_Info/Fibromyalgia/default.asp.
- Smythe H. Editorial: The symptom intensity scale, fibromyalgia, and the meaning of fibromyalgia-like symptoms. A review. Journal of Rheumatology. 2006;33:2113–2114. [PubMed] [Google Scholar]
- Toedter G, Hayden K, Wagner C, Brodmerkel C. Simultaneous detection of eight analytes in human serum by two commercially available platforms for multiplex cytokine analysis. Clinical and Vaccine Immunology. 2008;15:42–48. doi: 10.1128/CVI.00211-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Üçeyler N, Valenza R, Stock M, Schedel R, Sprotte G, Sommer C. Reduced levels of antiinflammatory cytokines in patients with chronic widespread pain. Arthritis & Rheumatism. 2006;54:2656–2664. doi: 10.1002/art.22026. [DOI] [PubMed] [Google Scholar]
- Wallace DJ, Linker-Israeli M, Hallegua D, Silverman S, Silver D, Weisman MH. Cytokines play an aetiopathogenetic role in fibromyalgia: A hypothesis and pilot study. Rheumatology (Oxford, England) 2001;40:743–749. doi: 10.1093/rheumatology/40.7.743. [DOI] [PubMed] [Google Scholar]
- Wallace DJ. Is there a role for cytokine based therapies in fibromyalgia. Current Pharmaceutical Design. 2006;12:17–22. [PubMed] [Google Scholar]
- Wallace DJ, Bowman RL, Wormsley SB, Peter JB. Cytokines and immune regulation in patients with fibrositis. Arthritis and Rheumatism. 1989;32:1334–1335. doi: 10.1002/anr.1780321024. [DOI] [PubMed] [Google Scholar]
- Wallace DJ, Margolin K, Waller P. Fibromyalgia and interleukin-2 therapy for malignancy. Annals of Internal Medicine. 1988;108:909. doi: 10.7326/0003-4819-108-6-909_1. [DOI] [PubMed] [Google Scholar]
- Webster JI, Tonelli L, Sternberg EM. Neuroendocrine regulation of immunity. Annual Review of Immunology. 2002;20:125–163. doi: 10.1146/annurev.immunol.20.082401.104914. [DOI] [PubMed] [Google Scholar]
- Wolfe F. Editorial: Stop using the American College of Rheumatology criteria. Journal of Rheumatology. 2003;30:1671–1672. [PubMed] [Google Scholar]
- Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Report of the multicenter criteria committee. Arthritis and Rheumatism. 1990;33:160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- Zautra AJ, Fasman R, Parish BP, Davis MC. Daily fatigue in women with osteoarthritis, rheumatoid arthritis, and fibromyalgia. Pain. 2007;128:128–135. doi: 10.1016/j.pain.2006.09.004. [DOI] [PubMed] [Google Scholar]