Abstract
Background
In cancer, tumor escape from the host immune system includes apoptosis of circulating CD3+CD8+ effector T lymphocytes. Here, we compare sensitivity to apoptosis of virus- with tumor-specific circulating CD8+ T cells in patients with head and neck cancer.
Methods
Wild-type p53 peptide-specific (p53264-272 and p53149-157) and viral peptide-specific (EBV BMLF259-267 and CMVpp65495-503) tetramers were used to measure the frequency of reactive T cells by flow cytometry. Annexin V (ANX) binding to circulating 7-amino-actinomycin D-negative but tetramer+CD8+ T cells in PBMC obtained from 21 patients with head and neck cancer and 11 normal controls (NC) was evaluated.
Results
In patients with head and neck cancer, a higher percentage of tetramer+CD8+ than tetramer−CD8+ T cells bound ANX (p < .023–.005). Although most tumor-epitope+CD8+ T cells bound ANX, lower percentages of virus-specific CD8+ T cells were ANX+ in the same patients.
Conclusions
Preferential demise of circulating tumor-specific CD8+ T cells and their paucity in head and neck cancer contribute to tumor escape.
Keywords: apoptosis, tetramers, effector T lymphocytes, tumor antigen-specific T cells
The ability of malignant tumors to engineer and execute an escape from the host immune system has been well documented in the literature.1,2 Tumor escape can involve a variety of mechanisms from the down-regulation or loss of surface molecules necessary for tumor recognition and antigen processing by immune cells to dysregulation of immune cell functions and/or the induction of apoptosis in effector T cells. As a result, tumors either remain unrecognized by the immune system or actively interfere with functions or the survival of effector cells.3,4 It is likely that these evasive strategies significantly contribute to tumor progression and negatively influence the outcome of immune therapies in patients with cancer. For this reason, tumor evasion and its mechanisms have captured the investigators’ attention and are currently considered to be an important factor in a design of novel cancer therapies.
We have previously reported that CD8+ T lymphocytes in the peripheral circulation of patients with head and neck cancer or melanoma readily undergo spontaneous apoptosis.3,4 Freshly isolated peripheral blood mononuclear cells (PBMC) of these patients contained elevated proportions and absolute numbers of CD8+ T lymphocytes that bound Annexin V (ANX). These ANX+CD8+ T cells did not stain with propidium iodide (PI) or 7-amino-actinomycin D (7-AAD),4 and thus were in the early stages of apoptosis. Further, the CD8+ T cells which bound ANX were CD95+ (Fas/Apo-1+), largely belonged to the effector-memory population,3–5 and included CD8+CD45RA+CD27− and CD8+CD45RA+CD28− T cells in patients with melanoma, breast carcinoma, or head and neck cancer.6,7 The reported decreases in the absolute number of circulating T lymphocytes in patients with head and neck cancer, including those not previously treated with cytotoxic therapies, could reflect apoptosis in these subsets of CD8+ T cells.8 In aggregate, our earlier data suggested that accelerated removal of effector T-cell subsets from the circulation occurring in patients with cancer might contribute to altered lymphocyte homeostasis.
Tumor associated antigen (TAA)-specific CD8+ T cells presumably represent only a very small fraction of antigen-committed T cells, and CD8+ effector cells undergoing spontaneous apoptosis in the peripheral circulation may have different specificities. To determine whether TAA-specific CD8+ effector T cells are preferentially targeted for apoptosis in the peripheral circulation of cancer patients, a sensitive and specific assay is required. We used major histocompatibility complex (MHC) class I peptide tetramers (tet) in multicolor flow cytometry, based on our previous data indicating that tetramer technology is sufficiently sensitive to detect such peptide-specific T cells present at relatively low frequencies in the peripheral circulation.9,10 The detection of tet+ T cells in the peripheral blood depends on a rare-event analysis performed under rigorous experimental conditions. Furthermore, measuring of ANX binding to tet+CD8+ T cells in the peripheral circulation involves a rapid analysis of freshly harvested cells that is equally demanding. ANX binding detects a “flip” in serine-phosphatidyl residue in the cell membrane11 and is considered to be one of the earliest apoptotic events.
We have adapted tetramer analysis established and validated in our laboratory9 to detection of ANX+tet+CD8+ cells in the peripheral circulation of patients with head and neck cancer. We show that in these patients, high percentages of tet+CD8+ T lymphocytes bind ANX, and that TAA-specific CD8+ T cells are more vulnerable to spontaneous apoptosis than the total CD8+ T cell population or EBV/CMV virus-specific tet+CD8+ T lymphocytes. Because these circulating tet+ANX+CD8+ T cells specific for TAA-derived epitopes are destined to undergo apoptosis, it suggests that their preferential demise represents a mechanism allows the tumor to escape from immune surveillance.
PATIENTS AND METHODS
Patients
Patients with head and neck cancer were seen in the outpatient clinic at the UPCI. Twenty-one patients with head and neck cancer included 4 patients who had active disease and 17 who were NED following therapy with intent to cure. The patients included 15 with stage I or II and 6 with stage III and IV disease. Nine patients had positive lymph nodes and 12 had no nodal involvement at the time of diagnosis. The cohort included 11 patients with oral cavity tumors, 8 with laryngeal tumors, and 1 with a hypopharyngeal tumor. One patient had esophageal tumor. The patients were not previously treated with biologic therapies and were not receiving any cytotoxic therapies at the time of blood draws for this study. All patients were HLA-A2, HLA-2+ as previously determined by sero-phenotyping of their PBMC using monoclonal antibodies (mAbs) BB7.2 and MA2.1 (produced by hybridomas obtained from American Type Culture Collection, Manassas, VA). Normal HLA-A2 donors (NC; n = 11) were recruited from among the laboratory personnel and were approximately matched in age with the patients. All patients and normal controls (NC) signed an informed consent, and the study was approved by the IRB (IRB # 991206; “The Specialized Project of Research Excellence in Head and Neck Cancer.”).
Cells
Peripheral venous blood (30–50 mL) was drawn into heparinized tubes and immediately delivered to the laboratory for lymphocyte recovery on Ficoll-Hypaque gradients. The elapsed time between phlebotomy and PBMC staining for flow cytometry was 1 to 2 hours. This time period was strictly adhered to for all specimens collected for the ANX binding studies. The recovered PBMC were washed, counted, and stained for flow cytometry.
Tetramers
The PE-labeled HLA-A*0201 tetramers for the wild-type (wt) sequence p53 peptides264-272 (LLGRNSFEV) and 149-157 (STPPPGTRV) referred to as p53 tet, as well as nonrelevant HLA-A*0201/HIVpol476-484 peptide (FLKEPVHGV) and Flu58-66 matrix peptide (GILGFVATL) tetramers were obtained through the National Institute of Allergy and Infectious Diseases Tetramer Facility in Atlanta, GA. HLA-A*0201 cytomegalovirus (CMVpp65495-503,NLVPMVATV) and Epstein-Barr virus (EBV) BMLF259-267 (GLCTLVAML) tetramers were purchased from Beckmann Coulter. Titrations of tetramers and specificity assays were as follows: (a) all tetramers were pretitered using the T-cell lines or clones specific for wt p53 peptides and available in our laboratories to determine optimal reagent concentrations and distinguish positive from negative signals; (b) to assure tetramer specificity, staining of peptide-specific versus irrelevant peptide-specific T-cell lines was performed and/or mix of tetramers was used to show that tet+ cells are single- and not double-stained; (c) negative controls, such as HLA-A*0201 HIV peptide tetramer, was used with HLA-A2 PBMC in all assays; (d) a cut-off for tetramer binding to HLA-A2-negative PBMC of normal donors (n = 10) was established, as previously described.9 The lower limit of detection (LLD) was at the upper 99th percentile of tet+CD8+ T cells in HLA-A2-negative individuals. For wt p53 tetramers, it corresponded to the frequency of 1/7800 cells or approximately 0.01%. This LLD was used for evaluating all tetramer results presented in this manuscript; (e) repeated staining of the same PBMC specimens was used whenever possible to confirm the repeatability of this rare event assay; (f) to ensure that ANX binding was not limited by a low number of gated events, comparisons of ANX binding to decreasing numbers of numbers tet+CD8+ T cells (from 4300 to 50) were performed using PBMC stained with different tetramers.
Antibodies
The mAbs used for surface staining of PBMC were purchased from Becton Coulter (Miami, FL) and included labeled mAbs to CD3, CD4, and CD8. The respective isotypes used as negative controls were also purchased from Becton Coulter.
Staining of Peripheral Blood Mononuclear Cells
For flow cytometry, PBMC were stained as previously described.7,9 Briefly, for p53 tet, aliquots of diluted stock (1/100) were added directly to cell pellets (5–7 × 106 cells). For tetramers purchased from Beckman Coulter, staining was performed as recommended by the manufacturer, and cells were labeled for 30 minutes at room temperature in the dark. Next, 5 μL aliquots of the labeled mAbs for surface staining were added, and cells were incubated for 30 minutes at 4°C in the dark. Cells were washed twice with flow buffer and resuspended in 100 μL of ANX-binding buffer followed by incubation with FITC-labeled ANX (Becton Dickinson) for 15 minutes at room temperature in the dark. 7-AAD was added as well to each tube. A 400-μL aliquot of binding buffer was added, and the samples were analyzed within 30 minutes.
ANX Binding
ANX binding to T cells was evaluated by multicolor flow cytometry as previously described.5,7 The data were acquired using a FACS Calibur (Becton Dickinson) equipped with a dual laser and Cell Quest software. The subsequent analysis was performed with Expo 32 ADC software (Beckman Coulter). A minimum of 1 × 106 and, when possible, up to 3 × 106 events were acquired for analysis. The percent of ANX-binding cells was scored after backgating on CD3+CD8+ cells. The gate was stringently set to eliminate cellular debris, and the cutoffs for ANX+ 7AAD− cells were established and used as previously described.5,7 This allowed for a clear-cut discrimination between viable nonapoptotic and apoptotic cells among subpopulations of T lymphocytes in the CD3+CD8+ gate.
Caspase Activation
Intracellular staining of activated caspases was performed using the pancaspase inhibitor FITC-VAD-FMK (Promega, Madison, WI) which serves as a marker of apoptosis independent of ANX binding. Binding of the inhibitor to caspases in the cells was quantified by flow cytometry as recommended by the manufacturer. Briefly, cells were suspended in PBS at 1 × 106 cells/mL, and FITC-VAD-FMK was added to the cells at a final concentration of 5 μM. The cells were incubated for 20 minutes at 37°C in a CO2 incubator, washed with PBS twice, fixed with 1% (v/v) paraformaldehyde in PBS and examined in a flow cytometer. In most cases, caspase activity was determined in CD8+tet+ or CD8+tet− T cells in parallel with ANX binding, using separate reaction tubes.
Statistical Analysis
Paired Student’s t tests were used to compare percentages of ANX+tet+ T cells with ANX+tet− T cells; ANX+tet+ with ANX-tet+ T cells and total ANX+CD8+ with total ANX-CD8+ T cells. Two sample Student’s t tests with corrections for unequal variances were also used to compare ANX+tet+CD8+ T cells in patients versus controls for individual viral- or TAA-specific tetramers. Reciprocal frequencies of tetramer counts were log transformed (base 10) and tested for differences with the paired t test. All statistical tests were 2 tailed.
RESULTS
Tetramer-Based Flow Cytometry
Tetramer analysis by flow cytometry detects rare events and requires special measures to assure its specificity and reliability. All tetramers used were pretiterd on T-cell lines specific for the HLA-A2 peptides incorporated in the tetramers, as previously described for wt p53 tetramers.12 In these experiments, a CD8+ T cell line recognizing wt p53264-272 did not bind the tetramer made with wt p53149-157 peptide, and vice versa. The tetramer incorporating CMV or EBV peptides failed to stain wt p53-specific T cell lines. LLD was established for wt p53 tetramers using cells obtained from HLA-A2 negative patients as 0.01% or 1/7800.9 For convenience, LLD used in this study was set at 1/7800. We have also tested the same PBMC more than once in the same and different assays to determine the assay reproducibility, and CV of 10% was established for the assay based on 20 determinations. On the basis of this CV, we determined that >1 × 106 cells or >100 events have to be acquired reliably to detect tetramer positive (tet+) cells when the LLD is set at 0.01%. In the described experiments, 3 × 106 cells or >300 events were acquired for analysis whenever possible. Another concern was that ANX binding might be compromised by a low number of tet+CD8+ T cells in the gate. After evaluating ANX binding to the gated cells ranging in numbers from 4300 to 50 for all viral- and TAA-specific tetramers, we established a cutoff of 200 tet+CD8+ T cells/gate as a limiting cell number for reproducible detection of ANX binding to these cells.
Detection of Circulating tet+CD8+ T Cells in Patients with Head and Neck Cancer
In patients with head and neck cancer, frequencies of tet+CD8+ T cells specific for wt p53264-272 or p53149-157 peptides were found to range from less than 1/7800 to 1/448 (0.2%) in the peripheral circulation (Table 1). On the basis of the established LLD, 13/21 patients with head and neck cancer had detectable frequencies of p53264-272 tet+CD8+ T cells (>1/7800 or >0.01%). The frequencies of CD8+ T cells specific for wt p53149-157 peptide appeared to be higher than those for wt p53264-272 -specific CD8+ T cells, as shown in Table 1; however, it was only possible to test the frequency in 5 patients due to limitations in available cell numbers. The data are consistent with our previously reported results12 and confirm the presence of wt p53 peptide-specific T cells in the circulation of patients with head and neck cancer albeit at low frequencies, as would be expected for these “self” tumor peptides.
Table 1.
Reciprocal frequencies of tet+CD8+ T lymphocytes specific for wt p53 peptides in the peripheral circulation of patients with head and neck cancer.*
| Patient number | p53264-272 | p53149-157 |
|---|---|---|
| 1 | 1878 | n.a. |
| 2 | 2883 | 776 |
| 3 | 2995 | n.a. |
| 4 | 3075 | n.a. |
| 5 | 3109 | n.a. |
| 6 | 3488 | n.a. |
| 7 | 5269 | n.a. |
| 8 | 5901 | 1006 |
| 9 | Neg | 2525 |
| 10 | Neg | 4087 |
| 11 | Neg | 2953 |
| 12 | Neg | n.a. |
| 13 | Neg | n.a. |
| 14 | 2872 | n.a. |
| 15 | 4483 | n.a. |
| 16 | 3451 | n.a. |
| 17 | 448 | n.a. |
| 18 | Neg | n.a. |
| 19 | 1740 | n.a. |
| 20 | Neg | n.a. |
| 21 | Neg | n.a. |
Tetramer frequencies were determined by flow cytometry as described in the Patients and Methods section. “Neg” means that the tetramer frequency was lower than 1/7800 which was set as LLD based on the results of previously reported experiments.9
In 7 patients with head and neck cancer, we determined the frequency of virus peptide-specific (EBV BMLF259 and CMVpp65495) CD8+ T cells in parallel with that of wt p53264-272 peptide-specific (ie, tet+) CD8+ T cells (Table 2). The representative flow cytometry data comparing the frequency of viral to wt p53 peptide tetramers in the peripheral blood of a representative patient with head and neck cancer are shown in Figure 1. As seen in Table 2 and Figure 1, these frequencies varied broadly between the patients and within the same patients.
Table 2.
Reciprocal frequencies of viral-specific tet+CD8+ T cells and wt p53264-272 peptide-specific tet+CD8+ T cells in the peripheral circulation of patients with head and neck cancer.*
| Patient number | EBV tet+ | CMV tet− | wt p53246 tet+ |
|---|---|---|---|
| 15 | 1634 | 2280 | 4483 |
| 16 | 2367 | 44 | 3451 |
| 17 | 690 | 121 | 448 |
| 18 | neg | 895 | neg |
| 19 | 1753 | 1752 | 1740 |
| 20 | 6826 | 3780 | neg |
| 21 | 1896 | 2281 | neg |
The data are reciprocal frequencies of tetramer+CD8+ T cells measured in the same patients.
FIGURE 1.
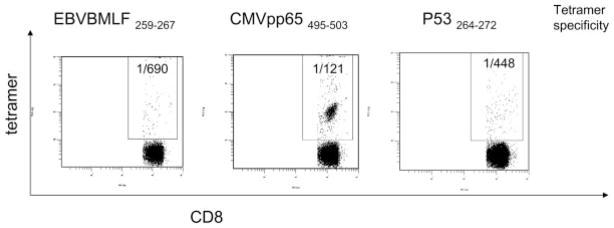
Four-color flow cytometry for the frequency of tetramer (tet)+CD3+CD8+ T cells in the peripheral circulation of a patient with HNC. The frequency of wt p53264-272 peptide-specific and viral peptide-specific T cells in the peripheral blood of a representative HLA-A*02+ patient with HNC is shown. The frequency values for tet+CD8+ T cells are indicated in the upper right quadrants.
The frequency of wt p53 peptide-reactive CD8+ T cells is low (approximately 1/5000) in the peripheral blood of NC. This is in agreement with the data reported by us earlier for wt p53264-272.10 However, we were only able to reliably measure the frequency of viral peptide-specific CD8+ T cells in these individuals. The frequency of CMVtet+CD8+ T cells ranged from 1/150 to <1/7800 and that for EBVtet+CD8+ T cells from 1/114 to 1/4502 in the peripheral blood of 11 NC. The frequencies for the HIV peptide-specific T cells ranged from 1/8000 to 1/10000 and those for the Flu peptide-specific T cells from 1/200 to 1/2000. The frequencies were similar in patients with head and neck cancer and in NC.
Binding of ANX to tet+CD8+ T Cells
To determine sensitivity of circulating tet+CD8+ T cells to apoptosis, ANX binding was also measured by flow cytometry. Because tetramers were added to cells prior to ANX, a concern existed that concentration-dependent tetramer binding could have induced membrane alterations allowing ANX binding. Therefore, using the CMV peptide-specific tetramers and normal PBMC, titration experiments were performed in which low versus high concentrations of tetramers were added to the same T cells. The percent of ANX+ cells remained constant (data not shown), indicating that ANX binding was unrelated to T cell receptor (TcR)-tetramer binding affinities. There was also a concern that tetramer binding induced ANX binding but not apoptosis. Therefore, pancaspase activation was measured in the same cells in parallel to ANX binding following tetramer staining. Although the mean percentage ± SD for ANX+tet−CD8+ T cells was 21 ± 11, the mean percentages of ANX+tet+CD8+ T cells were 70 ± 23 and 51 ± 23 for p53264-272 and p53149-157, respectively, in patients with head and neck cancer. In the same patients with head and neck cancer, caspase activation, as measured by FITC-VAD-FMK staining, was always observed in the proportion of tet+CD8+ cells that closely approached that of ANX-binding tet+CD8+ cells (data not shown). In PBMC of normal donors, ANX-V binding and caspase activity were seen in less than 10% of tet+CD8+ T lymphocytes or CD8+ T lymphocytes.
ANX was found to bind to a major proportion of circulating wt p53 peptide-specific tet+CD8+ T cells in most, but not all, patients with head and neck cancer. In 1 patient (#5), relatively few tet+CD8+ T cells bound ANX (Table 3). This patient, with primary base of tongue tumor, was staged as T1 N1. For all 14 patients with head and neck cancer, the percent of ANX+tet+CD8+ T cells was significantly higher than that of ANX+tet−CD8+ T cells (Table 3) indicating that a significantly greater proportion of tet+CD8+ T cells bound ANX than that of tet−CD8+ T cells (p < .005 to p < .0237). As illustrated for patient #8 with head and neck cancer in Figure 2, binding of ANX to tet+CD8+ T cells was at 67% relative to 17% ANX+tet−CD8+ T cells. The percentages of virus peptide-specific tet+CD8+ T cells which bound ANX were not significantly different from those for ANX+tet−CD8+ T cells (Table 4). However, the frequency of ANX-binding virus-specific CD8+ T cells was significantly lower than that of ANX+ wt p53 peptide-specific CD8+ T cells as illustrated for the patient #17 in Figure 3.
Table 3.
Annexin V binding to tet−CD8+ T cells and tet+CD8+ T cells in the peripheral circulation of patients with head and neck cancer.*
| Patient number | % Annexin V+ cells
|
||
|---|---|---|---|
| tet−CD8+ T cells | tet+CD8+ T cells
|
||
| p53264-272 | p53149-157 | ||
| 1 | 37 | 89 | n.a.† |
| 2 | 40 | 94 | 83 |
| 3 | 19 | 82 | n.a. |
| 4 | 7 | 50 | n.a. |
| 5 | 21 | 25 | n.a. |
| 6 | 33 | 85 | n.a. |
| 7 | 21 | 82 | n.a. |
| 8 | 17 | 77 | 67 |
| 9 | 28 | Neg‡ | 42 |
| 10 | 12 | Neg | 32 |
| 11 | 19 | Neg | 31 |
| 12 | 6 | Neg | n.a. |
| 13 | 29 | Neg | n.a. |
| 14 | 11 | 50 | n.a. |
|
|
|||
| p < .005 | |||
|
|
|||
| p < .0237 | |||
ANX binding to CD3+tet+CD8+ or tet−CD8+ T cells that were 7AAD− was measured by multicolor flow cytometry as described in the Patients and Methods section.
n.a., not available.
Neg, the tet+CD8+ T cell frequency was lower than LLD defined as 1/7800 (9).
FIGURE 2.
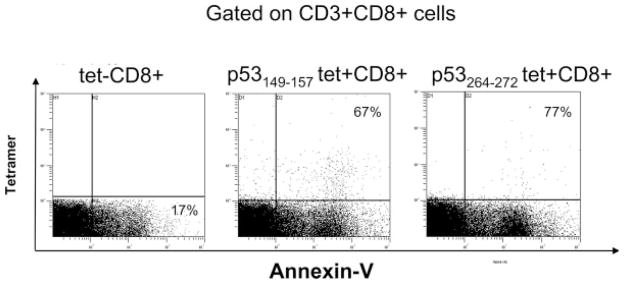
Flow cytometry for Annexin V binding to wt p53 peptide tet+CD8+ versus tet−CD8+ T cells in the peripheral circulation of HNC patient #8 with HNC. The percentages ANX+tet−CD8+ (lower right quadrant) and ANX+tet+CD8+ T cells (upper right quadrants) are shown.
Table 4.
Comparison of Annexin V binding to tet-CD8+ T cells and virus peptide- or wt p53264-272 peptide-specific (tet+) CD8+ T lymphocytes in the peripheral circulation of patients with head and neck cancer.*
| Patient number | % Annexin V+ cells
|
|||
|---|---|---|---|---|
| tet−CD8+ T cells | tet+CD8+ T cells
|
|||
| EBV | CMV | wt p53264 | ||
| 15 | 8 | 18 | 10 | 29 |
| 16 | 29 | 33 | 30 | 67 |
| 17 | 11 | 27 | 14 | 37 |
| 18 | 8 | Neg† | 9 | Neg |
| 19 | 7 | 12 | 13 | 9 |
| 20 | 5 | 9 | 10 | Neg |
| 21 | 9 | 6 | 9 | Neg |
|
|
||||
| NSD | ||||
|
|
||||
| NSD | ||||
|
|
||||
| p < .05 | ||||
ANX binding to CD3+CD8+tet+ or tet− T cells that were 7-AAD− was measured by multicolor flow cytometry as described in the Patients and Methods section.
Neg, the tet+CD8+ T cell frequency was lower than LLD defined as 1/7800 (9).
FIGURE 3.
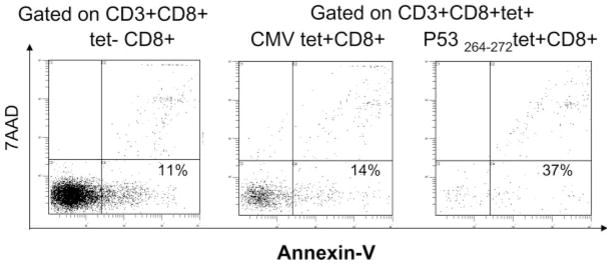
Flow cytometry for Annexin V binding and 7-AAD staining in virus-specific tet+CD8+ T cells and wt p53 tet+CD8+ T cells in the peripheral circulation of the HNC patient #17. The percentages of tet+CD8+ and tet−CD8+ T cells that bind ANX but are 7-AAD negative are shown in lower right quadrants.
In the peripheral blood of 5 NC, ANX binding to viral peptide-specific CD8+ T cells was determined and compared to that of tet−CD8+ T cells. The mean values ± SD for ANX+ CMV+CD8+ T cells were 20% ± 2% and for ANX+EBV+CD8+ T cells, 32% ± 3%. These percentages were significantly higher (p < .01) from those for tet−CD8+ cells (10% ± 3% and 6% ± 1%, respectively) in the circulation of these NC. Interestingly, the percentages of ANX-binding viral peptide-specific CD8+ T cells in NC were found to be comparable (NSD) to those seen in the circulation of patients with head and neck cancer (Table 4). Thus, it appears that viral peptide-specific CD8+ T cells are equally sensitive to spontaneous apoptosis in the blood of NC and patients with head and neck cancer.
In aggregate, the data for patients with head and neck cancer are consistent with the enhanced apoptosis of CD8+ T cells specific for TA epitopes as compared with CD8+ T cells specific peptides derived from commonly occurring viruses. ANX binding and intracytoplasmic caspase activity (not shown) were significantly lower in tet−CD8+ T cells than tet+CD8+ T cells. The data suggest that tumor epitope-specific CD8+ T lymphocytes in patients with head and neck cancer significantly differ in sensitivity to apoptosis as compared to virus-specific T cells or tet− T cells in the same patients, with TAA-specific tet+CD8+ T cells being particularly sensitive to spontaneous apoptosis.
DISCUSSION
Immunologic aberrations found in patients with head and neck cancer include elevated proportions of circulating CD95+ (Fas+) CD8+ T cells in the peripheral circulation,3,4,13,14 significantly decreased absolute numbers of T cells in the periphery,8 decreased numbers of naïve T cells which express few receptor excision circles (TREC)15 as well as cells with memory effector phenotype (CD8+CD45RA−CCR7−).16 These changes in circulating lymphocytes were previously linked to their rapid turnover due to apoptosis of activated effector CD8+ T cells.4,13 One of the hallmarks of early apoptosis in peripheral blood T lymphocytes is binding of ANX and the activation of intracellular caspases. We have previously demonstrated that in patients with cancer, ANX binding and changes in the mitochondrial membrane potential were largely targeted to CD8+ effector cell subsets.4,7,14 On the basis of these findings, we hypothesize that TAA epitope-specific CD8+ T cells in the peripheral circulation of patients with head and neck cancer might be preferentially eliminated and test the hypothesis by comparing ANXV binding to TAA specific CD8+ T cells versus tet−CD8+ T cells and viral tet+CD8+ T cells. This hypothesis is consistent with our earlier observations that phenotypically defined CD8+ effector T cells preferentially bound ANX in patients with melanoma, breast carcinoma, or head and neck cancer.3–7 In addition, we observed that CD8+ T cells which bind ANX have low expression of TCR-associated zeta chain and presumably do not signal normally. Low levels of the zeta chain expression in T cells of patients with cancer have been previously linked to apoptosis.17,18
Earlier reports of T-cell apoptosis at the tumor site19,20 suggested that in situ demise of tumor-infiltrating lymphocytes (TIL), generally considered to represent TAA-specific effector cells, is driven by the tumor or tumor-derived factors and facilitates tumor escape from the host immune system. Preferential death of tumor-specific immune cells would clearly favor tumor progression. However, since most tumor-specific antigens are “self,” the elimination of self-reactive T cells recognizing these antigens need not be restricted to the tumor site and could further contribute to the paucity of TAA-specific tet+CD8+ T cells in the patients’ circulation. The frequencies of viral peptide-specific tet+CD8+ T cells were often substantially higher in the same patients, suggesting that self-reactive, TAA-specific effector cells might be preferentially targeted for apoptosis by mechanisms maintaining tolerance to self. Although more than 1 mechanism may underlie the demise of TAA-specific effector cells, the net effect is an alteration in lymphocyte homeostasis and a decrease in antitumor surveillance.
In patients with head and neck cancer, T cells specific for endogenous wt sequence p53 peptides were highly susceptible to spontaneous apoptosis, with upwards of 80% to 90% of circulating p53 tet+CD8+ T cells binding ANX in some patients. Viral peptide-specific T cells were no more sensitive to apoptosis than the tet−CD8+ lymphocytes, which account for the vast majority of CD8+ T cells. Further, apoptosis of viral peptide-specific CD8+ T cells in patients with head and neck cancer was not significantly different from that in normal donors. Thus, the rapid turnover of TAA-specific CD8+ T cells due to spontaneous apoptosis in the patients’ circulation is not global but epitope-dependent. Also, it appears to be swifter and more pervasive than that of viral peptide-specific T cells in patients with head and neck cancer. The fact that tet+CD8+ T-cell frequency for wt p53264 was negative in 3 of 21 patients with head and neck cancer lends support to this conclusion. Although viral-specific CD8+ T cells in the circulation of normal donors also undergo spontaneous apoptosis, the latter occurs at a much-reduced level in comparison to that of the TAA peptide-specific CD8+ T cells in patients with head and neck cancer. On the basis of these data, a rapid turnover of TAA peptide-specific CD8+ T cells in patients with cancer is seen as evidence for substantially altered T-cell homeostasis, since clearance of apoptotic CD8+ T cells is likely to drive lymphocyte kinetics. The influx of naïve T cells from the thymus and their progression to the memory and effector memory compartment are known to occur in response to changes occurring in the periphery, including CD8+ T-cell apoptosis.15 The result of increasingly rapid progression is the paucity of effector T cells, including TAA-specific effector cells that are driven to apoptosis and are thus unable to perform or maintain antitumor functions necessary for tumor control. It is interesting that tet+CD8+ T cells bind ANX even in patients with head and neck cancer who are NED following oncologic therapy. This finding emphasizes the long-lasting nature of cancer-induced disturbances in T-cell homeostasis, and it confirms data previously reported by us.21 It also suggests that aberrations in T-cell homeostasis can occur in the absence of overt tumor growth in patients with NED after oncologic therapy.
The molecular mechanisms driving spontaneous apoptosis of circulating epitope-specific T cells in patients with cancer are unknown. Such mechanisms may involve the Fas/FasL pathway and simply represent propriocidal lymphocyte death, which is greatly exaggerated in cancer.22 They may involve tumor-derived factors responsible for disruption of signal transducing pathways in T cells. Recent discovery of FasL+ microvesicles in the circulation of patients with head and neck cancer, melanoma, and colon carcinoma suggests a potential mechanism for systemic elimination of CD95+ activated CD8+ T cells.23–25 However, neither the tumor origin of microvesicles present in patients’ sera nor their in vivo participation in T-cell apoptosis has been confirmed. Other mechanisms implicating tumor-derived products or molecules have been suggested as well.26,27 It is clear that the observed extensive and preferential elimination of antigen-committed effector T cells, as opposed to tet−CD8+ T cells, is of great importance for immune-mediated control of tumor progression and the success of immune interventions, including antitumor vaccines.
Acknowledgments
Contract grant sponsor: NIH; contract grant numbers: PO1-DE12321, PO1-CA109688.
References
- 1.Seliger B, Cabrera T, Garrido F, Ferrone S. HLA class I antigen abnormalities and immune escape by malignant cells. Sem Cancer Biol. 2002;12:3–13. doi: 10.1006/scbi.2001.0404. [DOI] [PubMed] [Google Scholar]
- 2.Whiteside TL. Tumor-induced death of immune cells: its mechanisms and consequences. Sem Cancer Biol. 2002;12:43–50. doi: 10.1006/scbi.2001.0402. [DOI] [PubMed] [Google Scholar]
- 3.Dworacki G, Meidenbauer N, Kuss I, et al. Decreased ζ expression and apoptosis in CD3+ peripheral blood T lymphocytes of patients with melanoma. Clin Cancer Res. 2001;7:947–957. [PubMed] [Google Scholar]
- 4.Hoffmann TK, Dworacki G, Meidenbauer N, Gooding W, Johnson JT, Whiteside TL. Spontaneous apoptosis of circulating T lymphocytes in patients with head and neck cancer and its clinical importance. Clin Cancer Res. 2002;8:2553–2562. [PubMed] [Google Scholar]
- 5.Saito T, Dworacki G, Gooding W, Lotze MT, Whiteside TL. Spontaneous apoptosis of CD8+ T lymphocytes in peripheral blood of patients with advanced melanoma. Clin Cancer Res. 2000;6:1351–1364. [PubMed] [Google Scholar]
- 6.Kuss I, Donnenberg A, Gooding W, Whiteside TL. Effector CD8+CD45RO−CD27− T cells have signaling defects in patients with head and neck cancer. Br J Cancer. 2003;88:223–230. doi: 10.1038/sj.bjc.6600694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsukishiro T, Donnenberg AD, Whiteside TL. Rapid turnover of the CD8+CD28− T-cell subset of effector cells in the circulation of patients with head and neck cancer. Cancer Immunol Immunother. 2003;52:599–607. doi: 10.1007/s00262-003-0395-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuss I, Hathaway B, Ferris RL, Gooding W, Whiteside TL. Imbalance in absolute counts of T lymphocyte subsets in patients with head and neck cancer and its relation to disease. Cancer Res Clin Oncol. 2003;129:S45. doi: 10.1159/000082506. [DOI] [PubMed] [Google Scholar]
- 9.Hoffmann TK, Donnenberg V, Friebe-Hoffmann U, et al. Competition of peptide-MHC class I tetrameric complexes with anti-CD3 provides evidence for specificity of peptide binding to the TCR complex. Cytometry. 2000;41:321–328. [PubMed] [Google Scholar]
- 10.Hoffmann TK, Donnenberg AD, Finkelstein SD, et al. Frequencies of tetramer+ T cells specific for the wild-type sequence p53264-272 peptide in the circulations of patients with head and neck cancer. Cancer Res. 2002;62:3521–3529. [PubMed] [Google Scholar]
- 11.Darzynkiewicz Z, Bruno S, Del Bino G, Gorczyca MA, Lassota P, Traganos F. Features of apoptotic cells measured by flow cytometry. Cytometry. 1992;13:795–808. doi: 10.1002/cyto.990130802. [DOI] [PubMed] [Google Scholar]
- 12.Albers A, Ferris RL, Kim GG, Chikamatsu K, DeLeo AB, Whiteside TL. Immune responses to p53 in patients with cancer: enrichment in tetramer+ p53 peptide-specific T cells and regulatory T cells at the tumor sites. Cancer Immunol Immunother. 2005;62:670–679. doi: 10.1007/s00262-005-0670-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whiteside TL. Apoptosis of immune cells in the tumor microenvironment and peripheral circulation of patients with cancer: implications for immunotherapy. Vaccine. 2002;20:A46–A51. doi: 10.1016/s0264-410x(02)00387-0. [DOI] [PubMed] [Google Scholar]
- 14.Kim J-W, Tsukishiro T, Johnson JT, Whiteside TL. Expression of pro- and anti-apoptotic proteins in circulating CD8+ T cells of patients with squamous cell carcinoma of the head and neck (SCCHN) Clin Cancer Res. 2004;10:5101–5110. doi: 10.1158/1078-0432.CCR-04-0309. [DOI] [PubMed] [Google Scholar]
- 15.Kuss I, Schaefer C, Godfrey TE, et al. Recent thymic emigrants and subsets of naïve and memory T cell in the circulation of patients with head and neck cancer. Clin Immunol. 2005;116:27–36. doi: 10.1016/j.clim.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 16.Kim J-W, Ferris RL, Whiteside TL. Chemokine receptor 7 (CCR7) expression and protection of circulating CD8+ T lymphocytes from apoptosis. Clin Cancer Res. 2005;11:7901–7910. doi: 10.1158/1078-0432.CCR-05-1346. [DOI] [PubMed] [Google Scholar]
- 17.Rabinowich H, Reichert TE, Kashii Y, Gastman BR, Bell MC, Whiteside TL. Lymphocyte apoptosis induced by Fas Ligand-expressing ovarian carcinoma cells. J Clin Invest. 1998;101:2579–2588. doi: 10.1172/JCI1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gastman BR, Johnson DE, Whiteside TL, Rabinowich H. Caspase-mediated degradation of TCR-ζ chain. Cancer Res. 1999;59:1422–1427. [PubMed] [Google Scholar]
- 19.Reichert TE, Strauss L, Wagner EM, Gooding W, White-side TL. Signaling abnormalities and reduced proliferation of circulating and tumor-infiltrating lymphocytes in patients with oral carcinoma. Clin Cancer Res. 2002;8:3137–3145. [PubMed] [Google Scholar]
- 20.Reichert TE, Rabinowich H, Johnson JT, Whiteside TL. Human immune cells in the tumor microenvironment: mechanisms responsible for signaling and functional defects. J Immunother. 1998;21:295–306. doi: 10.1097/00002371-199807000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Strauss L, Bergmann C, Gooding W, Johnson JT, White-side TL. The frequency and suppressor function of CD4+CD25highFoxp3+ T cells in the peripheral circulation of patients with squamous cell carcinoma of the head and neck. Clin Cancer Res. 2007;13:6301–6311. doi: 10.1158/1078-0432.CCR-07-1403. [DOI] [PubMed] [Google Scholar]
- 22.Whiteside TL. The role of death receptor ligands in shaping the tumor microenvironment. Immunol Invest. 2007;36:25–46. doi: 10.1080/08820130600991893. [DOI] [PubMed] [Google Scholar]
- 23.Kim J-W, Wieckowski E, Taylor DD, Reichert TE, Watkins S, Whiteside TL. FasL+ membraneous vesicles isolated from sera of patients with oral cancer induce apoptosis of activated T lymphocytes. Clin Cancer Res. 2005;11:1010–1020. [PubMed] [Google Scholar]
- 24.Andreola G, Rivoltini L, Castelli C, et al. Induction of lymphocyte apoptosis by tumor cell secretion of FasL-bearing microvesicles. J Exp Med. 2002;195:1303–1316. doi: 10.1084/jem.20011624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huber V, Fais S, Iero M, et al. Human colorectal cancer cells induce T-cell death through release of proapoptotic microvesicles: role in immune escape. Gastroenterology. 2005;128:1796–1804. doi: 10.1053/j.gastro.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 26.Whiteside TL, Campoli M, Ferrone S. Tumor induced immune suppression and immune escape: mechanisms and possible solutions. In: Nagorsen E, Marincola F, editors. Analyzing T cell responses. Springer; Dordrecht, The Netherlands: 2005. pp. 43–82. [Google Scholar]
- 27.Critchley-Thorne RJ, Yan N, Nacu S, Weber J, Holmes SP, Lee PP. Down-regulation of the interferon signaling pathway in T lymphocytes from patients with metastatic melanoma. PLoS Med. 2007;4:e176. doi: 10.1371/journal.pmed.0040176. [DOI] [PMC free article] [PubMed] [Google Scholar]


